Influence of Parental Health Literacy on Change over Time in the Oral Health of American Indian Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Secondary Data
2.2. PBC Participants and Procedures
2.3. Institutional Review
2.4. Measures
2.4.1. Health Literacy
2.4.2. Parental Oral Health Knowledge
2.4.3. Parental Oral Health Beliefs
2.4.4. Adherence to Recommended Parental Oral Health Behaviors
2.4.5. Oral Health Outcomes
2.4.6. Sociodemographic Characteristics
2.5. Statistical Methods
2.5.1. Change over Time in the Oral Health Constructs
2.5.2. Association of Health Literacy with Change over Time in the Oral Health Constructs
2.5.3. Data Analysis Time Points
2.5.4. Covariates
3. Results
3.1. Sample Characteristics
3.2. Initial Performance on Constructs of Interest
3.3. Change over Time in the Oral Health Constructs
3.4. Association of Health Literacy with Change over Time in the Oral Health Constructs
4. Discussion
5. Conclusions
5.1. Background and Summary of Findings
5.2. Future Research Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratzan, S.C.; Parker, R.M. Introduction. In National Library of Medicine Current Bibliographies in Medicine: Health Literacy; Selden, C.R., Zorn, M., Ratzan, S.C., Parker, R.M., Eds.; NLM Pub. No. CBM 2000-1; National Institutes of Health, United States Department of Health and Human Services: Bethesda, MD, USA, 2000. [Google Scholar]
- Vann, W.F., Jr.; Lee, J.Y.; Baker, D.; Divaris, K. Oral health literacy among female caregivers: Impact on oral health outcomes in early childhood. J. Dent. Res. 2010, 89, 1395–1400. [Google Scholar] [CrossRef]
- Brega, A.G.; Thomas, J.F.; Henderson, W.G.; Batliner, T.S.; Quissell, D.O.; Braun, P.A.; Wilson, A.; Bryant, L.L.; Nadeau, K.J.; Albino, J. Association of parental health literacy with oral health of Navajo Nation preschoolers. Health Educ. Res. 2016, 31, 70–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilella, K.D.; Alves, S.G.; de Souza, J.F.; Fraiz, F.C.; Assuncao, L.R. The association of oral health literacy and oral health knowledge with social determinants in pregnant Brazilian women. J. Community Health 2016, 41, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Brega, A.G.; Jiang, L.; Johnson, R.L.; Wilson, A.R.; Schmiege, S.J.; Albino, J. Health literacy and parental oral health knowledge, beliefs, behavior, and status among parents of American Indian newborns. J. Racial Ethn. Health Disparities 2020, 7, 598–608. [Google Scholar] [CrossRef]
- Miller, E.; Lee, J.Y.; DeWalt, D.A.; Vann, W.F., Jr. Impact of caregiver literacy on children’s oral health outcomes. Pediatrics 2010, 126, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrett, G.M.; Citi, A.M.; Gansky, S.A. Parental functional health literacy relates to skip pattern questionnaire error and to child oral health. J. Calif. Dent. Assoc. 2012, 40, 423–430. [Google Scholar]
- Bridges, S.M.; Parthasarathy, D.S.; Wong, H.M.; Yiu, C.K.; Au, T.K.; McGrath, C.P. The relationship between caregiver functional oral health literacy and child oral health status. Patient Educ. Couns. 2014, 94, 411–416. [Google Scholar] [CrossRef]
- Sorensen, K.; Van den Broucke, S.; Fullam, J.; Doyle, G.; Pelikan, J.; Slonska, Z.; Brand, H.; Consortium Health Literacy Project European. Health literacy and public health: A systematic review and integration of definitions and models. BMC Public Health 2012, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Phipps, K.R.; Ricks, T.L.; Mork, N.P.; Lozon, T.L. Indian Health Service Data Brief: The Oral Health of American Indian and Alaska Native Children Aged 1–5 Years: Results of the 2018–19 IHS Oral Health Survey; United States Department of Health and Human Services, Indian Health Service: Rockville, MD, USA, 2019. [Google Scholar]
- Phipps, K.R.; Ricks, T.L. Indian Health Service Data Brief: The Oral Health of American Indian and Alaska Native Children Aged 1–5 Years: Results of the 2014 IHS Oral Health Survey; United States Department of Health and Human Services, Indian Health Service: Rockville, MD, USA, 2015. [Google Scholar]
- Batliner, T.S.; Tiwari, T.; Henderson, W.G.; Wilson, A.R.; Gregorich, S.E.; Fehringer, K.A.; Brega, A.G.; Swyers, E.; Zacher, T.; Harper, M.M.; et al. Randomized trial of motivational interviewing to prevent early childhood caries in American Indian children. JDR Clin. Transl. Res. 2018, 3, 366–375. [Google Scholar] [CrossRef]
- Indian Health Service. The 2010 Indian Health Service Oral Health Survey of American Indian and Alaska Native Preschool Children; United States Department of Health and Human Services: Rockville, MD, USA, 2013. [Google Scholar]
- Kutner, M.; Greenberg, E.; Jin, Y.; Paulsen, C. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy (NCES 2006–483); U.S. Department of Education: Washington, DC, USA, 2006. [Google Scholar]
- Batliner, T.; Fehringer, K.A.; Tiwari, T.; Henderson, W.G.; Wilson, A.; Brega, A.G.; Albino, J. Motivational interviewing with American Indian mothers to prevent early childhood caries: Study design and methodology of a randomized control trial. Trials 2014, 15, 125. [Google Scholar] [CrossRef] [Green Version]
- American Academy of Pediatric Dentistry, Council on Clinical Affairs. Perinatal and Infant Oral Health Care. The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2016. [Google Scholar]
- American Academy of Pediatric Dentistry, Council on Clinical Affairs. Policy on Early Childhood Caries (ECC): Classifications, Consequences, and Preventive Strategies. The Reference Manual of Pediatric Dentistry; American Academy of Pediatric Dentistry: Chicago, IL, USA, 2016. [Google Scholar]
- Warren, J.J.; Fontana, M.; Blanchette, D.R.; Dawson, D.V.; Drake, D.R.; Levy, S.M.; Kolker, J.L.; Phipps, K.R. Timing of primary tooth emergence among U.S. racial and ethnic groups. J. Public Health Dent. 2016, 76, 259–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, W.R.; Rose, G.S. Toward a theory of motivational interviewing. Am. Psychol. 2009, 64, 527–537. [Google Scholar] [CrossRef]
- Borrelli, B.; Tooley, E.M.; Scott-Sheldon, L.A. Motivational interviewing for parent-child health interventions: A systematic review and meta-analysis. Pediatr. Dent. 2015, 37, 254–265. [Google Scholar] [PubMed]
- Albino, J.; Tiwari, T.; Gansky, S.A.; Henshaw, M.M.; Barker, J.C.; Brega, A.G.; Gregorich, S.E.; Heaton, B.; Batliner, T.S.; Borrelli, B.; et al. The Basic Research Factors Questionnaire for studying early childhood caries. BMC Oral Health 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Brega, A.G.; Batliner, T.S.; Henderson, W.; Campagna, E.J.; Fehringer, K.; Gallegos, J.; Daniels, D.; Albino, J. Assessment of parental oral health knowledge and behaviors among American Indians of a Northern Plains tribe. J. Public Health Dent. 2014, 74, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Wilson, A.R.; Brega, A.G.; Campagna, E.; Braun, P.A.; Henderson, W.G.; Bryant, L.L.; Batliner, T.S.; Quissell, D.O.; Albino, J. Validation and impact of caregivers’ oral health knowledge and behavior on children’s oral health status. Pediatr. Dent. 2016, 38, 47–54. [Google Scholar]
- Brega, A.G.; Jiang, L.; Beals, J.; Manson, S.M.; Acton, K.J.; Roubideaux, Y.; Special Diabetes Program for Indians Healthy Heart Demonstration Project. Special Diabetes Program for Indians: Reliability and validity of brief measures of print literacy and numeracy. Ethn. Dis. 2012, 22, 207–214. [Google Scholar]
- Wilson, A.R.; Brega, A.G.; Thomas, J.F.; Henderson, W.G.; Lind, K.E.; Braun, P.A.; Batliner, T.S.; Albino, J. Validity of measures assessing oral health beliefs of American Indian parents. J. Racial Ethn. Health Disparities 2018, 5, 1254–1263. [Google Scholar] [CrossRef]
- Pitts, N.B. Clinical diagnosis of dental caries: A European perspective. J. Dent. Educ. 2001, 65, 972–978. [Google Scholar] [CrossRef]
- Pitts, N.B. Modern concepts of caries measurement. J. Dent. Res. 2004, 83, C43–C47. [Google Scholar] [CrossRef]
- Warren, J.J.; Weber-Gasparoni, K.; Tinanoff, N.; Batliner, T.S.; Jue, B.; Santo, W.; Garcia, R.I.; Gansky, S.A.; Early Childhood Caries Collaborating Centers. Examination criteria and calibration procedures for prevention trials of the Early Childhood Caries Collaborating Centers. J. Public Health Dent. 2015, 75, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Wallace, L.S.; Rogers, E.S.; Roskos, S.E.; Holiday, D.B.; Weiss, B.D. Brief report: Screening items to identify patients with limited health literacy skills. J. Gen. Intern. Med. 2006, 21, 874–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, L.D.; Griffin, J.M.; Partin, M.R.; Noorbaloochi, S.; Grill, J.P.; Snyder, A.; Bradley, K.A.; Nugent, S.M.; Baines, A.D.; Vanryn, M. Validation of screening questions for limited health literacy in a large VA outpatient population. J. Gen. Intern. Med. 2008, 23, 561–566. [Google Scholar] [CrossRef] [Green Version]
- Chew, L.D.; Bradley, K.A.; Boyko, E.J. Brief questions to identify patients with inadequate health literacy. Fam. Med. 2004, 36, 588–594. [Google Scholar]
- Wallace, L.S.; Cassada, D.C.; Rogers, E.S.; Freeman, M.B.; Grandas, O.H.; Stevens, S.L.; Goldman, M.H. Can screening items identify surgery patients at risk of limited health literacy? J. Surg. Res. 2007, 140, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, U.; Schillinger, D.; Lopez, A.; Sudore, R. Validation of self-reported health literacy questions among diverse English and Spanish-speaking populations. J. Gen. Intern. Med. 2011, 26, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Powers, B.J.; Trinh, J.V.; Bosworth, H.B. Can this patient read and understand written health information? JAMA 2010, 304, 76–84. [Google Scholar] [CrossRef]
- Bishop, W.P.; Craddock Lee, S.J.; Skinner, C.S.; Jones, T.M.; McCallister, K.; Tiro, J.A. Validity of single-item screening for limited health literacy in English and Spanish speakers. Am. J. Public Health 2016, 106, 889–892. [Google Scholar] [CrossRef]
- Rosenstock, I.M. The Health Belief Model and preventive health behavior. Health Educ. Monogr. 1974, 2, 354–386. [Google Scholar] [CrossRef]
- Janz, N.K.; Becker, M.H. The Health Belief Model: A decade later. Health Educ. Behav. 1984, 11, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Reisine, S.; Litt, M. Social and psychological theories and their use for dental practice. Int. Dent. J. 1993, 43, 279–287. [Google Scholar] [PubMed]
- Rotter, J.B. Generalized expectancies for internal versus external control of reinforcement. Psychol. Monogr. Gen. Appl. 1966, 80, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Lencova, E.; Pikhart, H.; Broukal, Z.; Tsakos, G. Relationship between parental locus of control and caries experience in preschool children–cross-sectional survey. BMC Public Health 2008, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Carnahan, T.M. The Development and Validation of the Multidimensional Dental Locus of Control Scales; State University of New York: Buffalo, NY, USA, 1980. [Google Scholar]
- National Survey of Children’s Health. Child and Adolescent Health Measurement Initiative, Data Resource Center on Child and Adolescent Health. Available online: http://childhealthdata.org/learn/topics_questions/2007-nsch?itemid=K2Q01_D (accessed on 21 February 2013).
- Batliner, T.; Wilson, A.R.; Tiwari, T.; Glueck, D.; Henderson, W.; Thomas, J.; Braun, P.; Cudeii, D.; Quissell, D.; Albino, J. Oral health status in Navajo Nation Head Start children. J. Public Health Dent. 2014, 74, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, T.; Quissell, D.O.; Henderson, W.G.; Thomas, J.F.; Bryant, L.L.; Braun, P.A.; Albino, J.E. Factors associated with oral health status in American Indian children. J. Racial Ethn. Health Disparities 2014, 1, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.A.; Lee, J.Y.; Rozier, R.G.; Pahel, B.T.; Richman, J.A.; Vann, W.F., Jr. Development and testing of the Test of Functional Health Literacy in Dentistry (TOFHLiD). J. Public Health Dent. 2007, 67, 105–112. [Google Scholar] [CrossRef]
- Firmino, R.T.; Ferreira, F.M.; Martins, C.C.; Granville-Garcia, A.F.; Fraiz, F.C.; Paiva, S.M. Is parental oral health literacy a predictor of children’s oral health outcomes? Systematic review of the literature. Int. J. Paediatr. Dent. 2018, 28, 459–471. [Google Scholar] [CrossRef]
- Vann, W.F., Jr.; Divaris, K.; Gizlice, Z.; Baker, A.D.; Lee, J.Y. Caregivers’ health literacy and their young children’s oral-health-related expenditures. J. Dent. Res. 2013, 92, 55S–62S. [Google Scholar] [CrossRef] [Green Version]
- Richman, J.A.; Lee, J.Y.; Rozier, R.G.; Gong, D.A.; Pahel, B.T.; Vann, W.F., Jr. Evaluation of a word recognition instrument to test health literacy in dentistry: The REALD-99. J. Public Health Dent. 2007, 67, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Firmino, R.T.; Ferreira, F.M.; Paiva, S.M.; Granville-Garcia, A.F.; Fraiz, F.C.; Martins, C.C. Oral health literacy and associated oral conditions: A systematic review. J. Am. Dent. Assoc. 2017, 148, 604–613. [Google Scholar] [CrossRef]
- Berkman, N.D.; Sheridan, S.L.; Donahue, K.E.; Halpern, D.J.; Crotty, K. Low health literacy and health outcomes: An updated systematic review. Ann. Intern. Med. 2011, 155, 97–107. [Google Scholar] [CrossRef] [PubMed]
- DeWalt, D.A.; Berkman, N.D.; Sheridan, S.; Lohr, K.N.; Pignone, M.P. Literacy and health outcomes: A systematic review of the literature. J. Gen. Intern. Med. 2004, 19, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.C.; Brega, A.G.; Crutchfield, T.M.; Elasy, T.; Herr, H.; Kaphingst, K.; Karter, A.J.; Moreland-Russell, S.; Osborn, C.Y.; Pignone, M.; et al. Update on health literacy and diabetes. Diabetes Educ. 2014, 40, 581–604. [Google Scholar] [CrossRef]
- Mackey, L.M.; Doody, C.; Werner, E.L.; Fullen, B. Self-management skills in chronic disease management: What role does health literacy have? Med. Decis. Mak. 2016, 36, 741–759. [Google Scholar] [CrossRef]
- Brega, A.G.; Johnson, R.; Schmiege, S.J.; Wilson, A.R.; Jiang, L.; Albino, J. Pathways through which health literacy is linked to parental oral health behavior in an American Indian tribe. Ann. Behav. Med. 2021. [Google Scholar] [CrossRef]
- Vilella, K.D.; Fraiz, F.C.; Benelli, E.M.; Assuncao, L.R. Oral health literacy and retention of health information among pregnant women: A randomised controlled trial. Oral Health Prev. Dent. 2017, 15, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Brega, A.G.; Pratte, K.A.; Jiang, L.; Mitchell, C.; Stotz, S.; LoudHawk-Hedgepeth, C.; Morse, B.; Noe, T.; Moore, K.; Beals, J. Impact of targeted health promotion on cardiovascular knowledge among American Indians and Alaska Natives. Health Educ. Res. 2013, 28, 437–449. [Google Scholar] [CrossRef] [Green Version]
- Office of the Assistant Secretary-Indian Affairs. 2013 American Indian Population and Labor Force Report; United States Department of the Interior: Washington, DC, USA, 16 January 2014. [Google Scholar]
- United States Census Bureau. My Tribal Area: 2014–2018 American Community Survey 5-Year Estimates. Available online: https://www.census.gov/tribal/ (accessed on 15 June 2020).
- da Fonseca, M.A.; Avenetti, D. Social determinants of pediatric oral health. Dent. Clin. N. Am. 2017, 61, 519–532. [Google Scholar] [CrossRef]
- Phipps, K.R.; Ricks, T.L.; Manz, M.C.; Blahut, P. Prevalence and severity of dental caries among American Indian and Alaska Native preschool children. J. Public Health Dent. 2012, 72, 208–215. [Google Scholar] [CrossRef] [Green Version]
- Indian Health Service. The 1999 Oral Health Survey of American Indian and Alaska Native Dental Patients: Findings, Regional Differences and National Comparisons; United States Department of Health and Human Services: Rockville, MD, USA, 2001. [Google Scholar]
| Construct | Mean (SD) 2 | Adjusted Time Estimate (∆) (95% CI) | p Value |
|---|---|---|---|
| Health Literacy | 3.9 (0.8) | --- | --- |
| Parental Oral Health Knowledge | 75.7 (12.9) | <0.001 | |
| 12 Months | 3.12 (2.02, 4.22) | <0.001 | |
| 24 Months | 3.49 (2.19, 4.78) | <0.001 | |
| 36 Months | 4.74 (3.52, 5.97) | <0.001 | |
| Parental Oral Health Beliefs Extended Health Belief Model | |||
| Perceived Susceptibility | 2.9 (1.0) | 0.271 | |
| 12 Months | −0.09 (−0.19, 0.01) | 0.079 | |
| 24 Months | −0.04 (−0.14, 0.06) | 0.399 | |
| 36 Months | −0.01 (−0.11, 0.10) | 0.913 | |
| Perceived Severity | 4.4 (0.8) | 0.078 | |
| 12 Months | 0.03 (−0.04, 0.10) | 0.469 | |
| 24 Months | 0.00 (−0.07, 0.08) | 0.913 | |
| 36 Months | −0.07 (−0.15, 0.01) | 0.082 | |
| Perceived Barriers | 2.1 (0.9) | 0.143 | |
| 12 Months | 0.04 (−0.04, 0.12) | 0.364 | |
| 24 Months | 0.02 (−0.06, 0.10) | 0.646 | |
| 36 Months | 0.09 (0.01, 0.17) | 0.038 | |
| Perceived Benefits | 4.4 (0.8) | 0.484 | |
| 12 Months | 0.05 (−0.04, 0.13) | 0.284 | |
| 24 Months | 0.04 (−0.04, 0.11) | 0.357 | |
| 36 Months | 0.06 (−0.02, 0.15) | 0.127 | |
| Self-efficacy | 9.1 (4.1) | 0.547 | |
| 12 Months | 0.13 (−0.20, 0.46) | 0.431 | |
| 24 Months | −0.10 (−0.48, 0.28) | 0.598 | |
| 36 Months | −0.05 (−0.43, 0.33) | 0.795 | |
| Locus of Control (LOC) | |||
| Internal LOC | 4.1 (0.9) | 0.012 | |
| 12 Months | 0.12 (0.03, 0.21) | 0.010 | |
| 24 Months | 0.00 (−0.10, 0.10) | 0.980 | |
| 36 Months | 0.05 (−0.05, 0.10) | 0.338 | |
| External LOC—Powerful Others | 2.2 (1.1) | <0.001 | |
| 12 Months | −0.24 (−0.33, −0.14) | <0.001 | |
| 24 Months | −0.35 (−0.45, −0.26) | <0.001 | |
| 36 Months | −0.29 (−0.40, −0.19) | <0.001 | |
| External LOC–Chance | 2.4 (1.1) | 0.073 | |
| 12 Months | 0.00 (−0.09, 0.09) | 0.970 | |
| 24 Months | −0.11 (−0.21, −0.01) | 0.026 | |
| 36 Months | −0.07 (−0.17, 0.03) | 0.151 | |
| Parental Oral Health Behavior 3 | 49.2 (23.3) | <0.001 | |
| 24 Months | −7.71 (−9.56, −5.88) | <0.001 | |
| 36 Months | −7.85 (−9.74, −5.96) | <0.001 | |
| Pediatric Oral Health Outcomes | |||
| Oral Health Status | 1.6 (0.9) | <0.001 | |
| 12 Months | 0.31 (0.21, 0.41) | <0.001 | |
| 24 Months | 0.92 (0.80, 1.05) | <0.001 | |
| 36 Months | 1.14 (1.00, 1.27) | <0.001 | |
| dmfs 3 | 0.4 (1.9) | <0.001 | |
| 24 Months | 15.63 (10.63, 22.98) | <0.001 | |
| 36 Months | 68.92 (46.49, 102.17) | <0.001 |
| Oral Health Construct | Adjusted HL Z-Score Estimate (95% CI) | p Value | p Value for Adjusted HL Z-Score by Time Interaction |
|---|---|---|---|
| Parental Oral Health Knowledge 2 | 3.72 (2.67, 4.76) | <0.001 | 0.326 |
| Parental Oral Health Beliefs Extended Health Belief Model | |||
| Perceived Susceptibility | −0.19 (−0.28, −0.10) | <0.001 | 0.868 |
| Perceived Severity | 0.11 (0.05, 0.18) | <0.001 | 0.374 |
| Perceived Barriers | −0.24 (−0.31, −0.16) | <0.001 | 0.519 |
| Perceived Benefits | 0.12 (0.06, 0.18) | <0.001 | 0.529 |
| Self-efficacy | 0.92 (0.61, 1.24) | <0.001 | 0.581 |
| Locus of control (LOC) | |||
| Internal LOC | 0.09 (0.01, 0.16) | <0.001 | 0.770 |
| External LOC—Powerful Others 2 | −0.25 (−0.34, −0.15) | <0.001 | 0.407 |
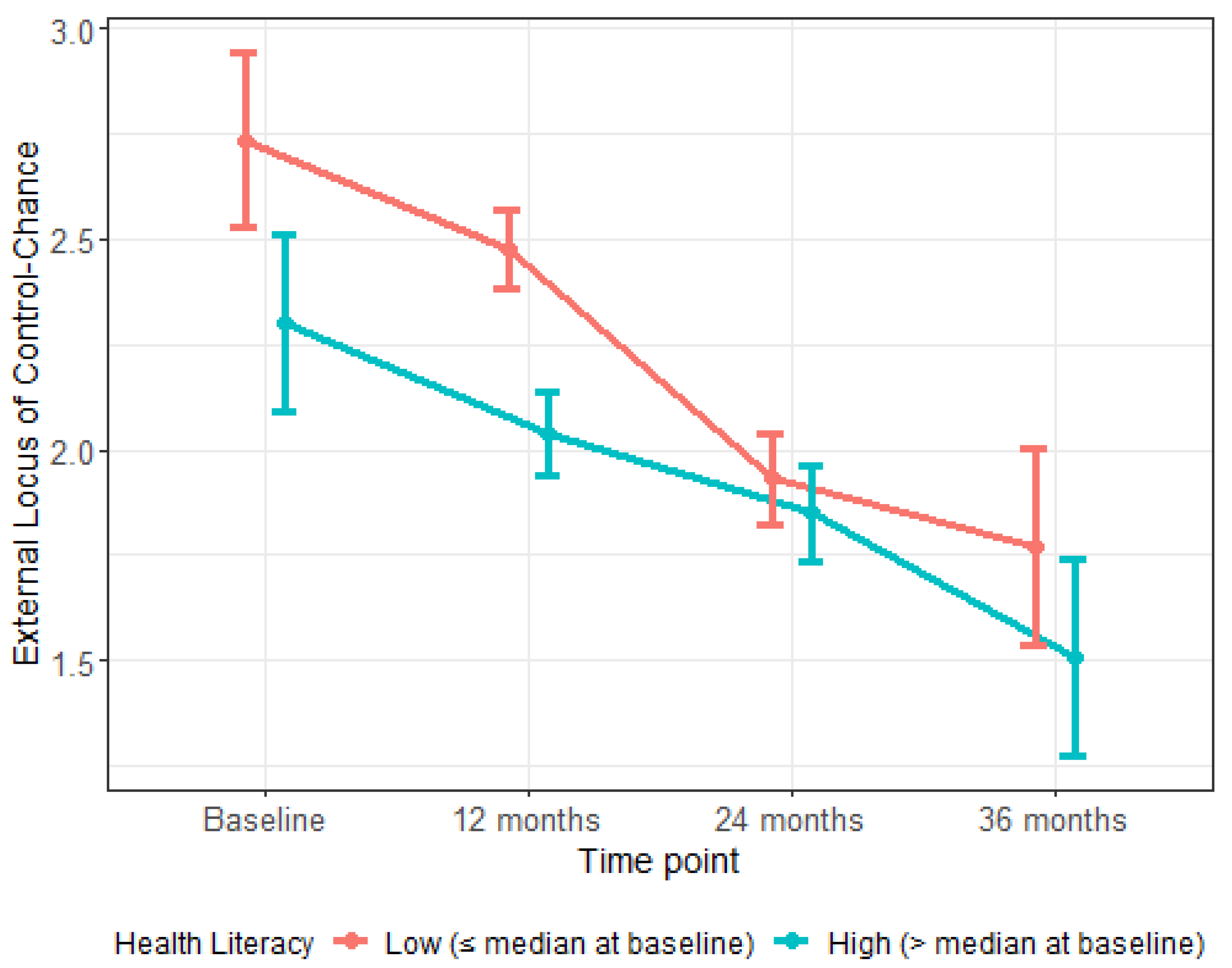
| External LOC—Chance | −0.29 (−0.38, −0.20) | <0.001 | <0.001 |
| Parental Oral Health Behavior 3 | 2.75 (0.97, 4.53) | <0.001 | 0.965 |
| Pediatric Oral Health Outcomes | |||
| Oral Health Status | −0.09 (−0.17, −0.002) | 0.004 | 0.747 |
| dmfs 3 | 1.15 (0.68, 1.95) | 0.838 | 0.332 |
| Oral Health Construct | Adjusted Time Estimate (∆) (95% CI) | p Value |
|---|---|---|
| Low Health Literacy Group | ||
| 12 Months | −0.03 (−0.13, 0.12) | 0.967 |
| 24 Months | −0.26 (−0.39, −0.14) | <0.001 |
| 36 Months | −0.15 (−0.29, −0.02) | 0.023 |
| High Health Literacy Group | ||
| 12 Months | −0.01 (−0.14, 0.13) | 0.907 |
| 24 Months | 0.09 (−0.05, 0.24) | 0.201 |
| 36 Months | 0.02 (−0.12, 0.17) | 0.769 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brega, A.G.; Johnson, R.L.; Jiang, L.; Wilson, A.R.; Schmiege, S.J.; Albino, J. Influence of Parental Health Literacy on Change over Time in the Oral Health of American Indian Children. Int. J. Environ. Res. Public Health 2021, 18, 5633. https://doi.org/10.3390/ijerph18115633
Brega AG, Johnson RL, Jiang L, Wilson AR, Schmiege SJ, Albino J. Influence of Parental Health Literacy on Change over Time in the Oral Health of American Indian Children. International Journal of Environmental Research and Public Health. 2021; 18(11):5633. https://doi.org/10.3390/ijerph18115633
Chicago/Turabian StyleBrega, Angela G., Rachel L. Johnson, Luohua Jiang, Anne R. Wilson, Sarah J. Schmiege, and Judith Albino. 2021. "Influence of Parental Health Literacy on Change over Time in the Oral Health of American Indian Children" International Journal of Environmental Research and Public Health 18, no. 11: 5633. https://doi.org/10.3390/ijerph18115633






