Role of Melatonin and Nitrogen Metabolism in Plants: Implications under Nitrogen-Excess or Nitrogen-Low
Abstract
1. Introduction
2. Methodology
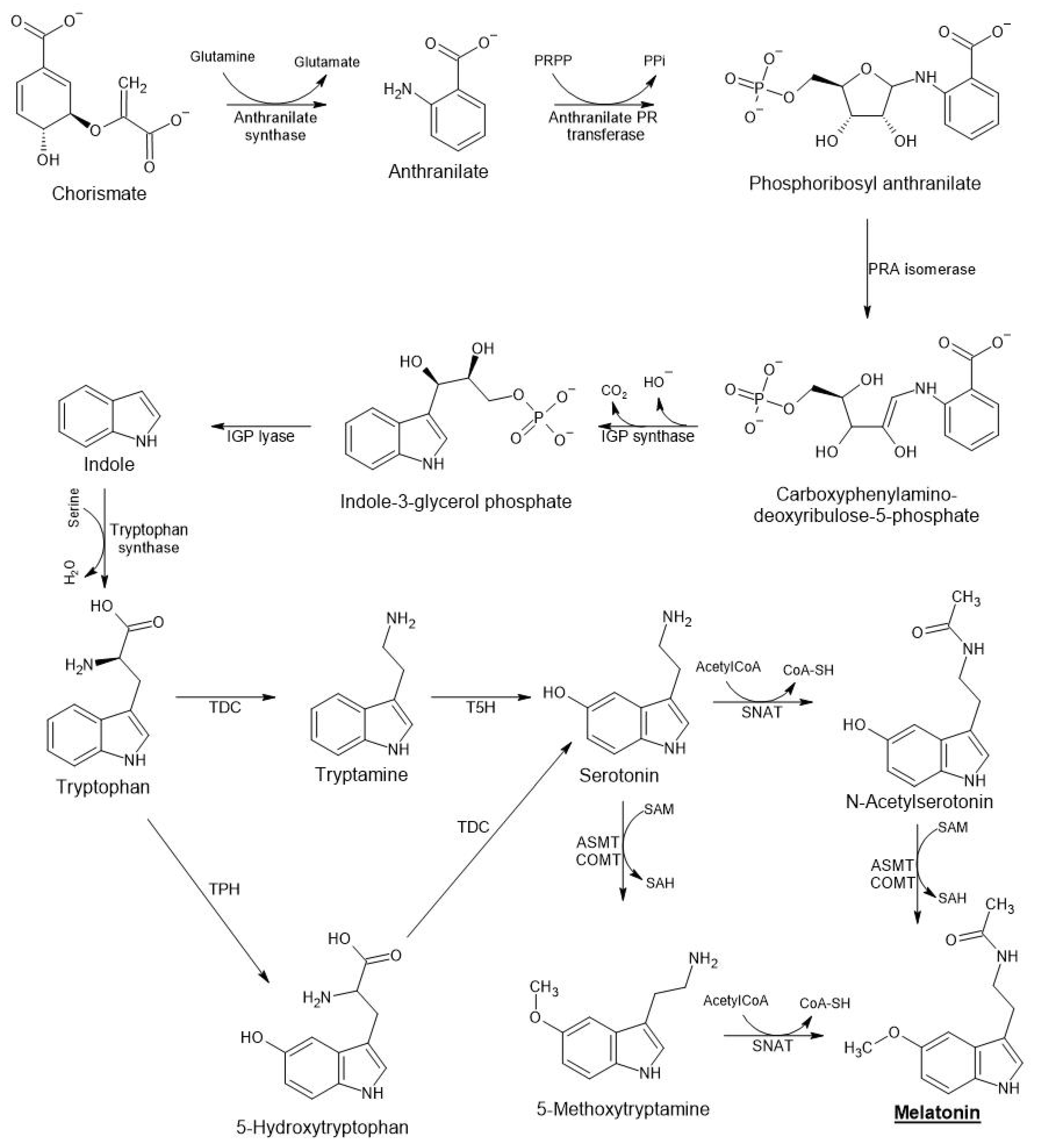
3. Melatonin Biosynthesis
4. Role of Melatonin in Nitrogen Metabolism
5. Melatonin in Osmoregulation and Redox Network
6. Melatonin, Nitrogen, and Implications in Crops
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aas | amino acids |
| AAP6a | amino acid permease 6a-like protein |
| ABA | abscisic acid |
| ACLA-A/B | citrate synthase |
| δ-ALAD | δ-aminolevulinic acid dehydratase |
| Anthranile PR transferase | anthranilate phosphoribosyl ltransferase |
| AMT | ammonium transporters |
| APX | ascorbate peroxidase |
| ASC | ascorbic acid |
| ASMT | acetylserotonin methyltransferase |
| BCAT | branched-chain-amino-acid amino transferase |
| CAO | chlorophyllide a oxygenase |
| CAT | catalase |
| Chl | chlorophyll |
| CHLG | chlorophyll synthase |
| COMT | caffeic-O-methyltransferase |
| COX | cytochrome c oxidase |
| CYSC | bifunctional L-3-cyanoalanine synthase/cysteine synthase |
| cytb6/f | cytochrome b6/f |
| FUM | fumarate hydratase |
| GDH | glutamate dehydrogenase |
| Gln | glutamine |
| Glu | glutamate |
| GOGAT (NADP/Fd dependent) | glutamate synthase |
| GPX | glutathione peroxidase |
| GR | glutathione reductase |
| GS | glutamine synthase |
| GSH | glutathione (reduced) |
| GSSH | glutathione (oxidized) |
| IAA | indole-3-acetic acid (auxin) |
| IDH | isocitrate dehydrogenase |
| IGP | indole-3-glycerol phosphate |
| MDH | malate dehydrogenase |
| (M)DHA | monodehydroascorbate |
| (M)DHAR | monodehydroascorbate reductase |
| MEL | melatonin |
| NO | nitric oxide |
| NOS-like | nitric oxide synthase |
| NiR | nitrite reductase |
| NPK | nitrogen/phosphorous/potassium |
| NR | nitrate reductase |
| NRT | nitrate transporter |
| NUE | nitrogen use efficiency |
| 2OG | 2-oxoglutarate (α-ketoglutarate) |
| P5CS2 | pyrroline-5-carboxylate synthetase |
| PDH | pyruvate dehydrogenase |
| PGPR | plant-growth-promoting rhizobacteria |
| POR | protochlorophyllide oxidoreductase |
| PPi | inorganic pyrophosphate |
| PSI | photosystem I |
| PSII | photosystem II |
| PSY | phytoene synthase |
| PRA | phosphoribosyl anthranilate isomerase |
| PROD | proline dehydrogenase |
| PRPP | phosphoribosyl pyrophosphate |
| RBOH | respiratory burst oxidase |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RWC | relative water content |
| SAH | S-adenosylhomocysteine |
| SAM | S-adenosylmethionine |
| SDH | succinate dehydrogenase |
| SHMT | serine hydroxymethyltransferase |
| SNAT | serotonin N-acetyltransferase |
| SOD | superoxide dismutase |
| T5H | tryptamine 5-hydroxylase |
| TCA | tricarboxylic acid cycle (Krebs cycle) |
| TDC | tryptophan decarboxylase |
| TPH | tryptophan hydroxylase |
References
- Frink, C.R.; Waggoner, P.E.; Ausubel, J.H. Nitrogen Fertilizer: Retrospect and Prospect. Proc. Natl. Acad. Sci. USA 1999, 96, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Skrzypczak, D.; Szopa, D.; Izydorczyk, G.; Moustakas, K.; Witek-Krowiak, A. Management of Biological Sewage Sludge: Fertilizer Nitrogen Recovery as the Solution to Fertilizer Crisis. J. Environ. Manag. 2022, 326, 116602. [Google Scholar] [CrossRef] [PubMed]
- Mayor, Á.; Beltran, E.; Cortina, J.L.; Valderrama, C. Nitrogen Flow Analysis in Spain: Perspectives to Increase Sustainability. Sci. Total Environ. 2022, 858, 160117. [Google Scholar] [CrossRef] [PubMed]
- Erisman, J.W.; Galloway, J.N.; Seitzinger, S.; Bleeker, A.; Dise, N.B.; Petrescu, A.M.R.; Leach, A.M.; de Vries, W. Consequences of Human Modification of the Global Nitrogen Cycle. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130116. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Mieulet, D.; Hubberten, H.-M.; Obata, T.; Hoefgen, R.; Fernie, A.R.; Fisahn, J.; San Segundo, B.; Guiderdoni, E.; Schippers, J.H.M.; et al. SALT-RESPONSIVE ERF1 Regulates Reactive Oxygen Species–Dependent Signaling during the Initial Response to Salt Stress in Rice. Plant Cell 2013, 25, 2115–2131. [Google Scholar] [CrossRef]
- Barraclough, P.B.; Howarth, J.R.; Jones, J.; Lopez-Bellido, R.; Parmar, S.; Shepherd, C.E.; Hawkesford, M.J. Nitrogen Efficiency of Wheat: Genotypic and Environmental Variation and Prospects for Improvement. Eur. J. Agron. 2010, 33, 1–11. [Google Scholar] [CrossRef]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate Reductase Regulates Plant Nitric Oxide Homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Scartazza, A.; Pompeiano, A.; Ciurli, A.; Lu, Y.; Guglielminetti, L.; Yamaguchi, J. Nitrate Reductase Modulation in Response to Changes in C/N Balance and Nitrogen Source in Arabidopsis. Plant Cell Physiol. 2018, 59, 1248–1254. [Google Scholar] [CrossRef]
- Yin, Y.; Ying, H.; Zheng, H.; Zhang, Q.; Xue, Y.; Cui, Z. Estimation of NPK Requirements for Rice Production in Diverse Chinese Environments under Optimal Fertilization Rates. Agric. For. Meteorol. 2019, 279, 107756. [Google Scholar] [CrossRef]
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can Less Yield More? Is Reducing Nutrient Input into the Environment Compatible with Maintaining Crop Production? Trends Plant Sci. 2004, 9, 597–605. [Google Scholar] [CrossRef]
- Congreves, K.A.; Otchere, O.; Ferland, D.; Farzadfar, S.; Williams, S.; Arcand, M.M. Nitrogen Use Efficiency Definitions of Today and Tomorrow. Front. Plant Sci. 2021, 12, 637108. [Google Scholar] [CrossRef]
- Tantray, A.Y.; Hazzazi, Y.; Ahmad, A. Physiological, Agronomical, and Proteomic Studies Reveal Crucial Players in Rice Nitrogen Use Efficiency under Low Nitrogen Supply. Int. J. Mol. Sci. 2022, 23, 6410. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Liu, H. Modification of Rhizosphere Microbial Communities: A Possible Mechanism of Plant Growth Promoting Rhizobacteria Enhancing Plant Growth and Fitness. Front. Plant Sci. 2022, 13, 920813. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root Exudates: Mechanistic Insight of Plant Growth Promoting Rhizobacteria for Sustainable Crop Production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Dai, M. Improving Crop Drought Resistance with Plant Growth Regulators and Rhizobacteria: Mechanisms, Applications, and Perspectives. Plant Comm. 2022, 3, 100228. [Google Scholar] [CrossRef]
- Hao, Q.; Shang, W.; Zhang, C.; Chen, H.; Chen, L.; Yuan, S.; Chen, S.; Zhang, X.; Zhou, X. Identification and Comparative Analysis of CBS Domain-Containing Proteins in Soybean (Glycine max) and the Primary Function of GmCBS21 in Enhanced Tolerance to Low Nitrogen Stress. Int. J. Mol. Sci. 2016, 17, 620. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, M.; Liu, Y.; Shi, L. Integration of the Metabolome and Transcriptome Reveals the Resistance Mechanism to Low Nitrogen in Wild Soybean Seedling Roots. Environ. Exp. Bot. 2020, 175, 104043. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, a Pineal Factor that Lightens Melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Heinzelmann, R.V. Structure of Melatonin. J. Am. Chem. Soc. 1959, 81, 6084–6085. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Mori, W.; Wright, M.R. Melatonin in Peripheral Nerve. Nature 1959, 183, 1821. [Google Scholar] [CrossRef]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in Edible Plants Identified by Radioimmunoassay and by HPLC-MS. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Kolar, J.; Machackova, I.; Illnerova, H.; Prinsen, E.; van Dongen, W.; van Onckelen, H. Melatonin in Higher Plant Determined by Radioimmunoassay and Liquid Chromatography-Mass Spectrometry. Biol. Rhythm Res. 1995, 26, 406–409. [Google Scholar]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J. Phytomelatonin: An Unexpected Molecule with Amazing Performances in Plants. J. Exp. Bot. 2022, 73, 5779–5800. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Regulatory Hub of Plant Hormone Levels and Action in Stress Situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a Plant Biostimulant in Crops and during Post-Harvest: A New Approach Is Needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin Against Environmental Plant Stressors: A Review. Curr. Protein Pept. Sci. 2022, 22, 413–429. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; et al. Phytomelatonin: An Overview of the Importance and Mediating Functions of Melatonin against Environmental Stresses. Physiol. Plant. 2021, 172, 820–846. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Mukherjee, S.; Flores, F.B.; Arnao, M.B.; Luo, Z.; Corpas, F.J. Functions of Melatonin during Postharvest of Horticultural Crops. Plant Cell Physiol. 2021, pcab175. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Acosta, M.; Cano, A.; Hernández-Ruiz, J.; Arnao, M.B. Melatonin as a Possible Natural Safener in Crops. Plants 2022, 11, 890. [Google Scholar] [CrossRef]
- Hernández-Ruiz, J.; Ruiz-Cano, D.; Giraldo-Acosta, M.; Cano, A.; Arnao, M. Melatonin in Brassicaceae: Role in Postharvest and Interesting Phytochemicals. Molecules 2022, 27, 1523. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R.J. Melatonin and Carbohydrate Metabolism in Plant Cells. Plants 2021, 10, 1917. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and Phytomelatonin: Chemistry, Biosynthesis, Metabolism, Distribution and Bioactivity in Plants and Animals—An Overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, J.; Arnao, M.B. Relationship of Melatonin and Salicylic Acid in Biotic/Abiotic Plant Stress Responses. Agronomy 2018, 8, 33. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Synthesis from Tryptophan and Its Role in Higher Plants. In Amino Acids in Higher Plants; D’ Mello, J., Ed.; CAB Intern.: Boston, MA, USA, 2015; pp. 390–435. ISBN 978-1-78064-263-5. [Google Scholar]
- Tan, D.X.; Reiter, R.J. An Evolutionary View of Melatonin Synthesis and Metabolism Related to Its Biological Functions in Plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef] [PubMed]
- Menhas, S.; Yang, X.; Hayat, K.; Aftab, T.; Bundschuh, J.; Arnao, M.B.; Zhou, Y.; Zhou, P. Exogenous Melatonin Enhances Cd Tolerance and Phytoremediation Efficiency by Ameliorating Cd-Induced Stress in Oilseed Crops: A Review. J. Plant Growth Regul. 2022, 41, 922–935. [Google Scholar] [CrossRef]
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Latorre-Jimenez, M.A.; Reiter, R.J. On the Significance of an Alternate Pathway of Melatonin Synthesis via 5-Methoxytryptamine: Comparisons across Species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin Biosynthesis in Plants: Multiple Pathways Catalyze Tryptophan to Melatonin in the Cytoplasm or Chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Back, K. Melatonin Metabolism, Signaling and Possible Roles in Plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef]
- Lee, H.Y.; Back, K. 2-Hydroxymelatonin, Rather Than Melatonin, Is Responsible for RBOH-Dependent Reactive Oxygen Species Production Leading to Premature Senescence in Plants. Antioxidants 2021, 10, 1728. [Google Scholar] [CrossRef]
- Byeon, Y.; Tan, D.X.; Reiter, R.J.; Back, K. Predominance of 2-Hydroxymelatonin over Melatonin in Plants. J. Pineal Res. 2015, 59, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Byeon, Y.; Back, K. Molecular Cloning of Melatonin 2-Hydroxylase Responsible for 2-Hydroxymelatonin Production in Rice (Oryza sativa). J. Pineal Res. 2015, 58, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. Effects of Melatonin on Seedling Growth, Mineral Nutrition, and Nitrogen Metabolism in Cucumber under Nitrate Stress. J. Pineal Res. 2017, 62, e12403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, A.; Hao, Y.; Su, W.; Sun, G.; Song, S.; Liu, H.; Chen, R. Nitric Oxide Is Essential for Melatonin to Enhance Nitrate Tolerance of Cucumber Seedlings. Molecules 2022, 27, 5806. [Google Scholar] [CrossRef]
- Zhao, N.; Sun, Y.; Wang, D.Y.; Zheng, J.X. Effects of Exogenous Melatonin on Nitrogen Metabolism in Cucumber Seedlings under High Temperature Stress. Zhiwu Shengli Xuebao/Plant Physiol. J. 2012, 48, 557–564. [Google Scholar]
- Chen, Z.; Cao, X.; Niu, J. Effects of Melatonin on Morphological Characteristics, Mineral Nutrition, Nitrogen Metabolism, and Energy Status in Alfalfa under High-Nitrate Stress. Front. Plant Sci. 2021, 12, 694179. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, C.; Cao, L.; Jin, X.; Wang, M.; Zhang, M.; Zhao, Q.; Li, H.; Zhang, Y.; Yu, G. The Mechanisms Underlying Melatonin Improved Soybean Seedling Growth at Different Nitrogen Levels. Funct. Plant Biol. 2021, 48, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-Term Exogenous Application of Melatonin Improves Nutrient Uptake Fluxes in Apple Plants under Moderate Drought Stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Qiao, Y.; Yin, L.; Wang, B.; Ke, Q.; Deng, X.; Wang, S. Melatonin Promotes Plant Growth by Increasing Nitrogen Uptake and Assimilation under Nitrogen Deficient Condition in Winter Wheat. Plant Physiol. Biochem. 2019, 139, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Erdal, S. Melatonin Promotes Plant Growth by Maintaining Integration and Coordination between Carbon and Nitrogen Metabolisms. Plant Cell Rep. 2019, 38, 1001–1012. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.; Chu, Y.-N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin Enhances Plant Growth and Abiotic Stress Tolerance in Soybean Plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef]
- Ren, S.; Jiang, G.-L.; Rutto, L. Melatonin Priming Enhances Symbiotic Nitrogen Fixation in Soybean, Glycine max L. J. Biotech Res. 2019, 10, 136–144. [Google Scholar]
- Wang, H.; Ren, C.; Cao, L.; Zhao, Q.; Jin, X.; Wang, M.; Zhang, M.; Yu, G.; Zhang, Y. Exogenous Melatonin Modulates Physiological Response to Nitrogen and Improves Yield in Nitrogen-Deficient Soybean (Glycine max L. Merr.). Front. Plant Sci. 2022, 13, 865758. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Qin, B.; Gong, Z.; Zhang, Y. Melatonin Improves Nitrogen Metabolism during Grain Filling under Drought Stress. Physiol. Mol. Biol. Plants 2022, 28, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Yu, H.; Yu, Q.; Jin, X.; Cao, L.; Wang, M.; Wang, M.; Ren, C.; Zhang, Y. Physiological and UPLC-MS/MS Widely Targeted Metabolites Mechanisms of Alleviation of Drought Stress-Induced Soybean Growth Inhibition by Melatonin. Ind. Crops Prod. 2021, 163, 113323. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Ren, J.; Yang, X.; Zhang, N.; Feng, L.; Ma, C.; Wang, Y.; Yang, Z.; Zhao, J. Melatonin Alleviates Aluminum-Induced Growth Inhibition by Modulating Carbon and Nitrogen Metabolism, and Reestablishing Redox Homeostasis in Zea mays L. J. Hazard. Mater. 2022, 423, 127159. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed Senescence of Apple Leaves by Exogenous Melatonin Treatment: Toward Regulating the Ascorbate-Glutathione Cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef]
- Arnao, M.; Hernández-Ruiz, J. Melatonin and Reactive Oxygen and Nitrogen Species: A Model for the Plant Redox Network. Melatonin Res. 2019, 2, 152–168. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Role of Melatonin to Enhance Phytoremediation Capacity. Appl. Sci. 2019, 9, 5293. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M.; et al. Role of Melatonin in Plant Tolerance to Soil Stressors: Salinity, PH and Heavy Metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative Roles of Nitric Oxide and Hydrogen Sulfide in Melatonin-Induced Tolerance of Pepper (Capsicum annuum L.) Plants to Iron Deficiency and Salt Stress Alone or in Combination. Physiol. Plant. 2020, 168, 256–277. [Google Scholar] [CrossRef] [PubMed]
- Feiyu, Y.; Wei, H.; Li, W.; Liu, Z.H.; Tang, S.; Chen, L.; Ding, C.Q.; Jiang, Y.; Ding, Y.; Li, G. Melatonin Improves K+ and Na+ Homeostasis in Rice under Salt Stress by Mediated Nitric Oxide. Ecotoxicol. Environ. Saf. 2020, 206, 111358. [Google Scholar]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Altaf, M.M.; Khan, L.U.; Shahid, S.; Jahan, M.S. Melatonin Alleviates Salt Damage in Tomato Seedling: A Root Architecture System, Photosynthetic Capacity, Ion Homeostasis, and Antioxidant Enzymes Analysis. Sci. Hort 2021, 285, 110145. [Google Scholar] [CrossRef]
- Hasan, M.K.; Liu, C.-X.; Pan, Y.-T.; Ahammed, G.J.; Qi, Z.-Y.; Zhou, J. Melatonin Alleviates Low-Sulfur Stress by Promoting Sulfur Homeostasis in Tomato Plants. Sci. Rep. 2018, 8, 10182. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Alsubaie, Q.D.; Ali, H.M.; Ibrahim, A.A.; Alsadon, A. Potential Roles of Melatonin and Sulfur in Alleviation of Lanthanum Toxicity in Tomato Seedlings. Ecotoxicol. Environ. Saf. 2019, 180, 656–667. [Google Scholar] [CrossRef]
- Hasan, M.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin Inhibits Cadmium Translocation and Enhances Plant Tolerance by Regulating Sulfur Uptake and Assimilation in Solanum lycopersicum L. J. Agr. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, S.; Ma, L.; Kong, L.; Pan, S.; Tang, X.; Tian, H.; Meiyang, D.; Mo, Z. Effect of Exogenous Melatonin Application on the Grain Yield and Antioxidant Capacity in Aromatic Rice under Combined Lead–Cadmium Stress. Antioxidants 2022, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Yang, W.; Li, Y.; Wang, S.; Yin, L.; Deng, X. Seed Pre-Soaking with Melatonin Improves Wheat Yield by Delaying Leaf Senescence and Promoting Root Development. Agronomy 2020, 10, 84. [Google Scholar] [CrossRef]
- Li, X.; Brestic, M.; Tan, D.X.; Zivcak, M.; Zhu, X.; Liu, S.; Song, F.; Reiter, R.J.; Liu, F. Melatonin Alleviates Low PS I-Limited Carbon Assimilation under Elevated CO2 and Enhances the Cold Tolerance of Offspring in Chlorophyll b-Deficient Mutant Wheat. J. Pineal Res. 2018, 64, e12453. [Google Scholar] [CrossRef]
- Zafar, S.; Hasnain, Z.; Anwar, S.; Perveen, S.; Iqbal, N.; Noman, A.; Ali, M. Influence of Melatonin on Antioxidant Defense System and Yield of Wheat (Triticum aestivum L.) Genotypes under Saline Condition. Pak. J. Bot. 2019, 51, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Abdalla, A.M.; bd Elhamid, E.M.; Ezzo, M.I. Role of Melatonin in Improving Growth, Yield Quantity and Quality of Moringa oleifera L. Plant under Drought Stress. Bull. Natl. Res. Cent. 2020, 44, 18. [Google Scholar] [CrossRef]
- Medina-Santamarina, J.; Serrano, M.; Lorente-Mento, J.; Pastor, M.E.; Zapata, P.; Valero, D.; Guillén, F. Melatonin Treatment of Pomegranate Trees Increases Crop Yield and Quality Parameters at Harvest and during Storage. Agronomy 2021, 11, 861. [Google Scholar] [CrossRef]
- Gul, M.; Khan, F.A.; Wani, S.A.; Bhat, S.A.; Mir, S.A.; Malik, A.A.; Kumar, A.; Narayan, S.; Stephen, K.; Lone, S.A. Effects of Foliar Application of Melatonin on Gas Exchange and Certain Biochemical Characteristics Broccoli Cv. Palam Samridhi. J. Appl. Nat. Sci. 2021, 13, 791–797. [Google Scholar] [CrossRef]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous Melatonin Application Delays Senescence of Kiwifruit Leaves by Regulating the Antioxidant Capacity and Biosynthesis of Flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Carrión-Antolí, A.; Lorente-Mento, J.; Valverde, J.; Castillo, S.; Valero, D.; Serrano, M. Effects of Melatonin Treatment on Sweet Cherry Tree Yield and Fruit Quality. Agronomy 2021, 12, 3. [Google Scholar] [CrossRef]
- Brysiewicz, A.; Hassan, I.F.; Murad, S.A.; Alam-Eldein, S.M.; Gaballah, M.S.; Ogbaga, C.C.; Bakr, B.M.M.; Mira, A. Does Melatonin Improve the Yield Attributes of Field-Droughted Banana under Egyptian Semi-Arid Conditions? J. Water Land Dev. 2022, 52, 221–231. [Google Scholar]
- Liu, J.; Yue, R.; Si, M.; Wu, M.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Effects of Exogenous Application of Melatonin on Quality and Sugar Metabolism in Zaosu Pear Fruit. J. Plant Growth Regul. 2019, 38, 1161–1169. [Google Scholar] [CrossRef]
- Jia, C.; Yu, X.; Zhang, M.; Liu, Z.; Zou, P.; Ma, J.; Xu, Y. Application of Melatonin-Enhanced Tolerance to High-Temperature Stress in Cherry Radish (Raphanus sativus L. Var. radculus pers). J. Plant Growth Regul. 2020, 39, 631–640. [Google Scholar] [CrossRef]
- Khan, M.N.; Khan, Z.; Luo, T.; Liu, J.; Rizwan, M.; Zhang, J.; Xu, Z.; Wu, H.; Hu, L. Seed Priming with Gibberellic Acid and Melatonin in Rapeseed: Consequences for Improving Yield and Seed Quality under Drought and Non-Stress Conditions. Ind. Crops Prod. 2020, 156, 112850. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. The Beneficial Effects of Exogenous Melatonin on Tomato Fruit Properties. Sci. Hort. 2016, 207, 14–20. [Google Scholar] [CrossRef]
- Madigan, A.P.; Egidi, E.; Bedon, F.; Franks, A.E.; Plummer, K.M. Bacterial and Fungal Communities Are Differentially Modified by Melatonin in Agricultural Soils under Abiotic Stress. Front. Microbiol. 2019, 10, 2616. [Google Scholar] [CrossRef]
- Asif, M.; Pervez, A.; Ahmad, R. Role of Melatonin and Plant-Growth-Promoting Rhizobacteria in the Growth and Development of Plants. Clean: Soil Air Water 2019, 47, 1800459. [Google Scholar] [CrossRef]
- Rostami, S.; Azhdarpoor, A.; Baghapour, M.A.; Dehghani, M.; Samaei, M.R.; Jaskulak, M.; Jafarpour, S.; Samare-Najaf, M. The Effects of Exogenous Application of Melatonin on the Degradation of Polycyclic Aromatic Hydrocarbons in the Rhizosphere of Festuca. Environ. Pollut. 2021, 274, 116559. [Google Scholar] [CrossRef]
- Xiao, R.; Han, Q.; Liu, Y.; Zhang, X.; Hao, Q.; Chai, Q.; Hao, Y.; Deng, J.; Li, X.; Ji, H. Melatonin Attenuates the Urea-Induced Yields Improvement Through Remodeling Transcriptome and Rhizosphere Microbial Community Structure in Soybean. Front. Microbiol. 2022, 13, 903467. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Jiang, M.; Zhang, P.; Liu, L.; Liu, S.; Zhao, C.; Li, X. Exogenous Melatonin Reprograms the Rhizosphere Microbial Community to Modulate the Responses of Barley to Drought Stress. Int. J. Mol. Sci. 2022, 23, 9665. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Ye, F.; Liu, F.; Brestic, M.; Li, X. Rhizosphere Melatonin Application Reprograms Nitrogen-Cycling Related Microorganisms to Modulate Low Temperature Response in Barley. Front. Plant Sci. 2022, 13, 998861. [Google Scholar] [CrossRef]
- Irshad, A.; Rehman, R.N.U.; Kareem, H.A.; Yang, P.; Hu, T. Addressing the Challenge of Cold Stress Resilience with the Synergistic Effect of Rhizobium Inoculation and Exogenous Melatonin Application in Medicago Truncatula. Ecotoxicol. Environ. Saf. 2021, 226, 112816. [Google Scholar] [CrossRef]
- Alinia, M.; Kazemeini, S.A.; Dadkhodaie, A.; Sepehri, M.; Pessarakli, M. Improving Salt Tolerance Threshold in Common Bean Cultivars Using Melatonin Priming: A Possible Mission? J. Plant Nutr. 2021, 44, 2691–2714. [Google Scholar] [CrossRef]
- Alinia, M.; Kazemeini, S.A.; Sepehri, M.; Dadkhodaie, A. Simultaneous Application of Rhizobium Strain and Melatonin Improves the Photosynthetic Capacity and Induces Antioxidant Defense System in Common Bean (Phaseolus vulgaris L.) under Salinity Stress. J. Plant Growth Regul. 2022, 41, 1367–1381. [Google Scholar] [CrossRef]
- Snyder, C.; Davidson, E.; Smith, P.; Venterea, R. Agriculture: Sustainable Crop and Animal Production to Help Mitigate Nitrous Oxide Emissions. Curr. Opin. Environ. Sustain. 2014, 9–10, 46–54. [Google Scholar] [CrossRef]
- Afridi, M.Z. Nitrogen Partitioning and Translocation in Wheat under Fertilizer-N Levels, Application Time and Decapitation Stress. J. Biol. Agric. Healthc. 2014, 4, 1–9. [Google Scholar]
- Ali, N. Review: Nitrogen Utilization Features in Cotton Crop. Am. J. Plant Sci. 2015, 6, 987–1002. [Google Scholar] [CrossRef]
- Khan, A.; Tan, D.K.Y.; Afridi, M.Z.; Luo, H.; Tung, S.A.; Ajab, M.; Fahad, S. Nitrogen Fertility and Abiotic Stresses Management in Cotton Crop: A Review. Environ. Sci. Pollut Res. 2017, 24, 14551–14566. [Google Scholar] [CrossRef] [PubMed]
- Chattha, M.S.; Ali, Q.; Haroon, M.; Afzal, M.J.; Javed, T.; Hussain, S.; Mahmood, T.; Solanki, M.K.; Umar, A.; Abbas, W.; et al. Enhancement of Nitrogen Use Efficiency through Agronomic and Molecular Based Approaches in Cotton. Front. Plant Sci. 2022, 13, 994306. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Yin, B.; Zhou, S.; Li, Z.; Zhang, X.; Cao, Y.; Han, R.; Shi, C.; Liang, B.; Xu, J. Melatonin and Dopamine Mediate the Regulation of Nitrogen Uptake and Metabolism at Low Ammonium Levels in Malus Hupehensis. Plant Physiol. Biochem. 2022, 171, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-W.; Yoshikuni, Y. Microbiome Engineering for Sustainable Agriculture: Using Synthetic Biology to Enhance Nitrogen Metabolism in Plant-Associated Microbes. Curr. Opin. Microbiol. 2022, 68, 102172. [Google Scholar] [CrossRef] [PubMed]
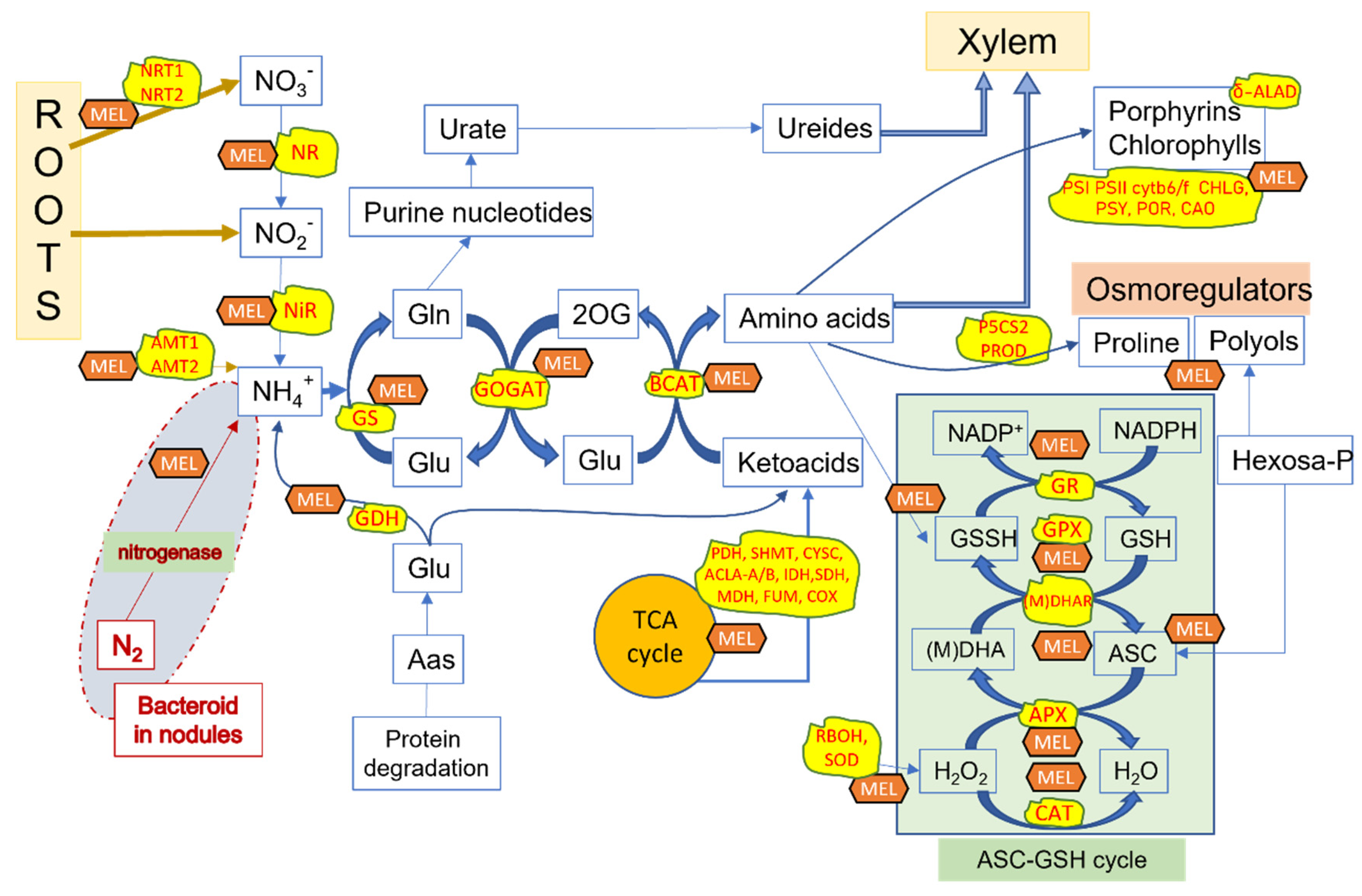
| Plant Species | Nitrogen Nutrition/Stress | Melatonin Treatment (µM) | Observed Effects | Reference |
|---|---|---|---|---|
| Cucumber | Normal High temperature | 100 | ↑ temperature tolerance, NR, GS, GOGAT, GDH, nitrate, ammonium restrained | [47] |
| Nitrate: N-excess | 100 | ↑ tolerance, growth, NPK balance, Ca, NR, GS, GOGAT, GDH ↓ damage, nitrate, ammonium | [45] | |
| Nitrate: N-excess | 2 | ↑ tolerance N excess, co-action with NO, lateral roots, root length, Ca, Mg, Fe, melatonin, NO, IAA, ABA, transcription levels of several genes of N metabolism, IAA, ABA and melatonin | [46] | |
| Apple | Normal (urea) Drought stress | 100 | ↑ drought tolerance, growth, photosynthesis, stomatal open, chls, RWC, NR, NiR, GS, GOGAT, N uptake genes (AMTs, NRTs), N, P, K, Ca, Mg, Cu, Zn, and B levels | [50] |
| Alfalfa | Nitrate: N-excess | 100 | ↑ tolerance N excess, shoot height, leaves (length, width, area), P, ATP, biomass, amino acids, energy charge, upregulates NR, GS, GOGAT, GDH ↓ total N, nitrate, ammonium, Na, K, Ca | [48] |
| Wheat | Nitrate and ammonium: N-low | 1 | ↑ N and nitrate, N absorption, N metabolism, NR, GS, growth, yield, in shoots and roots | [51] |
| Maize | Normal | 100 | ↑ nitrite, nitrate, NR, NiR, GS, GOGAT, GDH ↓ ammonium | [52] |
| Soybean | Normal Salt/drought stress | 50–100 | ↑ stress tolerance, growth, seed yield and fatty acid; up-regulates cell division, photosynthesis, carbohydrate, fatty acid, and ASC genes | [53] |
| Normal | 100 | ↑ number and size of nodules, fresh shoot biomass in 3 varieties | [54] | |
| Nitrate and ammonium: N-excess, N-normal and N-low | 100 | ↑ tolerance N-excess, N content in N-low, stem diameter, leaf area, nodule number, ATP, biomass in three-N conditions, antioxidant enzymes at N-excess, N-related genes | [49] | |
| Nitrate and ammonium: N-low | 100 | ↑ nodule number, total N fixed, tolerance to N deficiency, upregulating genes: NR2, NiR, GS1β, GOGAT, AAP6a, promoting enzyme activity: NR, GS, GOGAT, GDH, amino acids, protein, total N, chls, seed yield | [55] | |
| Normal Drought stress | 100 | ↑ N, NR, NiR, NRT, GS, GOGAT, GDH, protein, proline, ureides, N transport, growth, biomass | [56] | |
| Normal Drought stress | 100 | ↑ stress tolerance, growth, seed yield, amino acids, photosynthesis, antioxidants, regulates C/N ratio, and plant hormone levels | [57] |
| Plant Species | Melatonin Treatment (µM) | Reference |
|---|---|---|
| Rice | 50–200 | [70] |
| Wheat | 10–500 | [71] |
| 1000 | [72] | |
| 50–500 | [73] | |
| Moringa | 100 | [74] |
| Pomegranate | 100 | [75] |
| Maize | 10–1000 | [52] |
| Broccoli | 20–80 | [76] |
| Kiwifruit | 50–200 | [77] |
| Sweet cherry | 50–500 | [78] |
| Banana | 40–80 | [79] |
| Pepper | 100 | [64] |
| Pear tree | 100 | [80] |
| Radish | 50–300 | [81] |
| Rapeseed | 500 | [82] |
| Tomato | 100 | [83] |
| Soybean | 50–100 | [53,55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnao, M.B.; Hernández-Ruiz, J.; Cano, A. Role of Melatonin and Nitrogen Metabolism in Plants: Implications under Nitrogen-Excess or Nitrogen-Low. Int. J. Mol. Sci. 2022, 23, 15217. https://doi.org/10.3390/ijms232315217
Arnao MB, Hernández-Ruiz J, Cano A. Role of Melatonin and Nitrogen Metabolism in Plants: Implications under Nitrogen-Excess or Nitrogen-Low. International Journal of Molecular Sciences. 2022; 23(23):15217. https://doi.org/10.3390/ijms232315217
Chicago/Turabian StyleArnao, Marino B., Josefa Hernández-Ruiz, and Antonio Cano. 2022. "Role of Melatonin and Nitrogen Metabolism in Plants: Implications under Nitrogen-Excess or Nitrogen-Low" International Journal of Molecular Sciences 23, no. 23: 15217. https://doi.org/10.3390/ijms232315217
APA StyleArnao, M. B., Hernández-Ruiz, J., & Cano, A. (2022). Role of Melatonin and Nitrogen Metabolism in Plants: Implications under Nitrogen-Excess or Nitrogen-Low. International Journal of Molecular Sciences, 23(23), 15217. https://doi.org/10.3390/ijms232315217








