Evaluation of Static DNA Ploidy Analysis Using Conventional Brush Biopsy-Based Cytology Samples as an Adjuvant Diagnostic Tool for the Detection of a Malignant Transformation in Potentially Oral Malignant Diseases: A Prospective Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Material
2.1. Patients and Cell Collecting Procedure
- (a)
- Primary clinical diagnosis of OPMD or verified diagnosis of OSCC;
- (b)
- Both brushing and biopsy samples were obtained;
- (c)
- DNA content analysis was completed by DNA-ICM;
- (d)
- Histopathological examination by pathologist, or for the cytologically negative controls, clinical follow-up on average of 60 months by experienced oral surgeons.
2.2. Technical Approach of DNA-ICM
2.3. Criteria of Aneuploidy
- DNA stemline aneuploidy: The usual precision of recent DNA image cytometric measurements should at least allow DNA stemlines to be identified as abnormal (or aneuploid) if they deviate more than 10% from the diploid (2c) or tetraploid region (4c), i.e., if the modal values of DNA stemlines are outside 2c ± 0.2c or 4c ± 0.4c (examples: Figure 3 and Figure 4).



- 2.
- DNA single cell aneuploidy: Rare events in DNA histograms are abnormal cells often called 5c or 9c exceeding events, having a nuclear DNA content higher than the duplicate or quadruplicate region of a normal G1/G0 phase population, i.e., not belonging to the G2M phase. Accordingly, we defined “single cell aneuploidy” as the occurrence of at least one cell with a DNA content of >9c (example: Figure 3 and Figure 4).
2.4. Statistical Analysis
3. Results
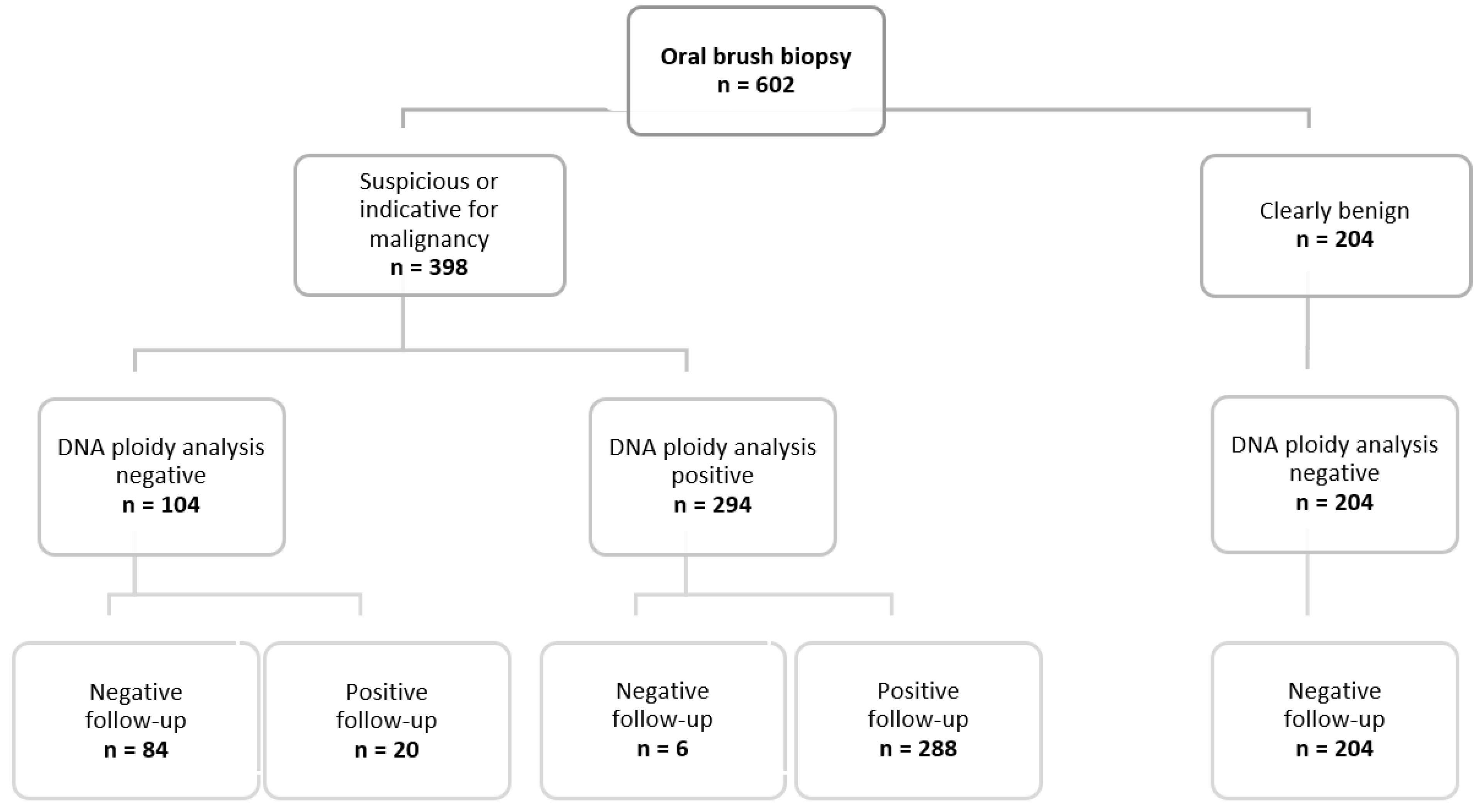
3.1. Enrolled Patients
3.2. Cytological Examination
3.3. DNA Image Cytometry
3.4. Logistic Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer. Lip and Oral Cavity Cancer Today; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Shrestha, A.D.; Vedsted, P.; Kallestrup, P.; Neupane, D. Prevalence and incidence of oral cancer in low- and middle-income countries: A scoping review. Eur. J. Cancer Care 2020, 29, e13207. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Lodi, G. Oral potentially malignant disorders: Proceedings from an expert symposium. Oral Dis. 2021, 27, 1859–1861. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Johnson, N.W.; van der Waal, I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J. Oral Pathol. Med. 2007, 36, 575–580. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Haroske, G.; Baak, J.P.; Danielsen, H.; Giroud, F.; Gschwendtner, A.; Oberholzer, M.; Reith, A.; Spieler, P.; Bocking, A. Fourth updated ESACP consensus report on diagnostic DNA image cytometry. Anal. Cell. Pathol. 2001, 23, 89–95. [Google Scholar] [CrossRef]
- Duesberg, P.; Fabarius, A.; Hehlmann, R. Aneuploidy, the primary cause of the multilateral genomic instability of neoplastic and preneoplastic cells. IUBMB Life 2004, 56, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Bocking, A.; Sproll, C.; Stocklein, N.; Naujoks, C.; Depprich, R.; Kubler, N.R.; Handschel, J. Role of brush biopsy and DNA cytometry for prevention, diagnosis, therapy, and followup care of oral cancer. J. Oncol. 2011, 2011, 875959. [Google Scholar] [CrossRef] [PubMed]
- Bocking, A.; Friedrich, D.; Schramm, M.; Palcic, B.; Erbeznik, G. DNA Karyometry for Automated Detection of Cancer Cells. Cancers 2022, 14, 4210. [Google Scholar] [CrossRef]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Laronde, D.; Palcic, B.; Guillaud, M. The role of DNA image cytometry in screening oral potentially malignant lesions using brushings: A systematic review. Oral Oncol. 2019, 96, 51–59. [Google Scholar] [CrossRef]
- Kammerer, P.W.; Koch, F.P.; Santoro, M.; Babaryka, G.; Biesterfeld, S.; Brieger, J.; Kunkel, M. Prospective, blinded comparison of cytology and DNA-image cytometry of brush biopsies for early detection of oral malignancy. Oral Oncol. 2013, 49, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, Y.; Li, C.; Liu, W. Current evidence on DNA aneuploidy cytology in noninvasive detection of oral cancer. Oral Oncol. 2020, 101, 104367. [Google Scholar] [CrossRef] [PubMed]
- Remmerbach, T.W.; Mathes, S.N.; Weidenbach, H.; Hemprich, A.; Bocking, A. [Noninvasive brush biopsy as an innovative tool for early detection of oral carcinomas]. Mund Kiefer Gesichtschir 2004, 8, 229–236. [Google Scholar] [CrossRef]
- Albertini, R.J.; Anderson, D.; Douglas, G.R.; Hagmar, L.; Hemminki, K.; Merlo, F.; Natarajan, A.T.; Norppa, H.; Shuker, D.E.; Tice, R.; et al. IPCS guidelines for the monitoring of genotoxic effects of carcinogens in humans. International Programme on Chemical Safety. Mutat. Res. 2000, 463, 111–172. [Google Scholar] [CrossRef] [PubMed]
- Norppa, H. Cytogenetic biomarkers and genetic polymorphisms. Toxicol. Lett. 2004, 149, 309–334. [Google Scholar] [CrossRef]
- Gordon, D.J.; Resio, B.; Pellman, D. Causes and consequences of aneuploidy in cancer. Nat. Rev. Genet. 2012, 13, 189–203. [Google Scholar] [CrossRef]
- Gupta, S.; Jawanda, M.K.; Madhushankari, G.S. Current challenges and the diagnostic pitfalls in the grading of epithelial dysplasia in oral potentially malignant disorders: A review. J. Oral Biol. Craniofac. Res. 2020, 10, 788–799. [Google Scholar] [CrossRef]
- Ma, J.M.; Zhou, T.J.; Wang, R.; Shan, J.; Wu, Y.N.; Song, X.L.; Gu, N.; Fan, Y. Brush biopsy with DNA-image cytometry: A useful and noninvasive method for monitoring malignant transformation of potentially malignant oral disorders. Eur. Arch. Otorhinolaryngol 2014, 271, 3291–3295. [Google Scholar] [CrossRef]
- Collins, B.M. The oral brush biopsy: An adjunct to early oral cancer detection. Pa. Dent. J. 2002, 69, 35–37. [Google Scholar]
- Velleuer, E.; Dietrich, R.; Pomjanski, N.; de Santana Almeida Araujo, I.K.; Silva de Araujo, B.E.; Sroka, I.; Biesterfeld, S.; Bocking, A.; Schramm, M. Diagnostic accuracy of brush biopsy-based cytology for the early detection of oral cancer and precursors in Fanconi anemia. Cancer Cytopathol. 2020, 128, 403–413. [Google Scholar] [CrossRef]
- Pektas, Z.O.; Keskin, A.; Gunhan, O.; Karslioglu, Y. Evaluation of nuclear morphometry and DNA ploidy status for detection of malignant and premalignant oral lesions: Quantitative cytologic assessment and review of methods for cytomorphometric measurements. J. Oral Maxillofac. Surg. 2006, 64, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Maraki, D.; Becker, J.; Boecking, A. Cytologic and DNA-cytometric very early diagnosis of oral cancer. J. Oral Pathol. Med. 2004, 33, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Reibel, J.; Bouquot, J.; Dabelsteen, E. Oral epithelial dysplasia classification systems: Predictive value, utility, weaknesses and scope for improvement. J. Oral Pathol. Med. 2008, 37, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Bocking, A.; Motherby, H. Assessment of cervical dysplasia with DNA image cytometry. Der Pathologe 1999, 20, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Deuerling, L.; Gaida, K.; Neumann, H.; Remmerbach, T.W. Evaluation of the Accuracy of Liquid-Based Oral Brush Cytology in Screening for Oral Squamous Cell Carcinoma. Cancers 2019, 11, 1813. [Google Scholar] [CrossRef]
- Poage, G.M.; Houseman, E.A.; Christensen, B.C.; Butler, R.A.; Avissar-Whiting, M.; McClean, M.D.; Waterboer, T.; Pawlita, M.; Marsit, C.J.; Kelsey, K.T. Global hypomethylation identifies Loci targeted for hypermethylation in head and neck cancer. Clin. Cancer Res. 2011, 17, 3579–3589. [Google Scholar] [CrossRef]
- Viet, C.T.; Zhang, X.; Xu, K.; Yu, G.; Asam, K.; Thomas, C.M.; Callahan, N.F.; Doan, C.; Walker, P.C.; Nguyen, K.; et al. Brush swab as a noninvasive surrogate for tissue biopsies in epigenomic profiling of oral cancer. Biomark. Res. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Viet, C.T.; Jordan, R.C.; Schmidt, B.L. DNA promoter hypermethylation in saliva for the early diagnosis of oral cancer. J. Calif. Dent. Assoc. 2007, 35, 844–849. [Google Scholar]
- Liao, P.H.; Chang, Y.C.; Huang, M.F.; Tai, K.W.; Chou, M.Y. Mutation of p53 gene codon 63 in saliva as a molecular marker for oral squamous cell carcinomas. Oral Oncol. 2000, 36, 272–276. [Google Scholar] [CrossRef]

| Clinical Diagnoses | Gender | Total | ||
|---|---|---|---|---|
| Female | Male | |||
| Oral Leukoplakia | number of cases | 68 | 44 | 112 |
| % | 60.7 | 39.3 | ||
| Oral Lichen planus | number of cases | 8 | 27 | 35 |
| % | 22.9 | 77.1 | ||
| Lichen erosivus | number of cases | 5 | 19 | 24 |
| % | 20.8 | 79.2 | ||
| OSCC * | number of cases | 228 | 73 | 301 |
| % | 75.7 | 24.3 | ||
| Ulceration | number of cases | 31 | 18 | 49 |
| % | 63.3 | 36.7 | ||
| Other lesions ** | number of cases | 40 | 41 | 81 |
| % | 49.4 | 50.6 | ||
| T-Status | (n) | N-Status | (n) | M-Status | (n) |
|---|---|---|---|---|---|
| Tx | 55 | Nx | 55 | Mx | 55 |
| Tis * | 1 | N0 | 139 | M0 | 186 |
| T1 | 84 | N1 | 43 | M1 | 71 |
| T2 | 85 | N2a | 14 | ||
| T3 | 37 | N2b | 42 | ||
| T4a | 46 | N2c | 12 | ||
| T4b | 0 | N3 | 3 |
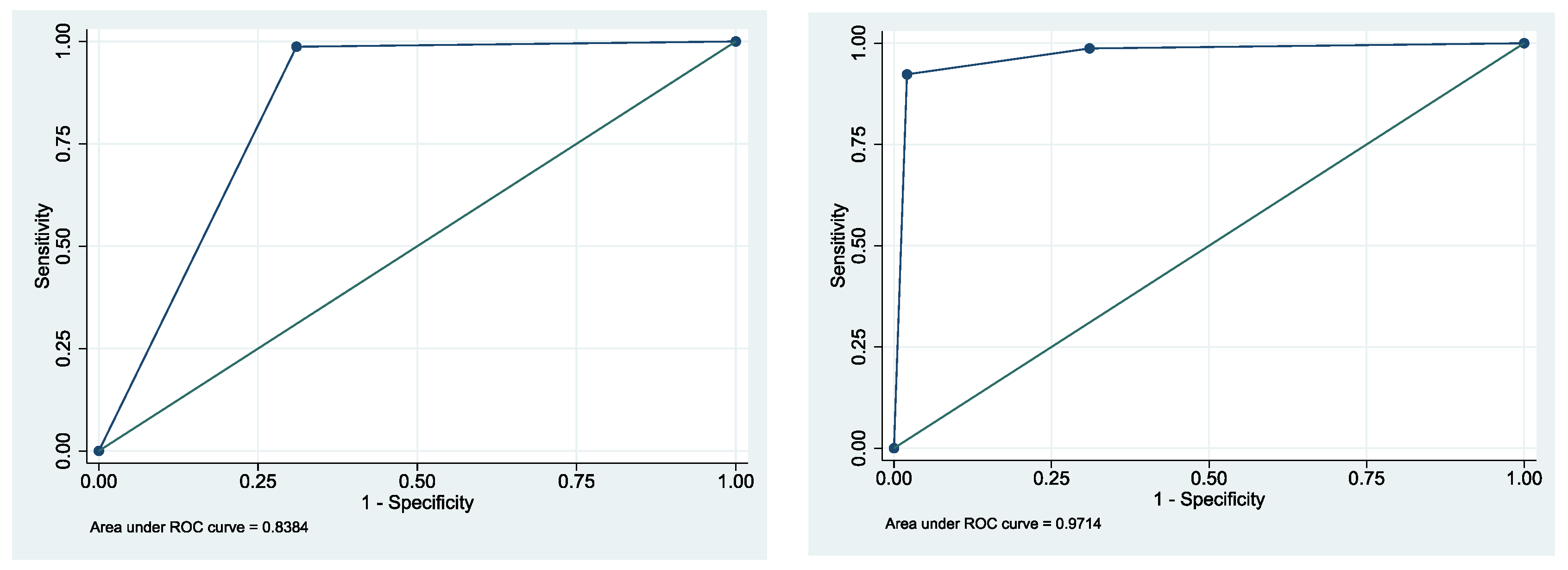
| Method | Specificity | Sensitivity | PPV | NPV | AUC | 95% CI (AUC) |
|---|---|---|---|---|---|---|
| Conventional Cytology | 69.4% | 100.0% | 77.4% | 100% | 0.85 | 0.82–0.87 |
| DNA-ICM | 98.0% | 93.5% | 98.0% | 93.5% | 0.96 | 0.94–0.97 |
| Cytological Examination | DNA Ploidy Analysis with DNA-ICM | |
|---|---|---|
| DNA Aneuploidy | No DNA Aneuploidy | |
| positive | 222 | 6 |
| suspicious | 49 | 14 |
| doubtful | 23 | 84 |
| negative | 0 | 204 |
| Aspects of DNA-Ploidy | Biological Behavior of the Lesion | |
|---|---|---|
| Malignant | Benign | |
| Normal stemline [1.8 < c < 2.2] x x = 1,2,3 | 20 | 288 |
| Atypical stemline [1.8 > c > 2.2] x x = 1,2,3 | 227 | 5 |
| 1 | 78 | 3 |
| 2 | 118 | 2 |
| >2 | 31 | |
| Cells > 9c | 301 | 4 |
| 1 to 3 | 112 | 3 |
| 4 to 10 | 86 | 1 |
| >10 | 103 | 0 |
| Atypical stemline and cells > 9c | 222 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bechstedt, N.; Pomjanski, N.; Schramm, M.; Remmerbach, T.W. Evaluation of Static DNA Ploidy Analysis Using Conventional Brush Biopsy-Based Cytology Samples as an Adjuvant Diagnostic Tool for the Detection of a Malignant Transformation in Potentially Oral Malignant Diseases: A Prospective Study. Cancers 2022, 14, 5828. https://doi.org/10.3390/cancers14235828
Bechstedt N, Pomjanski N, Schramm M, Remmerbach TW. Evaluation of Static DNA Ploidy Analysis Using Conventional Brush Biopsy-Based Cytology Samples as an Adjuvant Diagnostic Tool for the Detection of a Malignant Transformation in Potentially Oral Malignant Diseases: A Prospective Study. Cancers. 2022; 14(23):5828. https://doi.org/10.3390/cancers14235828
Chicago/Turabian StyleBechstedt, Natalie, Natalia Pomjanski, Martin Schramm, and Torsten W. Remmerbach. 2022. "Evaluation of Static DNA Ploidy Analysis Using Conventional Brush Biopsy-Based Cytology Samples as an Adjuvant Diagnostic Tool for the Detection of a Malignant Transformation in Potentially Oral Malignant Diseases: A Prospective Study" Cancers 14, no. 23: 5828. https://doi.org/10.3390/cancers14235828
APA StyleBechstedt, N., Pomjanski, N., Schramm, M., & Remmerbach, T. W. (2022). Evaluation of Static DNA Ploidy Analysis Using Conventional Brush Biopsy-Based Cytology Samples as an Adjuvant Diagnostic Tool for the Detection of a Malignant Transformation in Potentially Oral Malignant Diseases: A Prospective Study. Cancers, 14(23), 5828. https://doi.org/10.3390/cancers14235828





