C-Type Natriuretic Peptide (CNP) Could Improve Sperm Motility and Reproductive Function of Asthenozoospermia
Abstract
1. Introduction
2. Results
2.1. CNP Levels in Semen of Normal People and Patients with Asthenospermia
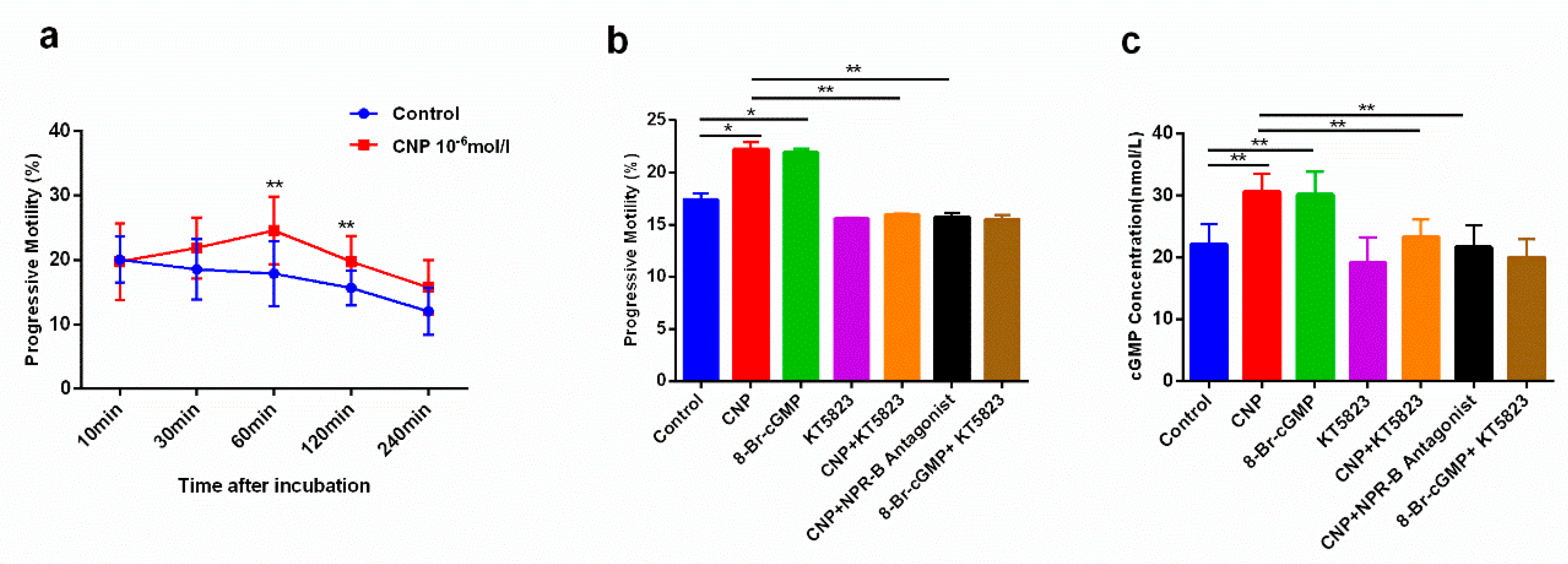
2.2. The Effect of CNP on Sperm Motility of Asthenospermia Patients In Vitro
2.3. The Effects of CNP on Reproductive System in Asthenospermia Mouse Model
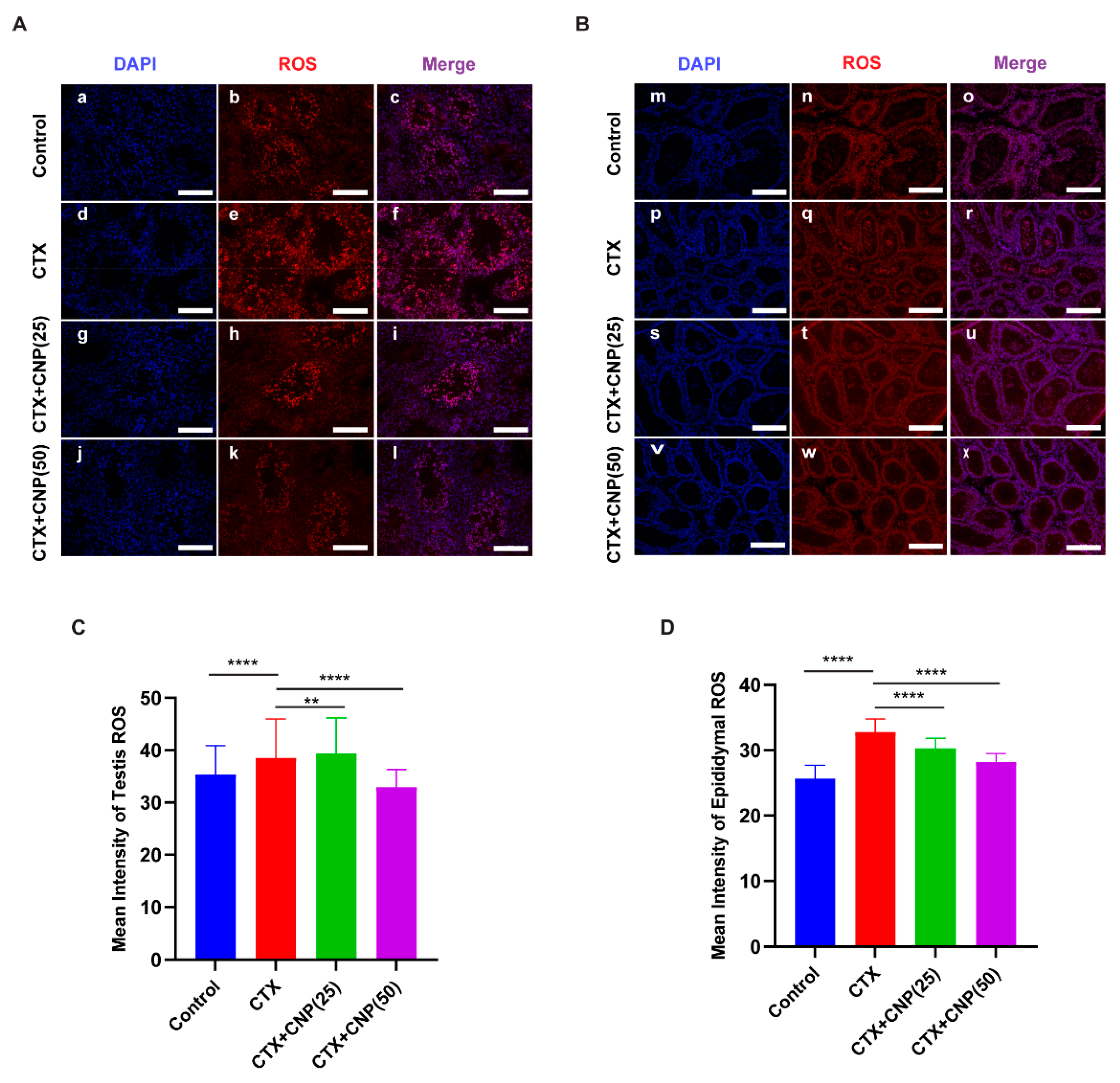
2.4. The Effects of CNP on ROS Level in Asthenospermia Mouse Model
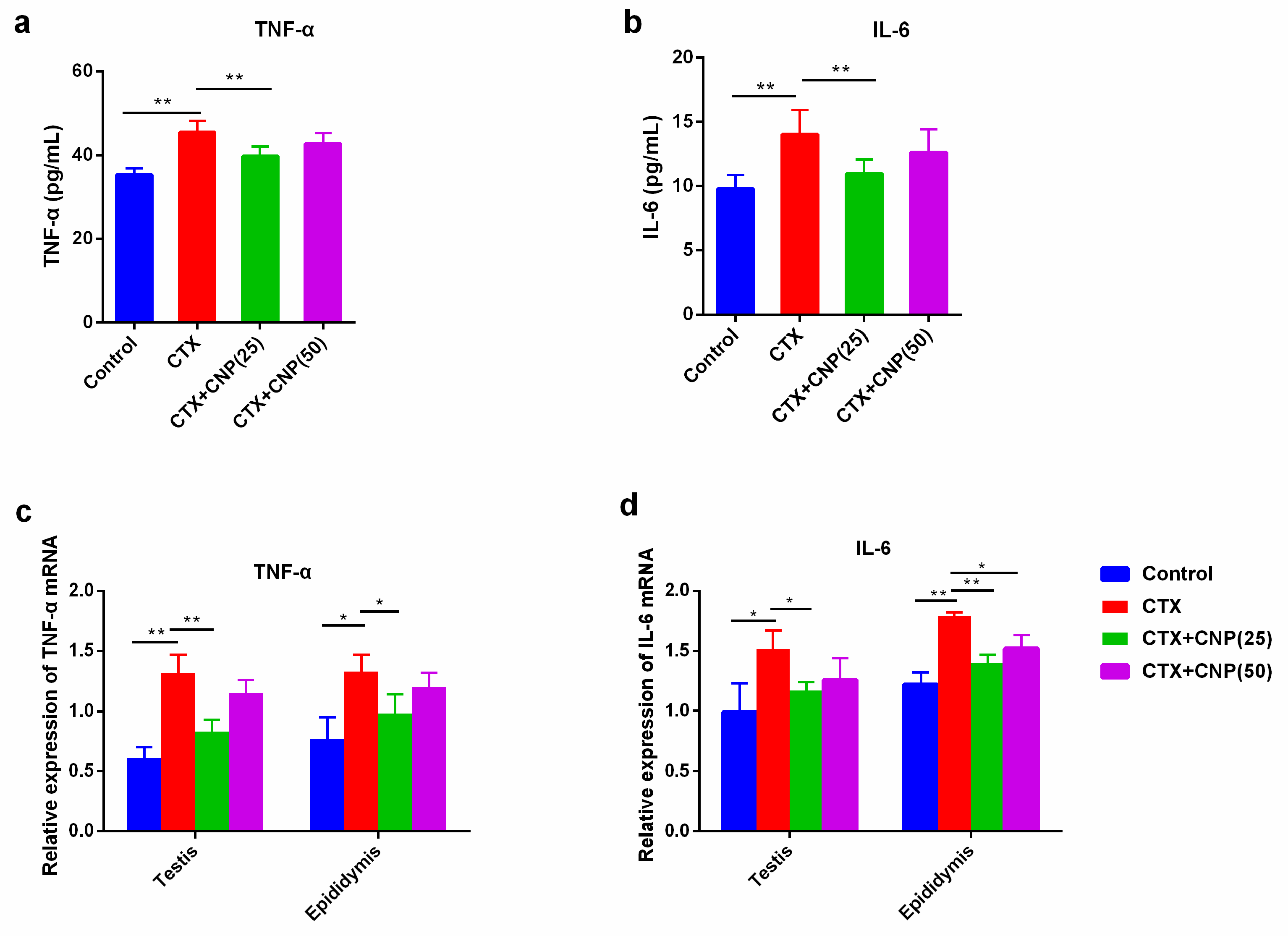
2.5. Effect of CNP on Immune Function in Asthenospermia Mouse Model
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Human Sperm Samples
4.3. Determination of cGMP Levels in Human Spermatozoa by ELISA
4.4. Establishment of Asthenospermia (Cyclophosphamide) Mouse Model
4.5. Statistics of Sperm Motility and Sperm Density
4.6. Measurement of Serum Testosterone and Oxidative Stress Levels by ELISA
4.7. Immunofluorescence
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobori, Y. Home testing for male factor infertility: A review of current options. Fertil. Steril. 2019, 111, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Punab, M.; Poolamets, O.; Paju, P.; Vihljajev, V.; Pomm, K.; Ladva, R.; Korrovits, P.; Laan, M. Causes of male infertility: A 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum. Reprod. 2016, 32, 18–31. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Wu, Z.G.; Chen, W.K.; Fei, Q.J.; Liu, Y.L.; Liu, X.D.; Huang, H.; Shang, X.J. Analysis of semen quality of 38,905 infertile male patients during 2008-2016 in Wenzhou, China. Asian J. Androl. 2021, 23, 314–318. [Google Scholar] [PubMed]
- Juyena, N.S.; Stelletta, C. Seminal Plasma: An Essential Attribute to Spermatozoa. J. Androl. 2011, 33, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Candenas, L.; Chianese, R. Exosome Composition and Seminal Plasma Proteome: A Promising Source of Biomarkers of Male Infertility. Int. J. Mol. Sci. 2020, 21, 7022. [Google Scholar] [CrossRef] [PubMed]
- Narciandi, F.; Lloyd, A.; Meade, K.G.; O’Farrelly, C. A novel subclass of bovine β-defensins links reproduction and immunology. Reprod. Fertil. Dev. 2014, 26, 769–777. [Google Scholar] [CrossRef]
- Zheng, P.; Zou, R.J.; Liu, Y.X. Source of plasminogen activator in rhesus monkey semen and its possible role in sperm capacitation. Sheng Li Xue Bao Acta Physiol. Sin. 2001, 53, 45–50. [Google Scholar]
- El-Sherbiny, H.R.; Fathi, M.; Samir, H.; Abdelnaby, E.A. Supplemental dietary curcumin improves testicular hemodynamics, testosterone levels, and semen quality in Baladi bucks in the non-breeding season. Theriogenology 2022, 188, 100–107. [Google Scholar] [CrossRef]
- Sudoh, T.; Minamino, N.; Kangawa, K.; Matsuo, H. C-Type natriuretic peptide (CNP): A new member of natriuretic peptide family identified in porcine brain. Biochem. Biophys. Res. Commun. 1990, 168, 863–870. [Google Scholar] [CrossRef]
- Chrisman, T.D.; Schulz, S.; Potter, L.R.; Garbers, D.L. Seminal plasma factors that cause large elevations in cellular cyclic GMP are C-type natriuretic peptides. J. Biol. Chem. 1993, 268, 3698–3703. [Google Scholar] [CrossRef]
- Nielsen, S.J.; Rehfeld, J.F.; Pedersen, F.; Kastrup, J.; Videbaek, R.; Goetze, J.P. Measurement of Pro-C-Type Natriuretic Peptide in Plasma. Clin. Chem. 2005, 51, 2173–2176. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nielsen, S.J.; Gøtze, J.P.; Jensen, H.L.; Rehfeld, J.F. ProCNP and CNP are expressed primarily in male genital organs. Regul. Pept. 2008, 146, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Xu, X.; Zhang, Y.; Wang, Y.; Hao, X.; Zhao, Y.; Qiao, J.; Xia, G.; Zhang, M. Natriuretic peptide type C induces sperm attraction for fertilization in mouse. Sci. Rep. 2017, 7, 39711. [Google Scholar] [CrossRef]
- Xia, H.; Chen, Y.; Wu, K.J.; Zhao, H.; Xiong, C.L.; Huang, D.H. Role of C-type natriuretic peptide in the function of normal human sperm. Asian J. Androl. 2016, 18, 80–84. [Google Scholar] [PubMed]
- Wu, K.; Mei, C.; Chen, Y.; Guo, L.; Yu, Y.; Huang, D. C-type natriuretic peptide regulates sperm capacitation by the cGMP/PKG signalling pathway via Ca2+ influx and tyrosine phosphorylation. Reprod. Biomed. Online 2019, 38, 289–299. [Google Scholar] [CrossRef]
- Huang, D.-H.; Zhang, S.-W.; Zhao, H.; Zhang, L. The role of C-type natriuretic peptide in rat testes during spermatogenesis. Asian J. Androl. 2010, 13, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Tomasiuk, R.; Faundez, R.; Cacko, M.; Mikaszewska-Sokolewicz, M.; Cacko, A.; Rabijewski, M. NT-proCNP as a new indicator of asthenozoospermia. Adv. Med. Sci. 2017, 62, 74–77. [Google Scholar] [CrossRef]
- Saberiyan, M.; Karimi, E.; Safi, A.; Movahhed, P.; Dehdehi, L.; Haririan, N.; Mirfakhraie, R. Circular RNAs: Novel Biomarkers in Spermatogenesis Defects and Male Infertility. Reprod. Sci. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Tu, C.; Wang, W.; Hu, T.; Lu, G.; Lin, G.; Tan, Y.Q. Genetic underpinnings of asthenozoospermia. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101472. [Google Scholar] [CrossRef]
- Shahrokhi, S.Z.; Salehi, P.; Alyasin, A.; Taghiyar, S.; Deemeh, M.R. Asthenozoospermia: Cellular and molecular contributing factors and treatment strategies. Andrologia 2019, 52, e13463. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Singh, R.; Cannarella, R.; Sengupta, P.; Dutta, S. Coenzyme Q10, oxidative stress, and male infertility: A review. Clin. Exp. Reprod. Med. 2021, 48, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Yasoda, A. Physiological and Pathophysiological Effects of C-Type Natriuretic Peptide on the Heart. Biology 2022, 11, 911. [Google Scholar] [CrossRef] [PubMed]
- Regan, J.T.; Mirczuk, S.M.; Scudder, C.J.; Stacey, E.; Khan, S.; Worwood, M.; Powles, T.; Dennis-Beron, J.S.; Ginley-Hidinger, M.; McGonnell, I.M.; et al. Sensitivity of the Natriuretic Peptide/cGMP System to Hyperammonaemia in Rat C6 Glioma Cells and GPNT Brain Endothelial Cells. Cells 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Pernomian, L.; do Prado, A.F.; Silva, B.R.; de Paula, T.D.; Grando, M.D.; Bendhack, L.M. C-type natriuretic peptide-induced relaxation through cGMP-dependent protein kinase and SERCA activation is impaired in two kidney-one clip rat aorta. Life Sci. 2021, 272, 119223. [Google Scholar] [CrossRef]
- Rignault-Clerc, S.; Bielmann, C.; Liaudet, L.; Waeber, B.; Feihl, F.; Rosenblatt-Velin, N. Natriuretic Peptide Receptor B modulates the proliferation of the cardiac cells expressing the Stem Cell Antigen-1. Sci. Rep. 2017, 7, 41936. [Google Scholar] [CrossRef]
- Lumsden, N.G.; Khambata, R.S.; Hobbs, A.J. C-type Natriuretic Peptide (CNP): Cardiovascular Roles and Potential as a Therapeutic Target. Curr. Pharm. Des. 2010, 16, 4080–4088. [Google Scholar] [CrossRef]
- Kulik-Rechberger, B.; Trojanowska-Szostek, M. C-type natriuretic peptide and its contribution to bone growth. Ann. Agric. Environ. Med. 2021, 29, 252–257. [Google Scholar] [CrossRef]
- Sato, Y.; Cheng, Y.; Kawamura, K.; Takae, S.; Hsueh, A.J. C-Type Natriuretic Peptide Stimulates Ovarian Follicle Development. Mol. Endocrinol. 2012, 26, 1158–1166. [Google Scholar] [CrossRef]
- Ghobadi, E.; Moloudizargari, M.; Asghari, M.H.; Abdollahi, M. The mechanisms of cyclophosphamide-induced testicular toxicity and the protective agents. Expert Opin. Drug Metab. Toxicol. 2016, 13, 525–536. [Google Scholar] [CrossRef]
- Wang, Y.N.; Wang, B.; Liang, M.; Han, C.Y.; Zhang, B.; Cai, J.; Sun, W.; Xing, G.G. Down-regulation of CatSper1 channel in epididymal spermatozoa contributes to the pathogenesis of asthenozoospermia, whereas up-regulation of the channel by Sheng-Jing-San treatment improves the sperm motility of asthenozoospermia in rats. Fertil. Steril. 2012, 99, 579–587. [Google Scholar] [CrossRef]
- Elangovan, N.; Chiou, T.J.; Tzeng, W.F.; Chu, S.T. Cyclophosphamide treatment causes impairment of sperm and its fertilizing ability in mice. Toxicology 2006, 222, 60–70. [Google Scholar] [CrossRef] [PubMed]
- El-Gehani, F.; Tena-Sempere, M.; Ruskoaho, H.; Huhtaniemi, I. Natriuretic Peptides Stimulate Steroidogenesis in the Fetal Rat Testis1. Biol. Reprod. 2001, 65, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lei, L.; Zhao, Q.; Gong, Y.; Guan, G.; Huang, S. C-Type Natriuretic Peptide/Natriuretic Peptide Receptor 2 Is Involved in Cell Proliferation and Testosterone Production in Mouse Leydig Cells. World J. Men’s Health 2019, 37, 186–198. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Nogueira-Ferreira, R.; Amado, F.; Alves, M.G.; Oliveira, P.F. Exploring the Role of Oxidative Stress in Sperm Motility: A Proteomic Network Approach. Antioxid. Redox Signal. 2021; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.N.; Colone, M.; Gambioli, R.; Stringaro, A.; Unfer, V. Oxidative Stress and Male Fertility: Role of Antioxidants and Inositols. Antioxidants 2021, 10, 1283. [Google Scholar] [CrossRef]
- Dorostghoal, M.; Kazeminejad, S.R.; Shahbazian, N.; Pourmehdi, M.; Jabbari, A. Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia 2017, 49, e12762. [Google Scholar] [CrossRef]
- Kopa, Z.; Keszthelyi, M.; Sofikitis, N. Administration of Antioxidants in the Infertile Male: When it may have a Beneficial Effect? Curr. Pharm. Des. 2021, 27, 2665–2668. [Google Scholar] [CrossRef]
- Zhenwei, J.; Xianhua, Z. Pre-IVM treatment with C-type natriuretic peptide in the presence of cysteamine enhances bovine oocytes antioxidant defense ability and developmental competence in vitro. Iran. J. Vet. Res. 2019, 20, 173–179. [Google Scholar]
- Comhaire, F.H.; Mahmoud, A.M.; Depuydt, C.E.; Zalata, A.A.; Christophe, A.B. Mechanisms and effects of male genital tract infection on sperm quality and fertilizing potential: The andrologist’s viewpoint. Hum. Reprod. Update 1999, 5, 393–398. [Google Scholar] [CrossRef]
- Azimi, A.S.; Soleimani Mehranjani, M.; Shariatzadeh, S.M.A.; Noshad Kamran, A.; Ghafarizadeh, A.A. Evaluating the therapeutic effect and toxicity of theophylline in infertile men with asthenoteratozoospermia: A double-blind, randomized clinical trial study. Drug Chem. Toxicol. 2021, 1–8. [Google Scholar] [CrossRef]
- Ďuračka, M.; Belić, L.; Tokárová, K.; Žiarovská, J.; Kačániová, M.; Lukáč, N.; Tvrdá, E. Bacterial communities in bovine ejaculates and their impact on the semen quality. Syst. Biol. Reprod. Med. 2021, 67, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Nojiri, T.; Hosoda, H.; Ishikane, S.; Shintani, Y.; Inoue, M.; Miyazato, M.; Okumura, M.; Kangawa, K. C-type natriuretic peptide attenuates lipopolysaccharide-induced acute lung injury in mice. J. Surg. Res. 2015, 194, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhao, J.; Yin, Y.; Wang, B.; Liu, Q.; Li, P.; Zhao, L.; Zhou, H. C-type natriuretic peptide attenuates LPS-induced endothelial activation: Involvement of p38, Akt, and NF-κB pathways. Amino Acids 2014, 46, 2653–2663. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Kang, Y.; Zhang, C.; He, C.; Liao, A.; Huang, D. C-Type Natriuretic Peptide Plays an Anti-Inflammatory Role in Rat Epididymitis Induced by UPEC. Front. Cell Infect. Microbiol. 2021, 11, 711842. [Google Scholar] [CrossRef] [PubMed]

| Basic Clinical Information | The Normal Group (n = 47) | The Asthenospermia Group (n = 45) |
|---|---|---|
| Age (years) | 30.20 ± 3.97 | 29.16 ± 5.01 |
| Semen volume (mL) | 3.63 ± 0.74 | 3.51 ± 0.97 |
| Sperm concentration (×106/mL) | 38.34 ± 11.64 | 27.79 ± 10.20 |
| Total number of sperm (×106) | 135.80 ± 41.78 | 95.75 ± 36.79 |
| Total motility (%) | 67.32 ± 14.55 | 29.42 ± 6.43 ** |
| VCL (μm/s) | 71.98 ± 20.58 | 56.47 ± 19.79 |
| VSL (μm/s) | 44.15 ± 15.18 | 32.19 ± 10.74 |
| VAP (μm/s) | 47.74 ± 14.64 | 37.20 ± 12.80 |
| CNP concentration (mean ± SD, pg/mL) | 259.75 ± 12.57 | 223.04 ± 20.91 ** |
| Control (n = 5) | CTX (n = 5) | CTX + CNP (25) (n = 5) | CTX + CNP (50) (n = 5) | |
|---|---|---|---|---|
| Body weight (g) | 24.22 ± 1.67 | 14.48 ± 1.78 | 17.04 ± 0.99 # | 15.45 ± 1.48 |
| Testis weight (mg) | 179.48 ± 9.48 | 104.50 ± 6.56 * | 131.55 ± 17.79 | 117.57 ± 27.86 |
| Testis index (mg/g b.w.) | 7.41 ± 0.71 | 7.23 ± 0.49 | 7.72 ± 0.98 | 7.61 ± 1.07 |
| Epididymis weight (mg) | 53.30 ± 6.46 | 21.04 ± 9.30 * | 34.59 ± 6.08 | 28.89 ± 13.72 |
| Epididymis index (mg/g b.w.) | 2.20 ± 0.25 | 1.45 ± 0.38 * | 2.03 ± 0.34 # | 1.87 ± 0.29 # |
| PR + NP (%) | 30.50 ± 2.30 | 18.55 ± 3.42 * | 24.25 ± 3.62 # | 22.81 ± 4.04 # |
| Sperm concentration (106 cells/mL/two epi.) | 3.61 ± 0.35 | 2.09 ± 0.41 * | 2.99 ± 0.48 # | 2.34 ± 0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Dong, X.; Fu, S.; Wang, X.; Li, H.; Song, G.; Huang, D. C-Type Natriuretic Peptide (CNP) Could Improve Sperm Motility and Reproductive Function of Asthenozoospermia. Int. J. Mol. Sci. 2022, 23, 10370. https://doi.org/10.3390/ijms231810370
Li N, Dong X, Fu S, Wang X, Li H, Song G, Huang D. C-Type Natriuretic Peptide (CNP) Could Improve Sperm Motility and Reproductive Function of Asthenozoospermia. International Journal of Molecular Sciences. 2022; 23(18):10370. https://doi.org/10.3390/ijms231810370
Chicago/Turabian StyleLi, Na, Xinyi Dong, Sen Fu, Xiaoyan Wang, Huaibiao Li, Ge Song, and Donghui Huang. 2022. "C-Type Natriuretic Peptide (CNP) Could Improve Sperm Motility and Reproductive Function of Asthenozoospermia" International Journal of Molecular Sciences 23, no. 18: 10370. https://doi.org/10.3390/ijms231810370
APA StyleLi, N., Dong, X., Fu, S., Wang, X., Li, H., Song, G., & Huang, D. (2022). C-Type Natriuretic Peptide (CNP) Could Improve Sperm Motility and Reproductive Function of Asthenozoospermia. International Journal of Molecular Sciences, 23(18), 10370. https://doi.org/10.3390/ijms231810370




