Receptor-Mediated AKT/PI3K Signalling and Behavioural Alterations in Zebrafish Larvae Reveal Association between Schizophrenia and Opioid Use Disorder
Abstract
:1. Introduction
2. Results
2.1. Systemic Toxicity
2.2. Startle Habituation Response
2.3. Effects of Drugs on Gene Expression
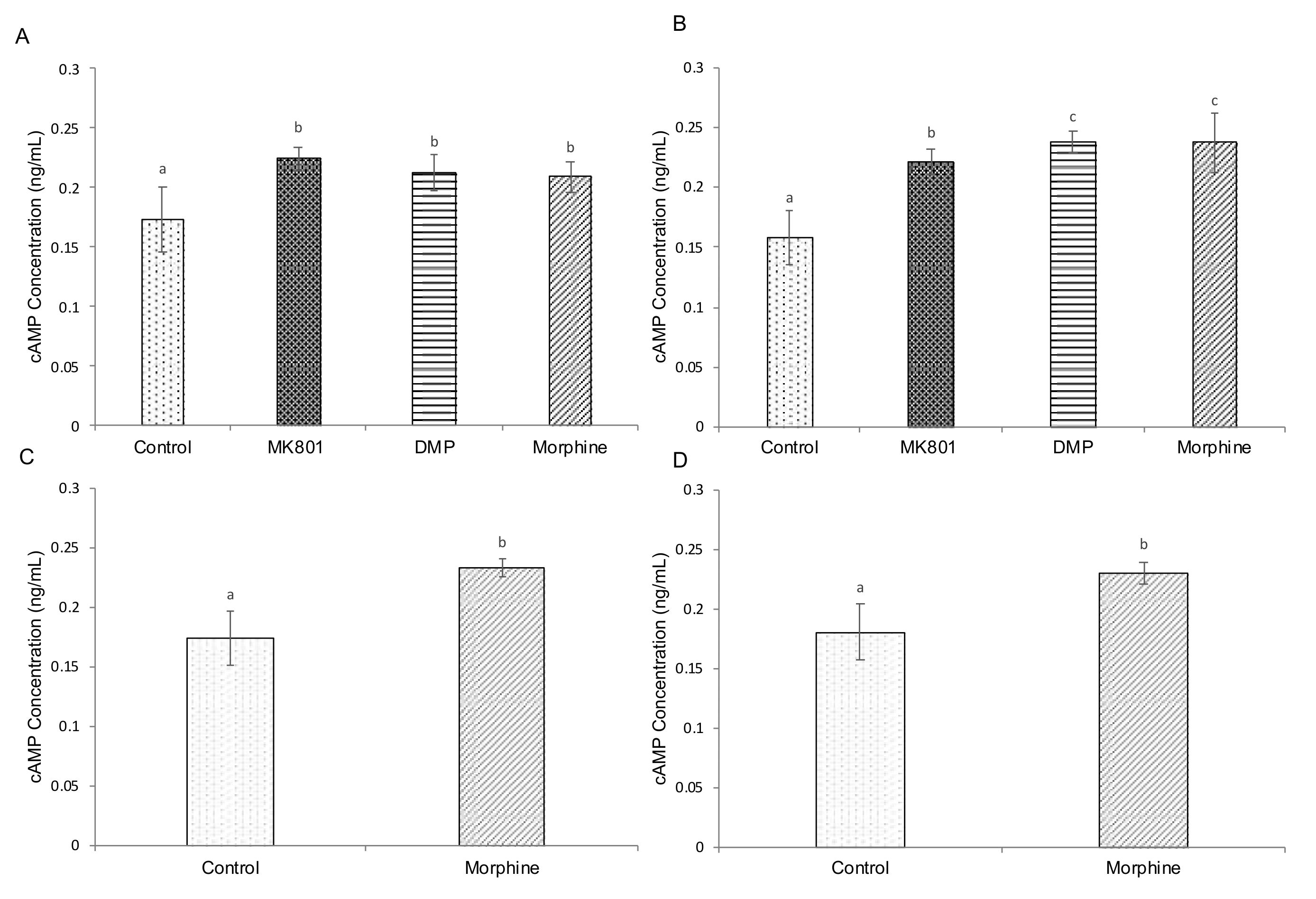
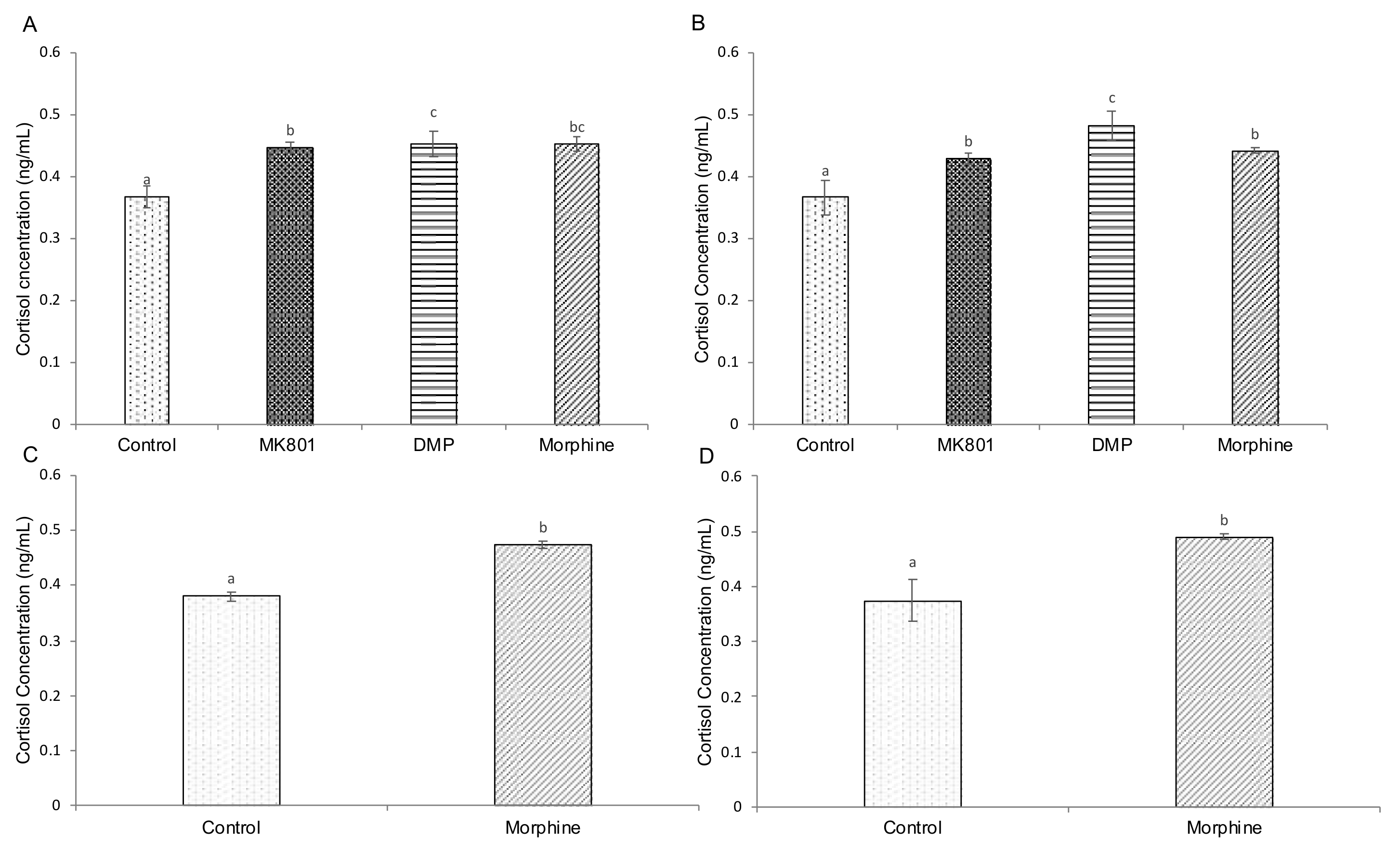
2.4. Effects of Drugs on cAMP, Cortisol and Serotonin-Based Signalling
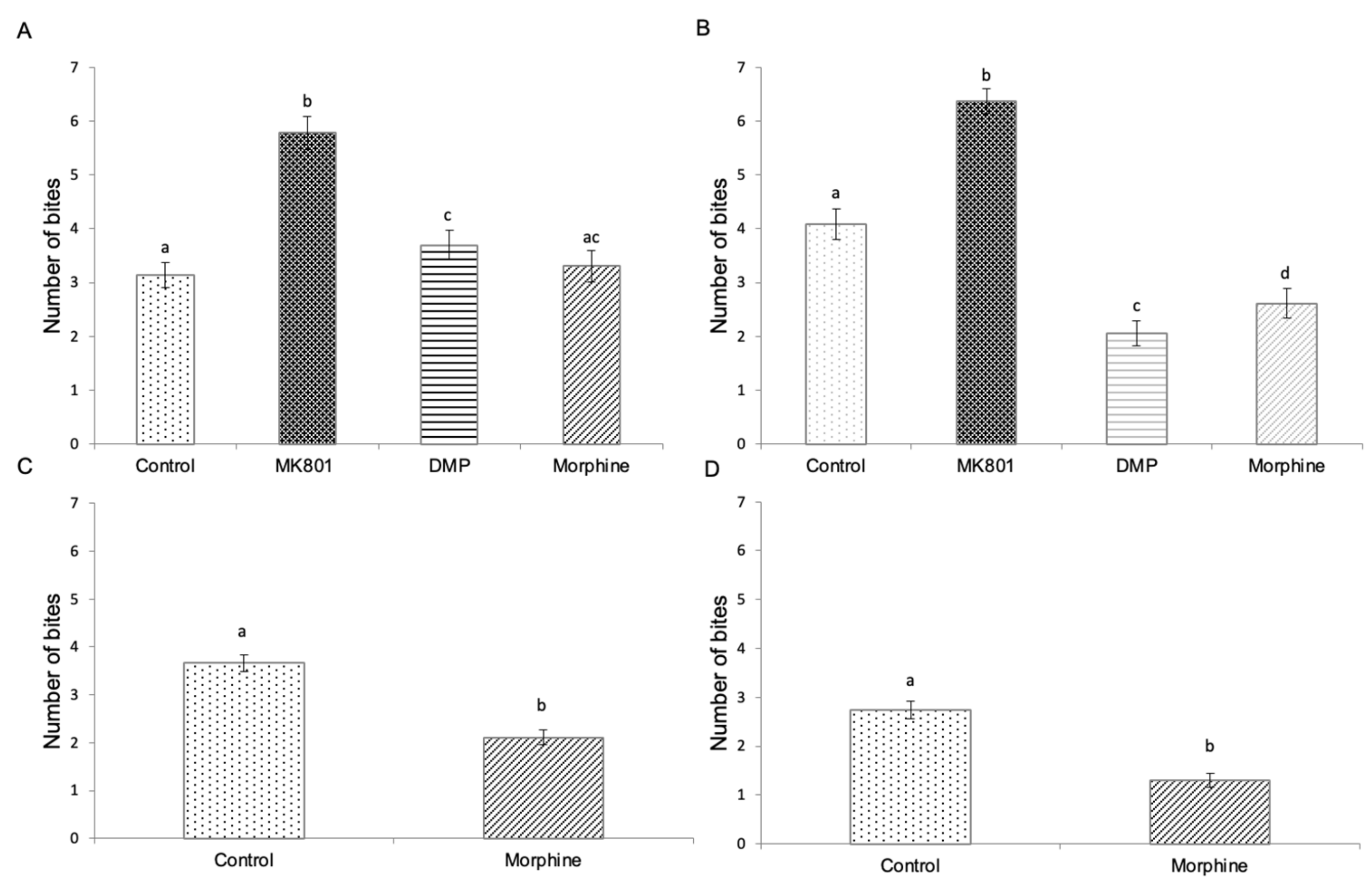
2.5. Effects of Drug Exposure and Morphine Withdrawal on Mirror Biting Behaviour
3. Discussion
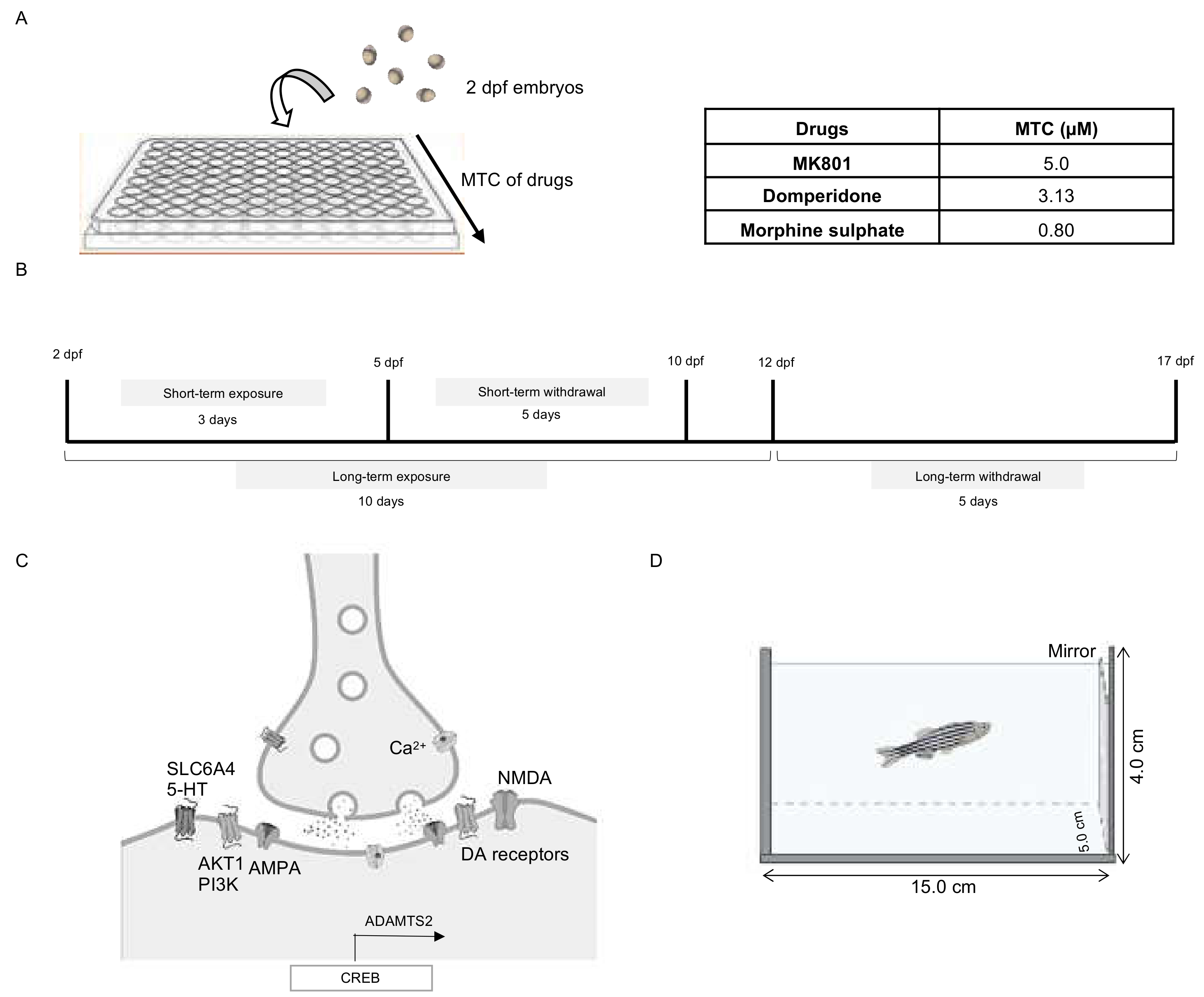
4. Materials and Methods
4.1. Zebrafish Strains and Housing Conditions
4.2. Optimization of Drug Dosages
4.3. Drug Treatments
4.4. RNA Extraction and Real-Time PCR
4.5. Total Protein Purification
4.6. Biochemical Assays
4.7. Behavioural Testing
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Stages (dpf)/ Drugs (𝜇M) | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|
| DMP | 50 | 50 | 50 | 25 | 12.5 | 6.25 | 3.13 | 3.13 | 3.13 |
| Morphine sulphate | 1.6 | 1.6 | 1.4 | 1.2 | 1.2 | 1.2 | 1.0 | 0.8 | 0.8 |
| Gene | Sequences | Specificity | References |
|---|---|---|---|
| Beta actin (actb1) | F:5′-AAGCTGTGACCCACCTCACG-3′ R:5′GGCTTTGCACATACCGGAGC-3′ | Danio rerio (zebrafish) Taxonomy ID:7955 | [10] |
| V-akt murine thymoma viral oncogene homolog 1 (akt1) | F:5′-GGCTATAAGGAGCGACCGCA-3′ R:5′-GGTGCGCTCAATGACAGTGG-3′ | Danio rerio (zebrafish) Taxonomy ID:7955 | [13] |
| Phosphoinositide 3-kinase (pi3k) | F:5′-GAGATTTTCTCGGCCCTGGCT-3′ R:5′-ACTCTTCCCATCTGTGTGAGGC-3′ | Danio rerio (zebrafish) Taxonomy ID:7955 | [13] |
| Solute carrier family 6 member 4 (slc6a4) | F:5′-GATGCTGGTCCCAGTCTGCT-3′ R:5′-CAACAGGTGGGGGAACTCGT-3′ | Danio rerio (zebrafish) Taxonomy ID:7955 | [10] |
| CAMP responsive element binding protein 1 (creb1) | F:5′-CGAGAACCAGCAGAGTGGA-3′ R:5′-CGGTGGGAGCAGATGATGTT-3′ | Danio rerio (zebrafish) Taxonomy ID:7955 | [13] |
| A disintegrin and metalloproteinase with thrombospondin motifs 2 (adamts2) | F:5′-CCTGACATCCTCAAACGGGA-3′ R:5′-GTGTGGGTTGTCACACTGGC-3′ | Danio rerio (zebrafish) Taxonomy ID:7955 | [10] |
References
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and treatment options. Peer Rev. J. Formul. Manag. 2014, 39, 638–645. [Google Scholar]
- Bell, C.C. DSM-IV: Diagnostic and statistical manual of mental disorders. JAMA 1994, 272, 828–829. [Google Scholar] [CrossRef]
- Winklbaur, B.; Ebner, N.; Sachs, G.; Thau, K.; Fischer, G. Substance abuse in patients with schizophrenia. Dialogues Clin. Neurosci. 2006, 8, 37. [Google Scholar] [CrossRef]
- Rashid, R.A.; Robson, N.; Sulaiman, A.H.; Salleh, R.; Zainal, N.Z.; Said, M.A.; Habil, M.H. Schizophrenia, substance use and aggressions: What are the relationships? ASEAN J. Psychiatr. 2010, 11, 72–78. [Google Scholar]
- Green, A.I.; Drake, R.E.; Brunette, M.F.; Noordsy, D.L. Schizophrenia and co-occurring substance use disorder. Am. J. Psychiatry 2007, 164, 402–408. [Google Scholar] [CrossRef]
- Chakraborty, R.; Chatterjee, A.; Chaudhury, S. Impact of substance use disorder on presentation and short-term course of schizophrenia. Psychiatry J. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Center for Substance Abuse Treatment. Physical detoxification services for withdrawal from specific sub-stances. In Detoxification and Substance Abuse Treatment; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2006; Treatment Improvement Protocol (TIP) Series No. 45; pp. 47–120. [Google Scholar]
- Flynn, P.M.; Brown, B.S. Co-occurring disorders in substance abuse treatment: Issues and prospects. J. Subst. Abus. Treat. 2008, 34, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Shreeram, S.; Dennison, S.; McDonald, T. Psychosis after ultrarapid opiate detoxification. Am. J. Psychiatry 2001, 158, 970. [Google Scholar] [CrossRef]
- Senay, E.C.; Adams, E.H.; Geller, A.; Inciardi, J.A.; Munoz, A.; Schnoll, S.H.; Woody, G.E.; Cicero, T.J. Physical dependence on Ultram®(tramadol hydrochloride): Both opioid-like and atypical withdrawal symptoms occur. Drug Alcohol. Depend. 2003, 69, 233–241. [Google Scholar] [CrossRef]
- Karila, L.; Berlin, I.; Benyamina, A.; Reynaud, M. Psychotic symptoms following buprenorphine withdrawal. Am. J. Psychiatry 2008, 165, 400–401. [Google Scholar] [CrossRef]
- Weibel, S.; Mallaret, M.; Bennouna-Greene, M.; Bertschy, G. A case of acute psychosis after buprenorphine withdrawal: Abrupt versus progressive discontinuation could make a difference. J. Clin. Psychiatry 2012, 73, 756. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Hsieh, F.Y.; Chiang, M.C.; Scotting, P.J.; Shih, H.Y.; Lin, S.J.; Wu, H.L.; Lee, H.T. Akt1 mediates neuronal differentiation in zebrafish via a reciprocal interaction with notch signaling. PLoS ONE 2013, 8, e54262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchesi, C.; Affaticati, A.; Monici, A.; De Panfilis, C.; Ossola, P.; Tonna, M. Predictors of symptomatic re-mission remission in patients with first-episode schizophrenia: A 16 years follow-up study. Compr. Psychiatry 2014, 55, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R. Chronic stress, drug use, and vulnerability to addiction. Ann. N. Y. Acad. Sci. 2008, 1141, 105–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Chiara, G.; Bassareo, V. Reward system and addiction: What dopamine does and doesn’t do. Curr. Opin. Pharmacol. 2007, 7, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Peselow, E. The neurobiology of addictive disorders. Clin. Neuropharmacol. 2009, 32, 269–276. [Google Scholar] [CrossRef]
- Carlsson, A.; Lindqvist, M. Effect of chlorpromazine and haloperidol on formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol. Toxicol. 1963, 20, 140–144. [Google Scholar] [CrossRef]
- Creese, I.; Burt, D.R.; Snyder, S.H. Dopamine receptor binding predicts clinical and pharmacological poten-cies potencies of antischizophrenic drugs. Science 1976, 192, 481–483. [Google Scholar] [CrossRef]
- Kebabian, J.W.; Calne, D.B. Multiple receptors for dopamine. Nature 1979, 277, 93–96. [Google Scholar] [CrossRef]
- Vallar, L.; Meldolesi, J. Mechanisms of signal transduction at the dopamine D2 receptor. Trends Pharmacol. Sci. 1989, 10, 74–77. [Google Scholar] [CrossRef]
- Spano, P.; Govoni, S.; Trabucchi, M. Studies on the pharmacological properties of dopamine receptors in various areas of the central nervous system. Adv. Biochem. Psychopharmacol. 1978, 19, 155–165. [Google Scholar] [PubMed]
- Xu, F.L.; Wang, B.J.; Yao, J. Association between the SLC6A4 gene and schizophrenia: An updated meta-analysis. Neuropsychiatr. Dis. Treat. 2019, 15, 143–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaulieu, J.-M. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J. Psychiatry Neurosci. 2012, 37, 7–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2000, 103, 211–225. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Barris, L.C.; Zurashvili, T.; Bayascas, J.R. Fine-tuning the intensity of the PKB/Akt signal enables diverse physiological responses. Cell Cycle 2014, 13, 3164–3168. [Google Scholar] [CrossRef] [Green Version]
- Alessi, D.R.; James, S.R.; Downes, C.; Holmes, A.B.; Gaffney, P.R.; Reese, C.B.; Cohen, P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 1997, 7, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Gai, H.; Su, R.; Deji, C.; Cui, J.; Lai, J.; Zhu, Y. PI3K-AKT-GSK3β-CREB signaling pathway regulates anxiety-like behavior in rats following alcohol withdrawal. J. Affect. Disord. 2018, 235, 96–104. [Google Scholar] [CrossRef]
- Ruso-Julve, F.; Pombero, A.; Pilar-Cuéllar, F.; García-Díaz, N.; Garcia-Lopez, R.; Juncal-Ruiz, M.; Castro, E.; Díaz, Á.; Vazquez-Bourgón, J.; García-Blanco, A.; et al. Dopaminergic control of ADAMTS2 expression through cAMP/CREB and ERK: Molecular effects of antipsychotics. Transl. Psychiatry 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Yuan, Q.-H.; Zhou, Q. Histamine H3 receptor antagonist Clobenpropit protects propofol-induced apoptosis of hippocampal neurons through PI3K/AKT pathway. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 8013–8020. [Google Scholar]
- Chambers, R.A.; Krystal, J.H.; Self, D.W. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol. Psychiatry 2001, 50, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Green, A.I.; Zimmet, S.V.; Straus, R.D.; Schildkraut, J.J. Clozapine for comorbid substance use disorder and schizophrenia: Do patients with schizophrenia have a reward-deficiency syndrome that can be ameliorated by clozapine? Harv. Rev. Psychiatry 1999, 6, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Karege, F.; Perroud, N.; Burkhardt, S.; Schwald, M.; Ballmann, E.; La Harpe, R.; Malafosse, A. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3β in ventral prefrontal cortex of depressed suicide victims. Biol. Psychiatry 2007, 61, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Balu, D.; Carlson, G.C.; Talbot, K.; Kazi, H.; Hill-Smith, T.E.; Easton, R.M.; Birnbaum, M.; Lucki, I. Akt1 deficiency in schizophrenia and impairment of hippocampal plasticity and function. Hippocampus 2010, 22, 230–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef]
- Neasta, J.; Ben Hamida, S.; Yowell, Q.V.; Carnicella, S.; Ron, D. AKT Signaling Pathway in the Nucleus Accumbens Mediates Excessive Alcohol Drinking Behaviors. Biol. Psychiatry 2011, 70, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szumlinski, K.K.; Ary, A.W.; Shin, C.B.; Wroten, M.G.; Courson, J.; Miller, B.W.; Ruppert-Majer, M.; Hiller, J.W.; Shahin, J.R.; Ben-Shahar, O.; et al. PI3K activation within ventromedial prefrontal cortex regulates the expression of drug-seeking in two rodent species. Addict. Biol. 2019, 24, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q.; An, Y.W.; Hu, A.Z.; Li, M.H.; Wu, J.L.; Liu, L.; Shi, Y.; Cui, G.H.; Chen, Y. Critical roles of the PI3K-Akt-mTOR signaling pathway in apoptosis and autophagy of astrocytes induced by methamphetamine. Open Chem. J. 2019, 17, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Norton, W.; Bally-Cuif, L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010, 11, 90. [Google Scholar] [CrossRef] [Green Version]
- Fero, K.; Yokogawa, T.; Burgess, H.A. The behavioral repertoire of larval zebrafish. In Zebrafish Models in Neurobehavioral Research; Kalueff, A.V., Cachat, J.M., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 249–291. [Google Scholar]
- Norton, W.H.J.; Stumpenhorst, K.; Faus-Kessler, T.; Folchert, A.; Rohner, N.; Harris, M.P.; Callebert, J.; Bally-Cuif, L. Modulation of Fgfr1a Signaling in Zebrafish Reveals a Genetic Basis for the Aggression-Boldness Syndrome. J. Neurosci. 2011, 31, 13796–13807. [Google Scholar] [CrossRef] [Green Version]
- Parker, M.O.; Brock, A.J.; Walton, R.T.; Brennan, C.H. The role of zebrafish (Danio rerio) in dissecting the genetics and neural circuits of executive function. Front. Neural Circuits 2013, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Pichler, F.B.; Laurenson, S.; Williams, L.C.; Dodd, A.; Copp, B.; Love, D. Chemical discovery and global gene expression analysis in zebrafish. Nat. Biotechnol. 2003, 21, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Bubenikova-Valesova, V.; Stuchlik, A.; Svoboda, J.; Bures, J.; Vales, K. Risperidone and ritanserin but not haloperidol block effect of dizocilpine on the active allothetic place avoidance task. Proc. Natl. Acad. Sci. USA 2008, 105, 1061–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, L.; Bregestovski, P.; Ascher, P.; Herbet, A.; Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 1984, 307, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.A.; Watson, D.J.G.; Fone, K.C.F. Animal models of schizophrenia. Br. J. Pharmacol. 2011, 164, 1162–1194. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Basu, A.; Benneyworth, M.; Balu, D.; Konopaske, G. Glutamatergic Glutamatergic synaptic dysregulation in schizophrenia: Therapeutic implications. Handb. Exp. Pharmacol. 2012, 267–295. [Google Scholar] [CrossRef] [Green Version]
- Hardingham, G.E.; Do, K.Q. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat. Rev. Neurosci. 2016, 17, 125–134. [Google Scholar] [CrossRef]
- Benvenutti, R.; Gallas-Lopes, M.; Sachett, A.; Marcon, M.; Strogulski, N.R.; Reis, C.G.; Chitolina, R.; Piato, A.; Herrmann, A.P. How do zebrafish (Danio rerio) respond to MK-801 and amphetamine? Relevance for assessing schizophrenia-related endophenotypes in alternative model organisms. J. Neurosci. Res. 2021, 99, 2844–2859. [Google Scholar] [CrossRef]
- Hussain, A.; Audira, G.; Siregar, P.; Lin, Y.C.; Villalobos, O.; Villaflores, O.; Wang, W.D.; Hsiao, C.D. Water-borne exposure of paclobutrazol at environmental relevant concentration induce locomotion hyperactivity in larvae and anxiolytic exploratory behavior in adult zebrafish. Int. J. Environ. Res. Public Health 2020, 17, 4632. [Google Scholar] [CrossRef]
- Muller, T.; Wulliman, M.F. Atlas of Early Zebrafish Brain Development. A Tool for Molecular Neurogenetics, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2005; pp. 1–183. [Google Scholar]
- Schweitzer, J.; Driever, W. Development of the dopamine systems in zebrafish. In Development and Engineering of Dopamine Neurons, Advances in Experimental Medicine and Biology; Pasterkamp, R.J., Smidt, M.P., Burbach, J.P.H., Eds.; Springer: New York, NY, USA, 2009; Volume 651, pp. 1–14. [Google Scholar]
- Fleming, A.; Diekmann, H.; Goldsmith, P. Functional Characterisation of the characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS ONE 2013, 8, e77548. [Google Scholar] [CrossRef]
- Champion, M.C.; Hartnett, M.; Yen, M. Domperidone, a new dopamine antagonist. Can. Med Assoc. J. 1986, 135, 457–461. [Google Scholar]
- Geyer, M.A.; Braff, D.L. Habituation of the Blink reflex in normals and schizophrenic patients. Psychophysiology 1982, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Abel, K.; Waikar, M.; Pedro, B.; Hemsley, D.; Geyer, M. Repeated testing of prepulse inhibition and habituation of the startle reflex: A study in healthy human controls. J. Psychopharmacol. 1998, 12, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Rothwell, J.; Thompson, P.D.; Britton, T.C.; Day, B.L.; Marsden, C.D. New observations on the normal auditory startle reflex in man. Brain 1991, 114, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish larvae as a behavioral model in neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braff, D.L.; Grillon, C.; Geyer, M.A. Gating and habituation of the startle reflex in schizophrenia patients. Arch. Gen. Psychiatry 1992, 49, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Grillon, C.; Ameli, R.; Charney, D.; Krystal, J.; Braff, D. Startle gating deficits occur across prepulse intensities in schizophrenic patients. Biol. Psychiatry 1992, 32, 939–943. [Google Scholar] [CrossRef]
- Kumari, V.; Soni, W.; Sharma, T. Normalization of information processing deficits in schizophrenia with clozapine. Am. J. Psychiatry 1999, 156. [Google Scholar] [CrossRef]
- Parwani, A.; Duncan, E.; Bartlett, E.; Madonick, S.; Efferen, T.R.; Rajan, R.; Sanfilipo, M.; Chappell, P.B.; Chakravorty, S.; Gonzenbach, S.; et al. Impaired prepulse inhibition of acoustic startle in schizophrenics. Biol. Psychiatry 2000, 47, 662–669. [Google Scholar] [CrossRef]
- Takahashi, H.; Iwase, M.; Ishii, R.; Ohi, K.; Fukumoto, M.; Azechi, M.; Ikezawa, K.; Kurimoto, R.; Canuet, L.; Nakahachi, T.; et al. Impaired prepulse inhibition and habituation of acous-tic acoustic startle response in Japanese patients with schizophrenia. Neurosci. Res. 2008, 62, 187–194. [Google Scholar] [CrossRef]
- Moriwaki, M.; Kishi, T.; Takahashi, H.; Hashimoto, R.; Kawashima, K.; Okochi, T.; Kitajima, T.; Furukawa, O.; Fujita, K.; Takeda, M.; et al. Prepulse inhibition of the startle response with chronic schizophrenia: A replication study. Neurosci. Res. 2009, 65, 259–262. [Google Scholar] [CrossRef]
- Walters, J.T.R.; Owen, M.J. Endophenotypes in psychiatric genetics. Mol. Psychiatry 2007, 12, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Light, G.A.; Swerdlow, N.R. Neurophysiological biomarkers informing the clinical neuroscience of schizophrenia: Mismatch negativity and prepulse inhibition of startle. Curr. Top. Behav. Neurosci. 2014, 21, 293–314. [Google Scholar] [PubMed] [Green Version]
- Mansbach, R.S.; Geyer, M.A.; Braff, D.L. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology 1988, 94, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Burgess, H.; Granato, M. Sensorimotor Gating in Larval Zebrafish. J. Neurosci. 2007, 27, 4984–4994. [Google Scholar] [CrossRef] [Green Version]
- Png, W.-Y.; Tang, P.-Y.; Ogawa, S.; Parhar, I.; Mok, S.-Y. Startle habituation: A tool for assessing information processing deficits in zebrafish model of schizophrenia. Sains Malays. 2021, 50, 201–206. [Google Scholar] [CrossRef]
- Potvin, S.; Stip, E.; Roy, J.Y. Schizophrenia and addiction: An evaluation of the self-medication hypothesis. L’Encephale 2003, 29, 193–203. [Google Scholar]
- Gandolfi, O.; Dall’Olio, R. Chronic treatment with MK-801 decreases D2 dopamine receptor function in rat striatum. Pharmacol. Biochem. Behav. 1993, 44, 683–687. [Google Scholar] [CrossRef]
- Georges, F.; Stinus, L.; Bloch, B.; Le Moine, C. Chronic morphine exposure and spontaneous withdrawal are associated with modifications of dopamine receptor and neuropeptide gene expression in the rat striatum. Eur. J. Neurosci. 1999, 11, 481–490. [Google Scholar] [CrossRef]
- Suzuki, S.; Chuang, L.F.; Doi, R.H.; Chuang, R.Y. Morphine suppresses lymphocyte apoptosis by blocking p53-mediated death signaling. Biochem. Biophys. Res. Commun. 2003, 308, 802–808. [Google Scholar] [CrossRef]
- Fahy, B.N.; Schlieman, M.; Virudachalam, S.; Bold, R.J. AKT inhibition is associated with chemosensitisation in the pancreatic cancer cell line MIA-PaCa-2. Br. J. Cancer 2003, 89, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, S.; Ikeda, Y.; Murakami, M.; Nakagawa, Y.; Tsuji, A.; Kitagishi, Y. Roles of PI3K/AKT/GSK3 pathway involved in psychiatric illnesses. Diseases 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emamian, E.S.; Hall, D.; Birnbaum, M.J.; Karayiorgou, M.; Gogos, J.A. Convergent evidence for impaired AKT1–GSK3beta signaling in schizophrenia. Nat. Genet. 2004, 36, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Stertz, L.; Di Re, J.; Pei, G.; Fries, G.R.; Mendez, E.; Li, S.; Smith-Callahan, L.; Raventos, H.; Tipo, J.; Cherukuru, R.; et al. Convergent genomic and pharmacological evidence of PI3K/GSK3 signaling alternations in neurons from schizophrenia patients. Neuropsychopharmacology 2021, 46, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Emamian, E.S. AKT/GSK3 signaling pathway and schizophrenia. Front. Mol. Neurosci. 2012, 5, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomkins, D.M.; Sellers, E.M. Addiction and the brain: The role of neurotransmitters in the cause and treatment of drug dependence. Can. Med. Assoc. J. 2001, 164, 817–821. [Google Scholar]
- Chao, J.; Nestler, E.J. Molecular neurobiology of drug addiction. Annu. Rev. Med. 2004, 55, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.T.; Christie, M.J.; Manzoni, O. Cellular and synaptic adaptations mediating opioid dependence. Physiol. Rev. 2001, 81, 299–343. [Google Scholar] [CrossRef]
- Salin, P.A.; Malenka, R.C.; Nicoll, R.A. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron 1996, 16, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Weiss, S.; Sebben, M.; García-Sáinz, J.A.; Bockaert, J. D2-dopamine receptor-mediated inhibition of cyclic AMP formation in striatal neurons in primary culture. Mol. Pharmacol. 1985, 27, 595–599. [Google Scholar]
- Crespo-Facorro, B.; Prieto, C.; Sainz, J. Schizophrenia gene expression profile reverted to normal levels by antipsychotics. Int. J. Neuropsychopharmacol. 2015, 18. [Google Scholar] [CrossRef]
- Pantazopoulos, H.; Katsel, P.; Haroutunian, V.; Chelini, G.; Klengel, T.; Berretta, S. Molecular signature of extracellular matrix pathology in schizophrenia. Eur. J. Neurosci. 2020, 53, 3960–3987. [Google Scholar] [CrossRef] [PubMed]
- Appelbaum, P.S.; Robbins, P.C.; Monahan, J. Violence and delusions: Data from the MacArthur violence risk assessment study. Am. J. Psychiatry 2000, 157, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Zabegalov, K.N.; Kolesnikova, T.O.; Khatsko, S.L.; Volgin, A.D.; Yakovlev, O.A.; Amstislavskaya, T.G.; Friend, A.J.; Bao, W.; Alekseeva, P.A.; Lakstygal, A.M.; et al. Understanding zebrafish aggressive behavior. Behav. Processes 2019, 158, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, J.D.; Roth, R.H. The neuropsychopharmacology of phencyclidine: From NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1999, 20, 201–225. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Han, M.H.; Mazei-Robison, M.; Iñiguez, S.D.; Ables, J.L.; Vialou, V.; Berton, O.; Ghose, S.; Covington III, H.E.; Wiley, M.D.; et al. AKT signaling within the ventral tegmental area regu-lates regulates cellular and behavioral responses to stressful stimuli. Biol. Psychiatry 2008, 64, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Luscher, C.; Isaac, J.T. The synapse: Center stage for many brain diseases. J. Physiol. 2009, 587, 727–729. [Google Scholar] [CrossRef]
- Zimmermann, F.F.; Gaspary, K.V.; Siebel, A.M.; Bonan, C.D. Oxytocin reversed MK801-induced social interaction and aggression deficits in zebrafish. Behav. Brain Res. 2016, 311, 368–374. [Google Scholar] [CrossRef]
- Seibt, K.J.; Piato, A.L.; Oliveira, R.D.L.; Capiotti, K.M.; Vianna, M.R.; Bonan, C.D. Antipsychotic drugs reverse MK-801-induced cognitive and social interaction deficits in zebrafish (Danio rerio). Behav. Brain Res. 2011, 224, 135–139. [Google Scholar] [CrossRef]
- Kamińska, K.; Rogóż, Z. The effect of combined treatment with risperidone and antidepressants on the MK-801-induced deficits in the social interaction test in rats. Pharmacol. Rep. 2015, 67, 1183–1187. [Google Scholar] [CrossRef]
- Beleslin, D.; Jovanović-Mićić, D.; Japundžić, N.; Terzić, A.; Samardžić, R. Behavioral, autonomic and motor effects of neuroleptic drugs in cats: Motor impairment and aggression. Brain Res. Bull. 1985, 15, 353–356. [Google Scholar] [CrossRef]
- Datla, K.P.; Sen, A.P.; Bhattacharya, S.K. Dopaminergic modulation of footshock induced aggression in paired rats. Indian J. Exp. Biol. 1992, 30, 587–591. [Google Scholar] [PubMed]
- Hemmelmann, M.; Knoth, C.; Schmitt, U.; Allmeroth, M.; Moderegger, D.; Barz, M.; Koynov, K.; Hiemke, C.; Rösch, F.; Zentel, R. HPMA based amphiphilic copolymers mediate central nervous effects of domperidone. Macromol. Rapid Commun. 2011, 32, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Woody, G.E.; Persky, H.; McLellan, A.T.; O’Brien, C.P.; Arndt, I. Psychoendocrine correlates of hostility and anxiety n in addicts. In Alcohol, Drug Abuse and Aggression; Gottheil, E., Druley, K.A., Skoloda, T.E., Waxman, H.M., Eds.; Charles C. Thomas: Springfield, IL, USA, 1983; pp. 227–244. [Google Scholar]
- Espert, R.; Navarro, J.F.; Salvador, A.; Simön, V.M. Effects of morphine hydrochloride on social encounters between male mice. Aggress. Behav. 1993, 19, 377–383. [Google Scholar] [CrossRef]
- Hoaken, P.N.; Stewart, S.H. Drugs of abuse and the elicitation of human aggressive behavior. Addict. Behav. 2003, 28, 1533–1554. [Google Scholar] [CrossRef] [Green Version]
- Miczek, K.A.; DeBold, J.F.; Haney, M.; Tidey, J.; Vivian, J.; Weerts, E.M. Alcohol, drugs of abuse, aggression, and violence. In Understanding and Preventing Violence; Reiss, A.J., Roth, J.A., Eds.; National Academy Press: Washington, DC, USA, 1994; Volume 3. [Google Scholar]
- Rodríguez-Arias, M.; Aguilar, M.A.; Simón, V.M. Drugs of abuse and aggression: A review in animal models. Curr. Top Pharmacol. 2005, 9, 1–27. [Google Scholar]
- Miczek, K.A.; Haney, M.; Tidey, J.; Vivian, J.; Weerts, E. Neurochemistry and pharmacotherapeutic man-agement of aggression and violence. In Understanding and Preventing Violence; Reiss, A.J., Miczek, K.A., Roth, J.A., Eds.; National Research Council; National Academy Press: Washington, DC, USA, 1994; Volume 2, Biobehavioral Influences; pp. 245–514. [Google Scholar]
- Kantak, K.M.; Miczek, K.A. Aggression during morphine withdrawal: Effects of method of withdrawal, fighting experience, and social role. Psychopharmacology 1986, 90, 451–456. [Google Scholar] [CrossRef]
- Tidey, J.W.; Miczek, K.A. Heightened aggressive behavior during morphine withdrawal: Effects of d-amphetamine. Psychopharmacology 1992, 107, 297–302. [Google Scholar] [CrossRef]
- Khor, B.-S.; Jamil, M.F.A.; Adenan, M.I.; Shu-Chien, A.C. Mitragynine attenuates withdrawal syndrome in morphine-withdrawn zebrafish. PLoS ONE 2011, 6, e28340. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.; Volgin, A.D.; Alpyshov, E.T.; Friend, A.; Strekalova, T.V.; de Abreu, M.S.; Collins, C.; Amstislavskaya, T.G.; Demin, K.A.; Kalueff, A.V. Opioid neurobiology, neurogenetics and neuropharmacology in zebrafish. Neuroscience 2019, 404, 218–232. [Google Scholar] [CrossRef]
- Miczek, K.A.; Covington, H.E., III; Nikulina, E.M., Jr.; Hammer, R.P. Aggression and defeat: Persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci. Biobehav. Rev. 2004, 27, 787–802. [Google Scholar] [CrossRef]
- Mondelli, V.; Dazzan, P.; Hepgul, N.; Di Forti, M.; Aas, M.; D’Albenzio, A.; Di Nicola, M.; Fisher, H.; Handley, R.; Marques, T.R.; et al. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: The role of stress and of antipsychotic treatment. Schizophr. Res. 2009, 116, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cachat, J.; Canavello, P.; Elegante, M.; Bartels, B.; Hart, P.; Bergner, C.; Egan, R.; Duncan, A.; Tien, D.; Chung, A.; et al. Modeling withdrawal syndrome in zebrafish. Behav. Brain Res. 2010, 208, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Steen, N.E.; Tesli, M.; Kähler, A.K.; Methlie, P.; Hope, S.; Barrett, E.A.; Larsson, S.; Mork, E.; Løvås, K.; Røssberg, J.I.; et al. SRD5A2 is associated with increased cortisol metabolism in schizophrenia spectrum disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Guest, P.C.; Schwarz, E.; Krishnamurthy, D.; Harris, L.W.; Leweke, F.M.; Rothermundt, M.; van Beveren, N.J.; Spain, M.; Barnes, A.; Steiner, J.; et al. Altered levels of circulating insulin and other neuro-endocrine hormones associated with the onset of schizophrenia. Psychoneuroendocrinology 2011, 36, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.H.; Lowry, C.A. Corticotropin-releasing factor-related peptides, serotonergic systems, and emotional behavior. Front. Neurosci. 2013, 7, 169. [Google Scholar] [CrossRef] [Green Version]
- Lesch, K.-P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Müller, C.R.; Hamer, D.H.; Murphy, D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef]
- Bradley, S.L.; Dodelzon, K.; Sandhu, H.K.; Philibert, R.A. Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2005, 136, 58–61. [Google Scholar] [CrossRef]
- Prats, E.; Gomez-Canela, C.; Ben-Lulu, S.; Ziv, T.; Padrós, F.; Tornero, D.; Garcia-Reyero, N.; Tauler, R.; Admon, A.; Raldúa, D. Modelling acrylamide acute neurotoxicity in zebrafish larvae. Sci. Rep. 2017, 7, 13952. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.; Zhang, H.; Klaminder, J.; Brodin, T.; Andersson, P.L.; Andersson, M. ToxTrac: A fast and robust software for tracking organisms. Methods Ecol. Evol. 2018, 9, 460–464. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, S.; Ahmad, S.; Madiha, S.; Khaliq, S.; Liaquat, L.; Sadir, S.; Rafiq, S.; Tabassum, S.; Batool, Z.; Haider, S. Dizocilpine induced psychosis-like behavior in rats: A possible animal model with full spectrum of schizophrenia. Pak. J. Pharm. Sci. 2017, 30, 2423–2427. [Google Scholar]
- Uttl, L.; Petrasek, T.; Sengul, H.; Svojanovska, M.; Lobellova, V.; Vales, K.; Radostova, D.; Tsenov, G.; Kubova, H.; Mikulecka, A.; et al. Chronic MK-801 application in adolescence and early adulthood: A spatial working memory deficit in adult Long-Evans rats but no changes in the hippocampal NMDA receptor subunits. Front. Pharmacol. 2018, 9, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, A.; Peters, B.; Schroeder, H.; Mann, T.; Huether, G.; Grecksch, G. Ketamine-induced changes in rat behaviour: A possible animal model of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 687–700. [Google Scholar] [CrossRef]
- Chomczynski, P.; Mackey, K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide-and proteoglycan-rich sources. Biotechniques 1995, 19, 942–945. [Google Scholar] [PubMed]
- Audira, G.; Sampurna, B.P.; Juniardi, S.; Liang, S.T.; Lai, Y.H.; Hsiao, C.D. A versatile setup for measuring multiple behavior endpoints in zebrafish. Inventions 2018, 3, 75. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiagarajan, S.K.; Mok, S.Y.; Ogawa, S.; Parhar, I.S.; Tang, P.Y. Receptor-Mediated AKT/PI3K Signalling and Behavioural Alterations in Zebrafish Larvae Reveal Association between Schizophrenia and Opioid Use Disorder. Int. J. Mol. Sci. 2022, 23, 4715. https://doi.org/10.3390/ijms23094715
Thiagarajan SK, Mok SY, Ogawa S, Parhar IS, Tang PY. Receptor-Mediated AKT/PI3K Signalling and Behavioural Alterations in Zebrafish Larvae Reveal Association between Schizophrenia and Opioid Use Disorder. International Journal of Molecular Sciences. 2022; 23(9):4715. https://doi.org/10.3390/ijms23094715
Chicago/Turabian StyleThiagarajan, Siroshini K., Siew Ying Mok, Satoshi Ogawa, Ishwar S. Parhar, and Pek Yee Tang. 2022. "Receptor-Mediated AKT/PI3K Signalling and Behavioural Alterations in Zebrafish Larvae Reveal Association between Schizophrenia and Opioid Use Disorder" International Journal of Molecular Sciences 23, no. 9: 4715. https://doi.org/10.3390/ijms23094715
APA StyleThiagarajan, S. K., Mok, S. Y., Ogawa, S., Parhar, I. S., & Tang, P. Y. (2022). Receptor-Mediated AKT/PI3K Signalling and Behavioural Alterations in Zebrafish Larvae Reveal Association between Schizophrenia and Opioid Use Disorder. International Journal of Molecular Sciences, 23(9), 4715. https://doi.org/10.3390/ijms23094715







