1. Introduction
Wheat bran, an important cereal industry by-product, is mostly used as fertilizer or discarded, resulting in a great waste of resources [
1]. Due to an increased interest in the health benefits of its bioactive phytochemicals, particularly phenolics, the use of bran as a functional food ingredient, is on the rise [
2,
3,
4]. Among different wheat varieties, black wheat was reported to possess the highest level of phenolic content, as well as antioxidant activity, compared with white and purple wheat [
5]. Around 80% of the total phenolics in whole grains exist in bound form and are distributed in the bran [
6,
7,
8]. Bound phenolics have shown significantly higher antioxidant capacity than free phenolics in vitro [
9,
10]. However, studies regarding phenolics are mostly focused on free phenolics rather than their bound form [
11,
12]. Moreover, the majority of published papers related to bound phenolics in foods mainly include fruits, vegetables, and legumes/seeds [
13]. There is a lack of knowledge regarding bound phenolics in grains, such as black wheat bran (BWB). In this respect, a recently developed black wheat Jizi439, which was hybrid from four different breeding lines [
14], was selected and investigated in the present study.
To maximize the TBPs yields of Jizi439 BWB and thereby improve their antioxidant activity, response surface methodology (RSM) was introduced and different extraction methods, including the traditional alkaline method, ultrasound-, and microwave- assisted extraction method, were compared in our study. The traditional acid or alkaline method was established in 1982 [
15]. Although acid treatment breaks glycosidic bonds of sugars and leaves ester bonds of bound phenolics intact [
16], the acid environment may lead to the degradation of flavanols [
17]. Compared with acid extraction, alkaline hydrolysis avoids flavanol degradation [
18] and reduces their loss [
19,
20]. However, both methods still have their drawbacks, such as low yield of phenolics and long production cycle. Therefore, a variety of new extraction techniques have been developed, such as ultrasound- [
21,
22,
23] and microwave-assisted extraction [
24,
25]. These two new green extraction techniques not only greatly shorten the extraction time, but also consume less solvents and energy [
26,
27]. The acoustic cavities, as well as the mechanical effect created by ultrasound waves, could disrupt the plant cell walls and release the bioactive compounds into solvent [
28]. Meanwhile, microwaves possess the ability to heat up the molecules in the plant by ionic conduction and dipole rotation, resulting in the rupture of cell walls, and accelerate the release of phenolic compounds [
29]. Extraction of bioactive compounds using ultrasound or microwave has been proven to be an efficient method that reduces the use of toxic chemicals [
26,
27].
Therefore, the objective of this study was: (1) to optimize UAE and MAE processes using RSM; (2) to compare the TBPC yields, AAs, and the profiles of bound phenolics obtained from UAE, MAE, and traditional alkaline extraction methods under the optimal extraction conditions; and (3) eventually to find an most efficiency way for obtaining more bound phenolics with high antioxidant activity. Taken together, our study fills the gap in optimization of extracting bound phenolics from BWB, provides a fundamental knowledge of bound phenolics profile in the Jizi439 BWB, and suggests an efficient extraction method for further use as a sustainable healthy food ingredient.
2. Materials and Methods
2.1. Materials and Reagents
The Jizi439 BWB was received from Yueqing Agricultural Science and Technology Co., Ltd. (Handan, China). Tris-HCl buffer was obtained from Solarbio Science and Technology Co. (Beijing, China). DPPH (2,2-diphenyl-1-picrylhydrazyl) and 2,2′-Azinobis (3-ethylbenzothiazoline-6-sulfonate) (ABTS) free radical were acquired from Sigma-Aldrich (St. Louis, MO, USA). Folin-Ciocalteu reagent and NaCO3 were purchased from Hushi chemical Co. (Shanghai, China). Standards, including Gallic acid, p-hydroxybenzoic acid, vanillic acid, p-coumaric acid, ferulic acid, syringic acid, caffeic acid, protocatechuic acid, chlorogenic acid, catechin, luteolin, and apigenin, were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China).
2.2. Preparation of Samples
Milling of Jizi439 BWB used a high-speed multifunctional pulverizer (RHP-100, Jinhua, China) before sieving through a 60-mesh screen. A total of 200 g of bran powder was then transferred to a flask, defatted with hexane at a ratio of 4:1 (v/w), and then kept on a thermostatic oscillator for 1 h at room temperature. After that, the defatted bran powder was dried in a hood overnight and stored at −20 °C before analysis.
2.3. Experimental Design
The scheme of the Jizi439 BWB extraction process, either by traditional, UAE, or MAE methods, was shown in
Figure 1. RSM was used to optimize the extraction conditions for UAE and MAE. The defatted bran powder acquired from
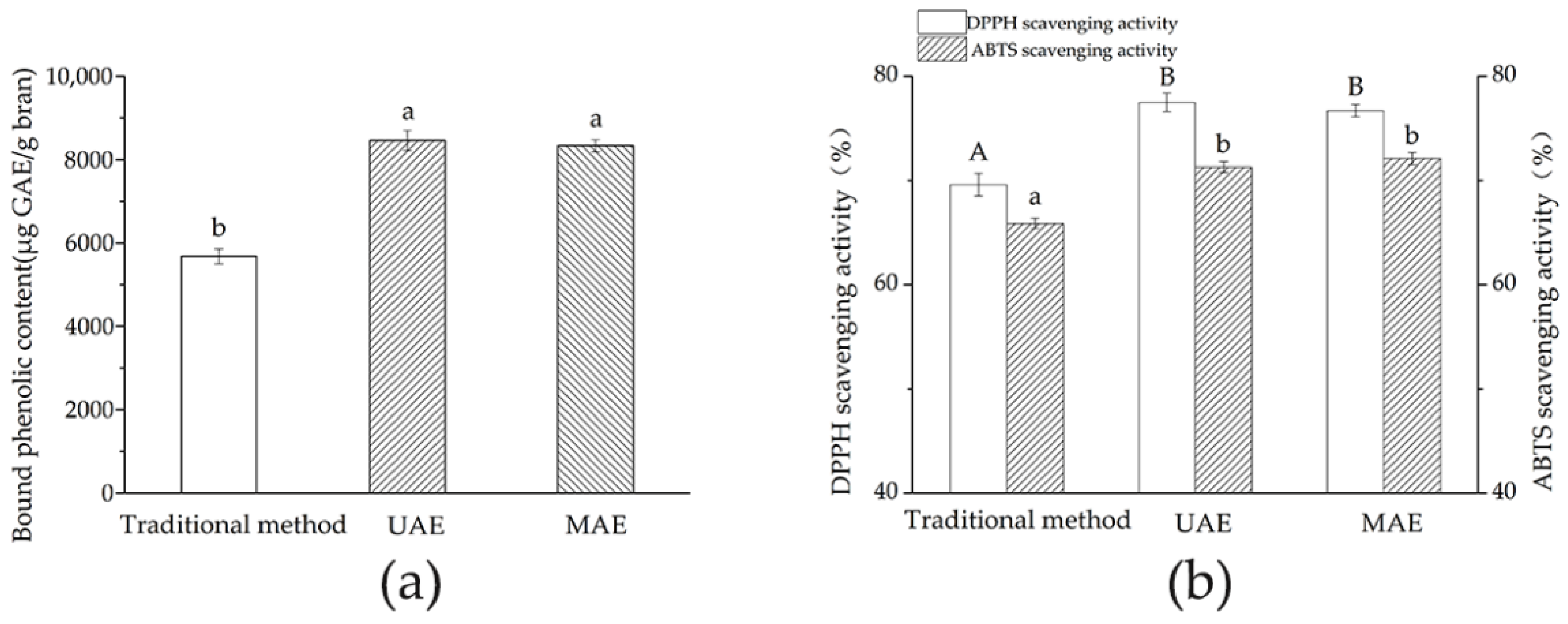
Section 2.2 was assigned to three groups, including: (1) the traditional alkaline extraction method group, where TBPs were extracted by the traditional alkaline method, without ultrasound or microwave treatment; (2) ultrasound-assisted extraction (UAE), where TBPs were extracted with ultrasound treatment; (3) microwave-assisted extraction (MAE), where TBPs were extracted with microwave treatment. TBPCs and their AAs (in terms of DPPH and ABTS scavenging activity) were compared in each group.
2.4. Traditional Alkaline Extraction Method
Bound phenolics were extracted according to Brij Verma et al. [
8], with modifications. One gram of defatted Jizi439 BWB was extracted two times with 80% ethanol before centrifugation at 2500×
g for 10 min. The supernatant was discarded, and the residue was mixed with 40 mL of NaOH (2 M), then left to stand for 4 h at room temperature. After that, the mixture was acidified to pH 2 with 6 M HCl. Bound phenolics were then extracted three times with diethyl ether (DE) and ethyl acetate (EA) (1:1
v/
v) by manually shaking before centrifuging at 2500×
g for 10 min. DE/EA layers were combined, and the supernatants were pooled and evaporated to less than 5 mL. The concentrated samples were reconstituted to a final volume of 15 mL with methanol, then stored at −20 °C until further use.
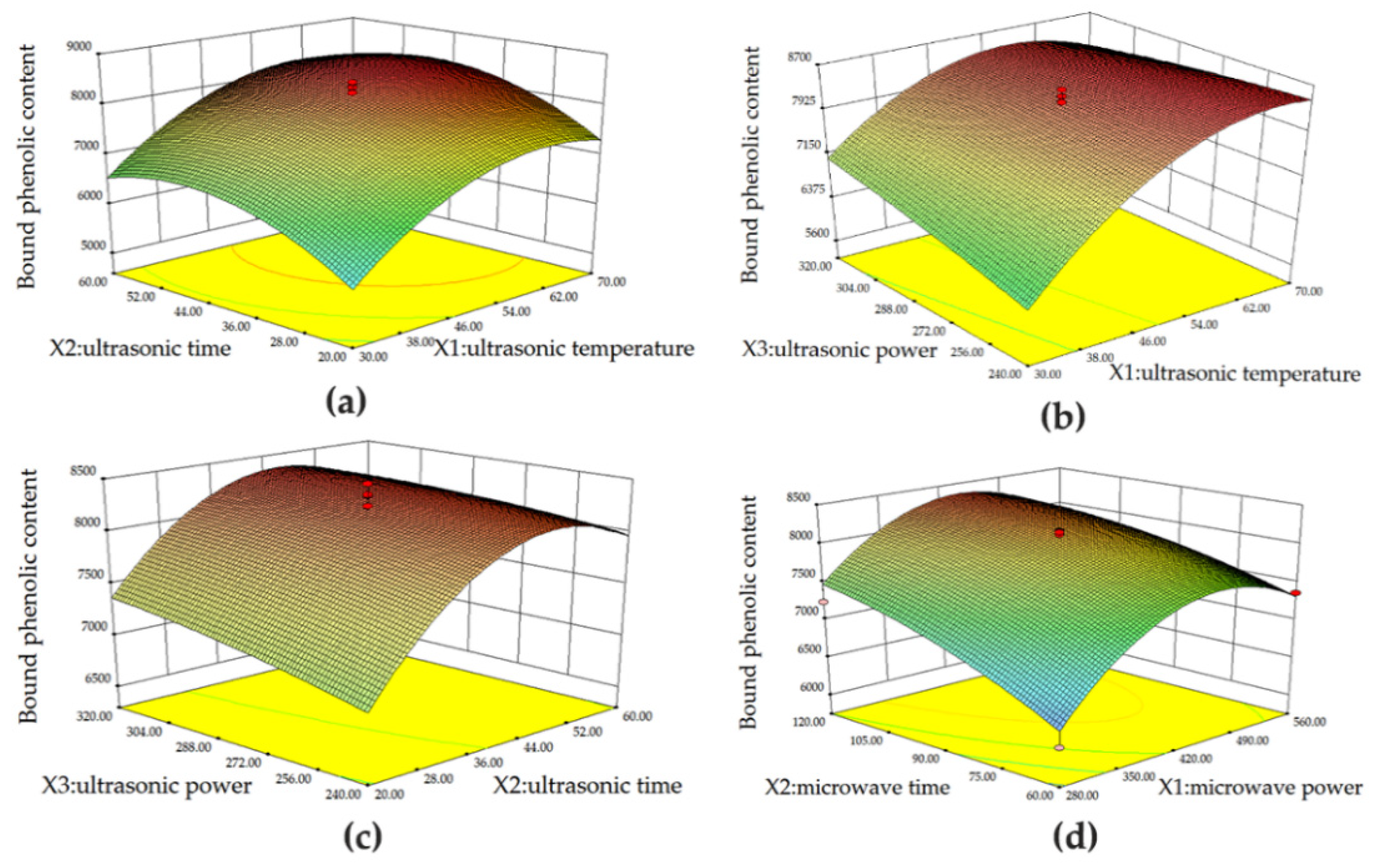
2.5. Response Surface Design for UAE and MAE
2.5.1. Ultrasound-Assisted Extraction (UAE)
Bound phenolics were extracted with 2 M NaOH and assisted with an ultrasound instrument (THC-2B, Jining Tianhua Ultrasound Electronic Instrument Co., Ltd., Jining, China). Ultrasonic temperature (
X1), ultrasonic time (
X2), and ultrasonic power (
X3) were selected as independent variables. TBPCs measured by the Folin-Ciocalteu method were used as a response value (
Y). The central composite design (CCD) was carried out for the levels of each independent variable. The ANOVA model was established by the Design-Expert version 8.0.6 software (Minneapolis, MN, USA). The response surface independent variables and levels design are shown in
Table 1.
2.5.2. Microwave-Assisted Extraction (MAE)
Bound phenolics were extracted from Jizi439 BWB with 2 M NaOH assisted with a microwave oven (700 W, Galanz Microwave Oven Manufacture Co., Ltd., Foshan, China). Microwave power (
X1) and microwave time (
X2) were selected as independent variables, and TBPCs measured by the Folin-Ciocalteu method were used as a response value (
Y). The central composite design (CCD) was carried out for the levels of each independent variable. The ANOVA model was established by the Design-Expert 8.0.6 software. The response surface independent variables and levels design are shown in
Table 2.
2.5.3. Model Fitting
Experimental data were fitted to a second-order polynomial model. The generalized second-order polynomial model used in the response surface analysis was as follows:
where
Y represented the measured response (TBPCs);
β0,
βi,
βii, and
βij were the regression coefficients for intercept, linear, quadratic, and interaction terms, respectively;
Xi and
Xj were the coded values of the
ith and
jth independent variables. The variable
XiXj represented the first order interaction between
Xi and
Xj for (
i <
j).
2.5.4. Verification of Model
Bound phenolics were extracted under optimized conditions of UAE or MAE, and the actual experimental data and the predicted value calculated by equation in
Section 2.5.3 were compared for the purpose of determining the validity of the model.
2.6. Determination of the Total Bound Phenolic Contents (TBPCs)
TPBCs were determined using Folin-Ciocalteu reagent, as adapted from Chen et al. [
30]. Briefly, in each well of the 96-well plate, an aliquot of the 20 µL sample solution or gallic acid standard solution (0–180 µg/mL) was mixed with 100 µL of the Folin-Ciocalteu reagent. The mixture reacted for 5 min at room temperature before the addition of 80 µL 7.5% NaCO
2 solution and then kept in the dark for 2 h at room temperature. The absorbance was measured at 750 nm using a microplate reader (EnSpire
® Multimode Plate reader, PerkinElmer Management Co., Ltd., Shanghai, China). Each well was repeated six times. The TBPCs was calculated and expressed as micrograms of gallic acid equivalent per gram of BWB (µg GAE/g).
2.7. Determination of Antioxidation Activity (AA)
DPPH assay: The DPPH scavenging activity of the Jizi439 BWB extracts was determined according to Shimamura et al. [
31]. In brief, the DPPH solution was freshly prepared before each use. In total, 20 μL of the sample solution and 80 μL of Tris-HCl buffer (0.1 M) were mixed before 100 μL of DPPH solution was added. The mixture was then allowed to stand for 30 min in the dark at room temperature. Absorbance was read at 515 nm using a microplate reader (EnSpire
® Multimode Plate reader, PerkinElmer Management Co., Ltd., Shanghai, China). The radical scavenging activity in terms of the inhibition ratio was calculated using the following equation:
where
As was the absorbance of DPPH solution and Tris-HCl buffer with the sample solution;
Ac was the absorbance of DPPH solution and Tris-HCl buffer with methanol;
A1 was the absorbance of Tris-HCl buffer and the sample solution, with methanol for background absorbance.
ABTS assay: This procedure was conducted according to Wu and Maria et al. [
32,
33], with some modification. The ABTS•
+ was generated by reacting an ABTS aqueous solution at 7.4 mM with a K
2S
2O
8 solution at 2.6 mM in the dark for 12−16 h and adjusting the Abs
734 nm to 0.700 (±0.020) with a pH 7.4 phosphate buffer solution. The sample solution of 10 μL was mixed with 190 μL of ABTS, and the absorbance was measured under 734 nm after avoiding light 20 min at room temperature. The solution was then shaken for 30 s during measurement to fully mix. The radical scavenging activity in terms of the inhibition ratio was calculated using the following equation:
where
A1 was the absorbance of the ABTS solution with the sample solution;
A2 was the absorbance of the sample solution with methanol;
A3 was the absorbance of the ABTS solution with methanol.
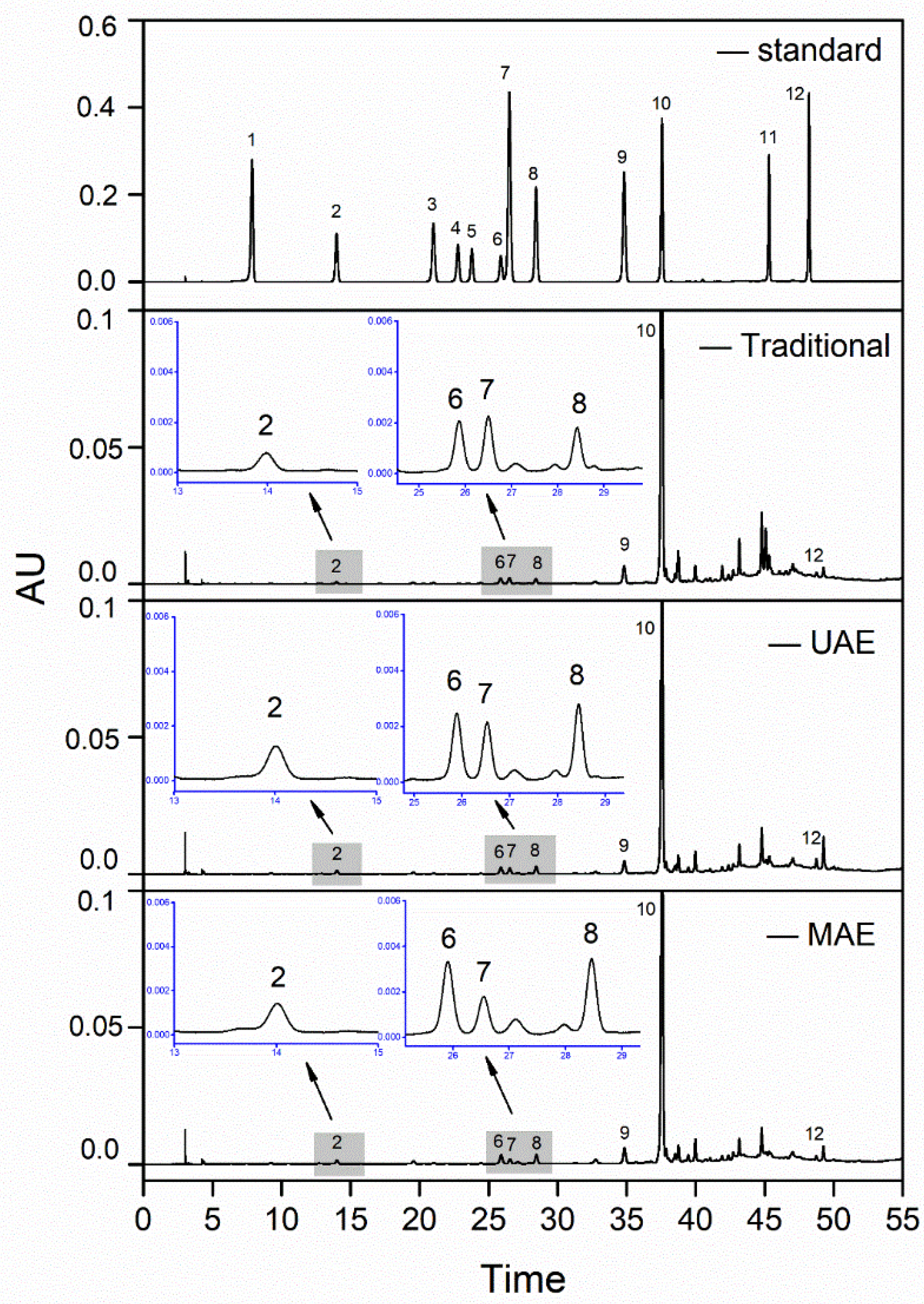
2.8. HPLC Analysis of Bound Phenolic Extracts from Jizi439 BWB
HPLC analysis was carried out according to Kim et al. [
18], with slight modifications. The profile of TBPs was analyzed using a Waters e2695 HPLC system equipped with an auto sampler, a Waters XSelect
® HSS T3 column (250 × 4.6 mm, 5 µm), and a UV detector (Milford, MA, USA). The mobile phase consisted of acetonitrile (solvent A) and 2% acetic acid solution(solvent B). The elution program was set as follows: 0–30 min, 0–15% A; 30–50 min, 15–50% A; 50–55 min, 50–100% A; 55–60 min, 100–0% A; 60–70 min, 0%A, at a flow rate of 1 mL/min. The injection volume and column temperature were set at 10 µL and 30 °C, respectively. Peaks were read at 280 nm. Retention times and chromatographic peaks were matched with those of the standards.
2.9. Statistical Analysis
All measurements were independently conducted in triplicate. Data were expressed as mean ± standard deviations (SD). The adequacy of the RSM model was predicted through the regression (R2) and ANOVA analysis. The CCD and the corresponding analysis of the data, including the generated 3D response surfaces and the determination of the optimal set of variables that maximize the yield of bound phenolics, were carried out using the software package Design Expert Version 8.0.6 software (Minneapolis, MN, USA).
4. Conclusions
The optimal UAE and MAE conditions generated by RSM models were ultrasonic temperature of 66 °C, ultrasonic time of 48 min, ultrasonic power of 240 W for UAE, microwave power of 420 W, and extraction time of 120 s for MAE, respectively. Under optimal conditions, the TBPCs and antioxidant activity of UAE and MAE were comparable with each other, and both were significantly higher than that of the traditional method. However, MAE possessed the economic advantage of a short extraction time with less energy consumption as compared to UAE and the traditional method. Based on our results, MAE is considered an efficient method to obtain large quantities of bound phenolics for further usage of a functional ingredient for food and pharmaceutical products.