Selected Methods of Resistance Training for Prevention and Treatment of Sarcopenia
Abstract
1. Introduction
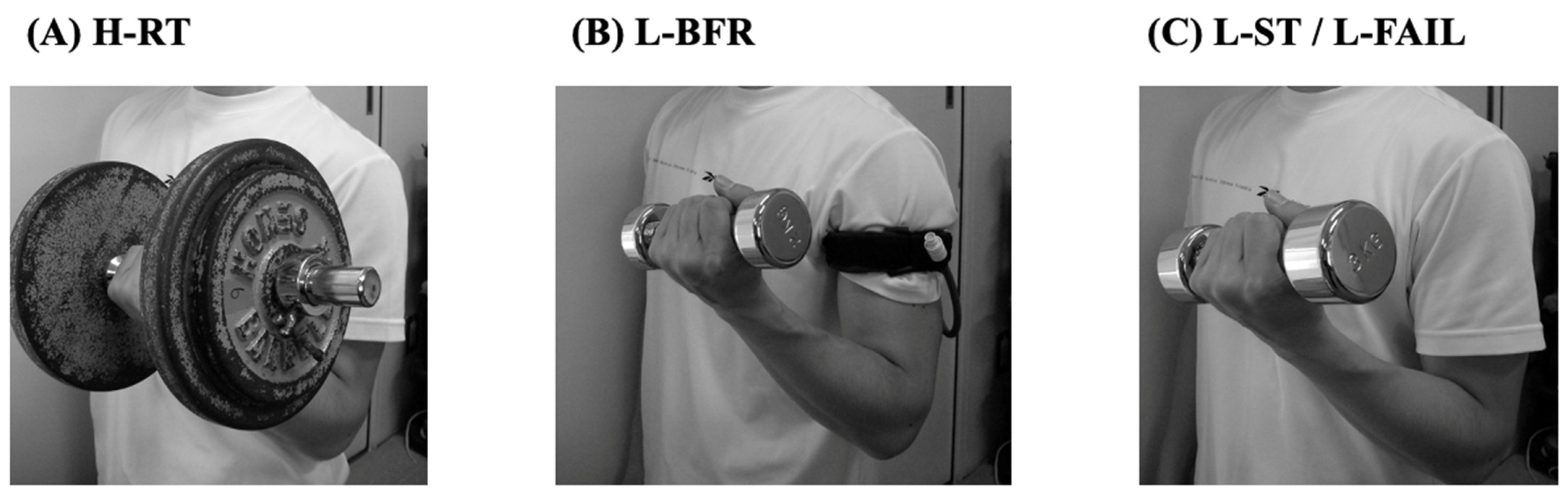
2. High-Load Resistance Training (H-RT)
2.1. Effects of H-RT
2.2. Safety of H-RT
2.3. Perspective of H-RT
3. Low-Load Resistance Training with Blood Flow Restriction (L-BFR)
3.1. Effects of L-BFR
3.2. Safety of L-BFR
3.3. Perspective of L-BFR
4. Low-Load Resistance Training with Relatively Slow Movement and Tonic Force Generation (L-ST)
4.1. Effects of L-ST
4.2. Safety of L-ST
4.3. Perspective of L-ST
5. Low-Load Resistance Training until Volitional Failure (L-FAIL)
5.1. Effects of L-FAIL
5.2. Safety of L-FAIL
5.3. Perspective of L-FAIL
6. Limitations
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Health, Labor and Welfare White Paper, 2020. Ministry of Health, Labor and Welfare Japan. Available online: https://www.mhlw.go.jp/english/wp/wp-hw13/dl/summary.pdf (accessed on 4 February 2022).
- Lexell, J.; Taylor, C.C.; Sjöström, M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J. Neurol. Sci. 1988, 84, 275–294. [Google Scholar] [CrossRef]
- Doherty, T.J. Invited review: Aging and sarcopenia. J. Appl. Physiol. 2003, 95, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Sakamaki, M.; Yasuda, T.; Bemben, M.G.; Kondo, M.; Kawakami, Y.; Fukunaga, T. Age-related, site-specific muscle loss in 1507 Japanese men and women aged 20 to 95 years. J. Sports Sci. Med. 2011, 10, 145–150. [Google Scholar]
- Miyatani, M.; Kanehisa, H.; Azuma, K.; Kuno, S.; Fukunaga, T. Site-related differences in muscle loss with aging: A cross sectional survey on the muscle thickness in Japanese men and Age-related muscle loss in Japanese adults 150 women aged 20 to 79 years. Int. J. Sport Health Sci. 2003, 1, 34–40. [Google Scholar] [CrossRef][Green Version]
- American College of Sports Medicine; Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Vikberg, S.; Sörlén, N.; Brandén, L.; Johansson, J.; Nordström, A.; Hult, A.; Nordström, P. Effects of resistance training on functional strength and muscle mass in 70-year-old individuals with pre-sarcopenia: A randomized controlled trial. J. Am. Med. Dir. Assoc. 2019, 20, 28–34. [Google Scholar] [CrossRef]
- Miyachi, M.; Kawano, H.; Sugawara, J.; Takahashi, K.; Hayashi, K.; Yamazaki, K.; Tabata, I.; Tanaka, H. Unfavorable effects of resistance training on central arterial compliance: A randomized intervention study. Circulation 2004, 110, 2858–2863. [Google Scholar] [CrossRef]
- Centner, C.; Wiegel, P.; Gollhofer, A.; König, D. Effects of blood flow restriction training on muscular strength and hypertrophy in older individuals: A systematic review and meta-analysis. Sports Med. 2019, 49, 95–108. [Google Scholar] [CrossRef]
- Evans, W.J. Effects of exercise on senescent muscle. Clin. Orthop. Relat. Res. 2002, 403, S211–S220. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.M.; Vandervoort, A.A.; Lexell, J. Aging of human muscle: Structure, function and adaptability. Scand. J. Med. Sci. Sports 1995, 5, 129–142. [Google Scholar] [CrossRef]
- Vandervoort, A.A. Aging of the human neuromuscular system. Muscle Nerve 2002, 25, 17–25. [Google Scholar] [CrossRef]
- American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med. Sci. Sports Exerc. 1998, 30, 975–991. [Google Scholar]
- National Strength and Conditioning Association. NSCA’s Essentials of Personal Training; Earle, R.W., Baechie, T.R., Eds.; Human Kinetics: Champaign, IL, USA, 2003. [Google Scholar]
- Campos, G.E.R.; Luecke, T.J.; Wendeln, H.K.; Toma, K.; Hagerman, F.C.; Murray, T.F.; Ragg, K.E.; Ratamess, N.A.; Kraemer, W.J.; Staron, R.S. Muscular adaptation in response to three different resistance-training regimens: Specificity of repetition maximum training. Eur. J. Appl. Physiol. 2002, 88, 50–60. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, M.J.; Davies, C.T. Adaptative response of mammalian skeletal muscle to exercise with high loads. Eur. J. Appl. Physiol. 1984, 52, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A. Fundamentals of resistance training: Progression and exercise prescription. Med. Sci. Sports Exerc. 2004, 36, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement From the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Karabulut, M.; Abe, T.; Sato, Y.; Bemben, M.G. The effects of low-intensity resistance training with vascular restriction on leg muscle strength in older men. Eur. J. Appl. Physiol. 2010, 108, 147–155. [Google Scholar] [CrossRef]
- Thiebaud, R.S.; Loenneke, J.P.; Fahs, C.A.; Rossow, L.M.; Kim, D.; Abe, T.; Anderson, M.A.; Young, K.C.; Bemben, D.A.; Bemben, M.G. The effects of elastic band resistance training combined with blood flow restriction on strength, total bone-free lean body mass and muscle thickness in postmenopausal women. Clin. Physiol. Funct. Imaging 2013, 33, 344–352. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Fukuda, T.; Uchida, Y.; Iida, H.; Meguro, M.; Sato, Y.; Yamasoba, T.; Nakajima, T. Muscle size and arterial stiffness after blood flow-restricted low-intensity resistance training in older adults. Scand. J. Med. Sci. Sports 2014, 24, 799–806. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Uchida, Y.; Koshi, H.; Iida, H.; Masamune, K.; Yamasoba, T.; Sato, Y.; Nakajima, T. Effects of low-load, elastic band resistance training combined with blood flow restriction on muscle size and arterial stiffness in older adults. J. Gerontol A Biol. Sci. Med. Sci. 2015, 70, 950–958. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Tomaru, T.; Nakajima, T. Thigh muscle size and vascular function after blood flow-restricted elastic band training in older women. Oncotarget 2016, 7, 33595–33607. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.B.; LaRoche, D.P.; Villa, M.R.; Barile, H.; Manini, T.M. Blood flow restricted resistance training in older adults at risk of mobility limitations. Exp. Gerontol. 2017, 99, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Mallorca, D.; Loaiza-Betancur, A.F.; Monteagudo, P.; Blasco-Lafarga, C.; Chulvi-Medrano, I. Resistance training with blood flow restriction compared to traditional resistance training on strength and muscle mass in non-active older adults: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 11441. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tanimoto, M.; Ohgane, A.; Sanada, K.; Miyachi, M.; Ishii, N. Increased muscle size and strength from slow-movement, low-intensity resistance exercise and tonic force generation. J. Aging Phys. Act. 2013, 21, 71–84. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tanimoto, M.; Oba, N.; Sanada, K.; Miyachi, M.; Ishii, N. Effect of resistance training using bodyweight in the elderly: Comparison of resistance exercise movement between slow and normal speed movement. Geriatr. Gerontol. Int. 2015, 15, 1270–1277. [Google Scholar] [CrossRef]
- Kanda, K.; Yoda, T.; Suzuki, H.; Okabe, Y.; Mori, Y.; Yamasaki, K.; Kitano, H.; Kanda, A.; Hirao, T. Effects of low-intensity bodyweight training with slow movement on motor function in frail elderly patients: A prospective observational study. Environ. Health Prev. Med. 2018, 23, 4. [Google Scholar] [CrossRef]
- Takenami, E.; Iwamoto, S.; Shiraishi, N.; Kato, A.; Watanabe, Y.; Yamada, Y.; Yamada, S.; Ishii, N. Effects of low-intensity resistance training on muscular function and glycemic control in older adults with type 2 diabetes. J. Diabetes Investig. 2019, 10, 331–338. [Google Scholar] [CrossRef]
- Van Roie, E.; Delecluse, C.; Coudyzer, W.; Boonen, S.; Bautmans, I. Strength training at high versus low external resistance in older adults: Effects on muscle volume, muscle strength, and force-velocity characteristics. Exp. Gerontol. 2013, 48, 1351–1361. [Google Scholar] [CrossRef]
- Hartman, J.W.; Moore, D.R.; Phillips, S.M. Resistance training reduces whole-body protein turnover and improves net protein retention in untrained young males. Appl. Physiol. Nutr. Metab. 2006, 31, 557–564. [Google Scholar] [CrossRef]
- Kosek, D.J.; Kim, J.S.; Petrella, J.K.; Cross, J.M.; Bamman, M.M. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J. Appl. Physiol. 2006, 101, 531–544. [Google Scholar] [CrossRef]
- Yarasheski, K.E.; Pak-Loduca, J.; Hasten, D.L.; Obert, K.A.; Brown, M.B.; Sinacore, D.R. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >/=76 yr old. Am. J. Physiol. 1999, 277, E118–E125. [Google Scholar] [CrossRef] [PubMed]
- Yarasheski, K.E.; Zachwieja, J.J.; Bier, D.M. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am. J. Physiol. 1993, 265, E210–E214. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; West, D.W.; Staples, A.W.; Atherton, P.J.; Baker, J.M.; Moore, D.R.; Holwerda, A.M.; Parise, G.; Rennie, M.J.; Baker, S.K.; et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men. PLoS ONE 2010, 5, e12033. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, R.; Fujita, S.; Hornberger, T.A.; Kitaoka, Y.; Makanae, Y.; Nakazato, K.; Naokata, I. The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci. Rep. 2016, 6, 31142. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, H.C.; Fujita, S.; Cadenas, J.G.; Chinkes, D.L.; Volpi, E.; Rasmussen, B.B. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J. Physiol. 2006, 576, 613–624. [Google Scholar] [CrossRef]
- Mitchell, C.J.; Churchward-Venne, T.A.; West, D.W.; Burd, N.A.; Breen, L.; Baker, S.K.; Phillips, S.M. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J. Appl. Physiol. 2012, 113, 71–77. [Google Scholar] [CrossRef]
- Burd, N.A.; Andrews, R.J.; West, D.W.; Little, J.P.; Cochran, A.J.; Hector, A.J.; Cashaback, J.G.; Gibala, M.J.; Potvin, J.R.; Baker, S.K.; et al. Muscle time under tension during resistance exercise stimulates differential muscle protein sub-fractional synthetic responses in men. J. Physiol. 2012, 590, 351–362. [Google Scholar] [CrossRef]
- Kumar, V.; Selby, A.; Rankin, D.; Patel, R.; Atherton, P.; Hildebrandt, W.; Williams, J.; Smith, K.; Seynnes, O.; Hiscock, N.; et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 2009, 587, 211–217. [Google Scholar] [CrossRef]
- Mayhew, D.L.; Kim, J.S.; Cross, J.M.; Ferrando, A.A.; Bamman, M.M. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J. Appl. Physiol. 2009, 107, 1655–1662. [Google Scholar] [CrossRef]
- Bolster, D.R.; Kubica, N.; Crozier, S.J.; Williamson, D.L.; Farrell, P.A.; Kimball, S.R.; Jefferson, L.S. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J. Physiol. 2003, 553, 213–220. [Google Scholar] [CrossRef]
- Fry, C.S.; Glynn, E.L.; Drummond, M.J.; Timmerman, K.L.; Fujita, S.; Abe, T.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J. Appl. Physiol. 2010, 108, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Yasuda, T.; Koide, S.; Yamasoba, T.; Obi, S.; Toyoda, S.; Sato, Y.; Inoue, T.; Kano, Y. Repetitive restriction of muscle blood flow enhances mTOR signaling pathways in a rat model. Heart Vessel. 2016, 31, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Ochi, E.; Hirose, T.; Hiranuma, K.; Min, S.K.; Ishii, N.; Nakazato, K. Elevation of myostatin and FOXOs in prolonged muscular impairment induced by eccentric contractions in rat medial gastrocnemius muscle. J. Appl. Physiol. 2010, 108, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Abe, T.; Drummond, M.J.; Cadenas, J.G.; Dreyer, H.C.; Sato, Y.; Volpi, E.; Rasmussen, B.B. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J. Appl. Physiol. 2007, 103, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Sudo, M.; Ando, S.; Poole, D.C.; Kano, Y. Blood flow restriction prevents muscle damage but not protein synthesis signaling following eccentric contractions. Physiol. Rep. 2015, 3, e12449. [Google Scholar] [CrossRef] [PubMed]
- Natsume, T.; Yoshihara, T.; Naito, H. Electromyostimulation with blood flow restriction enhances activation of mTOR and MAPK signaling pathways in rat gastrocnemius muscles. Appl. Physiol. Nutr. Metab. 2019, 44, 637–644. [Google Scholar] [CrossRef]
- Nakajima, T.; Koide, S.; Yasuda, T.; Hasegawa, T.; Yamasoba, T.; Obi, S.; Toyoda, S.; Nakamura, F.; Inoue, T.; Poole, D.C.; et al. Muscle hypertrophy following blood flow-restricted, low-force isometric electrical stimulation in rat tibialis anterior: Role for muscle hypoxia. J. Appl. Physiol. 2018, 125, 134–145. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morifuji, T.; Matsumoto, T.; Maeshige, N.; Tanaka, M.; Fujino, H. Effects of combined treatment with blood flow restriction and low-current electrical stimulation on muscle hypertrophy in rats. J. Appl. Physiol. 2019, 127, 1288–1296. [Google Scholar] [CrossRef]
- Fiatarone, M.A.; Marks, E.C.; Ryan, N.D.; Meredith, C.N.; Lipsitz, L.A.; Evans, W.J. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 1990, 263, 3029–3034. [Google Scholar] [CrossRef]
- Frontera, W.R.; Meredith, C.N.; O’Reilly, K.P.; Knuttgen, H.G.; Evans, W.J. Strength conditioning in older men: Skeletal muscle hypertrophy and improved function. J. Appl. Physiol. 1988, 64, 1038–1044. [Google Scholar] [CrossRef]
- Harridge, S.D.; Kryger, A.; Stensgaard, A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve 1999, 22, 831–839. [Google Scholar] [CrossRef]
- Hoffman, C.; Rice, D.; Sung, H. Persons with chronic conditions: Their prevalence and costs. JAMA 1996, 276, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Gheno, R.; Cepparo, J.M.; Rosca, C.E.; Cotton, A. Musculoskeletal disorders in the elderly. J. Clin. Imaging Sci. 2012, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Papa, E.V.; Dong, X.; Hassan, M. Skeletal muscle function deficits in the elderly: Current perspectives on resistance training. J. Nat. Sci. 2017, 3, e272. [Google Scholar] [PubMed]
- Lees, F.D.; Clarkr, P.G.; Nigg, C.R.; Newman, P. Barriers to exercise behavior among older adults: A focus-group study. J. Aging Phys. Act. 2005, 13, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Leveille, S.G.; Fried, L.P.; McMullen, W.; Guralnik, J.M. Advancing the taxonomy of disability in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 86–93. [Google Scholar] [CrossRef] [PubMed]
- American Geriatrics Society Panel on Exercise and Osteoarthritis. Exercise prescription for older adults with osteoarthritis pain: Consensus practice recommendations. A supplement to the AGS Clinical Practice Guidelines on the management of chronic pain in older adults. J. Am. Geriatr. Soc. 2001, 49, 808–823. [Google Scholar] [CrossRef]
- Ettinger, W.H., Jr.; Burns, R.; Messier, S.P.; Applegate, W.; Rejeski, W.J.; Morgan, T.; Shumaker, S.; Berry, M.J.; O’Toole, M.; Monu, J.; et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). JAMA 1997, 277, 25–31. [Google Scholar] [CrossRef]
- Abe, T.; Yasuda, T.; Midorikawa, T.; Sato, Y.; Kearns, C.F.; Inoue, K.; Koizumi, K.; Ishii, N. Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily “KAATSU” resistance training. Int. J. KAATSU Train. Res. 2005, 1, 6–12. [Google Scholar] [CrossRef]
- Thiebaud, R.S.; Yasuda, T.; Loenneke, J.P.; Abe, T. Effects of low-intensity concentric and eccentric exercise combined with blood flow restriction on indices of exercise-induced muscle damage. Interv. Med. Appl. Sci. 2013, 5, 53–59. [Google Scholar] [CrossRef]
- Fujita, T.; Brechue, W.F.; Kurita, K.; Sato, Y.; Abe, T. Increased muscle volume and strength following six days of low-intensity resistance training with restricted muscle blood flow. Int. J. KAATSU Train. Res. 2008, 4, 1–8. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Iida, H.; Nakajima, T. Effect of low-load resistance exercise with and without blood flow restriction to volitional fatigue on muscle swelling. Eur. J. Appl. Physiol. 2015, 115, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Sims, D.; Wolterstorff, C. A Case of Rhabdomyolysis Caused by Blood Flow-Restricted Resistance Training. J. Spec. Oper. Med. 2018, 18, 16–17. [Google Scholar]
- Tabata, S.; Suzuki, Y.; Azuma, K.; Matsumoto, H. Rhabdomyolysis After Performing Blood Flow Restriction Training: A Case Report. J. Strength Cond. Res. 2016, 30, 2064–2068. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, K.; Newton, M. Difference in the magnitude of muscle damage between maximal and submaximal eccentric loading. J. Strength Cond. Res. 2002, 16, 202–208. [Google Scholar] [PubMed]
- Shinohara, M.; Kouzaki, M.; Yoshihisa, T.; Fukunaga, T. Efficacy of tourniquet ischemia for strength training with low resistance. Eur. J. Appl. Physiol. 1998, 77, 189–191. [Google Scholar] [CrossRef]
- Takarada, Y.; Nakamura, Y.; Aruga, S.; Onda, T.; Miyazaki, S.; Ishii, N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J. Appl. Physiol. 2000, 88, 61–65. [Google Scholar] [CrossRef]
- Sato, Y. The history and future of KAATSU Training. Int. J. KAATSU Train. Res. 2005, 1, 1–5. [Google Scholar] [CrossRef]
- Yasuda, T.; Loenneke, J.P.; Thiebaud, R.S.; Abe, T. Effects of blood flow restricted low-intensity concentric or eccentric training on muscle size and strength. PLoS ONE 2012, 7, e52843. [Google Scholar] [CrossRef]
- Takarada, Y.; Takazawa, H.; Sato, Y.; Takebayashi, S.; Tanaka, Y.; Ishii, N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J. Appl. Physiol. 2000, 88, 2097–2106. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Fahs, C.A.; Rossow, L.M.; Abe, T.; Bemben, M.G. The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Med. Hypotheses 2012, 78, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Brechue, W.F.; Fujita, T.; Sato, Y.; Abe, T. Muscle activation during low-intensity muscle contractions with varying levels of external limb compression. J. Sports Sci. Med. 2008, 7, 467–474. [Google Scholar] [PubMed]
- Yasuda, T.; Brechue, W.F.; Fujita, T.; Shirakawa, J.; Sato, Y.; Abe, T. Muscle activation during low-intensity muscle contractions with restricted blood flow. J. Sports Sci. 2009, 27, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Lixandrão, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceição, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of muscle strength and mass adaptations between high-load resistance training versus low-load resistance training associated with blood-flow restriction: A systematic review and meta-analysis. Sports Med. 2018, 48, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Clark, B.C. Blood flow restricted exercise and skeletal muscle health. Exerc. Sport Sci. Rev. 2009, 37, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Bertovic, D.A.; Waddell, T.K.; Gatzka, C.D.; Cameron, J.D.; Dart, A.M.; Kingwell, B.A. Muscular strength training is associated with low arterial compliance and high pulse pressure. Hypertension 1999, 33, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Yasuda, T.; Ogasawara, R.; Sakamaki-Sunaga, M.; Naito, H.; Abe, T. Effects of high-intensity and blood flow-restricted low-intensity resistance training on carotid arterial compliance: Role of blood pressure during training sessions. Eur. J. Appl. Physiol. 2013, 113, 167–174. [Google Scholar] [CrossRef]
- London, G.M.; Guerin, A.P. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am. Heart J. 1999, 138, 220–224. [Google Scholar] [CrossRef]
- Yasuda, T.; Meguro, M.; Sato, Y.; Nakajima, T. Use and safety of KAATSU training: Results of a national survey in 2016. Int. J. KAATSU Train. Res. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Nakajima, T.; Kurano, M.; Iida, H.; Takano, H.; Oonuma, H.; Morita, T.; Meguro, K.; Sato, Y.; Nagata, T. Use and safety of KAATSU training: Results of a national survey. Int. J. KAATSU Train. Res. 2006, 2, 5–13. [Google Scholar] [CrossRef]
- Gronlund, C.; Christoffersen, K.S.; Thomsen, K.; Masud, T.; Jepsen, D.B.; Ryg, J. Effect of blood-flow restriction exercise on falls and fall related risk factors in older adults 60 years or above: A systematic review. J. Musculoskelet. Neuronal. Interact. 2020, 20, 513–525. [Google Scholar] [PubMed]
- Buford, T.W.; Fillingim, R.B.; Manini, T.M.; Sibille, K.T.; Vincent, K.R.; Wu, S.S. Kaatsu training to enhance physical function of older adults with knee osteoarthritis: Design of a randomized controlled trial. Contemp. Clin. Trials 2015, 43, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Pitsillides, A.; Stasinopoulos, D.; Mamais, I. Blood flow restriction training in patients with knee osteoarthritis: Systematic review of randomized controlled trials. J. Bodyw. Mov. Ther. 2021, 27, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Kurano, M.; Fukumura, K.; Yasuda, T.; Iida, H.; Morita, T.; Yamamoto, Y.; Takano, N.; Komuro, I.; Nakajima, T. Cardiac rehabilitation increases exercise capacity with a reduction of oxidative stress. Korean Circ. J. 2013, 43, 481–487. [Google Scholar] [CrossRef][Green Version]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef]
- Hiraizumi, Y.; Nakajima, T.; Sato, Y.; Imanishi, T. KAATSU training as a new effective exercise therapy in a case of femoral medial condyle osteonecrosis. Int. J. KAATSU Train. Res. 2016, 12, 1–4. [Google Scholar] [CrossRef][Green Version]
- Yasuda, T.; Oosumi, S.; Sugimoto, S.; Morita, T.; Sato, Y.; Ishii, M.; Nakajima, T. Effect of KAATSU training on thigh muscle size and safety for a patient with knee meniscectomy over 3 years. Int. J. KAATSU Train. Res. 2017, 13, 11–14. [Google Scholar] [CrossRef]
- Madarame, H.; Takano, H.; Iida, H.; Hashida, H.; Morita, T.; Nakajima, T. Blood flow-restricted exercise in a ballet dancer with Churg-Strauss syndrome. Gazz. Med. Ital. Arch. Sci. 2011, 170, 63–67. [Google Scholar]
- Bonde-Petersen, F.; Mork, A.L.; Nielsen, E. Local muscle blood flow and sustained contractions of human arm and back muscles. Eur. J. Appl. Physiol. Occup. Physiol. 1975, 34, 43–50. [Google Scholar] [CrossRef]
- Koba, S.; Hayashi, N.; Miura, A.; Endo, M.; Fukuba, Y.; Yoshida, T. Pressor response to static and dynamic knee extensions at equivalent workload in humans. Jpn. J. Physiol. 2004, 54, 471–481. [Google Scholar] [CrossRef]
- Tanimoto, M.; Ishii, N. Effects of low-intensity resistance exercise with slow movement and tonic force generation on muscular function in young men. J. Appl. Physiol. 2006, 100, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Madarame, H.; Ogasawara, R.; Nakazato, K.; Ishii, N. Effect of very low-intensity resistance training with slow movement on muscle size and strength in healthy older adults. Clin. Physiol. Funct. Imaging 2014, 34, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, M.; Sanada, K.; Yamamoto, K.; Kawano, H.; Gando, Y.; Tabata, I.; Ishii, N.; Miyachi, M. Effects of whole-body low-intensity resistance training with slow movement and tonic force generation on muscular size and strength in young men. J. Strength Cond. Res. 2008, 22, 1926–1938. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuku, S.; Kajioka, T.; Sakakibara, H.; Shimaoka, K. Slow movement resistance training using body weight improves muscle mass in the elderly: A randomized controlled trial. Scand. J. Med. Sci. Sports 2018, 28, 1339–1344. [Google Scholar] [CrossRef] [PubMed]
- Usui, S.; Maeo, S.; Tayashiki, K.; Nakatani, M.; Kanehisa, H. Low-load slow movement squat training increases muscle size and strength but not power. Int. J. Sports Med. 2016, 37, 305–312. [Google Scholar] [CrossRef]
- Ogasawara, R.; Loenneke, J.O.; Thiebaud, R.S.; Abe, T. Low-load bench press training to fatigue results in muscle hypertrophy similar to high-load bench press training. Int. J. Clin. Med. 2013, 4, 114–121. [Google Scholar] [CrossRef]
| Characteristics | Resistance Training | |||
|---|---|---|---|---|
| H-RT | L-BFR | L-ST | L-FAIL | |
| Load | 70–85% 1RM | 10–50% 1RM | BW, 30–50% 1RM | 20% 1RM |
| Frequency (day/week) | 2–3 | 2–3 | 2–7 | 3 |
| Sets x Repetitions | 1–3 x 8–15 | 1–4 x 15–30 | 1–3 x 5–15 | 1 x 80–100 |
| References | [6,14,17,18] | [9,19,20,21,22,23,24,25] | [26,27,28,29] | [30] |
| Resistance Training | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H-RT | L-BFR | L-ST | L-FAIL | |||||||||
| YH | OH | Rat | YH | OH | Rat | YH | OH | Rat | YH | OH | Rat | |
| mTOR signaling pathway | ||||||||||||
| PI3K/Akt | ↑ [35,41] | ↑ [36] | ↑ [43] | ↑ [44] | ↑ [45] | ↑ [35] | ||||||
| mTOR | ↑ [35,37,38] | ↑ [41] | ↑ [36] | ↑ [43] | ↑ [35,38] | |||||||
| S6K1/p70S6K | ↑ [37,38,39,40] | ↑ [36] | ↑ [46] | ↑ [43] | ↑ [44,47,48] | ↑ [45] | ↑ [35] | |||||
| rpS6 | ↑ [36,42] | ↑ [49,50] | ||||||||||
| 4E-BP1 | ↑ [35,37,40,41] | ↑ [36,42] | ↑ [35] | |||||||||
| Resistance Training | ||||
|---|---|---|---|---|
| H-RT | L-BFR | L-ST | L-FAIL | |
| Benefits 1 | Exercise repetition: Few [6,18,19] | Exercise load: Low [9,19,20,21,22,23,24,25] | Exercise load: Low [26,27] | Exercise load: Low [30] |
| Benefits 2 | Strength gain: Large [9,38] | Arterial stiffness: No change [21,22,23] | ||
| Benefits 3 | Versatility: High [9,22,25,61] | |||
| Potential complications 1 | Pain in bones and joints: Occurrence [9] | Muscle soreness and damage: Occurrence * [62,63] | Not applicable (Few reports) | Muscle soreness and damage: Occurrence * [64] |
| Potential complications 2 | Arterial stiffness: Increase * [8] | Rhabdomyolysis: Occurrence * [65,66] | Discomfort: Occurrence * [64] | |
| Potential complications 3 | Muscle soreness and damage: Occurrence [67] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, T. Selected Methods of Resistance Training for Prevention and Treatment of Sarcopenia. Cells 2022, 11, 1389. https://doi.org/10.3390/cells11091389
Yasuda T. Selected Methods of Resistance Training for Prevention and Treatment of Sarcopenia. Cells. 2022; 11(9):1389. https://doi.org/10.3390/cells11091389
Chicago/Turabian StyleYasuda, Tomohiro. 2022. "Selected Methods of Resistance Training for Prevention and Treatment of Sarcopenia" Cells 11, no. 9: 1389. https://doi.org/10.3390/cells11091389
APA StyleYasuda, T. (2022). Selected Methods of Resistance Training for Prevention and Treatment of Sarcopenia. Cells, 11(9), 1389. https://doi.org/10.3390/cells11091389






