Evaluation of Antimicrobial, Antiadhesive and Co-Aggregation Activity of a Multi-Strain Probiotic Composition against Different Urogenital Pathogens
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial and Antiadhesive Efficacy on HVE
2.2. Antibacterial and Antiadhesive Efficacy on HBE
2.3. Probiotic Microorganisms and Vaginal Pathogens Co-Aggregation
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. 3D Reconstructed Human Epithelia
4.3. Antimicrobial and Antiadhesive Efficacy on Reconstructed HVE
4.4. Antimicrobial and Antiadhesive Efficacy on HBE
4.5. Co-Aggregation Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, D.H. The microbiota of the vagina and its influence on women’s health and disease. Am. J. Med. Sci. 2012, 343, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farage, M.; Maibach, H. Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. 2006, 273, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, A.E. The Vaginal Microbiota and Urinary Tract Infection. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzny, C.A.; Łaniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef]
- Witkin, S.S.; Mendes-Soares, H.; Linhares, I.M.; Jayaram, A.; Ledger, W.J.; Forney, L.J. Influence of vaginal bacteria and D- and L-lactic acid isomers on vaginal extracellular matrix metalloproteinase inducer: Implications for protection against upper genital tract infections. mBio 2013, 4, e00460-13. [Google Scholar] [CrossRef] [Green Version]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 255. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Cortés, C.; Suarez, H.; Buitrago, G.; Nero, L.A.; Todorov, S.D. Enhanced Bacteriocin Production by Pediococcus pentosaceus 147 in Co-culture With Lactobacillus plantarum LE27 on Cheese Whey Broth. Front. Microbiol. 2018, 9, 2952. [Google Scholar] [CrossRef] [Green Version]
- Coman, M.M.; Verdenelli, M.C.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Caspani, M.; Mondello, F.; Cresci, A. In vitro evaluation on HeLa cells of protective mechanisms of probiotic lactobacilli against Candida clinical isolates. J. Appl. Microbiol. 2015, 119, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Vagios, S.; Hesham, H.; Mitchell, C. Understanding the potential of lactobacilli in recurrent UTI prevention. Microb. Pathog. 2020, 148, 104544. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [Green Version]
- Hattiholi, A.; Tendulkar, S.; Dodamani, S. An Update on the Probiotic Usage in Bacterial Vaginosis. In Probiotic Research in Therapeutics; Springer: Berlin/Heidelberg, Germany, 2021; pp. 191–213. [Google Scholar]
- Minardi, D.; d’Anzeo, G.; Cantoro, D.; Conti, A.; Muzzonigro, G. Urinary tract infections in women: Etiology and treatment options. Int. J. Gen. Med. 2011, 4, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Aldunate, M.; Srbinovski, D.; Hearps, A.C.; Latham, C.F.; Ramsland, P.A.; Gugasyan, R.; Cone, R.A.; Tachedjian, G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015, 6, 164. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Tariq, N.; Jaffery, T.; Ayub, R.; Alam, A.Y.; Javid, M.H.; Shafique, S. Frequency and antimicrobial susceptibility of aerobic bacterial vaginal isolates. J. Coll. Physicians Surg Pak. 2006, 16, 196–199. [Google Scholar]
- Córdoba, S.; Taverna, C.; Vivot, W.; Szusz, W.; Vivot, M.; Isla, G.; Davel, G. Emergence of Resistance to Fluconazole in Candida albicans Isolated From Vaginal Discharge. Curr. Fungal. Infect. Rep. 2018, 12, 155–160. [Google Scholar] [CrossRef]
- Vicariotto, F.; Mogna, L.; Del Piano, M. Effectiveness of the two microorganisms Lactobacillus fermentum LF15 and Lactobacillus plantarum LP01, formulated in slow-release vaginal tablets, in women affected by bacterial vaginosis: A pilot study. J. Clin. Gastroenterol. 2014, 48 (Suppl. S1), S106–S112. [Google Scholar] [CrossRef]
- Sobel, J.D.; Sobel, R. Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin. Pharmacother. 2018, 19, 971–977. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Sobel, J.D. Current Treatment of Bacterial Vaginosis-Limitations and Need for Innovation. J. Infect. Dis. 2016, 214 (Suppl. S1), S14–S20. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Park, Y.J. Probiotics in the Prevention and Treatment of Postmenopausal Vaginal Infections: Review Article. J. Menopausal. Med. 2017, 23, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef] [PubMed]
- De Seta, F.; Parazzini, F.; De Leo, R.; Banco, R.; Maso, G.P.; De Santo, D.; Sartore, A.; Stabile, G.; Inglese, S.; Tonon, M.; et al. Lactobacillus plantarum P17630 for preventing Candida vaginitis recurrence: A retrospective comparative study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.; Silva, J.; Teixeira, P. The role of Lactobacilli and probiotics in maintaining vaginal health. Arch. Gynecol. Obstet. 2014, 289, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Campana, R.; van Hemert, S.; Baffone, W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Measurement of aggregation properties between probiotics and pathogens: In vitro evaluation of different methods. J. Microbiol. Methods 2007, 71, 71–74. [Google Scholar] [CrossRef]
- Presti, I.; D’Orazio, G.; Labra, M.; La Ferla, B.; Mezzasalma, V.; Bizzaro, G.; Giardina, S.; Michelotti, A.; Tursi, F.; Vassallo, M.; et al. Evaluation of the probiotic properties of new Lactobacillus and Bifidobacterium strains and their in vitro effect. Appl. Microbiol. Biotechnol. 2015, 99, 5613–5626. [Google Scholar] [CrossRef]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Sandionigi, A.; La Ferla, B.; Schiano, I.; Michelotti, A.; Nobile, V.; Labra, M.; Di Gennaro, P. A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation. Biomed. Res. Int. 2016, 2016, 4740907. [Google Scholar] [CrossRef] [Green Version]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Boccarusso, M.; Di Gennaro, P.; Schiano, I.; Michelotti, A.; Labra, M. Orally administered multispecies probiotic formulations to prevent uro-genital infections: A randomized placebo-controlled pilot study. Arch. Gynecol. Obstet. 2017, 295, 163–172. [Google Scholar] [CrossRef]
- Barrientos-Durán, A.; Fuentes-López, A.; de Salazar, A.; Plaza-Díaz, J.; García, F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients 2020, 12, 419. [Google Scholar] [CrossRef] [Green Version]
- Scillato, M.; Spitale, A.; Mongelli, G.; Privitera, G.F.; Mangano, K.; Cianci, A.; Stefani, S.; Santagati, M. Antimicrobial properties of Lactobacillus cell-free supernatants against multidrug-resistant urogenital pathogens. Microbiologyopen 2021, 10, e1173. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef] [Green Version]
- Thänert, R.; Reske, K.A.; Hink, T.; Wallace, M.A.; Wang, B.; Schwartz, D.J.; Seiler, S.; Cass, C.; Burnham, C.A.; Dubberke, E.R.; et al. Comparative Genomics of Antibiotic-Resistant Uropathogens Implicates Three Routes for Recurrence of Urinary Tract Infections. mBio 2019, 10, e01977-19. [Google Scholar] [CrossRef] [Green Version]
- Meštrović, T.; Matijašić, M.; Perić, M.; Čipčić Paljetak, H.; Barešić, A.; Verbanac, D. The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics 2020, 11, 7. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. Female Gut and Genital Tract Microbiota-Induced Crosstalk and Differential Effects of Short-Chain Fatty Acids on Immune Sequelae. Front. Immunol. 2020, 11, 2184. [Google Scholar] [CrossRef]
- Murina, F.; Vicariotto, F. Evaluation of an Orally Administered Multistrain Probiotic Supplement in Reducing Recurrences Rate of Bacterial Vaginosis: A Clinical and Microbiological Study. Adv. Inf. Dis. 2019, 9, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Javanshir, N.; Hosseini, G.N.G.; Sadeghi, M.; Esmaeili, R.; Satarikia, F.; Ahmadian, G.; Allahyari, N. Evaluation of the Function of Probiotics, Emphasizing the Role of their Binding to the Intestinal Epithelium in the Stability and their Effects on the Immune System. Biol. Proced. Online 2021, 23, 23. [Google Scholar] [CrossRef]
- Liévin-Le Moal, V.; Servin, A.L. Anti-infective activities of lactobacillus strains in the human intestinal microbiota: From probiotics to gastrointestinal anti-infectious biotherapeutic agents. Clin. Microbiol. Rev. 2014, 27, 167–199. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, R.J.; Nygaard, M. Interbacterial aggregation of plaque bacteria. Arch. Oral Biol. 1970, 15, 1397–1400. [Google Scholar] [CrossRef]
- Vlková, E.; Rada, V.; Smehilová, M.; Killer, J. Auto-aggregation and co-aggregation ability in Bifidobacteria and Clostridia. Folia. Microbiol. 2008, 53, 263–269. [Google Scholar] [CrossRef]
- Atassi, F.; Servin, A.L. Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC933 and vaginal strain Lactobacillus gasseri KS120.1 against enteric, uropathogenic and vaginosis-associated pathogens. FEMS Microbiol. Lett. 2010, 304, 29–38. [Google Scholar]
- Bujnáková, D.; Vlková, E.; Rada, V.; Kmet, V. Aggregation of Lactobacilli and Bifidobacteria with Escherichia coli O157. Folia. Microbiol. 2004, 49, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Kos, B.; Susković, J.; Vuković, S.; Simpraga, M.; Frece, J.; Matosić, S. Adhesion and aggregation ability of probiotic strain Lactobacillus acidophilus M92. J. Appl. Microbiol. 2003, 94, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Adhesion and aggregation properties of probioticand pathogen strains. Eur. Food Res. Technol. 2008, 226, 1065–1073. [Google Scholar] [CrossRef]
- Sengupta, R.; Altermann, E.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Roy, N.C. The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediat. Inflamm. 2013, 2013, 237921. [Google Scholar] [CrossRef]
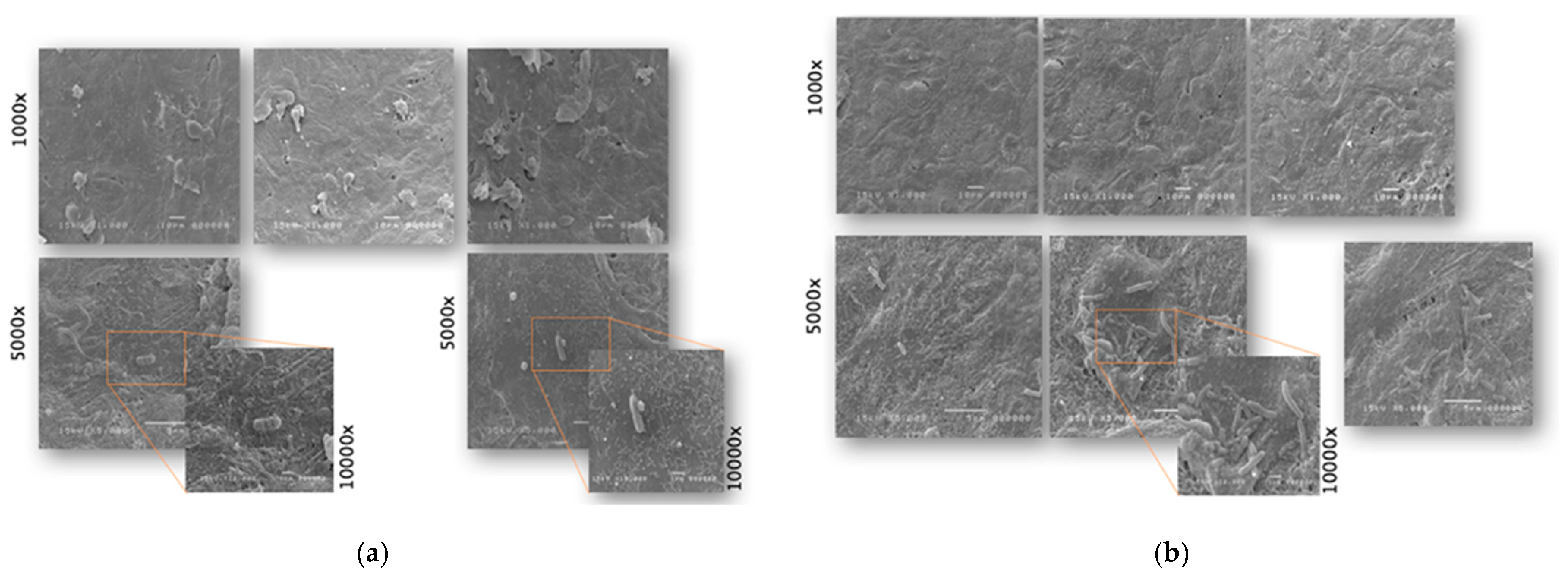
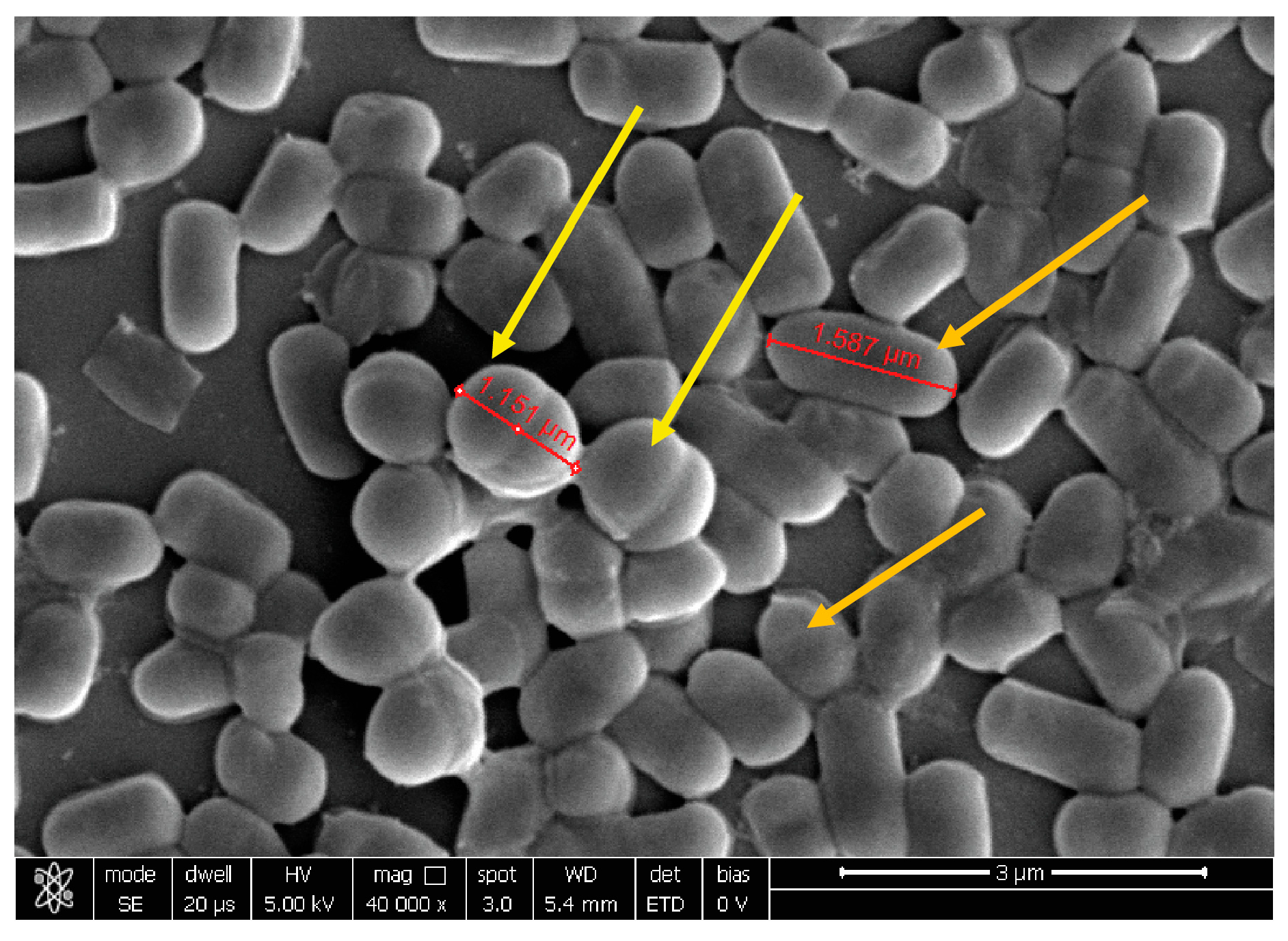
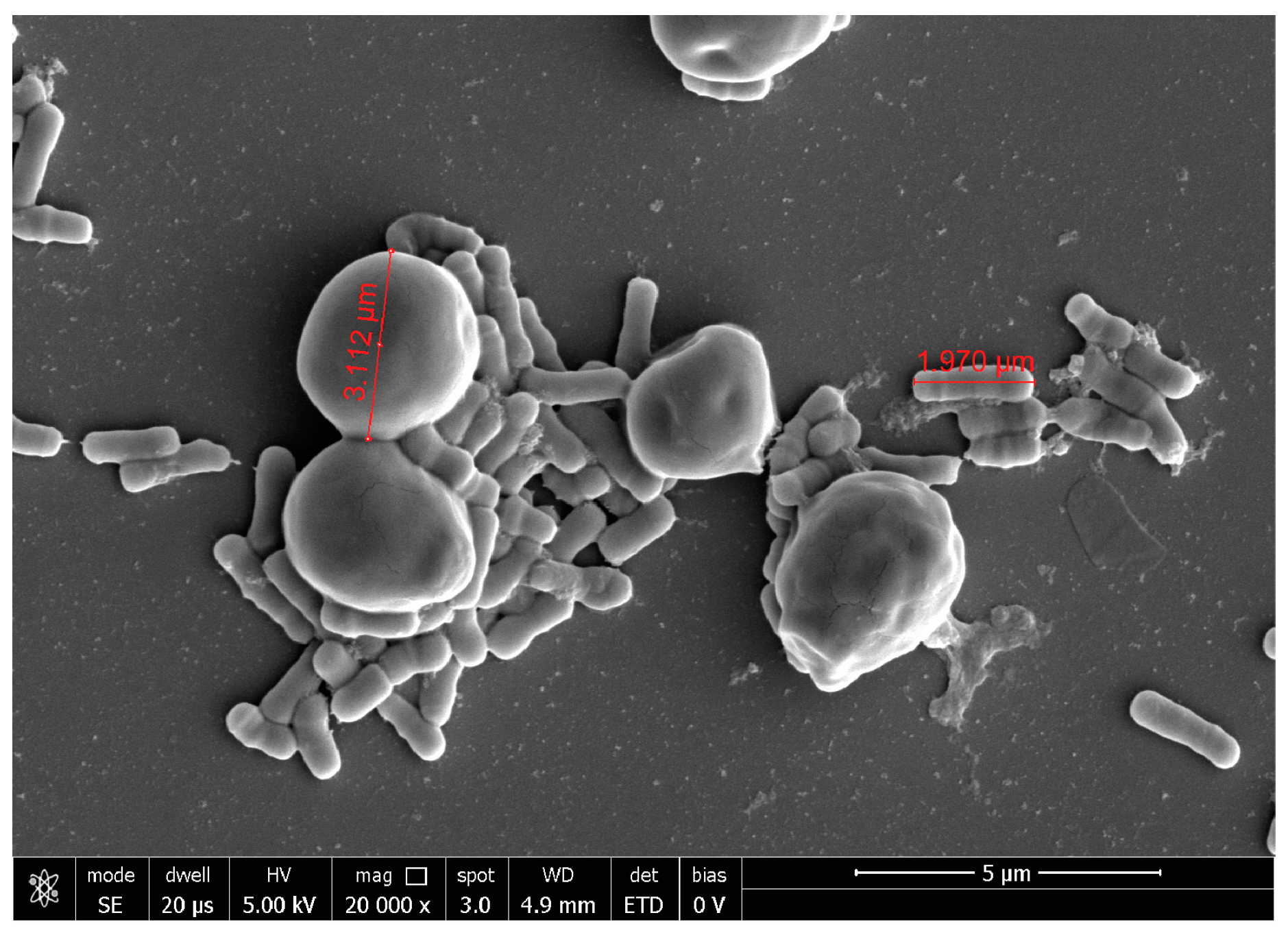


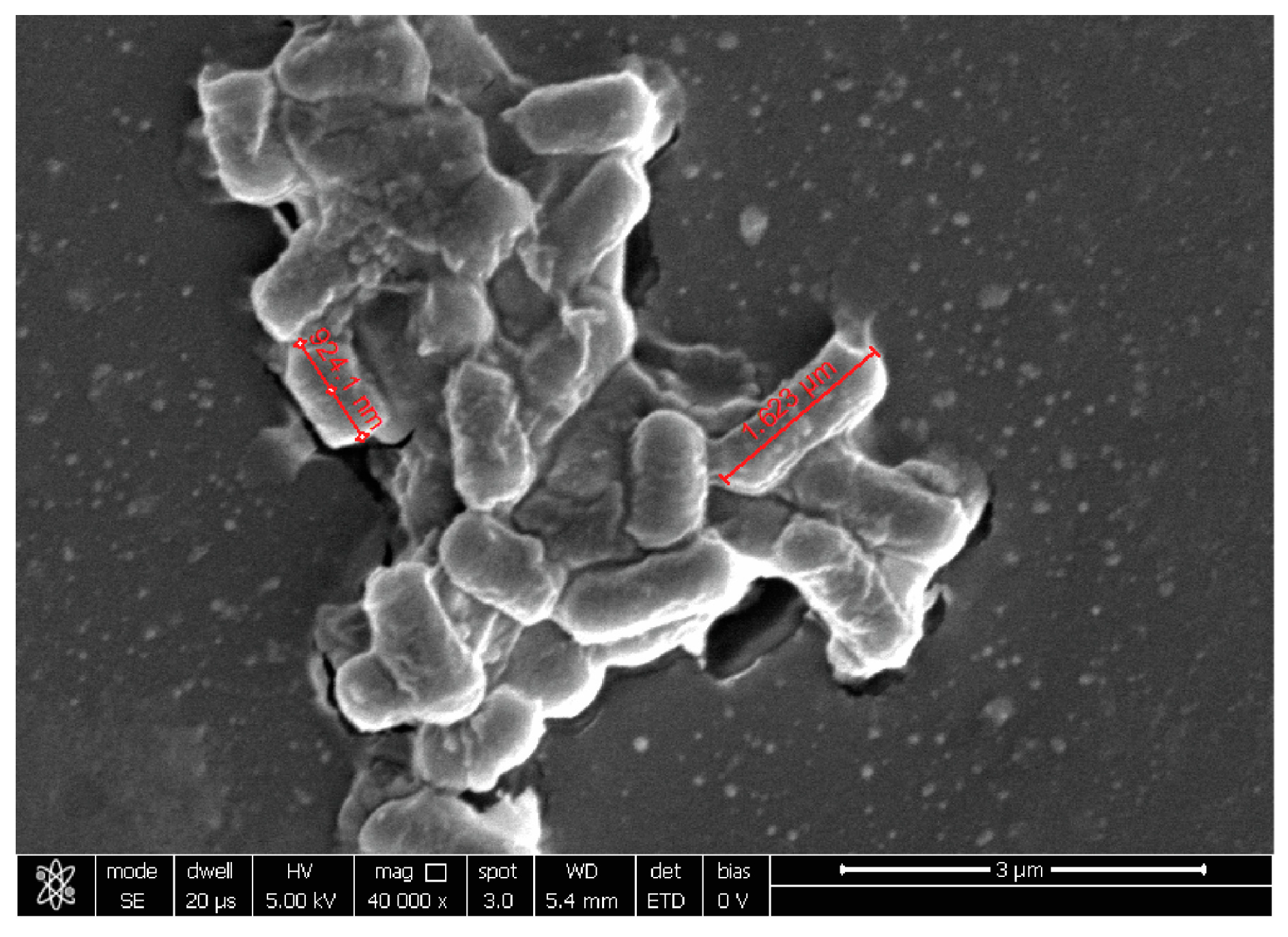
| Protocol A | SynBalance® Femme | C. glabrata | SynBalance® Femme | N. gonhorreae | SynBalance® Femme | T. vaginalis |
|---|---|---|---|---|---|---|
| Initial count | 7.74 | 7.75 | 7.47 | 7.81 | 7.96 | 7.49 |
| Non-adhered microrganism (washing) | 6.68 | 7.75 | 0 | 0 | 4.60 | 0 |
| Adhered microrganism (homogenates) | 7.30 | 5.43 | 6.10 | 4.69 | 6.68 | 0 |
| Microrganism penetrated into the epithelium (medium) | 0 | 0 | 0 | 0 | 0 | 0 |
| Epithelium viability (%) | 99.0 | - | 98.7 | - | 98.7 | - |
| Epithelium integrity (TEER, Ω) | 173.3 | - | 175.4 | - | 173.8 | - |
| Protocol B | SynBalance® Femme | C. glabrata | SynBalance® Femme | N. gonhorreae | SynBalance® Femme | T. vaginalis |
|---|---|---|---|---|---|---|
| Initial count | 7.74 | 7.75 | 7.47 | 7.81 | 7.96 | 7.49 |
| Non-adhered microrganism (washing) | 6.20 | 6.17 | 0 | 0 | 4.84 | 2.17 |
| Adhered microrganism (homogenated) | 7.83 | 0 | 5.95 | 5.14 | 3.95 | 3.48 |
| Microrganism penetrated into epithelium (medium) | 0 | 0 | 0 | 0 | 0 | 0 |
| Epithelium viability (%) | 99.1 | - | 96.8 | - | 95.1 | - |
| Epithelium integrity (TEER, Ω) | 170.7 | - | 171.0 | - | 170.9 | - |
| Protocol A | Protocol B | |||
|---|---|---|---|---|
| APICAL log10 CFU/mL | HOMOGENATE log10 CFU/mL | APICAL log10 CFU/mL | HOMOGENATE log10 CFU/mL | |
| E. coli | 7.18 | 5.90 | 6.56 | 5.96 |
| B. animalis subsp. lactis BL050 | 0.00 | 4.38 | 6.38 | 4.08 |
| L. plantarum PBS067 | 4.63 | 0.00 | 6.63 | 4.93 |
| L. rhamnosus LRH020 | 5.11 | 0.00 | 5.45 | 0.00 |
| Protocol A | Protocol B | |||
|---|---|---|---|---|
| Baseline | T = 20 h | Baseline | T = 20 h | |
| Negative control | 71.00 ± 0.94 | 76.25 ± 0.35 | 71.08 ± 1.53 | 75.58 ± 0.59 |
| E. coli | 71.08 ± 1.77 | 73.83 ± 1.41 | 71.42 ± 1.06 | 69.92 ± 3.18 |
| B. animalis subsp. lactis BL050 | - | 71.58 ± 4.12 | - | 68.33 ± 1.65 |
| L. plantarum PBS067 | - | 73.08 ± 2.24 | - | 70.75 ± 5.30 |
| L. rhamnosus LRH020 | - | 69.25 ± 1.06 | - | 72.17 ± 1.18 |
| Protocol A | Protocol B | |||
|---|---|---|---|---|
| Strain Tested | APICAL | HOMOGENATE | APICAL | HOMOGENATE |
| B. animalis supsp. lactis BL050 | >7 log10 (total depletion) | >1 log10 | No variation | >1 log10 |
| L. plantarum PBS067 | >2 log10 | >5 log10 (total depletion) | No variation | 1 log10 |
| L. rhamnosus LRH020 | >1 log10 | >5 log10 (total depletion) | >1 log10 | >5 log10 (total depletion) |
| Probiotic Strain/Formulation | Pathogen | Precipitate Formation | Flocculation Time |
|---|---|---|---|
| B. animalis subsp. lactis BL050 | Gardnerella vaginalis | + | >30 min |
| Escherichia coli | + | >30 min | |
| Candida albicans | ++ | 15 < min <30 | |
| L. plantarum PBS067 | Gardnerella vaginalis | − | >30 min |
| Escherichia coli | − | >30 min | |
| Candida albicans | − | >30 min | |
| L. rhamnosus LRH020 | Gardnerella vaginalis | +++ | <15 min |
| Escherichia coli | +++ | <15 min | |
| Candida albicans | +++ | <15 min | |
| SynBalance® Femme | Gardnerella vaginalis | +++ | <15 min |
| Escherichia coli | +++ | <15 min | |
| Candida albicans | +++ | <15 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malfa, P.; Brambilla, L.; Giardina, S.; Masciarelli, M.; Squarzanti, D.F.; Carlomagno, F.; Meloni, M. Evaluation of Antimicrobial, Antiadhesive and Co-Aggregation Activity of a Multi-Strain Probiotic Composition against Different Urogenital Pathogens. Int. J. Mol. Sci. 2023, 24, 1323. https://doi.org/10.3390/ijms24021323
Malfa P, Brambilla L, Giardina S, Masciarelli M, Squarzanti DF, Carlomagno F, Meloni M. Evaluation of Antimicrobial, Antiadhesive and Co-Aggregation Activity of a Multi-Strain Probiotic Composition against Different Urogenital Pathogens. International Journal of Molecular Sciences. 2023; 24(2):1323. https://doi.org/10.3390/ijms24021323
Chicago/Turabian StyleMalfa, Patrizia, Laura Brambilla, Silvana Giardina, Martina Masciarelli, Diletta Francesca Squarzanti, Federica Carlomagno, and Marisa Meloni. 2023. "Evaluation of Antimicrobial, Antiadhesive and Co-Aggregation Activity of a Multi-Strain Probiotic Composition against Different Urogenital Pathogens" International Journal of Molecular Sciences 24, no. 2: 1323. https://doi.org/10.3390/ijms24021323
APA StyleMalfa, P., Brambilla, L., Giardina, S., Masciarelli, M., Squarzanti, D. F., Carlomagno, F., & Meloni, M. (2023). Evaluation of Antimicrobial, Antiadhesive and Co-Aggregation Activity of a Multi-Strain Probiotic Composition against Different Urogenital Pathogens. International Journal of Molecular Sciences, 24(2), 1323. https://doi.org/10.3390/ijms24021323







