Effect of Electrical Accelerated Aging on DC Resistivity of Mineral Oil Used in Power Transformers
Abstract
:1. Introduction
2. Electrical Conduction in Mineral Oil
3. Experimental Method
3.1. Accelerated Aging Method
3.1.1. Method Implementation Principle
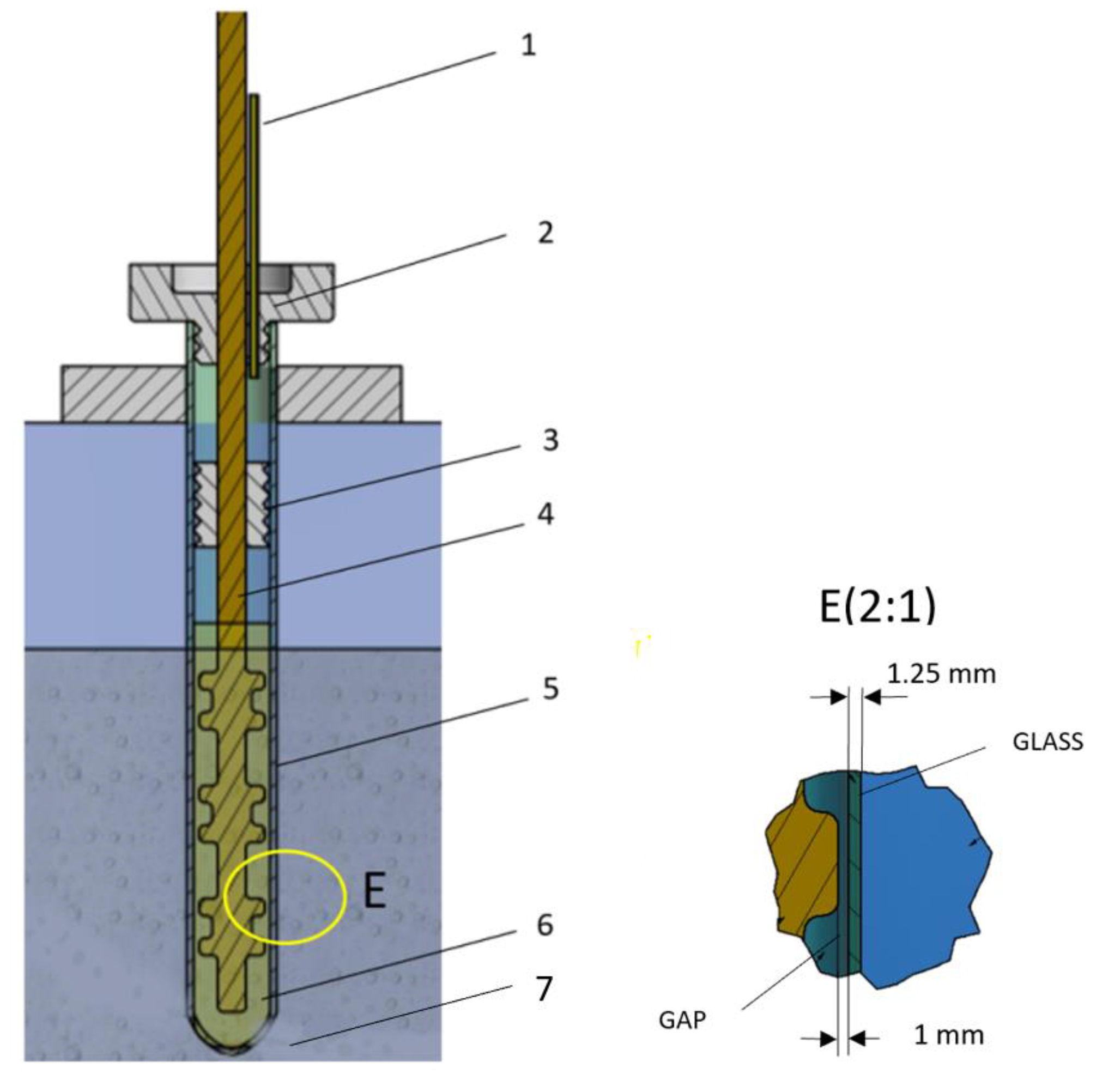
3.1.2. Accelerated Aging Cell Design
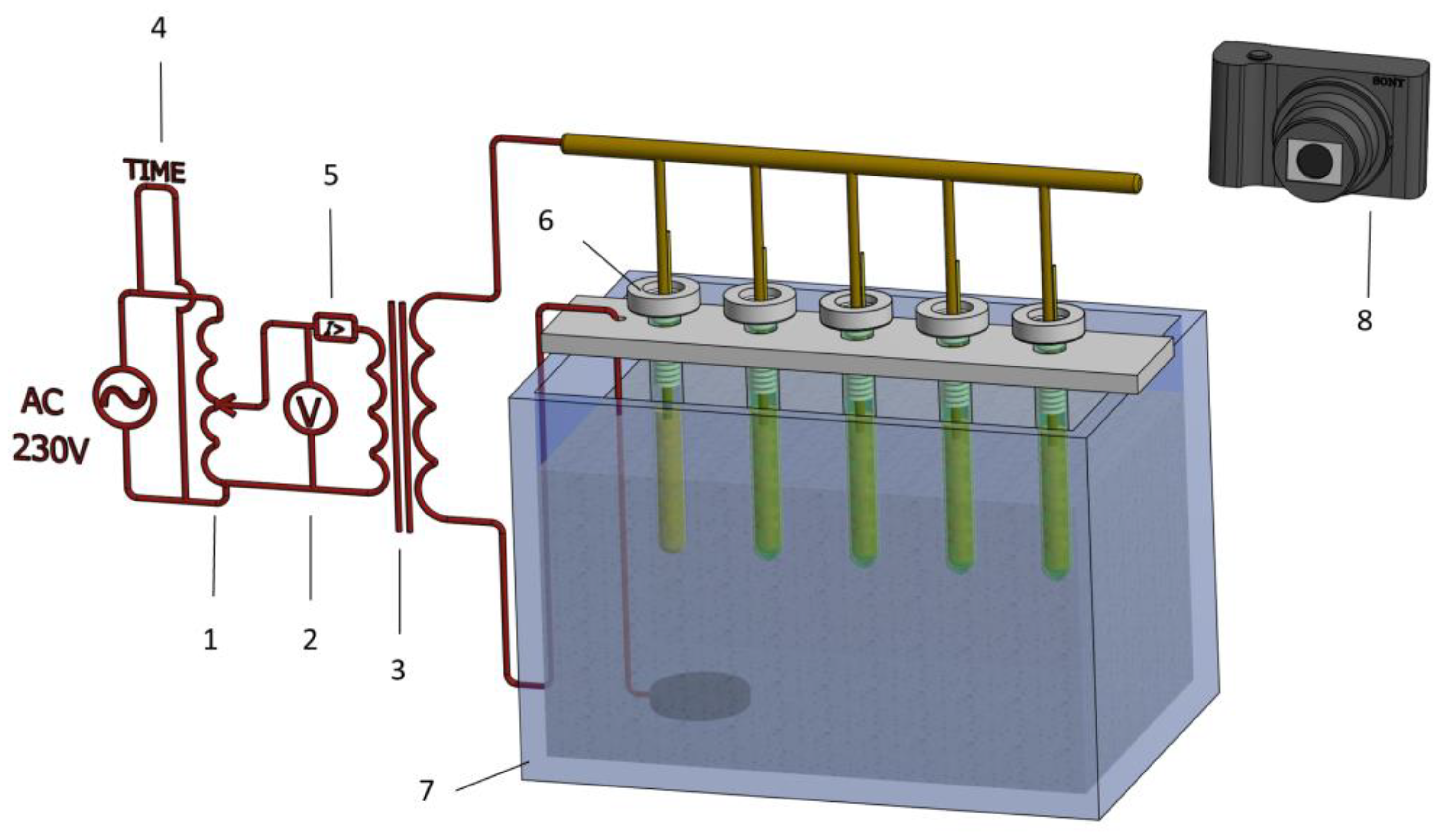
3.1.3. Experimental Aging Setup
3.2. Absorption and Resorption Currents Measurement
3.2.1. Absorption and Resorption Currents
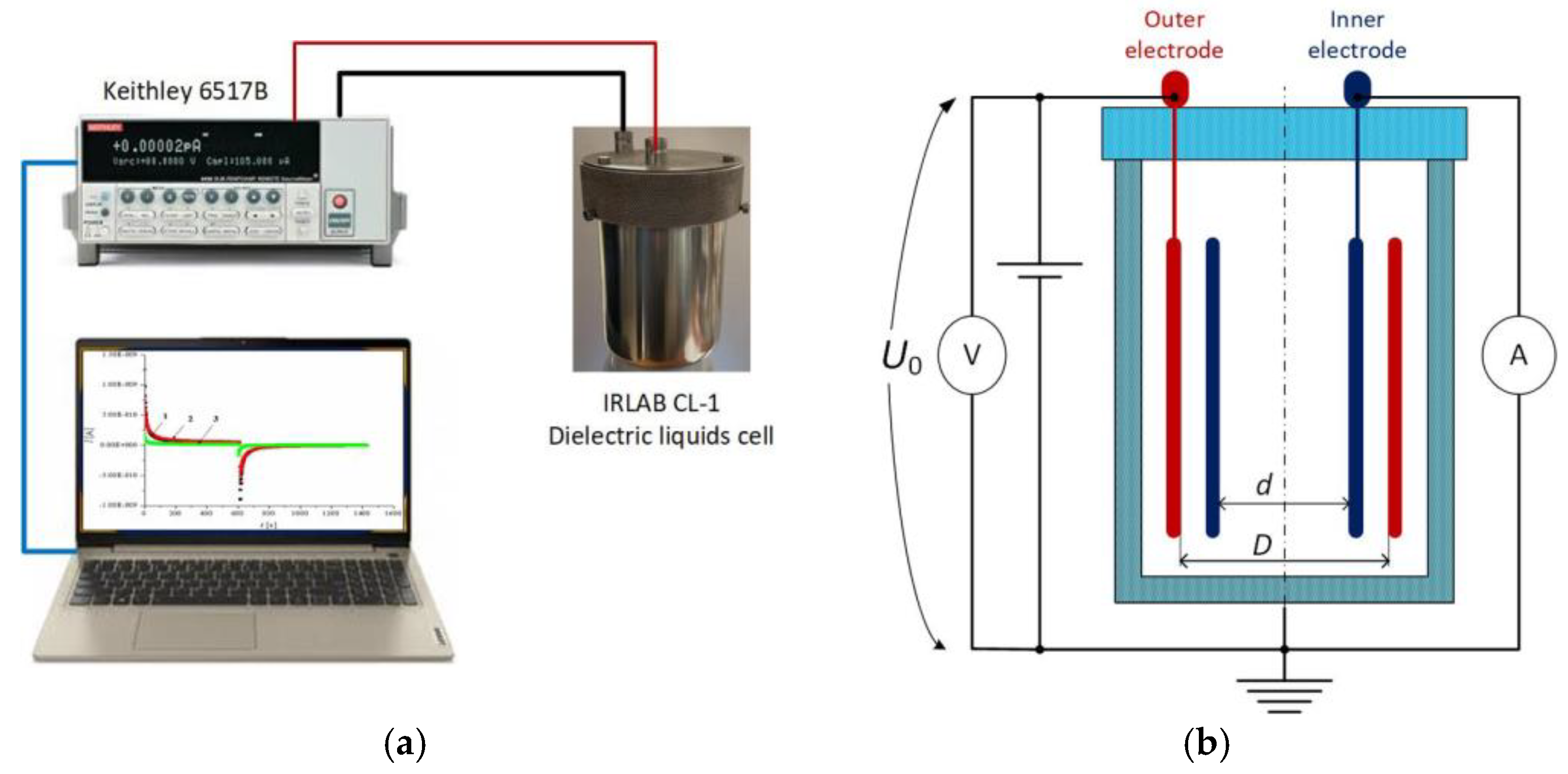
3.2.2. Absorption and Resorption Currents Testing Bench Setup
3.2.3. Mineral Oil Samples
4. Experimental Results and Discussion
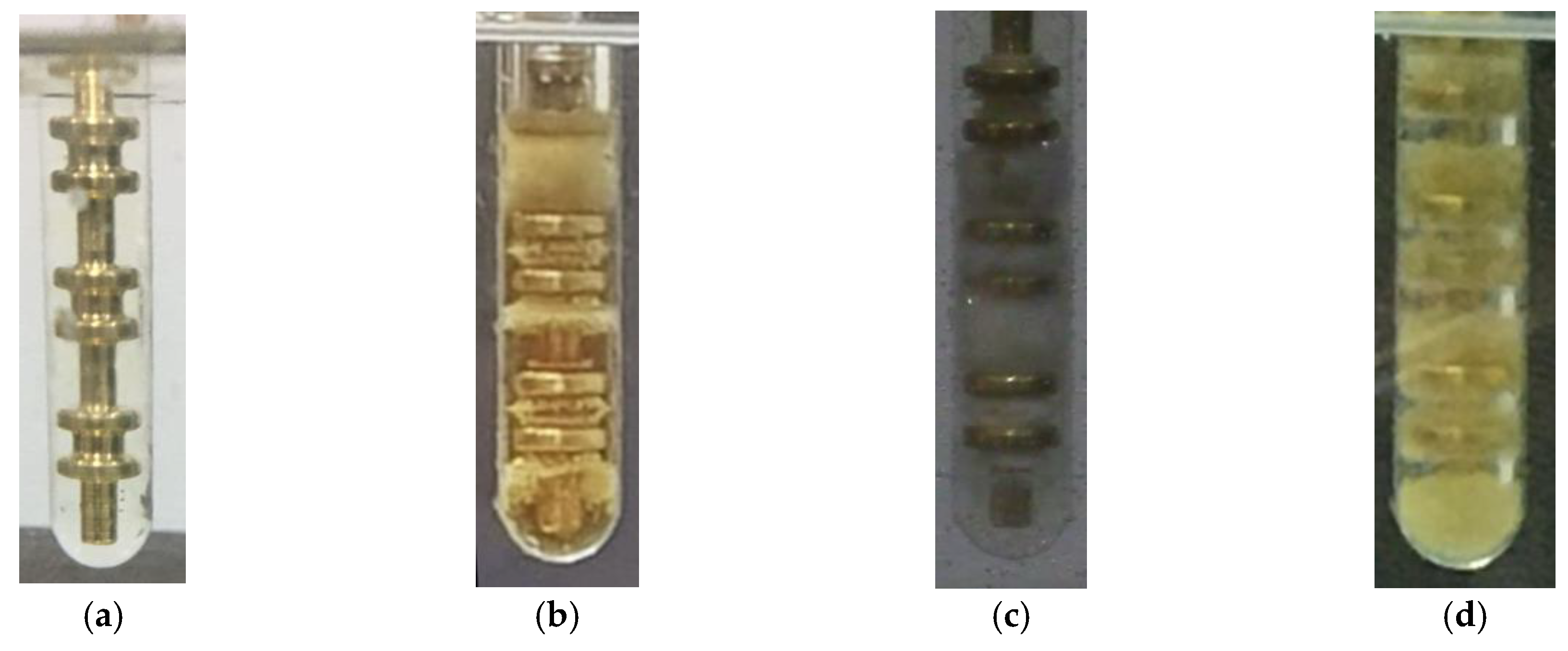
4.1. Experimental Aging Process Observation
4.2. Absorption and Resorption Currents and Resistivity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- North American Electric Reliability Corporation, Special Report: Spare Equipment Database System. October 2011. Available online: https://drosheim.files.wordpress.com/2012/10/sedtf_special_report_october_2011.pdf (accessed on 19 December 2022).
- Schwarz, R.; Muhr, M. Diagnostic Methods for Transformers. In Proceedings of the International Conference on Condition Monitoring and Diagnosis, Pekin, China, 21–24 April 2008; pp. 974–977. [Google Scholar]
- Saha, T.K.; Pradhan, M.K.; Yew, J.H. Optimal Time Selection for the Polarisation and Depolarisation Current Measurement for Power Transformer Insulation Diagnosis. In Proceedings of the Power Engineering Society General Meeting, Tampa, FL, USA, 24–28 June 2007; pp. 1–7. [Google Scholar]
- Gubanski, S.; Boss, P.; Csépes, G.; Der Houhanessian, V.; Filippini, J.; Guuinic, P.; Gäfvert, U.; Karius, V.; Lapworth, J.; Urbani, G.; et al. Dielectric Response Methods for Diagnostics of Power Transformers. CIGRE 2002, 254, 1–40. [Google Scholar]
- Zhang, X.; Gockenbach, E. Asset-Management of Transformers Based on Condition Monitoring and Standard Diagnosis. IEEE Electr. Insul. Mag. 2008, 24, 26–40. [Google Scholar] [CrossRef]
- Tenbohlen, S.; Vahidi, F.; Gebauer, J.; Krüger, M.; Müller, P. Assessment of Power Transformer Reliability. In Proceedings of the XVII International Symposium on High Voltage Engineering, Hannover, Germany, 22–26 August 2011. [Google Scholar]
- Metwally, I.A. Failures, monitoring and new trends of power transformers. IEEE Potentials 2011, 30, 36–43. [Google Scholar] [CrossRef]
- Jahromi, A.; Piercy, R.; Cress, S.; Service, J.; Fan, W. An Approach to Power Transformer Asset Management Using Health Index. IEEE Electr. Insul. Mag. 2009, 25, 20–34. [Google Scholar] [CrossRef]
- Notingher, P.; Badicu, L.; Dumitran, L.; Setnescu, R.; Setnescu, T. Transformerboard Lifetime Estimation using Activation Energy. In Proceedings of the ISEF 2011—XV International Symposium on Electromagnetic Fields in Mechatronics, Electrical, and Electronic Engineering, Funchal, Portugal, 1–3 September 2011. [Google Scholar]
- CIGRE Brochure 323, Ageing of Cellulose in Mineral-Oil Insulated Transformers. 2007. Available online: https://e-cigre.org/publication/323-ageing-of-cellulose-in-mineral-oil-insulated-transformers (accessed on 19 December 2022).
- Du, Y.; Zahn, M.; Lesieutre, B.; Mamishev, A.; Lindgren, S. Moisture Equilibrium in Transformer Paper-Oil Systems. IEEE Electr. Insul. Mag. 1999, 15, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Fabre, J.; Pichon, A. Deteriorating Processes and Products of Paper in Oil. Application to Transformers, In Proceedings of the 1960 International Conference on Large High Voltage Electric System (CIGRE), Paris, France, 15–25 June 1960; p. 137.
- Lelekakis, N.; Guo, W.; Martin, D.; Wijaya, J.; Susa, D. A Field Study of Aging in Paper-Oil Insulation Systems. IEEE Electr. Insul. Mag. 2012, 28, 12–19. [Google Scholar] [CrossRef]
- Manea, A.; Dumitran, L.; Borzea, C.; Cazacu, E. Accelerated Ageing Method of Mineral Oil Under High Electric Field and Partial Discharges. In Proceedings of the 12th International Symposium on Advanced Topics in Electrical Engineering (ATEE 2021), Bucharest, Hungary, 25–27 March 2021. [Google Scholar]
- Felici, N. High-Field Conduction in Dielectric Liquids Revisited. IEEE Trans. Elect. Insul. 1985, EI-20, 233–238. [Google Scholar] [CrossRef]
- Felici, N.; Gosse, J. Injection d’ions par des électrodes métalliques dans les hydrocarbures liquides de résistivité élevée. Rev. Phys. Appliquée 1979, 14, 629–633. [Google Scholar] [CrossRef]
- Onsager, L. Deviations from Ohm’s Law in Weak Electrolytes. J. Chem. Phys. 1934, 2, 599–615. [Google Scholar] [CrossRef]
- Badicu, L.V. Diagnosis, and Monitoring of Insulation Systems of Power Transformers. Ph.D. Thesis, University Politehnica of Bucharest, Bucharest, Hungary, 2011. [Google Scholar]
- Lewis, T.J. Basic electrical processes in dielectric liquids. IEEE Trans. Dielect. Electr. Insul. 1994, 1, 630–643. [Google Scholar] [CrossRef]
- Minday, R.M. Excess Electrons in Liquid Hydrocarbons. J. Chem. Phys. 1971, 54, 3112. [Google Scholar] [CrossRef]
- Küchler, A.; Piowan, U.; Ananstova-Höhlein, I.; Berglund, M.; Chen, G.; Fabian, J.; Fritsche, R.; Grav, T.; Gubanski, S.; Jaufer, S.; et al. HVDC Transformer Insulation: Oil conductivity. CIGRÉ Technol. Broch. 646 2016. [Google Scholar]
- ASTM D 6180–05; Standard Test Method for Stability of Insulating Oils of Petroleum Origin under Electrical Discharge. ASTM International: West Conshohocken, PA, USA, 2005.
- Loiselle, L.; Rao, U.M.; Fofana, I. Influence of aging on oil degradation and gassing tendency under high-energy electrical discharge faults for mineral oil and synthetic ester. IET J. High Volt. 2020, 5, 731–738. [Google Scholar] [CrossRef]
- Badicu, L.; Gorgan, B.; Busoi, S.; Dumitran, L.; Notingher, P. Use of time-domain spectroscopy for assessment of oil impregnated paper condition. U.P.B. Sci. Bull. Ser. C 2011, 73, 267–282. [Google Scholar]

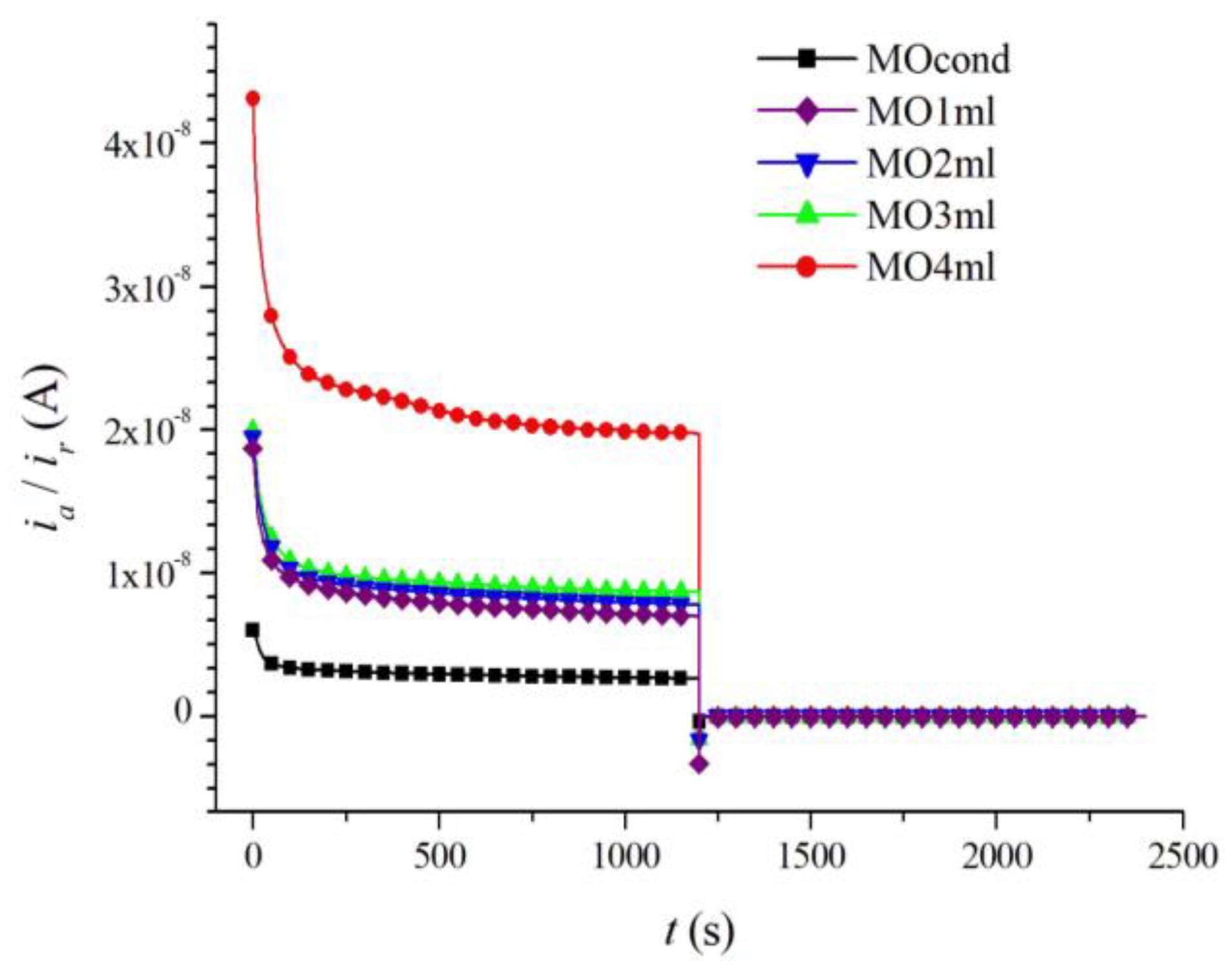
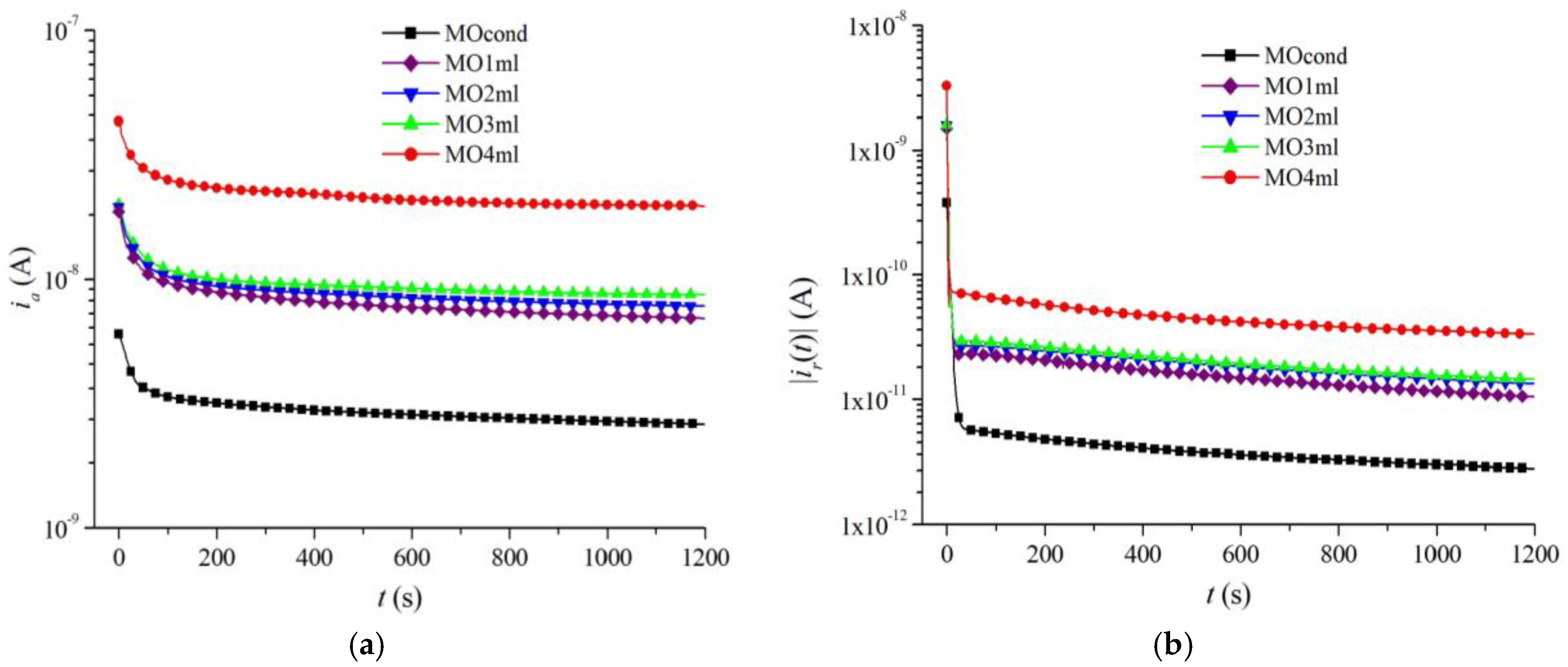
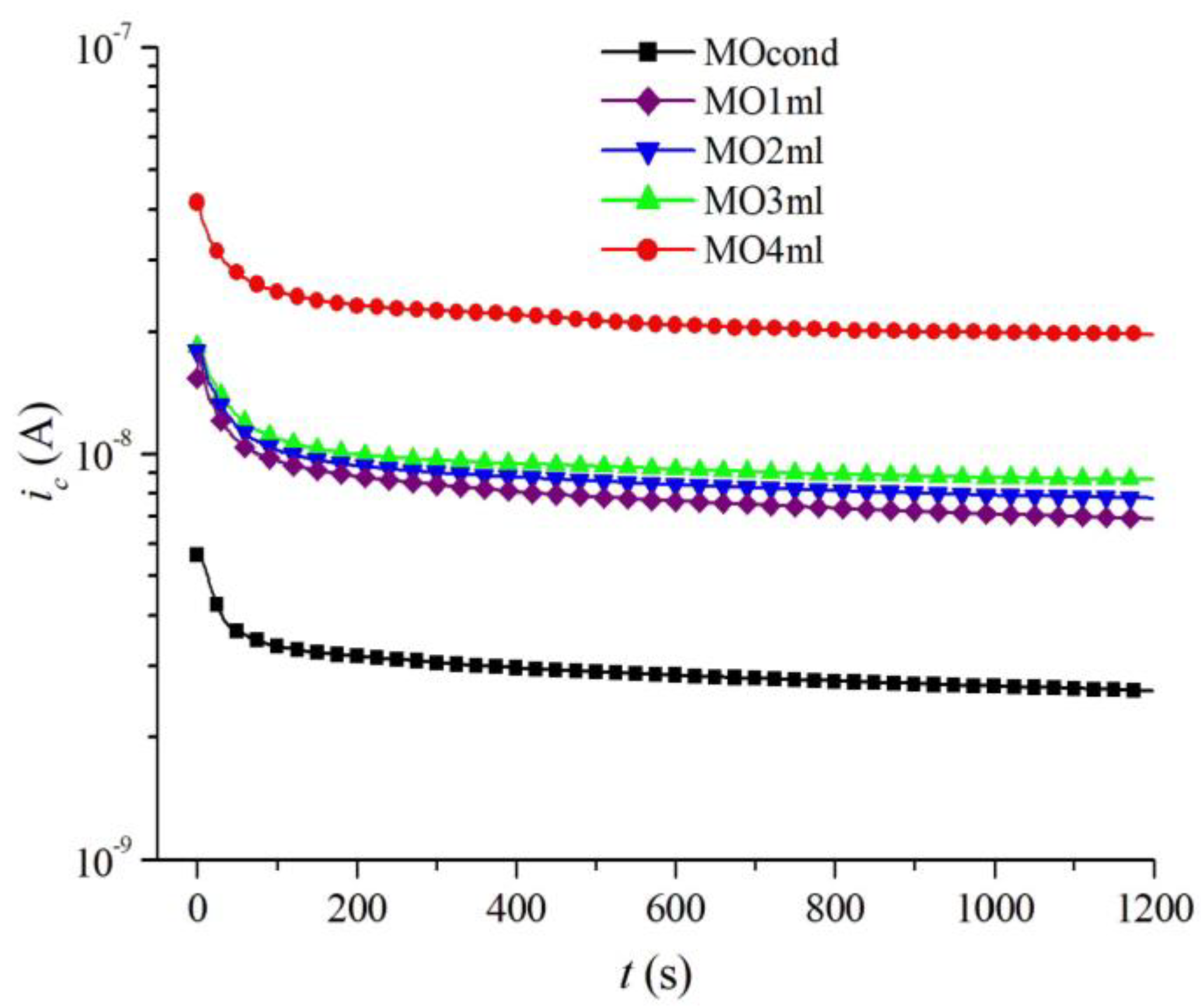

| Sample | Composition |
|---|---|
| MOcond | 150 mL unaged oil |
| MO1ml | 150 mL unaged oil + 1 mL aged oil |
| MO2ml | 150 mL unaged oil + 2 mL aged oil |
| MO3ml | 150 mL unaged oil + 3 mL aged oil |
| MO4ml | 150 mL unaged oil + 4 mL aged oil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manea, A.; Gorjanu, T.; Lazeanu, A.; Dumitran, L.M. Effect of Electrical Accelerated Aging on DC Resistivity of Mineral Oil Used in Power Transformers. Energies 2023, 16, 294. https://doi.org/10.3390/en16010294
Manea A, Gorjanu T, Lazeanu A, Dumitran LM. Effect of Electrical Accelerated Aging on DC Resistivity of Mineral Oil Used in Power Transformers. Energies. 2023; 16(1):294. https://doi.org/10.3390/en16010294
Chicago/Turabian StyleManea, Andrei, Teodora Gorjanu, Andreea Lazeanu, and Laurentiu Marius Dumitran. 2023. "Effect of Electrical Accelerated Aging on DC Resistivity of Mineral Oil Used in Power Transformers" Energies 16, no. 1: 294. https://doi.org/10.3390/en16010294





