Finding the Right Heavy Chains for Immunostimulatory Antibodies
Abstract
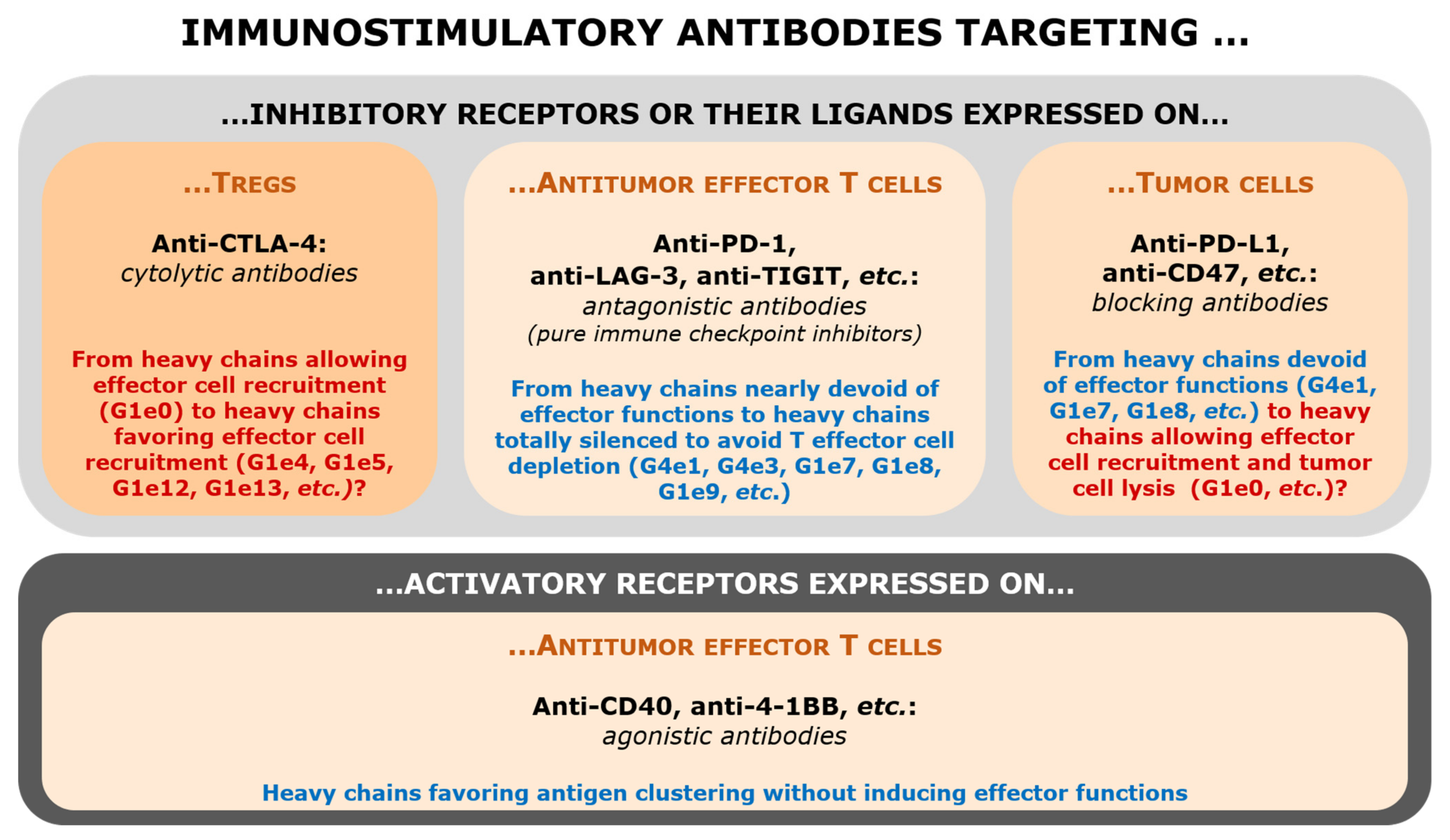
1. Introduction: The Perimeter of Immunostimulatory Antibodies
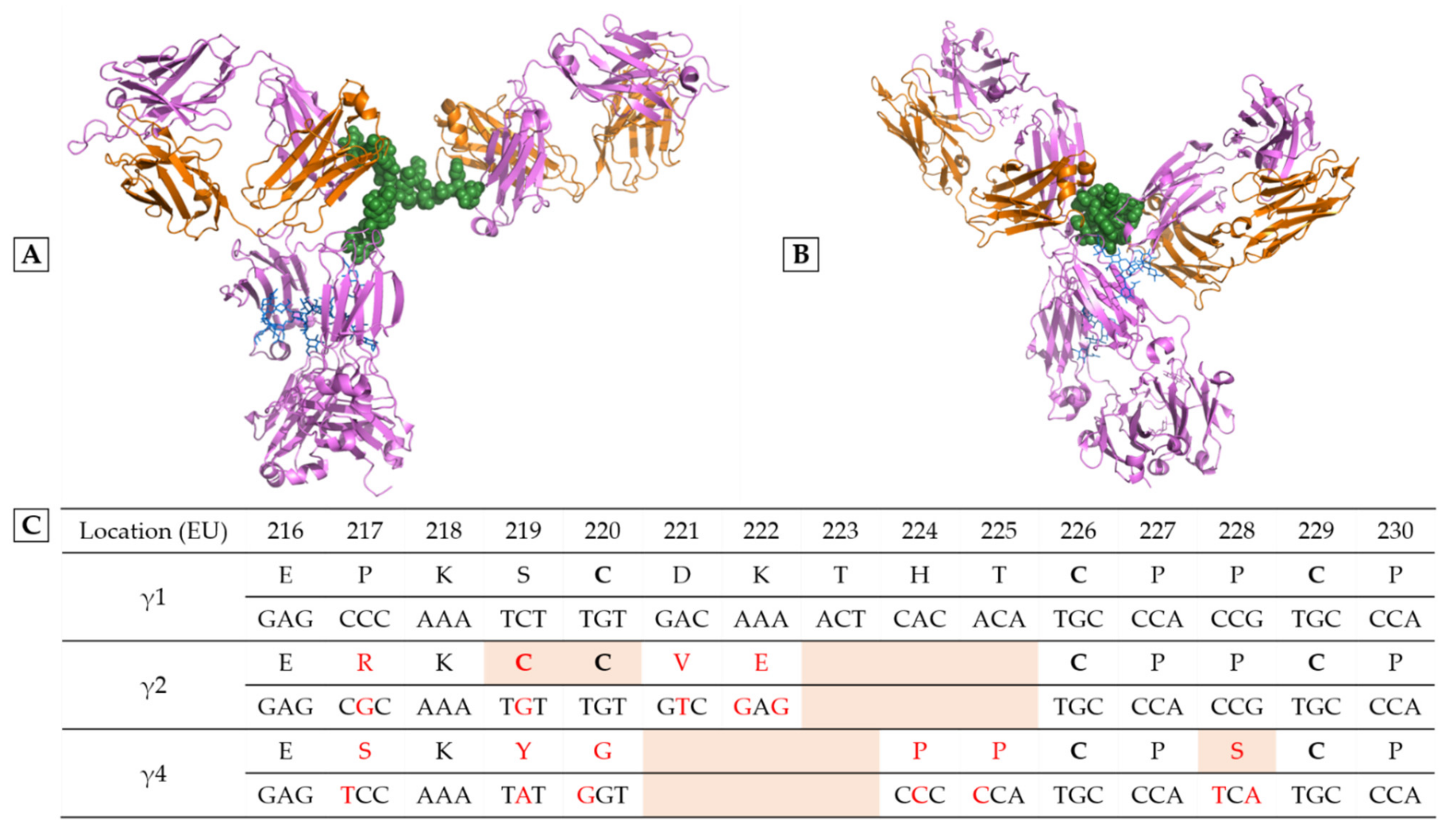
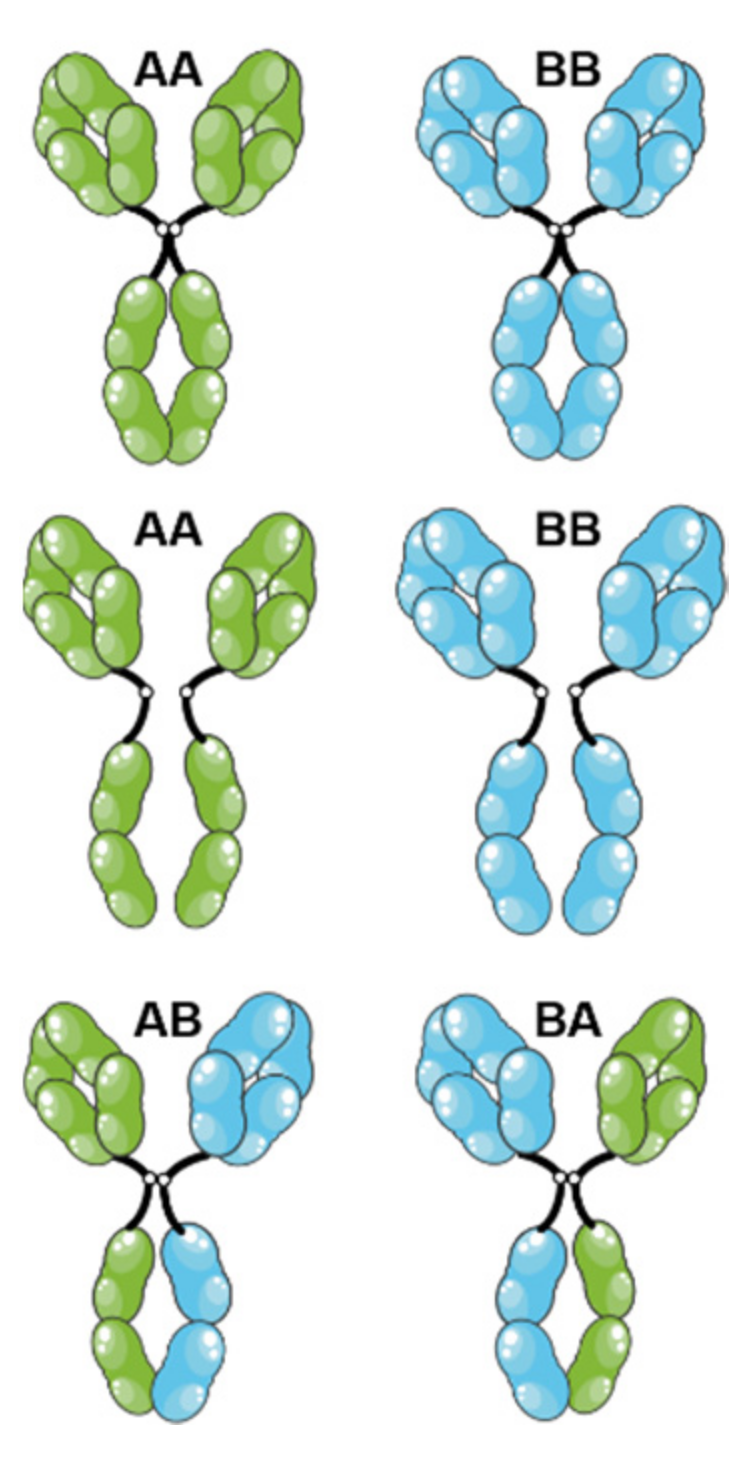
2. Playing with IgG Subclasses to Adjust the Pharmacological Effects
3. Targeting of Inhibitory Receptors (Immune Checkpoints) or Their Ligands
3.1. Anti-CTLA-4 Antibodies
3.2. Anti-PD-1 Antibodies
3.3. Anti-TIGIT and Anti-LAG-3 Antibodies: Antagonist Antibodies?
3.4. Anti-PD-L1 (and Anti-CD47) Antibodies
4. Agonistic Antibodies Targeting Lymphocyte Activation Receptors
4.1. Anti-CD40 Antibodies
4.2. Anti-4-1BB Antibodies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watier, H. Biotherapies, immunotherapies, targeted therapies, biopharmaceuticals… which word should be used? Med. Sci. 2014, 30, 567–575. [Google Scholar] [CrossRef][Green Version]
- Abès, R.; Gélizé, E.; Fridman, W.H.; Teillaud, J.-L. Long-Lasting Antitumor Protection by Anti-CD20 Antibody through Cellular Immune Response. Blood 2010, 116, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Tabs- Therapeutic Antibody Database. Available online: https://tabs.craic.com/ (accessed on 28 July 2022).
- IMGT/MAb-DB. Available online: https://www.imgt.org/mAb-DB/ (accessed on 28 July 2022).
- Lists of Recommended and Proposed INNs. Available online: https://www.who.int/teams/health-product-and-policy-standards/inn/inn-lists (accessed on 28 July 2022).
- ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ (accessed on 28 July 2022).
- Vermorken, J.B.; Stöhlmacher-Williams, J.; Davidenko, I.; Licitra, L.; Winquist, E.; Villanueva, C.; Foa, P.; Rottey, S.; Skladowski, K.; Tahara, M.; et al. Cisplatin and Fluorouracil with or without Panitumumab in Patients with Recurrent or Metastatic Squamous-Cell Carcinoma of the Head and Neck (SPECTRUM): An Open-Label Phase 3 Randomised Trial. Lancet Oncol. 2013, 14, 697–710. [Google Scholar] [CrossRef]
- Isaacs, J.D.; Wing, M.G.; Greenwood, J.D.; Hazleman, B.L.; Hale, G.; Waldmann, H. A Therapeutic Human IgG4 Monoclonal Antibody That Depletes Target Cells in Humans. Clin. Exp. Immunol. 1996, 106, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Pottier, J.; Chastang, R.; Dumet, C.; Watier, H. Rethinking the INN System for Therapeutic Antibodies. MAbs 2017, 9, 5–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ribas, A. Anti-CTLA4 Antibody Clinical Trials in Melanoma. Update Cancer Ther. 2007, 2, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Keler, T.; Halk, E.; Vitale, L.; O’Neill, T.; Blanset, D.; Lee, S.; Srinivasan, M.; Graziano, R.F.; Davis, T.; Lonberg, N.; et al. Activity and Safety of CTLA-4 Blockade Combined with Vaccines in Cynomolgus Macaques. J. Immunol. 2003, 171, 6251–6259. [Google Scholar] [CrossRef]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 Antibodies of IgG2a Isotype Enhance Antitumor Activity through Reduction of Intratumoral Regulatory T Cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef]
- Ingram, J.R.; Blomberg, O.S.; Rashidian, M.; Ali, L.; Garforth, S.; Fedorov, E.; Fedorov, A.A.; Bonanno, J.B.; Le Gall, C.; Crowley, S.; et al. Anti–CTLA-4 Therapy Requires an Fc Domain for Efficacy. Proc. Natl. Acad. Sci. USA 2018, 115, 3912–3917. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Tang, F.; Liu, M.; Su, J.; Zhang, Y.; Wu, W.; Devenport, M.; Lazarski, C.A.; Zhang, P.; Wang, X.; et al. A Reappraisal of CTLA-4 Checkpoint Blockade in Cancer Immunotherapy. Cell Res. 2018, 28, 416–432. [Google Scholar] [CrossRef]
- Romano, E.; Kusio-Kobialka, M.; Foukas, P.G.; Baumgaertner, P.; Meyer, C.; Ballabeni, P.; Michielin, O.; Weide, B.; Romero, P.; Speiser, D.E. Ipilimumab-Dependent Cell-Mediated Cytotoxicity of Regulatory T Cells Ex Vivo by Nonclassical Monocytes in Melanoma Patients. Proc. Natl. Acad. Sci. USA 2015, 112, 6140–6145. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Subudhi, S.K.; Blando, J.; Scutti, J.; Vence, L.; Wargo, J.; Allison, J.P.; Ribas, A.; Sharma, P. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3+ Regulatory T Cells (Tregs) in Human Cancers. Clin. Cancer Res. 2019, 25, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Quezada, S.A.; Peggs, K.S. Lost in Translation: Deciphering the Mechanism of Action of Anti-Human CTLA-4. Clin. Cancer Res. 2019, 25, 1130–1132. [Google Scholar] [CrossRef]
- Cartron, G.; Dacheux, L.; Salles, G.; Solal-Celigny, P.; Bardos, P.; Colombat, P.; Watier, H. Therapeutic Activity of Humanized Anti-CD20 Monoclonal Antibody and Polymorphism in IgG Fc Receptor FcgammaRIIIa Gene. Blood 2002, 99, 754–758. [Google Scholar] [CrossRef]
- Arce Vargas, F.; Furness, A.J.S.; Litchfield, K.; Joshi, K.; Rosenthal, R.; Ghorani, E.; Solomon, I.; Lesko, M.H.; Ruef, N.; Roddie, C.; et al. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell 2018, 33, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Sanseviero, E.; O’Brien, E.M.; Karras, J.R.; Shabaneh, T.B.; Aksoy, B.A.; Xu, W.; Zheng, C.; Yin, X.; Xu, X.; Karakousis, G.C.; et al. Anti-CTLA-4 Activates Intratumoral NK Cells and Combined with IL15/IL15Rα Complexes Enhances Tumor Control. Cancer Immunol. Res. 2019, 7, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Tanaka, A.; Kibayashi, T.; Tanemura, A.; Sugiyama, D.; Wing, J.B.; Lim, E.L.; Teng, K.W.W.; Adeegbe, D.; Newell, E.W.; et al. Differential Control of Human Treg and Effector T Cells in Tumor Immunity by Fc-Engineered Anti-CTLA-4 Antibody. Proc. Natl. Acad. Sci. USA 2019, 116, 609–618. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.; Bullock, A.; Tsimberidou, A.; Mahadevan, D.; Wilky, B.; Twardowski, P.; Bockorny, B.; Moser, J.; Feliu, W.O.; Grossman, J.; et al. 479 AGEN1181, an Fc-Enhanced Anti-CTLA-4 Antibody, Alone and in Combination with Balstilimab (Anti-PD-1) in Patients with Advanced Solid Tumors: Initial Phase I Results. J. Immunother. Cancer 2021, 9, 479. [Google Scholar] [CrossRef]
- Waight, J.; Manrique, M.; Gombos, R.; Costa, M.; Iyer, P.; Vincent, S.; Ward, R.; Paltrinieri, E.; Chand, D.; Wilson, N.; et al. Preclinical Functional Characterization of AGEN1181, a Clinical Stage Fc-Engineered Anti-CTLA-4 Antibody for the Treatment of Patients with Early and Advanced Malignancies. JCO 2019, 37, e14126. [Google Scholar] [CrossRef]
- Waight, J.D.; Chand, D.; Dietrich, S.; Gombos, R.; Horn, T.; Gonzalez, A.M.; Manrique, M.; Swiech, L.; Morin, B.; Brittsan, C.; et al. Selective FcγR Co-Engagement on APCs Modulates the Activity of Therapeutic Antibodies Targeting T Cell Antigens. Cancer Cell 2018, 33, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Oldham, R.J.; Teal, E.; Beers, S.A.; Cragg, M.S. Fc-Engineering for Modulated Effector Functions—Improving Antibodies for Cancer Treatment. Antibodies 2020, 9, 64. [Google Scholar] [CrossRef] [PubMed]
- Arce Vargas, F.; Furness, A.J.S.; Solomon, I.; Joshi, K.; Mekkaoui, L.; Lesko, M.H.; Miranda Rota, E.; Dahan, R.; Georgiou, A.; Sledzinska, A.; et al. Fc-Optimized Anti-CD25 Depletes Tumor-Infiltrating Regulatory T Cells and Synergizes with PD-1 Blockade to Eradicate Established Tumors. Immunity 2017, 46, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Bulliard, Y.; Jolicoeur, R.; Zhang, J.; Dranoff, G.; Wilson, N.S.; Brogdon, J.L. OX40 Engagement Depletes Intratumoral Tregs via Activating FcγRs, Leading to Antitumor Efficacy. Immunol. Cell Biol. 2014, 92, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Dahan, R.; Sega, E.; Engelhardt, J.; Selby, M.; Korman, A.J.; Ravetch, J.V. FcγRs Modulate the Anti-Tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell 2015, 28, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Song, X.; Xu, L.; Ma, J.; Zhang, Y.; Gong, W.; Zhang, Y.; Zhou, X.; Wang, Z.; Wang, Y.; et al. The Binding of an Anti-PD-1 Antibody to FcγRΙ Has a Profound Impact on Its Biological Functions. Cancer Immunol. Immunother. 2018, 67, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Pang, X.; Zhong, T.; Qu, T.; Chen, N.; Ma, S.; He, X.; Xia, D.; Wang, M.; Xia, M.; et al. Penpulimab, an Fc-Engineered IgG1 Anti-PD-1 Antibody, With Improved Efficacy and Low Incidence of Immune-Related Adverse Events. Front. Immunol. 2022, 13, 924542. [Google Scholar] [CrossRef]
- Dumet, C.; Pottier, J.; Gouilleux-Gruart, V.; Watier, H. Insights into the IgG Heavy Chain Engineering Patent Landscape as Applied to IgG4 Antibody Development. MAbs 2019, 11, 1341–1350. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Buijsse, A.O.; van den Bremer, E.T.J.; Verwilligen, A.Y.W.; Bleeker, W.K.; Thorpe, S.J.; Killestein, J.; Polman, C.H.; Aalberse, R.C.; Schuurman, J.; et al. Therapeutic IgG4 Antibodies Engage in Fab-Arm Exchange with Endogenous Human IgG4 in Vivo. Nat. Biotechnol. 2009, 27, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.; Duruisseaux, M.; Brevet, M.; Dumontet, C. How Can Immune Checkpoint Inhibitors Cause Hyperprogression in Solid Tumors? Front. Immunol. 2020, 11, 492. [Google Scholar] [CrossRef]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.; Okazaki, T. LAG-3: From Molecular Functions to Clinical Applications. J. Immunother. Cancer 2020, 8, e001014. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Burova, E.; Hermann, A.; Dai, J.; Ullman, E.; Halasz, G.; Potocky, T.; Hong, S.; Liu, M.; Allbritton, O.; Woodruff, A.; et al. Preclinical Development of the Anti-LAG-3 Antibody REGN3767: Characterization and Activity in Combination with the Anti-PD-1 Antibody Cemiplimab in Human PD-1xLAG-3–Knockin Mice. Mol. Cancer Ther. 2019, 18, 2051–2062. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan Nair, V.; Elkord, E. Immune Checkpoint Inhibitors in Cancer Therapy: A Focus on T-Regulatory Cells. Immunol. Cell Biol. 2018, 96, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive Correlates of Response to the Anti-PD-L1 Antibody MPDL3280A in Cancer Patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Oganesyan, V.; Gao, C.; Shirinian, L.; Wu, H.; Dall’Acqua, W.F. Structural Characterization of a Human Fc Fragment Engineered for Lack of Effector Functions. Acta Cryst. D Biol. Cryst. 2008, 64, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; Morrow, M.; Hammond, S.A.; Mulgrew, K.; Marcus, D.; Poon, E.; Watkins, A.; Mullins, S.; Chodorge, M.; Andrews, J.; et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol. Res. 2015, 3, 1052–1062. [Google Scholar] [CrossRef]
- Clynes, R.A.; Towers, T.L.; Presta, L.G.; Ravetch, J.V. Inhibitory Fc Receptors Modulate in Vivo Cytotoxicity against Tumor Targets. Nat. Med. 2000, 6, 443–446. [Google Scholar] [CrossRef]
- Boyerinas, B.; Jochems, C.; Fantini, M.; Heery, C.R.; Gulley, J.L.; Tsang, K.Y.; Schlom, J. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol. Res. 2015, 3, 1148–1157. [Google Scholar] [CrossRef]
- Neumann, M.; Murphy, N.; Seetharamu, N. The Evolving Role of PD-L1 Inhibition in Non-Small Cell Lung Cancer: A Review of Durvalumab and Avelumab. Cancer Med. J. 2022, 5, 31–45. [Google Scholar]
- Beaver, J.A.; Pazdur, R. The Wild West of Checkpoint Inhibitor Development. N. Engl. J. Med. 2022, 386, 1297–1301. [Google Scholar] [CrossRef]
- Goletz, C.; Lischke, T.; Harnack, U.; Schiele, P.; Danielczyk, A.; Rühmann, J.; Goletz, S. Glyco-Engineered Anti-Human Programmed Death-Ligand 1 Antibody Mediates Stronger CD8 T Cell Activation Than Its Normal Glycosylated and Non-Glycosylated Counterparts. Front. Immunol. 2018, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.S.; Viller, N.N.; Wong, M.; Pang, X.; Lin, G.H.Y.; Dodge, K.; Chai, V.; Chen, H.; Lee, V.; House, V.; et al. TTI-621 (SIRPαFc): A CD47-Blocking Innate Immune Checkpoint Inhibitor with Broad Antitumor Activity and Minimal Erythrocyte Binding. Clin. Cancer Res. 2017, 23, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, S.M.; Foran, J.M.; Maris, M.; Sawas, A.; Okada, C.; Feldman, T.A.; Minden, M.D.; Sokol, L.; Mei, M.; Flinn, I.W.; et al. Updates from Ongoing, First-in-Human Phase 1 Dose Escalation and Expansion Study of TTI-621, a Novel Biologic Targeting CD47, in Patients with Relapsed or Refractory Hematologic Malignancies. Blood 2020, 136, 41–43. [Google Scholar] [CrossRef]
- Wilson, N.S.; Yang, B.; Yang, A.; Loeser, S.; Marsters, S.; Lawrence, D.; Li, Y.; Pitti, R.; Totpal, K.; Yee, S.; et al. An Fcγ Receptor-Dependent Mechanism Drives Antibody-Mediated Target-Receptor Signaling in Cancer Cells. Cancer Cell 2011, 19, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chan, H.T.C.; Orr, C.M.; Dadas, O.; Booth, S.G.; Dahal, L.N.; Penfold, C.A.; O’Brien, L.; Mockridge, C.I.; French, R.R.; et al. Complex Interplay between Epitope Specificity and Isotype Dictates the Biological Activity of Anti-Human CD40 Antibodies. Cancer Cell 2018, 33, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Bartholomaeus, P.; Semmler, L.Y.; Bukur, T.; Boisguerin, V.; Römer, P.S.; Tabares, P.; Chuvpilo, S.; Tyrsin, D.Y.; Matskevich, A.; Hengel, H.; et al. Cell Contact-Dependent Priming and Fc Interaction with CD32+ Immune Cells Contribute to the TGN1412-Triggered Cytokine Response. J. Immunol. 2014, 192, 2091–2098. [Google Scholar] [CrossRef]
- Barlesi, F.; Lolkema, M.; Rohrberg, K.S.; Hierro, C.; Marabelle, A.; Razak, A.A.; Teixeira, L.; Boni, V.; Miller, W.H.; Aggarwal, C.; et al. 291 Phase Ib Study of Selicrelumab (CD40 Agonist) in Combination with Atezolizumab (Anti-PD-L1) in Patients with Advanced Solid Tumors. J. Immunother. Cancer 2020, 8, A178. [Google Scholar] [CrossRef]
- White, A.L.; Chan, H.T.C.; French, R.R.; Willoughby, J.; Mockridge, C.I.; Roghanian, A.; Penfold, C.A.; Booth, S.G.; Dodhy, A.; Polak, M.E.; et al. Conformation of the Human Immunoglobulin G2 Hinge Imparts Superagonistic Properties to Immunostimulatory Anticancer Antibodies. Cancer Cell 2015, 27, 138–148. [Google Scholar] [CrossRef]
- Dahan, R.; Barnhart, B.C.; Li, F.; Yamniuk, A.P.; Korman, A.J.; Ravetch, J.V. Therapeutic Activity of Agonistic, Human Anti-CD40 Monoclonal Antibodies Requires Selective FcγR Engagement. Cancer Cell 2016, 29, 820–831. [Google Scholar] [CrossRef]
- De Vos, S.; Forero-Torres, A.; Ansell, S.M.; Kahl, B.; Cheson, B.D.; Bartlett, N.L.; Furman, R.R.; Winter, J.N.; Kaplan, H.; Timmerman, J.; et al. A Phase II Study of Dacetuzumab (SGN-40) in Patients with Relapsed Diffuse Large B-Cell Lymphoma (DLBCL) and Correlative Analyses of Patient-Specific Factors. J. Hematol. Oncol. 2014, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.M.; Kimberlin, C.R.; Roe-Zurz, Z.; Zhang, P.; Xu, A.; Liao-Chan, S.; Sen, D.; Nager, A.R.; Oakdale, N.S.; Brown, C.; et al. Structure of the 4-1BB/4-1BBL Complex and Distinct Binding and Functional Properties of Utomilumab and Urelumab. Nat. Commun. 2018, 9, 4679. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, F.; Wu, Y.; Cheng, C.; Han, P.; Wang, J.; Yang, X. Optimization of 4-1BB Antibody for Cancer Immunotherapy by Balancing Agonistic Strength with FcγR Affinity. Nat. Commun. 2019, 10, 2141. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Harb, W.A.; Patel, S.P.; Lu, C.; Halperin, D.M.; Hsu, Y.H.; Shi, N.; Yamamura, Y.; Tang, T.; Jiang, L.; et al. Early Safety and Efficacy from a Phase I Open-Label Clinical Trial of CD137(4-1BB) Agonistic Antibody LVGN6051 as Monotherapy and in Combination with Pembrolizumab. JCO 2021, 39, 2521. [Google Scholar] [CrossRef]
- Zhang, D.; Goldberg, M.V.; Chiu, M.L. Fc Engineering Approaches to Enhance the Agonism and Effector Functions of an Anti-OX40 Antibody. J. Biol. Chem. 2016, 291, 27134–27146. [Google Scholar] [CrossRef]
- Lejeune, J.; Brachet, G.; Watier, H. Evolutionary Story of the Low/Medium-Affinity IgG Fc Receptor Gene Cluster. Front. Immunol. 2019, 10, 1297. [Google Scholar] [CrossRef]
- Ramdani, Y.; Lamamy, J.; Watier, H.; Gouilleux-Gruart, V. Monoclonal Antibody Engineering and Design to Modulate FcRn Activities: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 9604. [Google Scholar] [CrossRef]
| Ge Numbering | Heavy Chain Variations (EU Numbering) 2 | INN (Year of First Approval in the EU, USA or JP) |
|---|---|---|
| G1e0 | No variation | Many examples, notably: IPILIMUMAB (2011), AVELUMAB (2017) |
| G1e1 | Lacks CH1 + C220S C226S C229S P238S substitutions | abatacept (2005), belatacept (2011) |
| G1e2 | Lacks CH1 + Production in E.coli (aglycosylation) | romiplostim (2008) |
| G1e3 | Lack CH1 + Deletion of the five first amino acids of the hinge region | aflibercept (2011), efmoroctocog α (2014), eftrenonacog α (2014) |
| G1e4 | Hypo- or afucosylation | mogamulizumab (2012), benralizumab (2017), naxitamab (2021), inebilizumab (2022) |
| G1e5 | Addition of a bisecting GlcNAc also indirectly inducing hypo- or afucosylation | obinutuzumab (2013) |
| G1e6 | F126L substitution | ramucirumab (2014), necitumumab (2015) |
| G1e7 | L235A G237A substitutions | vedolizumab (2014) |
| G1e8 | N297A substitution | ATEZOLIZUMAB (2016) |
| G1e9 | L234F L235E P331S substitutions | DURVALUMAB (2017) |
| G1e10 | L234A L235A substitutions | etesevimab (2021), risankizumab (2019) |
| G1e11 | K213A N297A substitutions | eptinezumab (2020) |
| G1e12 | S239D K274Q Y296F Y300F L309V I332E A339T V397M substitutions | tafasitamab (2020) |
| G1e13 | L235V F243L R292P Y300L P396L substitutions | margetuximab (2020) |
| G1e14 | F405L substitution and afucosylation | amivantamab chain A (2021) |
| K409R substitution and afucosylation | amivantamab chain B (2021) | |
| G1e15 | L234F L235E M252Y S254T T256E P331S substitutions | tixagevimab/cilgavimab (2021) |
| G1e16 | M428L N434S substitutions | sotrovimab (2021) |
| G1e17 | M252Y S254T T256E H433K N434F susbtitutions | efgartigimod (2021) |
| G1e18 | N297G T366W substitutions (chain A) | mosunetuzumab (2022) |
| N297G T366S L368A Y407V substitutions (chain B) |
| Ge Numbering | Heavy Chain Variations (EU Numbering) 2 | INN (Year of First Approval in the EU, USA or JP) |
|---|---|---|
| G4e0 | No variation | natalizumab (2004) |
| G4e1 | S228P substitution | gemtuzumab ozogamicin (2000), PEMBROLIZUMAB (2014), NIVOLUMAB (2014), CEMIPLIMAB (2018), etc. |
| G4e2 | Hybrid IgG2 (up to T260)/IgG4 | eculizumab (2017) |
| G4e3 | S228P, F234A and L235A substitutions | dulaglutide (2014), galcanezumab (2018) |
| G4e4 | K196Q, S228P, F296Y, K439E and L445P substitutions plus removal of G446 | emicizumab chain A (2017) |
| K196Q, S228P, F296Y, E356K, H435R and L445P substitutions plus removal of G446 | emicizumab chain B (2017) | |
| G4e5 | Hybrid IgG2 (before T260)/IgG4 (after) and M428L, N434S substitutions | ravulizumab (2018) |
| G4e6 | S228P, L235E substitutions | SUTIMLIMAB (2022) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boulard, P.; Gouilleux-Gruart, V.; Watier, H. Finding the Right Heavy Chains for Immunostimulatory Antibodies. Int. J. Mol. Sci. 2022, 23, 10367. https://doi.org/10.3390/ijms231810367
Boulard P, Gouilleux-Gruart V, Watier H. Finding the Right Heavy Chains for Immunostimulatory Antibodies. International Journal of Molecular Sciences. 2022; 23(18):10367. https://doi.org/10.3390/ijms231810367
Chicago/Turabian StyleBoulard, Pierre, Valérie Gouilleux-Gruart, and Hervé Watier. 2022. "Finding the Right Heavy Chains for Immunostimulatory Antibodies" International Journal of Molecular Sciences 23, no. 18: 10367. https://doi.org/10.3390/ijms231810367
APA StyleBoulard, P., Gouilleux-Gruart, V., & Watier, H. (2022). Finding the Right Heavy Chains for Immunostimulatory Antibodies. International Journal of Molecular Sciences, 23(18), 10367. https://doi.org/10.3390/ijms231810367








