Mass Spectrometry Imaging of Lipids in the Scent Glands of Muskrat (Ondatra zibethicus) in Different Reproductive Statuses
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Collection
2.3. MALDI-TOF/TOF MS/MS Analysis
2.4. Metabolites Extraction
2.5. LC-MS/MS Analysis
2.6. GC-MS Analysis
2.7. H&E Staining
2.8. Quantitative Real-Time PCR (qRT-PCR)
2.9. Statistical Analysis
3. Results
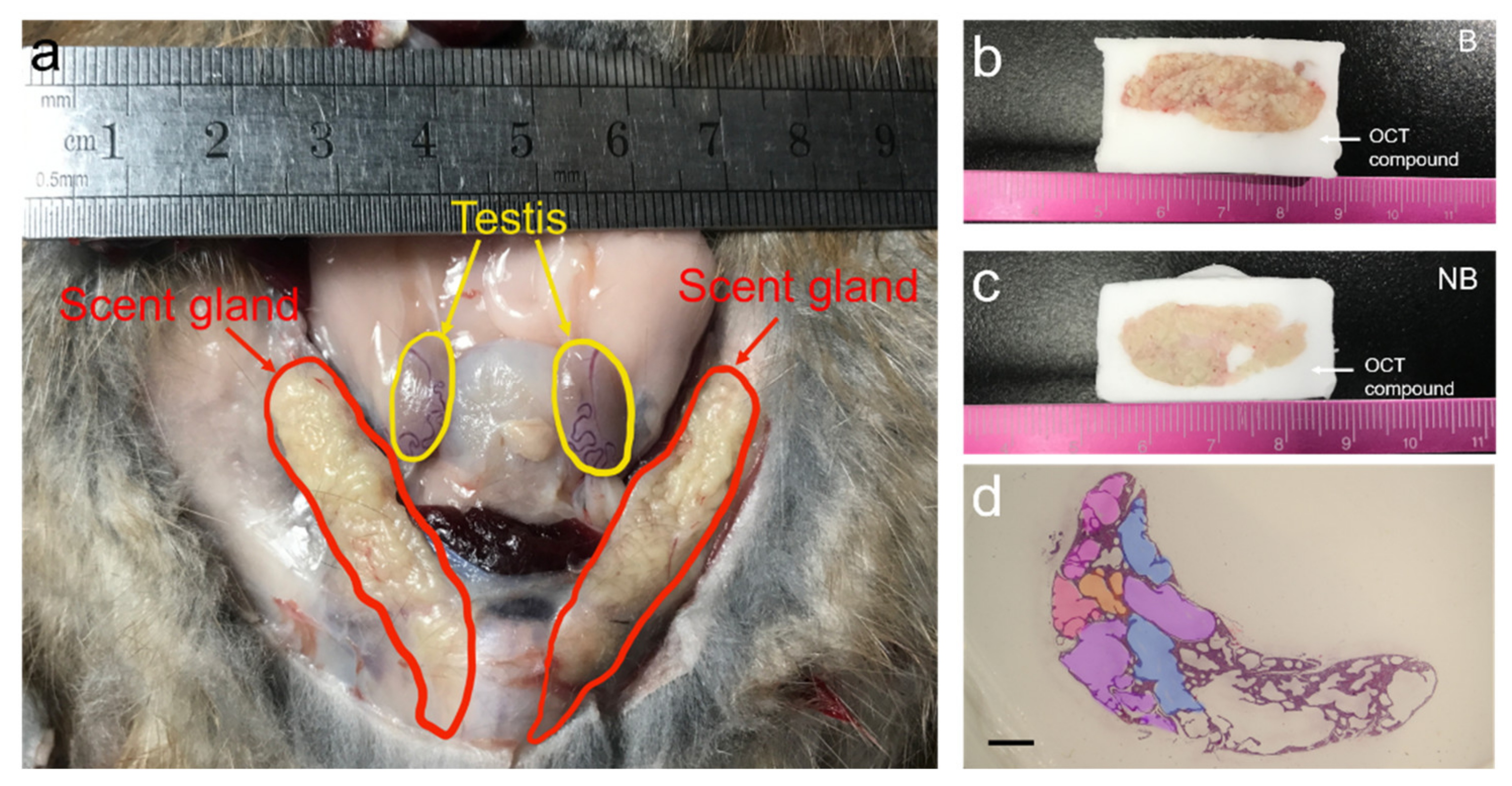
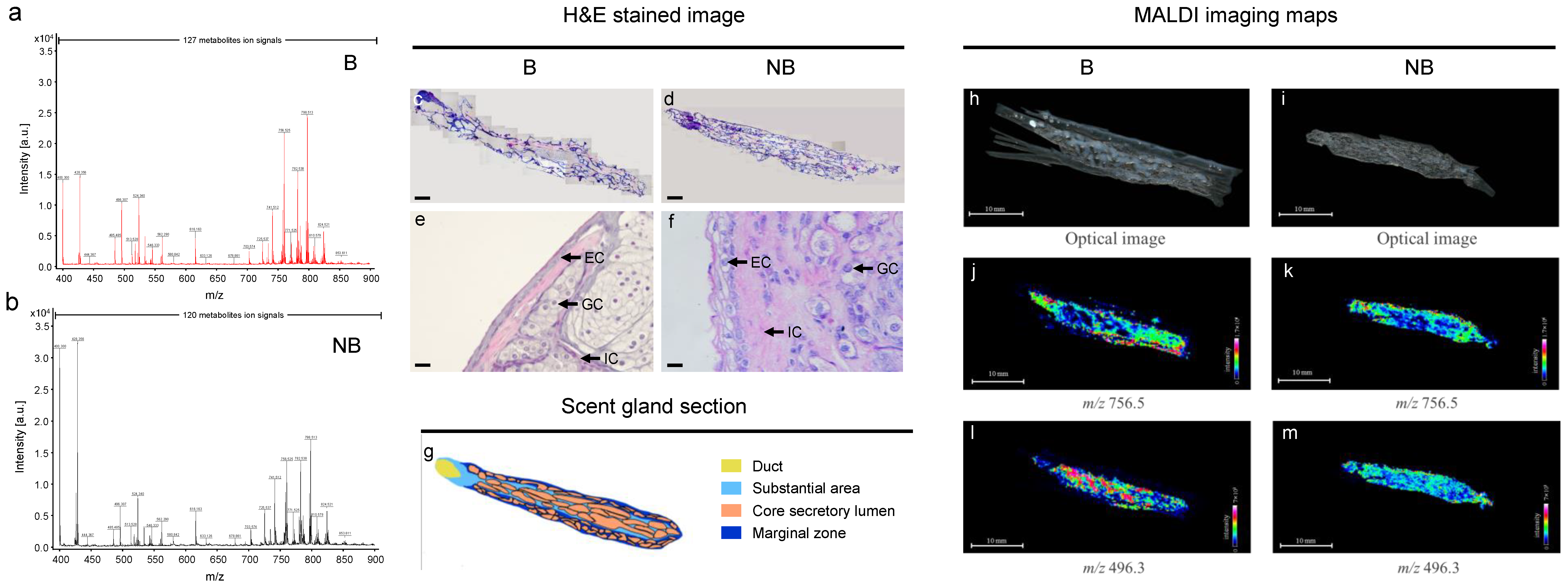
3.1. Anatomic Localization and Tissue Sections of the Scent Glands of Muskrats
3.2. Main Compounds Based on LC-MS/MS and GC-MS
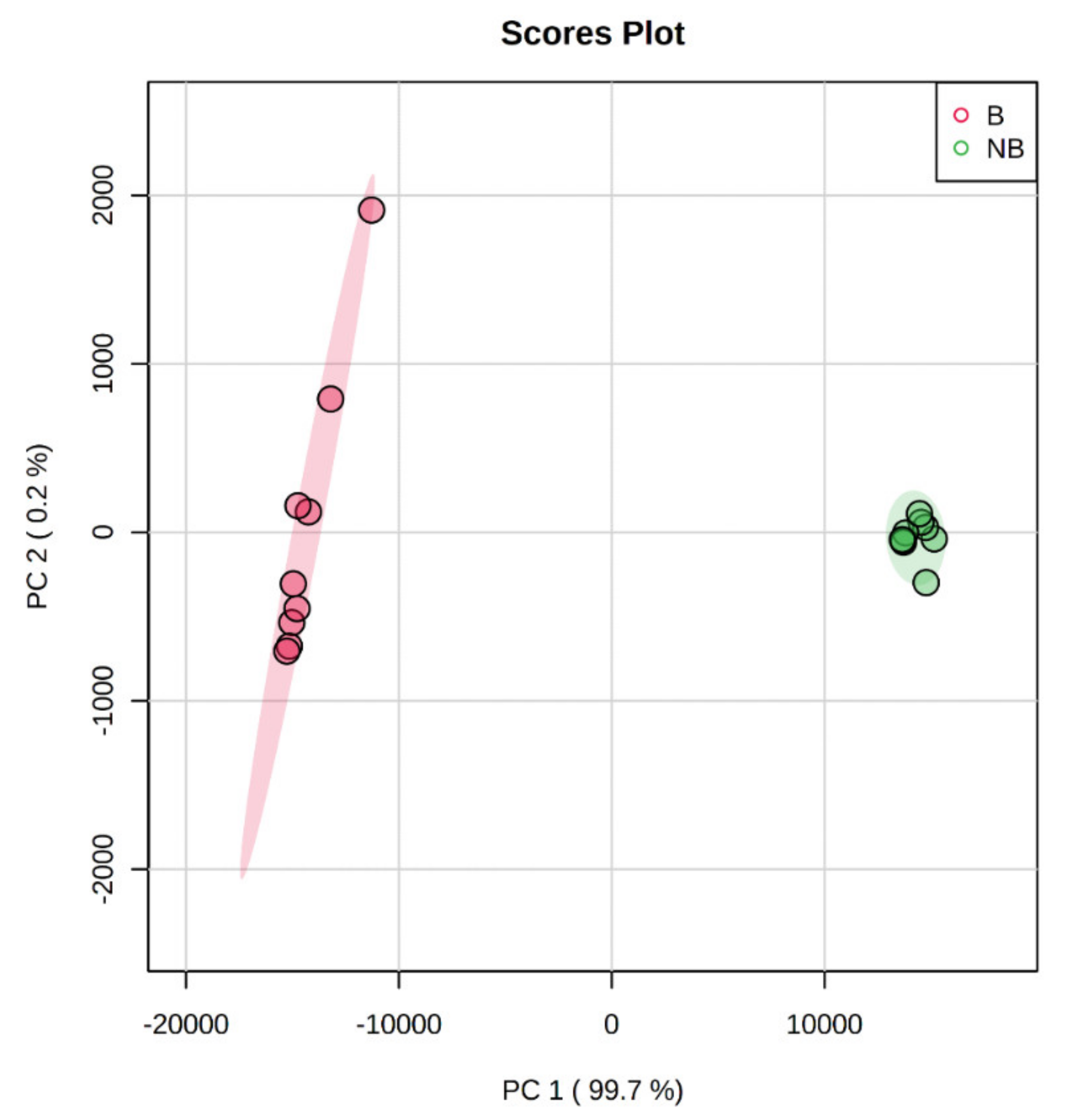
3.3. Principal Component Analysis (PCA) Based on MALDI-TOF/TOF MS/MS
3.4. MALDI-MS In Situ Detection and Imaging of Different Metabolites in the Scent Glands of Muskrats during Different Reproductive Statuses
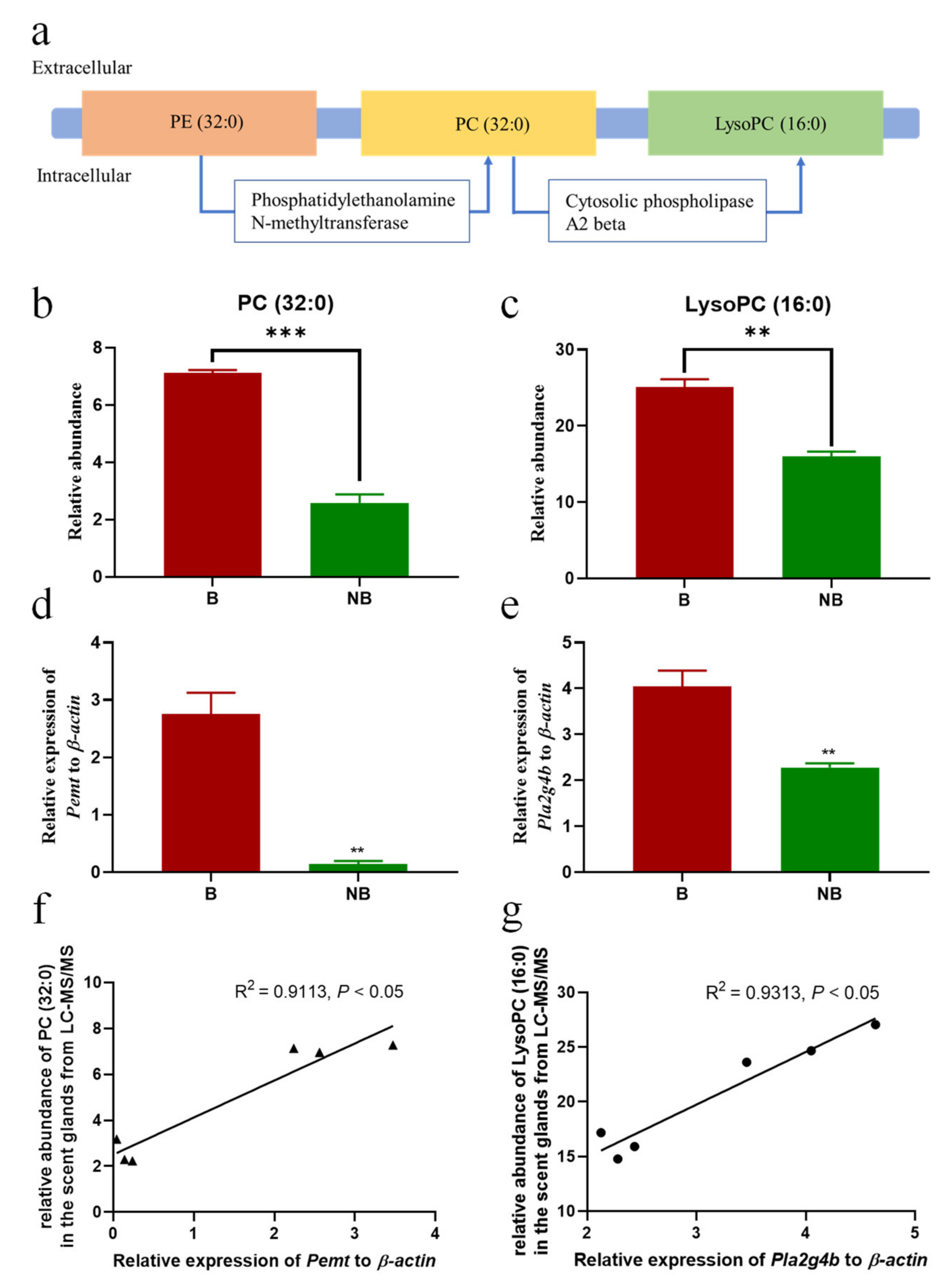
3.5. Schematic Diagram of Metabolic Pathway and Expression Diagram of Metabolite Synthase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid Classification, Structures and Tools. Biochim. Biophys. Acta 2011, 1811, 637–647. [Google Scholar] [CrossRef]
- Storck, E.M.; Ozbalci, C.; Eggert, U.S. Lipid Cell Biology: A Focus on Lipids in Cell Division. Annu. Rev. Biochem. 2018, 87, 839–869. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Brown, H.A.; Glass, C.K.; Merrill Jr, A.H.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; Seyama, Y.; Shaw, W.; et al. A Comprehensive Classification System for Lipids. J. Lipid. Res. 2005, 46, 839–861. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, S. Mammalian Lipids: Structure, Synthesis and Function. Essays Biochem. 2021, 65, 813–845. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Nakamura, Y.; Harwood, J. Lipids: From Chemical Structures, Biosynthesis, and Analyses to Industrial Applications. Subcell. Biochem. 2016, 86, 1–18. [Google Scholar]
- White, S.W.; Zheng, J.; Zhang, Y.M.; Rock. The Structural Biology of Type II Fatty Acid Biosynthesis. Annu. Rev. Biochem. 2005, 74, 791–831. [Google Scholar] [CrossRef]
- de Carvalho, C.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Asturias, F.J.; Chadick, J.Z.; Cheung, I.K.; Stark, H.; Witkowski, A.; Joshi, A.K.; Smith, S. Structure and Molecular Organization of Mammalian Fatty Acid Synthase. Nat. Struct. Mol. Biol. 2005, 12, 225–232. [Google Scholar] [CrossRef]
- Cartocci, V.; Segatto, M.; Di Tunno, I.; Leone, S.; Pfrieger, F.W.; Pallottini, V. Modulation of the Isoprenoid/Cholesterol Biosynthetic Pathway During Neuronal Differentiation in Vitro. J. Cell. Biochem. 2016, 117, 2036–2044. [Google Scholar] [CrossRef]
- Roy, D.; Tedeschi, A. The Role of Lipids, Lipid Metabolism and Ectopic Lipid Accumulation in Axon Growth, Regeneration and Repair after Cns Injury and Disease. Cells 2021, 10, 1078. [Google Scholar] [CrossRef]
- Wu, Z.; Bagarolo, G.I.; Thoröe-Boveleth, S.; Jankowski, J. “Lipidomics”: Mass Spectrometric and Chemometric Analyses of Lipids. Adv. Drug. Deliv. Rev. 2020, 159, 294–307. [Google Scholar] [CrossRef]
- Lee, H.C.; Yokomizo, T. Applications of Mass Spectrometry-Based Targeted and Non-Targeted Lipidomics. Biochem. Biophys. Res. Commun. 2018, 504, 576–581. [Google Scholar] [CrossRef]
- Feider, C.L.; Krieger, A.; DeHoog, R.J.; Eberlin, L.S. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal. Chem. 2019, 91, 4266–4290. [Google Scholar] [CrossRef]
- Hart, P.J.; Clench, M.R. MALDI-MSI of Lipids in Human Skin. Methods Mol. Biol. 2017, 1618, 29–36. [Google Scholar]
- Lee, G.K.; Lee, H.S.; Park, Y.S.; Lee, J.H.; Lee, S.C.; Lee, J.H.; Lee, S.J.; Shanta, S.R.; Park, H.M.; Kim, H.R.; et al. Lipid MALDI Profile Classifies Non-Small Cell Lung Cancers According to the Histologic Type. Lung Cancer 2012, 76, 197–203. [Google Scholar] [CrossRef]
- Buchberger, A.R.; DeLaney, K.; Johnson, J.; Li, L. Mass Spectrometry Imaging: A Review of Emerging Advancements and Future Insights. Anal. Chem. 2018, 90, 240–265. [Google Scholar] [CrossRef]
- Chughtai, K.; Heeren, R.M. Mass Spectrometric Imaging for Biomedical Tissue Analysis. Chem. Rev. 2010, 110, 3237–3277. [Google Scholar] [CrossRef]
- Norris, J.L.; Caprioli, R.M. Analysis of Tissue Specimens by Matrix-Assisted Laser Desorption/Ionization Imaging Mass Spectrometry in Biological and Clinical Research. Chem. Rev. 2013, 113, 2309–2342. [Google Scholar] [CrossRef]
- Schnackenberg, L.K.; Thorn, D.A.; Barnette, D.; Jones, E.E. MALDI imaging mass spectrometry: An emerging tool in neurology. Metab. Brain Dis. 2022, 37, 105–121. [Google Scholar] [CrossRef]
- Berry, K.A.; Hankin, J.A.; Barkley, R.M.; Spraggins, J.M.; Caprioli, R.M.; Murphy, R.C. MALDI Imaging of Lipid Biochemistry in Tissues by Mass Spectrometry. Chem. Rev. 2011, 111, 6491–6512. [Google Scholar] [CrossRef]
- Cerruti, C.D.; Benabdellah, F.; Laprevote, O.; Touboul, D.; Brunelle, A. MALDI Imaging and Structural Analysis of Rat Brain Lipid Negative Ions with 9-Aminoacridine Matrix. Anal. Chem. 2012, 84, 2164–2171. [Google Scholar] [CrossRef]
- Shanta, S.R.; Choi, C.S.; Lee, J.H.; Shin, C.Y.; Kim, Y.J.; Kim, K.H.; Kim, K.P. Global Changes in Phospholipids Identified by MALDI MS in Rats with Focal Cerebral Ischemia. J. Lipid. Res. 2012, 53, 1823–1831. [Google Scholar] [CrossRef]
- Caras, R.A. North American Mammals: Fur-Bearing Animals of the United States and Canada; Meredith Press: Minneapolis, MN, USA, 1967. [Google Scholar]
- Lu, L.; Liu, S.; Li, Q.; Huang, S.; Bao, L.; Sheng, X.; Han, Y.; Watanabe, G.; Taya, K.; Weng, Q. Seasonal Expression of Androgen Receptor in Scented Gland of Muskrat (Ondatra Zibethicus). Gen. Comp. Endocrinol. 2014, 204, 1–7. [Google Scholar] [CrossRef]
- Han, W.; Xie, W.; Zhang, Y.; Zhang, F.; Zhang, H.; Han, Y.; Yuan, Z.; Weng, Q. Seasonal Expression of P450c17 and 5alpha-Reductase-2 in the Scented Gland of Male Muskrats (Ondatra Zibethicus). Gen. Comp. Endocrinol. 2017, 254, 60–67. [Google Scholar] [CrossRef]
- Li, Q.; Weng, J.; Zhang, H.; Lu, L.; Ma, X.; Wang, Q.; Cao, H.; Liu, S.; Xu, M.; Weng, Q.; et al. Immunohistochemical Evidence: Testicular and Scented Glandular Androgen Synthesis in Muskrats (Ondatra Zibethicus) During the Breeding Season. Eur. J. Histochem. 2011, 55, e32. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, W.; Zhao, M.; Chang, Z.; Zhang, Y.; Ma, D. Histological Observation on Musk-Secreting Scented Gland in Muskrat. Chin. J. Zool. 2007, 2, 91–95. (In Chinese) [Google Scholar]
- Liu, Q.; Qin, J.; Chen, Q.; Wang, D.; Shi, D. Fertility Control of Rattus Nitidus Using Quinestrol: Effects on Reproductive Organs and Social Behavior. Integr. Zool. 2013, 8 (Suppl. 1), 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, H.; Weng, J.; Sheng, X.; Lu, L.; Hu, X.; Liu, S.; Xu, M.; Weng, Q.; Watanabe, G.; et al. Immunolocalization of Inhibin/Activin Subunit Proteins During the Breeding Season in Testes and Scented Glands of Muskrats (Ondatra Zibethicus). J. Vet. Med. Sci. 2011, 73, 1199–1205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, H.; Zhang, F.; Zhu, M.; Wang, J.; Sheng, X.; Yuan, Z.; Han, Y.; Watanabe, G.; Taya, K.; Weng, Q. Seasonal Expressions of Follicle-Stimulating Hormone Receptor and Luteinizing Hormone Receptor in the Scented Gland of the Male Muskrat (Ondatra Zibethicus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R569–R574. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Tang, Z.; Guo, Y.; Zhang, C.; Zhang, H.; Han, Y.; Yuan, Z.; Weng, Q. Seasonal Expressions of Growth Hormone Receptor, Insulin-Like Growth Factor 1 and Insulin-Like Growth Factor 1 Receptor in the Scented Glands of the Muskrats (Ondatra Zibethicus). Gen. Comp. Endocrinol. 2019, 281, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, Z.; Liang, D.; Yu, H.; Zheng, X.; Huang, Y. GC-MS Analysis of the Differences in the Chemical Components of Muskrat and Musk. Nat. Prod. Res. Dev. 2021, 10, 1643–1648. (In Chinese) [Google Scholar]
- Zhao, W.; Chen, Y.; Zhao, M.; Li, X.; Liu, D. Study on the Comparison for the Muskrat Per Fume Secretion of Fragrant Gland Cell Cultured in Vitro and That in Nature. Spec. Wild Econ. Anim. Plant Res. 2009, 4, 50–53. (In Chinese) [Google Scholar]
- Liu, K.; Li, Z.; Zhang, H.; Yu, Y.; Liu, D. Study on Chemical Constituents and Pharmacological Action of Muskrat. J. Chang. Univ. Tradit. Chin. Med. 2008, 2, 1. (In Chinese) [Google Scholar]
- Chen, J.; Liu, Z.; Song, F.; Liu, S. Analysis of Chemical Constitutes in Lipid from Musk-Rat Musk by Gas Chromatography/Mass Spectrometry. Chin. J. Anal. Chem. 1998, 9, 1142–1145. (In Chinese) [Google Scholar]
- Jiang, Y.; Han, X.; Feng, N.; Jin, W.; Zhang, T.; Zhang, M.; Shi, M.; Zhang, B.; Liu, S.; Hu, D. Androgen Plays an Important Role in Regulating the Synthesis of Pheromone in the Scent Gland of Muskrat. J. Steroid. Biochem. Mol. Biol. 2022, 217, 106026. [Google Scholar] [CrossRef]
- Xie, W.; Zhao, X.; Guo, L.; Han, Y.; Yuan, Z.; Zhang, H.; Weng, Q. Seasonal Expressions of Eralpha, Erbeta, Egf, Egfr, Pi3k and Akt in the Scent Glands of the Muskrats (Ondatra Zibethicus). J. Steroid. Biochem. Mol. Biol. 2021, 213, 105961. [Google Scholar] [CrossRef]
- Muro, E.; Atilla-Gokcumen, G.E.; Eggert, U.S. Lipids in Cell Biology: How Can We Understand Them Better? Mol. Biol. Cell 2014, 25, 1819–1823. [Google Scholar] [CrossRef]
- Jackson, S.N.; Wang, H.Y.; Woods, A.S. Direct Profiling of Lipid Distribution in Brain Tissue Using MALDI-TOFMS. Anal. Chem. 2005, 77, 4523–4527. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, L.; Sun, J.; Chen, L.; Jia, L.; Zhao, J.; Yang, H.; Xue, K.; Wang, X.; Sang, W. Metabolite changes associated with earthworms (Eisenia fetida) graphene exposure revealed by matrix-assisted laser desorption/ionization mass spectrometry imaging. Ecotoxicol. Environ. Saf. 2020, 205, 111102. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, S.; Shi, M.; Zhang, S.; Zhang, T.; Li, Y.; Xu, S.; Cha, M.; Meng, Y.; Lin, S.; et al. Regulatory Roles of Peroxisomal Metabolic Pathways Involved in Musk Secretion in Muskrats. J. Membr. Biol. 2019, 252, 61–75. [Google Scholar] [CrossRef]
- Xie, W.; Tang, Z.; Xu, L.; Zhong, J.; Zhang, H.; Han, Y.; Yuan, Z.; Weng, Q. Seasonal Expressions of Sf-1, Star and P450scc in the Scent Glands of the Muskrats (Ondatra Zibethicus). J. Steroid. Biochem. Mol. Biol. 2020, 204, 105766. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, M.; Shi, M.; Zhang, T.; Qi, L.; Yu, J.; Li, X.; Lin, S.; Huang, Z.; Yang, S.; et al. Sex Hormones Play Roles in Determining Musk Composition During the Early Stages of Musk Secretion by Musk Deer (Moschus Berezovskii). Endocr. J. 2018, 65, 1111–1120. [Google Scholar] [CrossRef]
- Furse, S.; de Kroon, A.I. Phosphatidylcholine’s Functions Beyond That of a Membrane Brick. Mol. Membr. Biol. 2015, 32, 117–119. [Google Scholar] [CrossRef]
- Luehr, T.C.; Koide, E.M.; Wang, X.; Han, J.; Borchers, C.H.; Helbing, C.C. Metabolomic Insights into the Effects of Thyroid Hormone on Rana [Lithobates] Catesbeiana Metamorphosis Using Whole-Body Matrix Assisted Laser Desorption/Ionization-Mass Spectrometry Imaging (MALDI-MSI). Gen. Comp. Endocrinol. 2018, 265, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Kurabe, N.; Hayasaka, T.; Igarashi, H.; Mori, H.; Sekihara, K.; Tao, H.; Yamada, H.; Kahyo, T.; Onishi, I.; Tsukui, H.; et al. Visualization of Phosphatidylcholine (16:0/16:0) in Type II Alveolar Epithelial Cells in the Human Lung Using Imaging Mass Spectrometry. Pathol. Int. 2013, 63, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Xie, C.; Zheng, J.; Dong, Q.; Si, T.; Zhang, J.; Hou, S.T. An Imbalanced Ratio between PC (16:0/16:0) and LPC (16:0) Revealed by Lipidomics Supports the Role of the Lands Cycle in Ischemic Brain Injury. J. Biol. Chem. 2021, 296, 100151. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, V.E.; Kagan, M.Z.; Vasilieva, V.S.; Prihodko, V.I.; Zinkevich, E.P. Musk Deer (Moschus Moschiferus): Reinvestigation of Main Lipid Components from Preputial Gland Secretion. J. Chem. Ecol. 1987, 13, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Phosphatidylserine and Phosphatidylethanolamine in Mammalian Cells: Two Metabolically Related Aminophospholipids. J. Lipid. Res. 2008, 49, 1377–1387. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Moitra, S.; Basu, S.; Pawlowic, M.; Hsu, F.-f.; Zhang, K. De Novo Synthesis of Phosphatidylcholine Is Essential for the Promastigote but not Amastigote Stage in Leishmania Major. Front. Cell. Infect. Microbiol. 2021, 11, 647870. [Google Scholar] [CrossRef]
- Li, Z.; Vance, D.E. Phosphatidylcholine and Choline Homeostasis. J. Lipid. Res. 2008, 49, 1187–1194. [Google Scholar] [CrossRef]
- Pynn, C.J.; Henderson, N.G.; Clark, H.; Koster, G.; Bernhard, W.; Postle, A.D. Specificity and Rate of Human and Mouse Liver and Plasma Phosphatidylcholine Synthesis Analyzed in Vivo. J. Lipid. Res. 2011, 52, 399–407. [Google Scholar] [CrossRef]
- Murakami, M.; Taketomi, Y.; Miki, Y.; Sato, H.; Hirabayashi, T.; Yamamoto, K. Recent Progress in Phospholipase a2 Research: From Cells to Animals to Humans. Prog. Lipid. Res. 2011, 50, 152–192. [Google Scholar] [CrossRef]
- Liu, H.; Zuo, F.; Wu, H. Blockage of Cytosolic Phospholipase A2 Alpha by Monoclonal Antibody Attenuates Focal Ischemic Brain Damage in Mice. Biosci. Trends. 2017, 11, 439–449. [Google Scholar] [CrossRef][Green Version]
- Hishikawa, D.; Hashidate, T.; Shimizu, T.; Shindou, H. Diversity and Function of Membrane Glycerophospholipids Generated by the Remodeling Pathway in Mammalian Cells. J. Lipid. Res. 2014, 55, 799–807. [Google Scholar] [CrossRef]
- Giroud, S.; Chery, I.; Bertile, F.; Bertrand-Michel, J.; Tascher, G.; Gauquelin-Koch, G.; Arnemo, J.M.; Swenson, J.E.; Singh, N.J.; Lefai, E.; et al. Lipidomics Reveals Seasonal Shifts in a Large-Bodied Hibernator, the Brown Bear. Front. Physiol. 2019, 10, 389. [Google Scholar] [CrossRef]
- Giroud, S.; Frare, C.; Strijkstra, A.; Boerema, A.; Arnold, W.; Ruf, T. Membrane Phospholipid Fatty Acid Composition Regulates Cardiac Serca Activity in a Hibernator, the Syrian Hamster (Mesocricetus Auratus). PLoS ONE 2013, 8, e63111. [Google Scholar] [CrossRef]
- Chayama, Y.; Ando, L.; Sato, Y.; Shigenobu, S.; Anegawa, D.; Fujimoto, T.; Taii, H.; Tamura, Y.; Miura, M.; Yamaguchi, Y. Molecular basis of white adipose tissue remodeling that precedes and coincides with hibernation in the syrian hamster, a food-storing hibernator. Front. Physiol. 2018, 9, 1973. [Google Scholar] [CrossRef]
- Watts, A.J.; Logan, S.M.; Kübber-Heiss, A.; Posautz, A.; Stalder, G.; Painer, J.; Gasch, K.; Giroud, S.; Storey, K.B. Regulation of Peroxisome Proliferator-Activated Receptor Pathway During Torpor in the Garden Dormouse, Eliomys Quercinus. Front. Physiol. 2020, 11, 615025. [Google Scholar] [CrossRef]

| Gene Name | Primer Sequence (5′->3′) | Product Length (bp) |
|---|---|---|
| Pemt | F: CCACATTTCCCTTCAGCGTG | 175 |
| R: CGGTAGATCTCCGCAGTGAA | ||
| Pla2g4b | F: GTGTTGAGCATGACTAGATCCCA | 191 |
| R: AGACCACTCTGGGTCCTCAT | ||
| Actb | F: GACTCGTCGTACTCCTGCTT | 223 |
| R: AAGACCTCTATGCCAACACC |
| Compound Name | m/z | RT (Min) | Mean B | Mean NB | p Value | Fold Change |
|---|---|---|---|---|---|---|
| PC(16:0/16:0) | 756.525 | 2.354 | 7.124 | 2.568 | 0.031 | 1.791 |
| Phosphatidylethanolamine | 748.571 | 2.122 | 0.167 | 0.097 | 0.025 | 1.723 |
| 1-Stearoyl-2-arachidonoyl-sn-glycerol | 627.529 | 3.108 | 0.085 | 0.134 | 0.000 | 0.634 |
| LysoPC(16:0) | 496.313 | 3.026 | 25.106 | 15.954 | 0.028 | 2.493 |
| Glycochenodeoxycholate | 467.351 | 3.098 | 0.029 | 0.009 | 0.048 | 3.279 |
| Nervonic acid | 430.375 | 2.748 | 0.171 | 0.100 | 0.015 | 1.700 |
| Stearoylcarnitine | 428.370 | 2.543 | 12.765 | 7.198 | 0.001 | 1.773 |
| 16-Hydroxypalmitic acid | 314.266 | 0.743 | 0.095 | 0.055 | 0.007 | 1.727 |
| Linoleic acid | 298.271 | 0.603 | 0.552 | 0.303 | 0.046 | 1.821 |
| sn-Glycerol 3-phosphoethanolamine | 216.061 | 6.404 | 0.238 | 0.080 | 0.032 | 2.967 |
| Compound Name | m/z | RT (Min) | Mean B | Mean NB | p Value | Fold Change |
|---|---|---|---|---|---|---|
| L-palmitoylcarnitine | 458.348 | 2.652 | 0.610 | 0.225 | 0.002 | 2.718 |
| 1-Oleoyl-sn-glycerol 3-phosphate | 435.251 | 3.095 | 0.013 | 0.005 | 0.019 | 2.449 |
| Tetracosanoic acid | 427.378 | 0.782 | 0.021 | 0.011 | 0.012 | 1.868 |
| Stearoylcarnitine | 426.358 | 2.616 | 0.098 | 0.049 | 0.001 | 2.001 |
| Palmitic acid | 255.234 | 0.750 | 89.598 | 63.163 | 0.023 | 1.419 |
| cis-9-Palmitoleic acid | 253.218 | 0.744 | 43.476 | 15.598 | 0.031 | 2.787 |
| Azelaic acid | 187.097 | 5.581 | 0.023 | 0.042 | 0.007 | 0.546 |
| L-Malic acid | 133.014 | 7.117 | 0.515 | 0.310 | 0.021 | 1.664 |
| Hydroxyisocaproic acid | 131.071 | 2.470 | 0.494 | 0.253 | 0.033 | 1.951 |
| 2-Hydroxy-3-methylbutyric acid | 117.056 | 2.704 | 0.912 | 0.316 | 0.006 | 2.888 |
| Measured Value (m/z) | Theoretical Value (m/z) | Error/ppm 1 | Molecular Formula | Precursor Type 2 | RT (Min) | Compound Name |
|---|---|---|---|---|---|---|
| 756.525 | 756.5513 | 3.5 | C40H80NO8P | [M + Na]+ | 2.354 | PC (32:0) |
| 496.313 | 496.3325 | 3.9 | C24H50NO7P | [M + H]+ | 3.026 | LysoPC (16:0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Mu, S.; Zhong, J.; Zhang, C.; Zhang, H.; Wang, X.; Weng, Q. Mass Spectrometry Imaging of Lipids in the Scent Glands of Muskrat (Ondatra zibethicus) in Different Reproductive Statuses. Cells 2022, 11, 2228. https://doi.org/10.3390/cells11142228
Xie W, Mu S, Zhong J, Zhang C, Zhang H, Wang X, Weng Q. Mass Spectrometry Imaging of Lipids in the Scent Glands of Muskrat (Ondatra zibethicus) in Different Reproductive Statuses. Cells. 2022; 11(14):2228. https://doi.org/10.3390/cells11142228
Chicago/Turabian StyleXie, Wenqian, Shengheng Mu, Jinkun Zhong, Chaoran Zhang, Haolin Zhang, Xiaodong Wang, and Qiang Weng. 2022. "Mass Spectrometry Imaging of Lipids in the Scent Glands of Muskrat (Ondatra zibethicus) in Different Reproductive Statuses" Cells 11, no. 14: 2228. https://doi.org/10.3390/cells11142228
APA StyleXie, W., Mu, S., Zhong, J., Zhang, C., Zhang, H., Wang, X., & Weng, Q. (2022). Mass Spectrometry Imaging of Lipids in the Scent Glands of Muskrat (Ondatra zibethicus) in Different Reproductive Statuses. Cells, 11(14), 2228. https://doi.org/10.3390/cells11142228







