Surgical Extent for Oral Cancer: Emphasis on a Cut-Off Value for the Resection Margin Status: A Narrative Literature Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Method
3. Results
4. Discussion
4.1. Surgical Concept for Oral Cancer
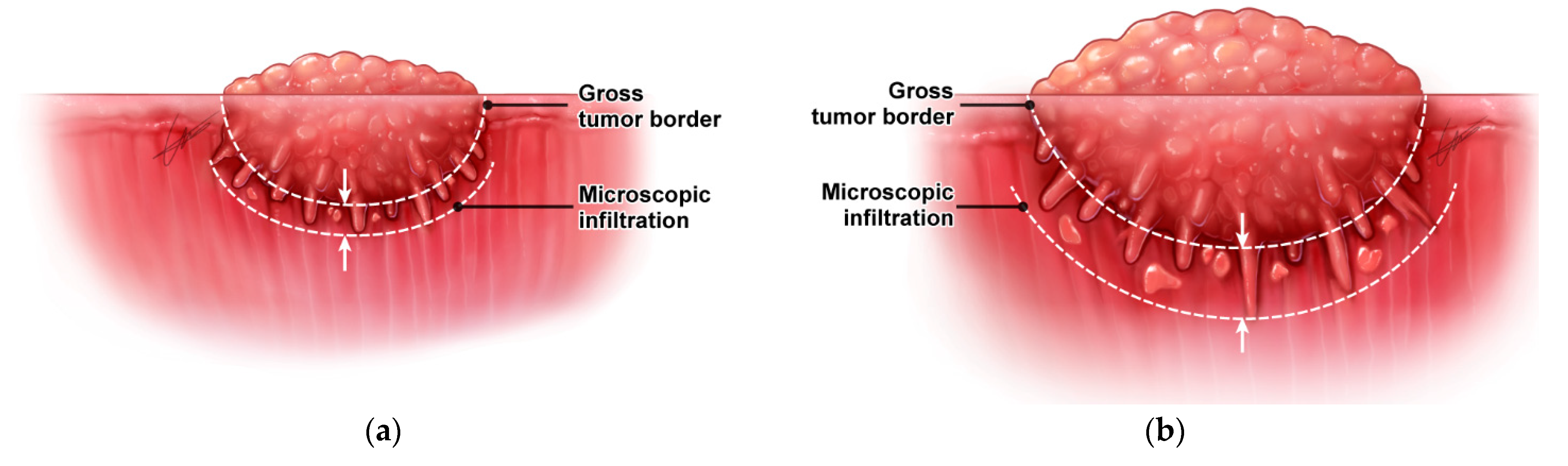
4.1.1. Irregular Three-Dimensional Growth of Primary Tumors
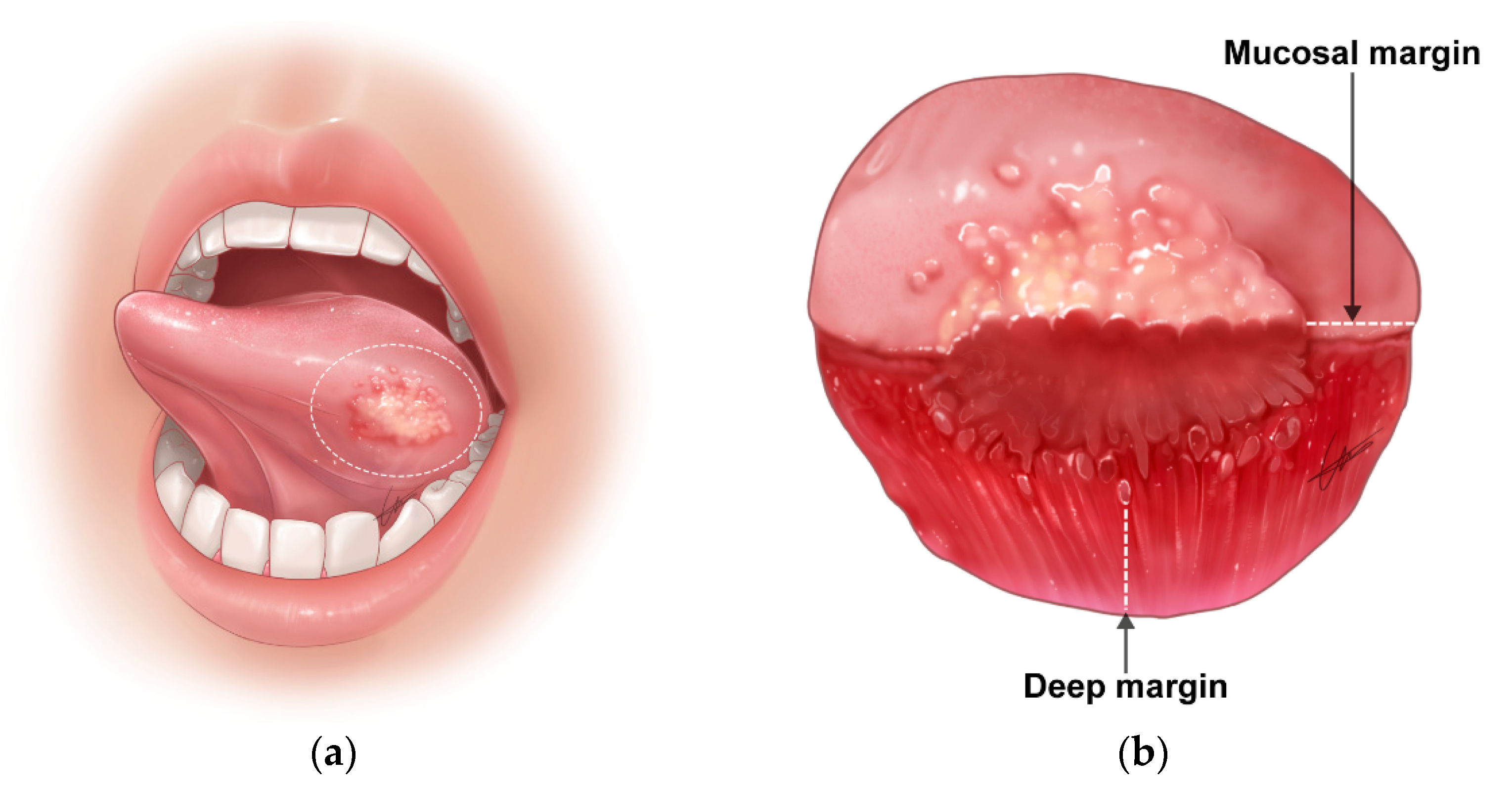
4.1.2. Difficulty in the Delineation of Normal to Tumor Margin during Surgery
4.1.3. Removal of Potential (Future) Malignant Sources
4.2. Resection Margin Status and Risk Stratification
- (1)
- Clear margin: defined as the distance from the invasive tumor front of 5 mm or more from the resected margin on final histopathology;
- (2)
- Close margin: defined as the distance from the invasive tumor front to the resected margin less than 5 mm on final histopathology;
- (3)
- Positive margin: defined as carcinoma in situ or invasive carcinoma at the margin of resection.
4.3. Criteria for a Close Resection Margin and Its Clinical Significance
4.4. Dynamic Cut-Off Values for Postoperative Risk Stratification
| Published Article First Author | Publication Year | Study Design and Number of Patients | Cut-Off Points of the Resection Margin | Local Recurrence Rate | Disease-Free Survival | Overall Survival (5Y) |
|---|---|---|---|---|---|---|
| Loree [41] | 1990 | Retrospective single center (N = 398) | <5 mm | 52.0% | ||
| ≥5 mm | 60.0% | |||||
| Sutton [33] | 2003 | Retrospective single center (N = 200) | Involved | 55% | 11.0% | |
| <5 mm | 33% | 36.0% | ||||
| ≥5 mm | 12% | 60.0% | ||||
| Weijers [34] | 2004 | Retrospective single center (N = 68) | ≤5 mm >5 mm | 6.7% 7.9% (No difference) | ||
| Garzino-Demo [37] | 2006 | Retrospective single center (N = 245) | <5 mm | 48.0% | ||
| ≥5 mm | 65.0% | |||||
| Binahmed [43] | 2007 | Retrospective single center (N = 425) | Involved | 38.7% | ||
| <2 mm | 58.3% | |||||
| ≥2 mm | 68.4% | |||||
| Liao [40] | 2008 | Retrospective single center (N = 827) | 3–11 mm in 1 mm intervals | On multivariate analysis, resection margin ≤ 7 mm was significantly associated with decreased local disease control | ||
| Nason [27] | 2009 | Retrospective single center (N = 277) | Involved | 48.3% | 38.6% | |
| <2 mm | 48.5% | 62.6% | ||||
| 3–4 mm | 69.5% | 69.6% | ||||
| ≥5 mm | 70.5% | 72.9% | ||||
| Kurita [14] | 2010 | Retrospective single center (N = 148) | 1 mm | 33.3% | ||
| 2 mm | 11.1% | |||||
| 3 mm | 33.3% | |||||
| Severe dysplasia | 42.9% | |||||
| Mild/mod dysplasia | 0.0% | |||||
| Tasche [44] | 2017 | Retrospective single center (N = 432) | Involved | 44.0% | ||
| <1 mm | 28.0% | |||||
| 1 mm | 17.0% | |||||
| 2 mm | 13.0% | |||||
| 3 mm | 13.0% | |||||
| 4 mm | 14.0% | |||||
| ≥5 mm | 11.0% | |||||
| Singh [45] | 2020 | Retrospective single center (N = 451) | ≤2 mm | 88.9% | ||
| 3–7 mm | 49.8% | |||||
| ≥8 mm | 35.3% | |||||
| Jain [46] | 2020 | Retrospective single center (N = 612) | Involved | 57.7% | 38.5% | |
| <2 mm | 60.0% | 60.0% | ||||
| 2–4 mm | 76.8% | 66.7% | ||||
| ≥5mm | 72.1% | 76.3% | ||||
| Lin [47] | 2021 | Taiwan Cancer Registry (N = 15,654) | Involved | 46.7% | ||
| <1 mm | 69.5% | |||||
| 1 mm | 66.0% | |||||
| 2 mm | 71.8% | |||||
| 3 mm | 73.9% | |||||
| 4 mm | 74.8% | |||||
| ≥5 mm | 76.1% | |||||
| Tumor Variables (Reference No.) | Number of Patients | Findings | p-Value | p-Value | |
| Tumor status [55] | 90 | Microscopic infiltration | |||
| T1–2 | 0.96 ± 0.54 mm | ||||
| T3–4 | 1.76 ± 1.20 mm | <0.001 | |||
| Depth of invasion [57] | 100 | PNI | LVI | ||
| <4 mm | 2% | 10% | |||
| ≥4 mm | 38% | <0.01 | 28% | 0.02 | |
| MTR in deep resection [58] | 501 | Two-year locoregional control rate | |||
| >0.3 | 94% | ||||
| ≤0.3 | 87% | ||||
| Log MTR [59] | 302 | Five-year disease-specific survival | |||
| >33% | HR 1 | ||||
| ≤33% | HR 2.48 | <0.001 |
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, D.K.; Montero, P.H.; Migliacci, J.C.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Survival outcomes after treatment of cancer of the oral cavity (1985–2015). Oral Oncol. 2019, 90, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Head and Neck Cancers. Version 3. 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 27 July 2021).
- Patel, V.; Galloway, T.J.; Liu, J.C. The impact of positive margin on survival in oral cavity squamous cell carcinoma. Oral Oncol. 2021, 122, 105499. [Google Scholar] [CrossRef]
- D’Cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef]
- Pantvaidya, G.; Rao, K.; D’Cruz, A. Management of the neck in oral cancers. Oral Oncol. 2020, 100, 104476. [Google Scholar] [CrossRef]
- Civantos, F.J.; Zitsch, R.P.; Schuller, D.E.; Agrawal, A.; Smith, R.B.; Nason, R.; Petruzelli, G.; Gourin, C.G.; Wong, R.J.; Ferris, R.L.; et al. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: Results of a prospective multi-institutional trial. J. Clin. Oncol. 2010, 28, 1395–1400. [Google Scholar] [CrossRef]
- Garrel, R.; Poissonnet, G.; Moya Plana, A.; Fakhry, N.; Dolivet, G.; Lallemant, B.; Sarini, J.; Vergez, S.; Guelfucci, B.; Choussy, O.; et al. Equivalence randomized trial to compare treatment on the basis of sentinel node biopsy versus neck node dissection in operable T1-T2N0 oral and oropharyngeal cancer. J. Clin. Oncol. 2020, 38, 4010–4018. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Tsukahara, K.; Yoshimoto, S.; Miura, K.; Yokoyama, J.; Hirano, S.; Uemura, H.; Sugasawa, M.; Yoshizaki, T.; Homma, A.; et al. Neck dissections based on sentinel lymph node navigation versus elective neck dissections in early oral cancers: A randomized, multicenter, and noninferiority trial. J. Clin. Oncol. 2021, 39, 2025–2036. [Google Scholar] [CrossRef]
- Cramer, J.D.; Sridharan, S.; Ferris, R.L.; Duvvuri, U.; Samant, S. Sentinel lymph node biopsy versus elective neck dissection for stage I to II oral cavity cancer. Laryngoscope 2019, 129, 162–169. [Google Scholar] [CrossRef]
- Bowe, C.M.; Shastri, M.; Gulati, A.; Norris, P.; Corrigan, A.; Barrett, A.W.; Bisase, B. Challenges and outcomes in establishing a sentinel lymph node biopsy service for oral squamous cell carcinoma in a regional district specialist hospital. Br. J. Oral Maxillofac. Surg. 2021, 59, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.D.; Oliver, D.A.; Varvares, M.A. Surgical margin determination in head and neck oncology: Current clinical practice. The results of an International American Head and Neck Society Member Survey. Head Neck 2005, 27, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Kurita, H.; Nakanishi, Y.; Nishizawa, R.; Xiao, T.; Kamata, T.; Koike, T.; Kobayashi, H. Impact of different surgical margin conditions on local recurrence of oral squamous cell carcinoma. Oral Oncol. 2010, 46, 814–817. [Google Scholar] [CrossRef]
- Bulbul, M.G.; Zenga, J.; Tarabichi, O.; Parikh, A.S.; Sethi, R.K.; Robbins, K.T.; Puram, S.V.; Varvares, M.A. Margin practices in oral cavity cancer resections: Survey of American head and neck society members. Laryngoscope 2021, 131, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Salama, A.M.; Valero, C.; Yuan, A.; Khimraj, A.; Saliba, M.; Zanoni, D.K.; Ganly, I.; Patel, S.G.; Katabi, N.; et al. The prognostic role of histologic grade, worst pattern of invasion, and tumor budding in early oral tongue squamous cell carcinoma: A comparative study. Virchows Arch. 2021, 479, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Kain, J.J.; Birkeland, A.C.; Udayakumar, N.; Morlandt, A.B.; Stevens, T.M.; Carroll, W.R.; Rosenthal, E.L.; Warram, J.M. Surgical margins in oral cavity squamous cell carcinoma: Current practices and future directions. Laryngoscope 2020, 130, 128–138. [Google Scholar] [CrossRef]
- Tarabichi, O.; Bulbul, M.G.; Kanumuri, V.V.; Faquin, W.C.; Juliano, A.F.; Cunnane, M.E.; Varvares, M.A. Utility of intraoral ultrasound in managing oral tongue squamous cell carcinoma: Systematic review. Laryngoscope 2019, 129, 662–670. [Google Scholar] [CrossRef]
- de Koning, K.J.; Koppes, S.A.; de Bree, R.; Dankbaar, J.W.; Willems, S.M.; van Es, R.J.J.; Noorlag, R. Feasibility study of ultrasound-guided resection of tongue cancer with immediate specimen examination to improve margin control–comparison with conventional treatment. Oral Oncol. 2021, 116, 105249. [Google Scholar] [CrossRef]
- Fatakdawala, H.; Poti, S.; Zhou, F.; Sun, Y.; Bec, J.; Liu, J.; Yankelevich, D.R.; Tinling, S.P.; Gandour-Edwards, R.F.; Farwell, D.G.; et al. Multimodal in vivo imaging of oral cancer using fluorescence lifetime, photoacoustic and ultrasound techniques. Biomed. Opt. Express 2013, 4, 1724–1741. [Google Scholar] [CrossRef] [Green Version]
- Noorlag, R.; de Bree, R.; Witjes, M.J.H. Image-guided surgery in oral cancer: Toward improved margin control. Curr. Opin. Oncol. 2022, 34, 170–176. [Google Scholar] [CrossRef]
- Wu, C.; Gleysteen, J.; Teraphongphom, N.T.; Li, Y.; Rosenthal, E. In-vivo optical imaging in head and neck oncology: Basic principles, clinical applications and future directions. Int. J. Oral Sci. 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, K.; Ma, E.; Kejriwal, S.; Nielsen, T.; Aulakh, S.S.; Birkeland, A.C. Intraoperative in vivo imaging modalities in head and neck cancer surgical margin delineation: A systematic review. Cancers 2022, 14, 3416. [Google Scholar] [CrossRef]
- Brennan, J.A.; Mao, L.; Hruban, R.H.; Boyle, J.O.; Eby, Y.J.; Koch, W.M.; Goodman, S.N.; Sidransky, D. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 1995, 332, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Mistry, R.C.; Qureshi, S.S.; Kumaran, C. Post-resection mucosal margin shrinkage in oral cancer: Quantification and significance. J. Surg. Oncol. 2005, 91, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.E.; Sigman, J.D.; Funk, G.F.; Robinson, R.A.; Hoffman, H.T. Quantification of surgical margin shrinkage in the oral cavity. Head Neck 1997, 19, 281–286. [Google Scholar] [CrossRef]
- Nason, R.W.; Binahmed, A.; Pathak, K.A.; Abdoh, A.A.; Sándor, G.K. What is the adequate margin of surgical resection in oral cancer? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 625–629. [Google Scholar] [CrossRef]
- Cheng, A.; Cox, D.; Schmidt, B.L. Oral squamous cell carcinoma margin discrepancy after resection and pathologic processing. J. Oral Maxillofac. Surg. 2008, 66, 523–529. [Google Scholar] [CrossRef]
- Alicandri-Ciufelli, M.; Bonali, M.; Piccinini, A.; Marra, L.; Ghidini, A.; Cunsolo, E.M.; Maiorana, A.; Presutti, L.; Conte, P.F. Surgical margins in head and neck squamous cell carcinoma: What is ‘close’? Eur. Arch. Otorhinolaryngol. 2013, 270, 2603–2609. [Google Scholar] [CrossRef]
- Dixit, S.; Vyas, R.K.; Toparani, R.B.; Baboo, H.A.; Patel, D.D. Surgery versus surgery and postoperative radiotherapy in squamous cell carcinoma of the buccal mucosa: A comparative study. Ann. Surg. Oncol. 1998, 5, 502–510. [Google Scholar] [CrossRef]
- Hinni, M.L.; Ferlito, A.; Brandwein-Gensler, M.S.; Takes, R.P.; Silver, C.E.; Westra, W.H.; Seethala, R.R.; Rodrigo, J.P.; Corry, J.; Bradford, C.R.; et al. Surgical margins in head and neck cancer: A contemporary review. Head Neck 2013, 35, 1362–1370. [Google Scholar] [CrossRef]
- Oliver, R.J.; Clarkson, J.E.; Conway, D.I.; Glenny, A.; Macluskey, M.; Pavitt, S.; Sloan, P.; Panel, C.E.; Worthington, H.V. Interventions for the treatment of oral and oropharyngeal cancers: Surgical treatment. Cochrane Database Syst. Rev. 2007, 17, CD006205. [Google Scholar] [CrossRef]
- Sutton, D.N.; Brown, J.S.; Rogers, S.N.; Vaughan, E.D.; Woolgar, J.A. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2003, 32, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Weijers, M.; Snow, G.B.; Bezemer, D.P.; van dr Wal, J.E.; van der Waal, I. The status of the deep surgical margins in tongue and floor of mouth squamous cell carcinoma and risk of local recurrence; an analysis of 68 patients. Int. J. Oral Maxillofac. Surg. 2004, 33, 146–149. [Google Scholar] [CrossRef]
- Wong, L.S.; McMahon, J.; Devine, J.; McLellan, D.; Thompson, E.; Farrow, A.; Moos, K.; Ayoub, A. Influence of close resection margins on local recurrence and disease-specific survival in oral and oropharyngeal carcinoma. Br. J. Oral Maxillofac. Surg. 2012, 50, 102–108. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, G.; Kandil, A.; Jamshed, A.; Khafaga, Y.; Saleem, M.; Allam, A.; Al-Rajhi, N.; Al-Amro, A.; Rostom, A.Y.; Abuzeid, M.; et al. Squamous cell carcinoma of the oral tongue: An analysis of prognostic factors. Br. J. Oral Maxillofac. Surg. 2000, 38, 193–199. [Google Scholar] [CrossRef]
- Garzino-Demo, P.; Dell’Acqua, A.; Dalmasso, P.; Fasolis, M.; La Terra Maggiore, G.M.; Ramieri, G.; Berrone, S.; Rampino, M.; Schena, M. Clinicopathological parameters and outcome of 245 patients operated for oral squamous cell carcinoma. J. Craniomaxillofac. Surg. 2006, 34, 344–350. [Google Scholar] [CrossRef]
- Hicks, W.L., Jr.; North, J.H., Jr.; Loree, T.R.; Maamoun, S.; Mullins, A.; Orner, J.B.; Bakamjian, V.Y.; Shedd, D.P. Surgery as a single modality therapy for squamous cell carcinoma of the oral tongue. Am. J. Otolaryngol. 1998, 19, 24–28. [Google Scholar] [CrossRef]
- Kademani, D.; Bell, R.B.; Bagheri, S.; Holmgren, E.; Dierks, E.; Potter, B.; Homer, L. Prognostic factors in intraoral squamous cell carcinoma: The influence of histologic grade. J. Oral Maxillofac. Surg. 2005, 63, 1599–1605. [Google Scholar] [CrossRef]
- Liao, C.T.; Chang, J.T.; Wang, H.M.; Ng, S.H.; Hsueh, C.; Lee, L.Y.; Lin, C.H.; Chen, I.H.; Huang, S.F.; Cheng, A.J.; et al. Analysis of risk factors of predictive local tumor control in oral cavity cancer. Ann. Surg. Oncol. 2008, 15, 915–922. [Google Scholar] [CrossRef]
- Loree, T.R.; Strong, E.W. Significance of positive margins in oral cavity squamous carcinoma. Am. J. Surg. 1990, 160, 410–414. [Google Scholar] [CrossRef]
- Anderson, C.R.; Sisson, K.; Moncrieff, M. A meta-analysis of margin size and local recurrence in oral squamous cell carcinoma. Oral Oncol. 2015, 51, 464–469. [Google Scholar] [CrossRef]
- Binahmed, A.; Nason, R.W.; Abdoh, A.A. The clinical significance of the positive surgical margin in oral cancer. Oral Oncol. 2007, 43, 780–784. [Google Scholar] [CrossRef] [Green Version]
- Tasche, K.K.; Buchakjian, M.R.; Pagedar, N.A.; Sperry, S.M. Definition of “close margin” in oral cancer surgery and association of margin distance with local recurrence rate. JAMA Otolaryngol. Head Neck Surg. 2017, 143, 1166–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Mishra, A.; Singhvi, H.; Sharin, F.; Bal, M.; Laskar, S.G.; Prabhash, K.; Chaturvedi, P. Optimum surgical margins in squamous cell carcinoma of the oral tongue: Is the current definition adequate? Oral Oncol. 2020, 111, 104938. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.V.; Sharan, R.; Manikantan, K.; Clark, G.M.; Chatterjee, S.; Mallick, I.; Roy, P.; Arun, P. Redefining adequate margins in oral squamous cell carcinoma: Outcomes from close and positive margins. Eur. Arch. Otorhinolaryngol. 2020, 277, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Leu, Y.S.; Chiang, C.J.; Ko, J.Y.; Wang, C.P.; Yang, T.L.; Chen, T.C.; Chen, C.N.; Chen, H.L.; Liao, C.T.; et al. Adequate surgical margins for oral cancer: A Taiwan cancer registry national database analysis. Oral Oncol. 2021, 119, 105358. [Google Scholar] [CrossRef]
- Barry, C.P.; Ahmed, F.; Rogers, S.N.; Lowe, D.; Bekiroglu, F.; Brown, J.S.; Shaw, R.J. Influence of surgical margins on local recurrence in T1/T2 oral squamous cell carcinoma. Head Neck 2015, 37, 1176–1180. [Google Scholar] [CrossRef]
- Barry, C.P.; Katre, C.; Papa, E.; Brown, J.S.; Shaw, R.J.; Bekiroglu, F.; Lowe, D.; Rogers, S.N. De-escalation of surgery for early oral cancer–is it oncologically safe? Br. J. Oral Maxillofac. Surg. 2013, 51, 30–36. [Google Scholar] [CrossRef]
- Chen, T.C.; Wang, C.P.; Ko, J.Y.; Yang, T.L.; Lou, P.J. The impact of pathologic close margin on the survival of patients with early stage oral squamous cell carcinoma. Oral Oncol. 2012, 48, 623–628. [Google Scholar] [CrossRef]
- Yanamoto, S.; Yamada, S.; Takahashi, H.; Yoshitomi, I.; Kawasaki, G.; Ikeda, H.; Minamizato, T.; Shiraishi, T.; Fujita, S.; Ikeda, T.; et al. Clinicopathological risk factors for local recurrence in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2012, 41, 1195–1200. [Google Scholar] [CrossRef]
- Nichols, A.C.; Theurer, J.; Prisman, E.; Read, N.; Berthelet, E.; Tran, E.; Fung, K.; de Almeida, J.R.; Bayley, A.; Goldstein, D.P.; et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): An open-label, phase 2, randomised trial. Lancet Oncol. 2019, 20, 1349–1359. [Google Scholar] [CrossRef]
- Ferris, R.L.; Flamand, Y.; Weinstein, G.S.; Li, S.L.; Quon, H.; Mehra, R.; Garcia, J.J.; Chung, C.H.; Gillison, M.L.; Duvvuri, U.; et al. Transoral robotic surgical resection followed by randomization to low- or standard-dose IMRT in resectable p16+locally advanced oropharynx cancer: A trial of the ECOG-ACRIN Cancer Research Group (E3311). J. Clin. Oncol. 2020, 38, 6500. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.; Hwang, N.; Choi, S.Y.; Park, W.; Choi, N.; Son, Y.I.; Cho, J.; Jeong, H.S. Tumor dimension-dependent microscopic extensions of hypopharyngeal cancer: Therapeutic implications for larynx-preserving hypopharyngectomy. J. Surg. Oncol. 2021, 123, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Choi, N.; Ko, Y.H.; Chung, M.K.; Son, Y.I.; Baek, C.H.; Baek, K.H.; Jeong, H.S. Differential impact of close surgical margin on local recurrence according to primary tumor size in oral squamous cell carcinoma. Ann. Surg. Oncol. 2017, 24, 1698–1706. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoon, N.; Choi, S.Y.; Moon, J.H.; Chung, M.K.; Son, Y.I.; Ko, Y.H.; Jeong, H.S.; Baek, C.H. Extent of local invasion and safe resection in cT1-2 tonsil cancer. J. Surg. Oncol. 2013, 107, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.R.; Kemmer, J.; Formeister, E.; El-Sayed, I.; Ha, P.; George, J.; Ryan, W.; Chan, E.; Heaton, C. Beyond depth of invasion: Adverse pathologic tumor features in early oral tongue squamous cell carcinoma. Laryngoscope 2020, 130, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Heiduschka, G.; Virk, S.A.; Palme, C.E.; Ch’ng, S.; Elliot, M.; Gupta, R.; Clark, J. Margin to tumor thickness ratio—A predictor of local recurrence and survival in oral squamous cell carcinoma. Oral Oncol. 2016, 55, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lin, Y.S.; Kang, B.H.; Chang, K.P.; Chi, C.C.; Lin, M.Y.; Su, H.H.; Chang, T.S.; Lee, H.P.; Lee, C.C. Log margin-to-thickness ratio improves disease-specific survival prediction in oral cancer: A single cancer centre database. Clin. Otolaryngol. 2019, 44, 63–69. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.Y.; Choi, N.; Jeong, H.-S. Surgical Extent for Oral Cancer: Emphasis on a Cut-Off Value for the Resection Margin Status: A Narrative Literature Review. Cancers 2022, 14, 5702. https://doi.org/10.3390/cancers14225702
Jang JY, Choi N, Jeong H-S. Surgical Extent for Oral Cancer: Emphasis on a Cut-Off Value for the Resection Margin Status: A Narrative Literature Review. Cancers. 2022; 14(22):5702. https://doi.org/10.3390/cancers14225702
Chicago/Turabian StyleJang, Jeon Yeob, Nayeon Choi, and Han-Sin Jeong. 2022. "Surgical Extent for Oral Cancer: Emphasis on a Cut-Off Value for the Resection Margin Status: A Narrative Literature Review" Cancers 14, no. 22: 5702. https://doi.org/10.3390/cancers14225702





