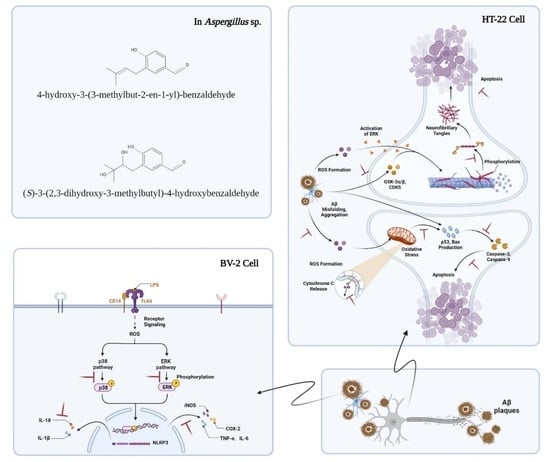
The Mechanism of Two Benzaldehydes from Aspergillus terreus C23-3 Improve Neuroinflammatory and Neuronal Damage to Delay the Progression of Alzheimer’s Disease
Abstract
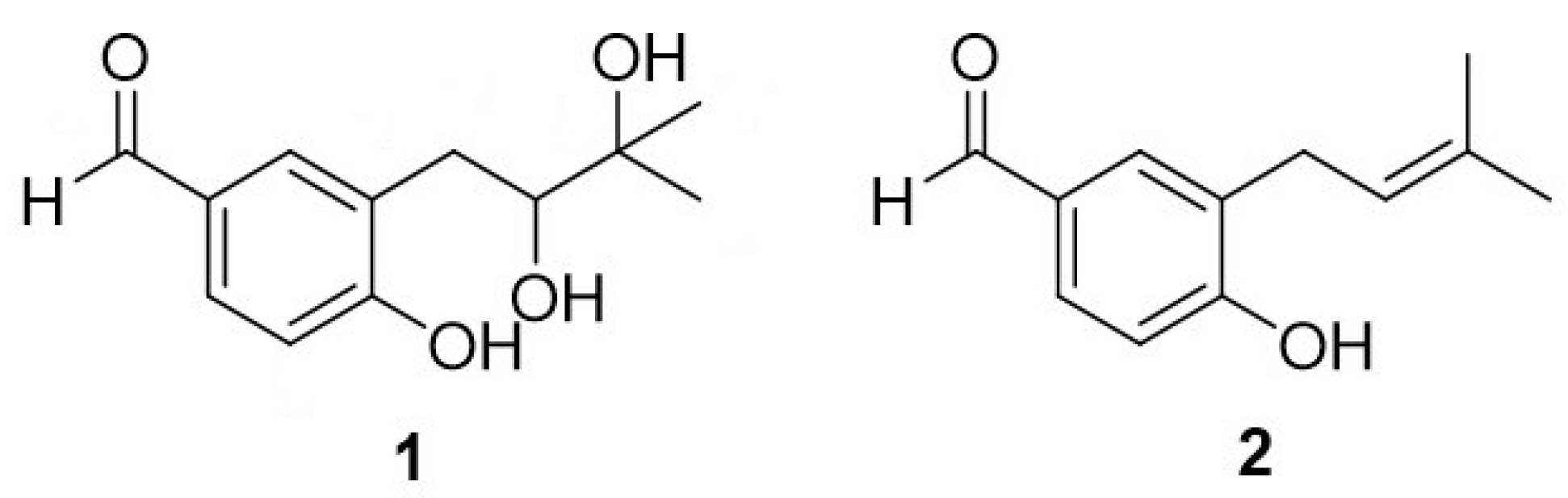
1. Introduction
2. Results
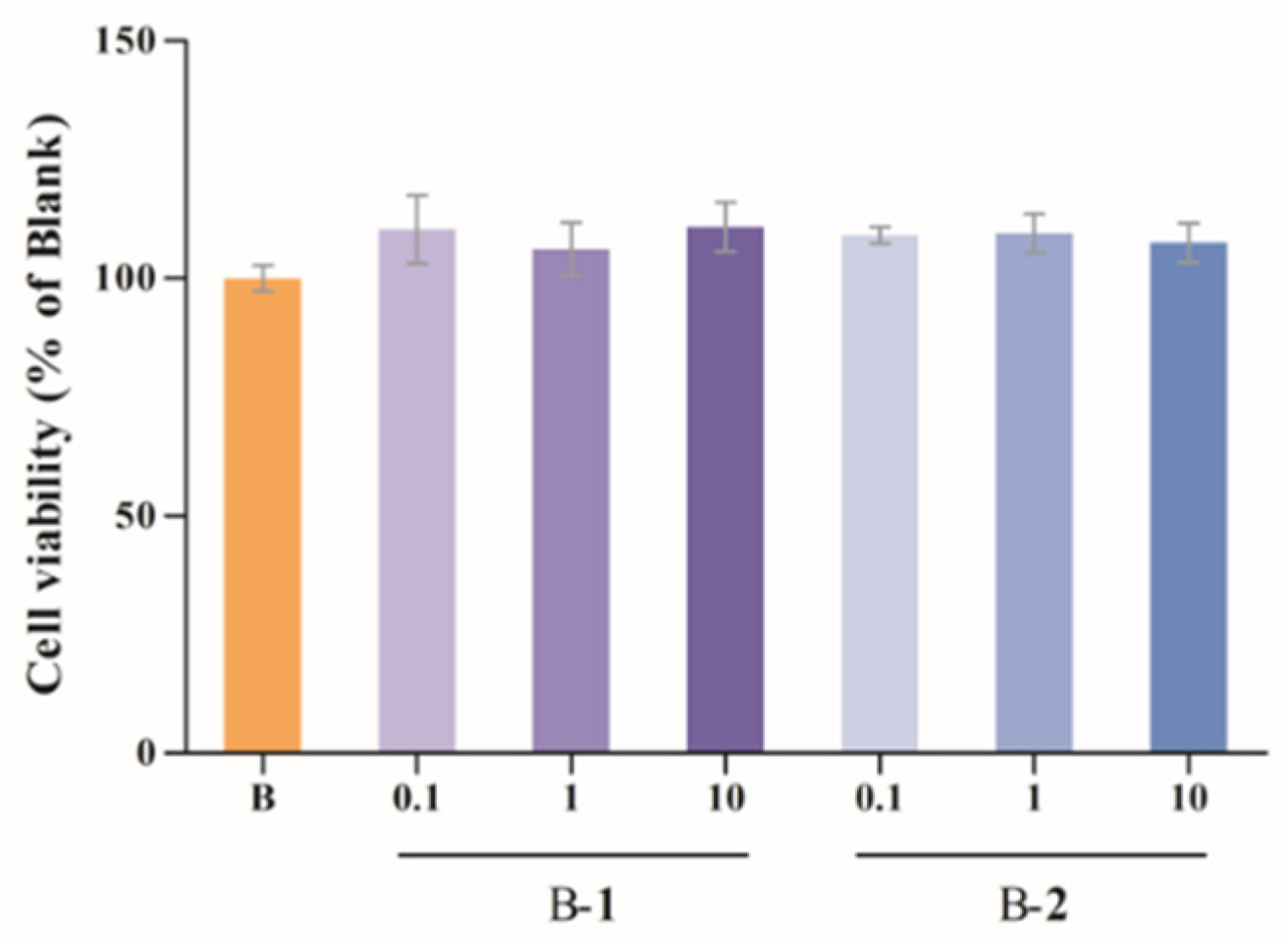
2.1. Cytotoxic Assessment
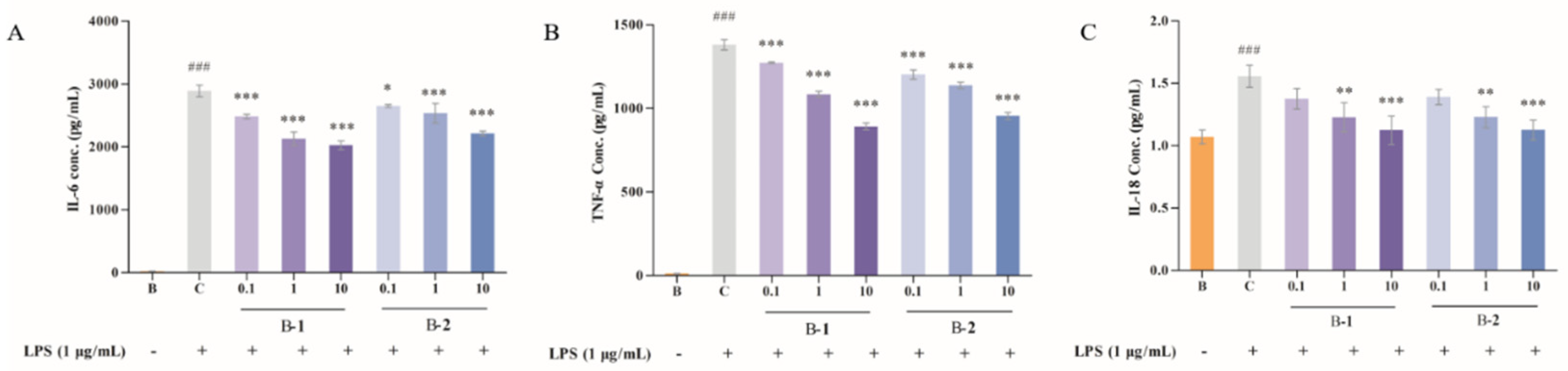
2.2. Effect of Two Benzaldehydes (1 and 2) on Levels of IL-6, TNF-α and IL-18
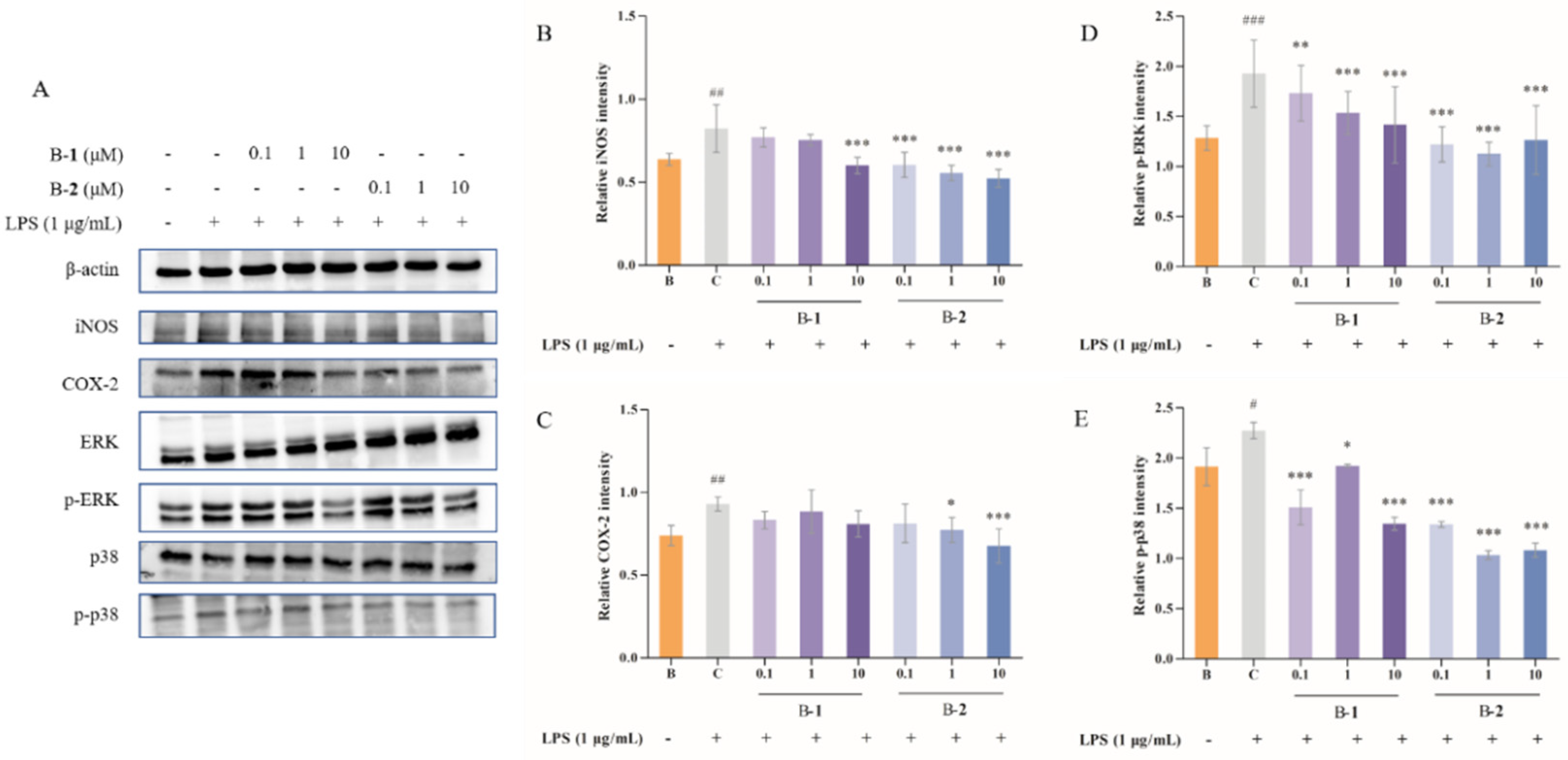
2.3. Effect of Two Benzaldehydes (1 and 2) on Expression of iNOS, COX-2 and MAPKs Signaling Pathways
2.4. Effect of Benzaldehydes 2 on ROS Production
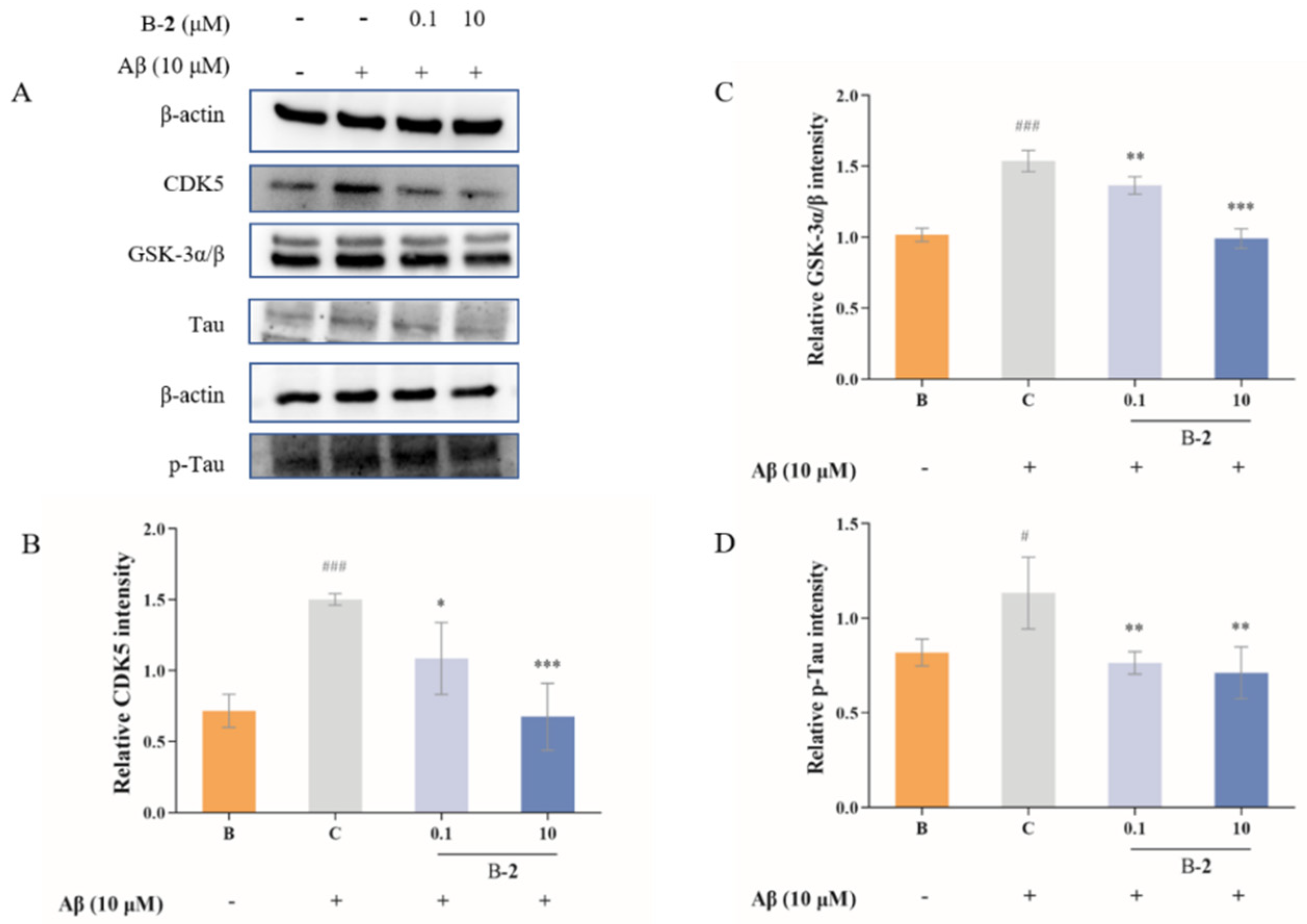
2.5. Effect of Benzaldehydes 2 on Tau Related Pathway
2.6. Effect of Benzaldehyde 2 on Mitochondrial Membrane Potential
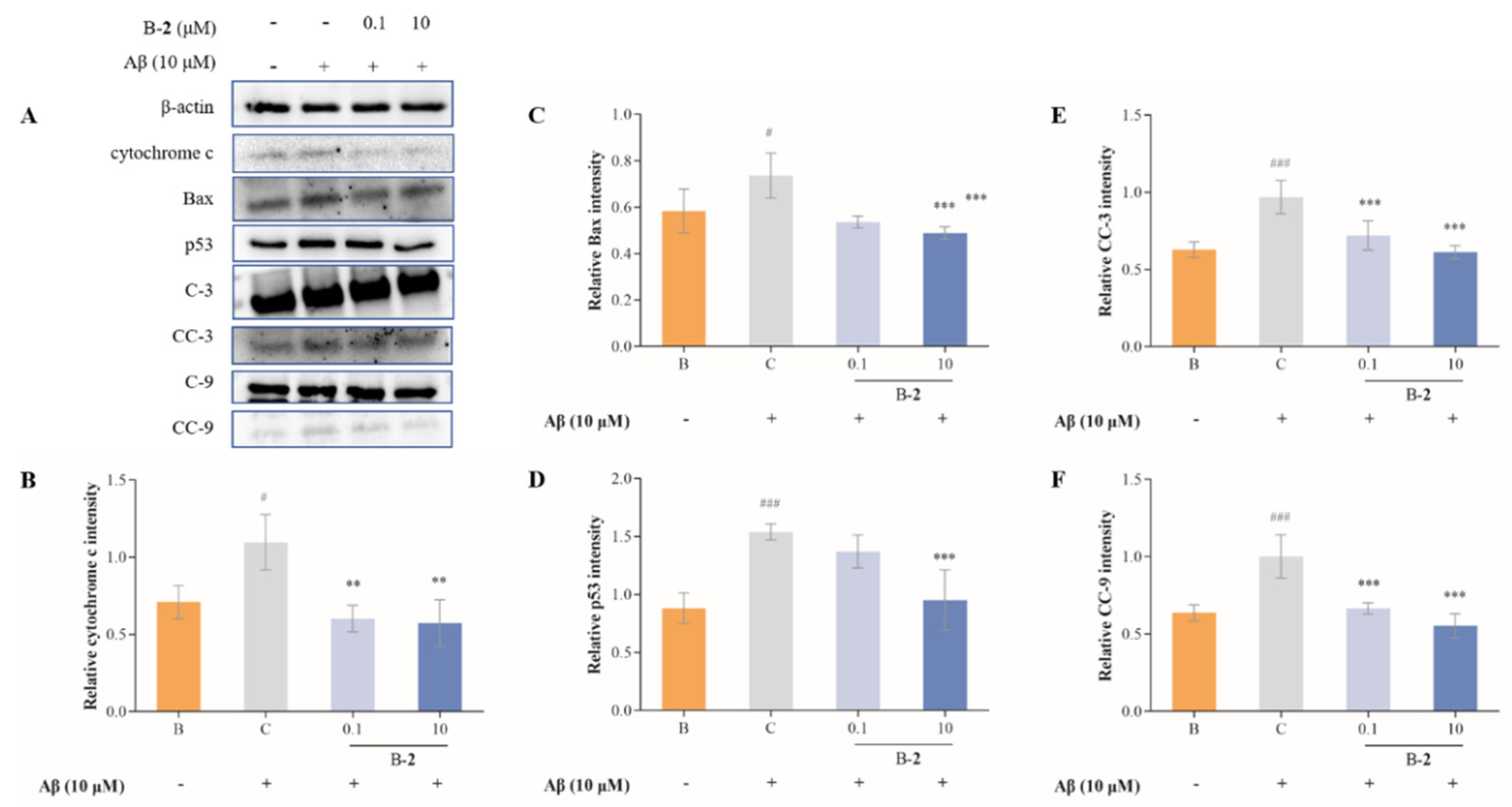
2.7. Effect of Benzaldehydes 2 on Expression of Cytochrome C and Caspase Family Related Pathway
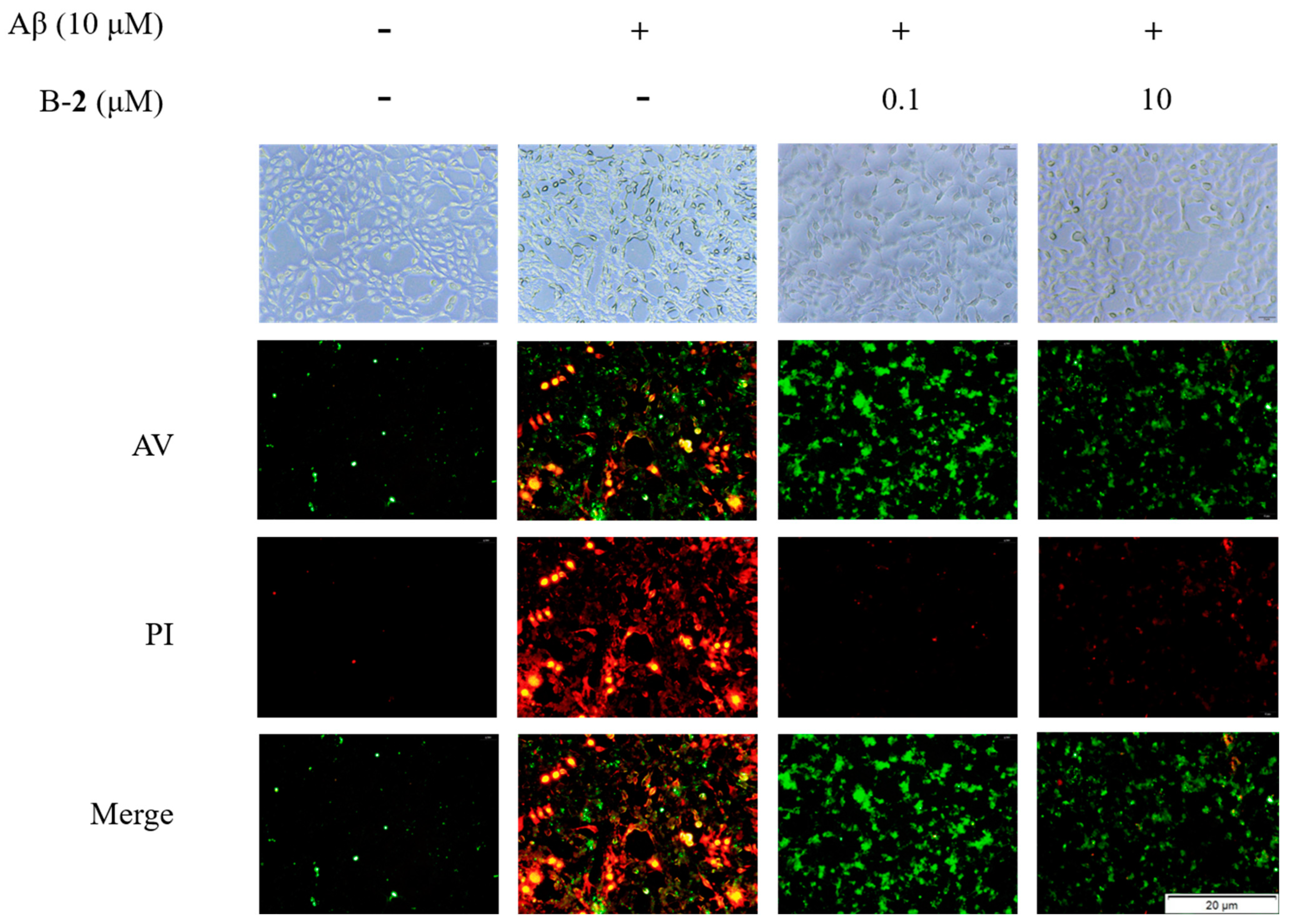
2.8. Effect of Benzaldehyde 2 on Cell Cycle and Apoptosis
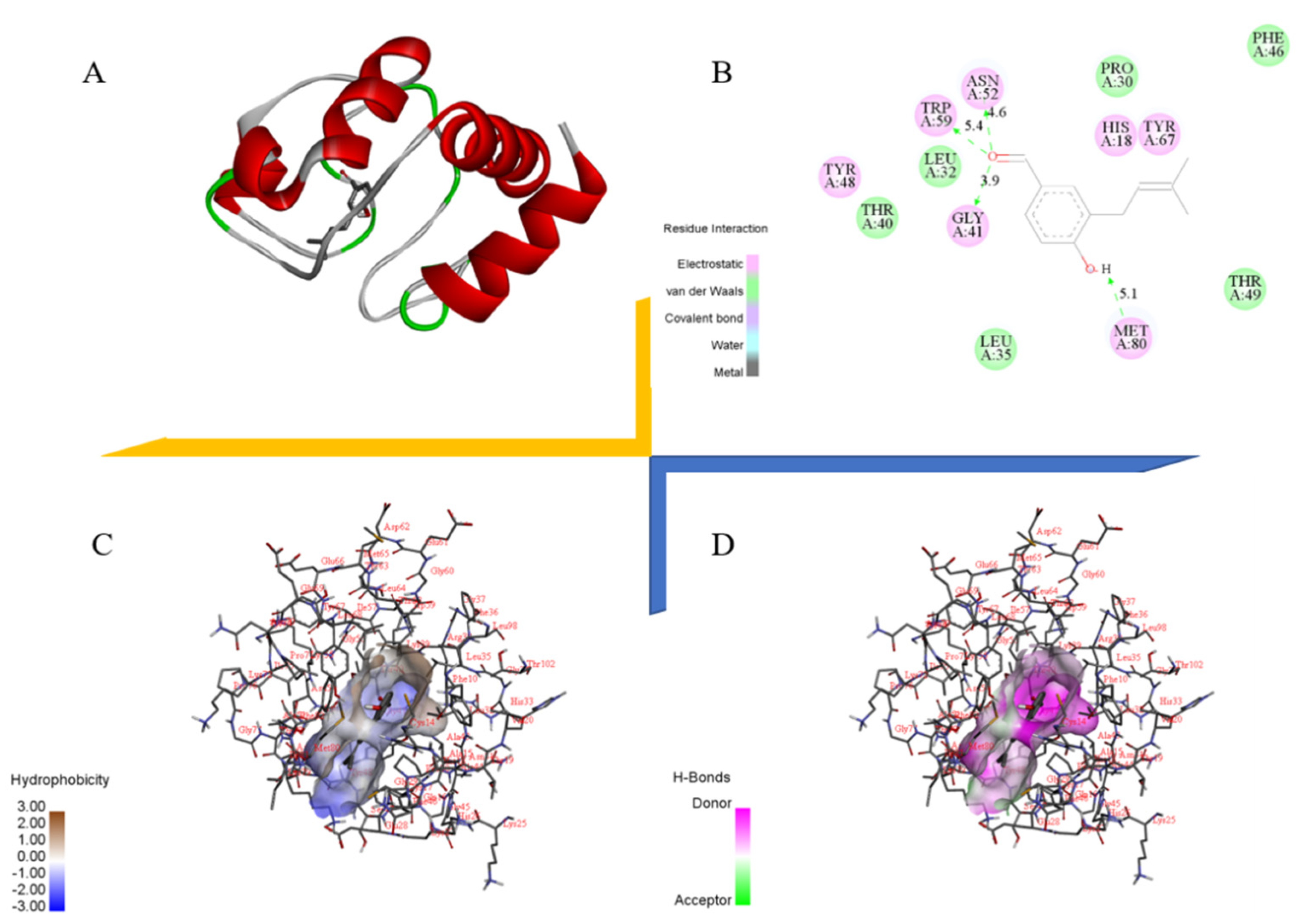
2.9. Docking Study of Cytochrome C with Benzaldehyde 2
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Cell Culture
4.3. Cytotoxic Assessment
4.4. IL-6, TNF-α and IL-18 Determination
4.5. Western Blotting
4.6. ROS Determination
4.7. Measurement of Mitochondrial Membrane Potential
4.8. Cell Cycle and Apoptosis Analysis
4.9. Molecular Docking
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holtzman, D.M.; Morris, J.C.; Goate, A.M. Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 2011, 3, 77. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Castellani, R.J.; Rolston, R.K.; Smith, M.A. Alzheimer disease. Disease-a-Month 2010, 56, 484–546. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Corbett, A.; Ballard, C. Emerging treatments for Alzheimer’s disease for non-amyloid and non-tau targets. Expert Rev. Neurother. 2017, 17, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Liu, F.; Gong, C.-X.; Grundke-Iqbal, I. Tau in Alzheimer Disease and Related Tauopathies. Curr. Alzheimer Res. 2010, 7, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Venkatramani, A.; Panda, D. Regulation of neuronal microtubule dynamics by tau: Implications for tauopathies. Int. J. Biol. Macromol. 2019, 133, 473–483. [Google Scholar] [CrossRef]
- Marcus, J.N.; Schachter, J. Targeting post-translational modifications on tau as a therapeutic strategy for Alzheimer’s disease. J. Neurogenet. 2011, 25, 127–133. [Google Scholar] [CrossRef]
- Chen, J.X.; Yan, S.S. Role of mitochondrial amyloid-β in Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, S569–S578. [Google Scholar] [CrossRef]
- Swerdlow, R.H. The Neurodegenerative Mitochondriopathies. J. Alzheimer’s Dis. 2009, 17, 737–751. [Google Scholar] [CrossRef]
- Suzuki, A.; Tsutomi, Y.; Yamamoto, N.; Shibutani, T.; Akahane, K. Mitochondrial Regulation of Cell Death: Mitochondria Are Essential for Procaspase 3-p21 Complex Formation to Resist Fas-Mediated Cell Death. Mol. Cell. Biol. 1999, 19, 3842–3847. [Google Scholar] [CrossRef]
- McGeer, P.L.; Itagaki, S.; Tago, H.; McGeer, E.G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 1987, 79, 195–200. [Google Scholar] [CrossRef]
- Itagaki, S.; McGeer, P.L.; Akiyama, H.; Zhu, S.; Selkoe, D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J. Neuroimmunol. 1989, 24, 173–182. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Rogers, J. Inflammation in Alzheimer Disease—A Brief Review of the Basic Science and Clinical Literature. Cold Spring Harb. Perspect. Med. 2012, 2, a006346. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Mandrekar-Colucci, S.; Landreth, G.E. Microglia and Inflammation in Alzheimers Disease. CNS Neurol. Disord. Drug Targets 2012, 9, 156–167. [Google Scholar] [CrossRef]
- Tay, T.L.; Hagemeyer, N.; Prinz, M. The force awakens: Insights into the origin and formation of microglia. Curr. Opin. Neurobiol. 2016, 39, 30–37. [Google Scholar] [CrossRef]
- Kim, B.W.; Koppula, S.; Kumar, H.; Park, J.Y.; Kim, I.W.; More, S.V.; Kim, I.S.; Han, S.D.; Kim, S.K.; Yoon, S.H.; et al. α-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology 2015, 97, 46–57. [Google Scholar] [CrossRef]
- Chen, M.; Liang, J.; Wang, Y.; Liu, Y.; Zhou, C.; Hong, P.; Zhang, Y.; Qian, Z.J. A new benzaldehyde from the coral-derived fungus Aspergillus terreus C23-3 and its anti-inflammatory effects via suppression of MAPK signaling pathway in RAW264.7 cells. J. Zhejiang Univ. Sci. B 2022, 23, 230–240. [Google Scholar] [CrossRef]
- Yang, J.M.; Liu, Y.Y.; Yang, W.C.; Ma, X.X.; Zhang, Y. An anti-inflammatory isoflavone from soybean inoculated with a marine fungus Aspergillus terreus C23-3. Biosci. Biotechnol. Biochem. 2020, 84, 1546–1553. [Google Scholar] [CrossRef]
- Nie, Y.; Yang, W.; Liu, Y.; Yang, J.; Lei, X.; Gerwick, W.H.; Zhang, Y. Acetylcholinesterase inhibitors and antioxidants mining from marine fungi: Bioassays, bioactivity coupled LC–MS/MS analyses and molecular networking. Mar. Life Sci. Technol. 2020, 2, 386–397. [Google Scholar] [CrossRef]
- Yang, J.M.; Yang, W.C.; Liu, Y.Y.; Nie, Y.Y.; Lei, X.L.; Zhang, Y. Influence of chemical induction on the secondary metabolites and biological activities of a marine-derived fungal strain Aspergillus terreus C23-3. Microbiol. China 2019, 46, 441–452. [Google Scholar] [CrossRef]
- Machado, F.P.; Kumla, D.; Pereira, J.A.; Sousa, E.; Kijjoa, A. Prenylated phenylbutyrolactones from cultures of a marine sponge-associated fungus Aspergillus flavipes KUFA1152. Phytochemistry 2021, 185, 112709. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Cui, X.; Lee, D.-S.; Ko, W.; Sohn, J.; Yim, J.; An, R.-B.; Kim, Y.-C.; Oh, H. Inhibitory effects of benzaldehyde derivatives from the marine fungus eurotium sp. SF-5989 on inflammatory mediators via the induction of heme oxygenase-1 in lipopolysaccharide-stimulated RAW264.7 macrophages. Int. J. Mol. Sci. 2014, 15, 23749–23765. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.-M.; Teuscher, F.; Li, D.-L.; Diesel, A.; Ebel, R.; Proksch, P.; Wang, B. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006, 69, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Yamashita, T.; Saito, M.; Sekita, S.; Takahashi, C. Chemical and cytotoxicity survey on the metabolites of toxic fungi. Jpn. J. Exp. Med. 1974, 44, 83–96. [Google Scholar]
- Yamamoto, Y.; Nishimura, K.; Kiriyama, N. Studies on the metabolic products of Aspergillus terreus. I. Metabolites of the strain IFO 6123. Chem. Pharm. Bull. 1976, 24, 1853–1859. [Google Scholar] [CrossRef]
- Geng, Y.; Zhu, S.; Cheng, P.; Lu, Z.M.; Xu, H.Y.; Shi, J.S.; Xu, Z.H. Bioassay-guided fractionation of ethyl acetate extract from Armillaria mellea attenuates inflammatory response in lipopolysaccharide (LPS) stimulated BV-2 microglia. Phytomedicine 2017, 26, 55–61. [Google Scholar] [CrossRef]
- Wood, L.B.; Winslow, A.R.; Proctor, E.A.; McGuone, D.; Mordes, D.A.; Frosch, M.P.; Hyman, B.T.; Lauffenburger, D.A.; Haigis, K.M. Identification of neurotoxic cytokines by profiling Alzheimer’s disease tissues and neuron culture viability screening. Sci. Reports 2015, 5, 16622. [Google Scholar] [CrossRef]
- Hwang, J.H.; Wu, S.J.; Wu, P.L.; Shih, Y.Y.; Chan, Y.C. Neuroprotective effect of tempeh against lipopolysaccharide-induced damage in BV-2 microglial cells. Nutr. Neurosci. 2018, 22, 840–849. [Google Scholar] [CrossRef]
- Amir, M.; Javed, S.A.; Kumar, H. Synthesis of some newer analogues of 4-hydroxyphenyl acetic acid as potent anti-inflammatory agents. J. Chin. Chem. Soc. 2008, 55, 201–208. [Google Scholar] [CrossRef]
- Eckermann, K.; Mocanu, M.M.; Khlistunova, I.; Biernat, J.; Nissen, A.; Hofmann, A.; Schönig, K.; Bujard, H.; Haemisch, A.; Mandelkow, E.; et al. The β-propensity of tau determines aggregation and synaptic loss in inducible mouse models of tauopathy. J. Biol. Chem. 2007, 282, 31755–31765. [Google Scholar] [CrossRef]
- Piedrahita, D.; Hernández, I.; López-Tobón, A.; Fedorov, D.; Obara, B.; Manjunath, B.S.; Boudreau, R.L.; Davidson, B.; LaFerla, F.; Gallego-Gómez, J.C.; et al. Silencing of CDK5 reduces neurofibrillary tangles in transgenic Alzheimer’s mice. J. Neurosci. 2010, 30, 13966–13976. [Google Scholar] [CrossRef]
- Sun, K.H.; De Pablo, Y.; Vincent, F.; Shah, K. Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. J. Neurochem. 2008, 107, 265–278. [Google Scholar] [CrossRef]
- Lopes, J.P.; Oliveira, C.R.; Agostinho, P. Neurodegeneration in an Aβ-induced model of Alzheimer’s disease: The role of Cdk5. Aging Cell 2010, 9, 64–77. [Google Scholar] [CrossRef]
- Meredith, J.E.; Sankaranarayanan, S.; Guss, V.; Lanzetti, A.J.; Berisha, F.; Neely, R.J.; Slemmon, J.R.; Portelius, E.; Zetterberg, H.; Blennow, K.; et al. Characterization of Novel CSF Tau and ptau Biomarkers for Alzheimer’s Disease. PLoS ONE 2013, 8, e76523. [Google Scholar] [CrossRef]
- Martinou, J.C.; Desagher, S.; Antonsson, B. Cytochrome c release from mitochondria: All or nothing. Nat. Cell Biol. 2000, 2, E41–E43. [Google Scholar] [CrossRef]
- Himaya, S.W.A.; Ryu, B.; Qian, Z.J.; Li, Y.; Kim, S.K. 1-(5-bromo-2-hydroxy-4-methoxyphenyl)ethanone [SE1] suppresses pro-inflammatory responses by blocking NF-κB and MAPK signaling pathways in activated microglia. Eur. J. Pharmacol. 2011, 670, 608–616. [Google Scholar] [CrossRef]
- Lin, Y.H.; Shah, S.; Salem, N. Altered essential fatty acid metabolism and composition in rat liver, plasma, heart and brain after microalgal DHA addition to the diet. J. Nutr. Biochem. 2011, 22, 758–765. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; Al-Qahtani, J.M.; El-Safty, S.A. Omega-3 polyunsaturated fatty acids in large doses attenuate seizures, cognitive impairment, and hippocampal oxidative DNA damage in young kindled rats. Neurosci. Lett. 2015, 584, 173–177. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property re-sulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Liang, J.; Liu, Y.; Liu, Y.; Zhou, C.; Hong, P.; Zhang, Y.; Qian, Z.-J. The Mechanism of Two Benzaldehydes from Aspergillus terreus C23-3 Improve Neuroinflammatory and Neuronal Damage to Delay the Progression of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 905. https://doi.org/10.3390/ijms24020905
Chen M, Liang J, Liu Y, Liu Y, Zhou C, Hong P, Zhang Y, Qian Z-J. The Mechanism of Two Benzaldehydes from Aspergillus terreus C23-3 Improve Neuroinflammatory and Neuronal Damage to Delay the Progression of Alzheimer’s Disease. International Journal of Molecular Sciences. 2023; 24(2):905. https://doi.org/10.3390/ijms24020905
Chicago/Turabian StyleChen, Minqi, Jinyue Liang, Yi Liu, Yayue Liu, Chunxia Zhou, Pengzhi Hong, Yi Zhang, and Zhong-Ji Qian. 2023. "The Mechanism of Two Benzaldehydes from Aspergillus terreus C23-3 Improve Neuroinflammatory and Neuronal Damage to Delay the Progression of Alzheimer’s Disease" International Journal of Molecular Sciences 24, no. 2: 905. https://doi.org/10.3390/ijms24020905
APA StyleChen, M., Liang, J., Liu, Y., Liu, Y., Zhou, C., Hong, P., Zhang, Y., & Qian, Z.-J. (2023). The Mechanism of Two Benzaldehydes from Aspergillus terreus C23-3 Improve Neuroinflammatory and Neuronal Damage to Delay the Progression of Alzheimer’s Disease. International Journal of Molecular Sciences, 24(2), 905. https://doi.org/10.3390/ijms24020905