Multi-Parameter Detection of Urine Based on Electropolymerized PANI: PSS/AuNPs/SPCE
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.1.1. Reagents
2.1.2. Instrument
2.2. Preparation of PANI: PSS-Based NH4+-Selective Electrodes
2.3. Preparation of Urea Electrode and Creatinine Based on NH4+ Detection
2.4. Test Method for Electrochemical Characteristics of Electrodes
3. Results and Discussion
3.1. Characterization of PANI: PSS Films
3.1.1. Scanning Electron Micrograph of PANI: PSS Film
3.1.2. Transmission Electron Micrograph (TEM) of PANI: PSS Film
3.1.3. Fourier Infrared Spectroscopy (FTIR) of PANI: PSS Films
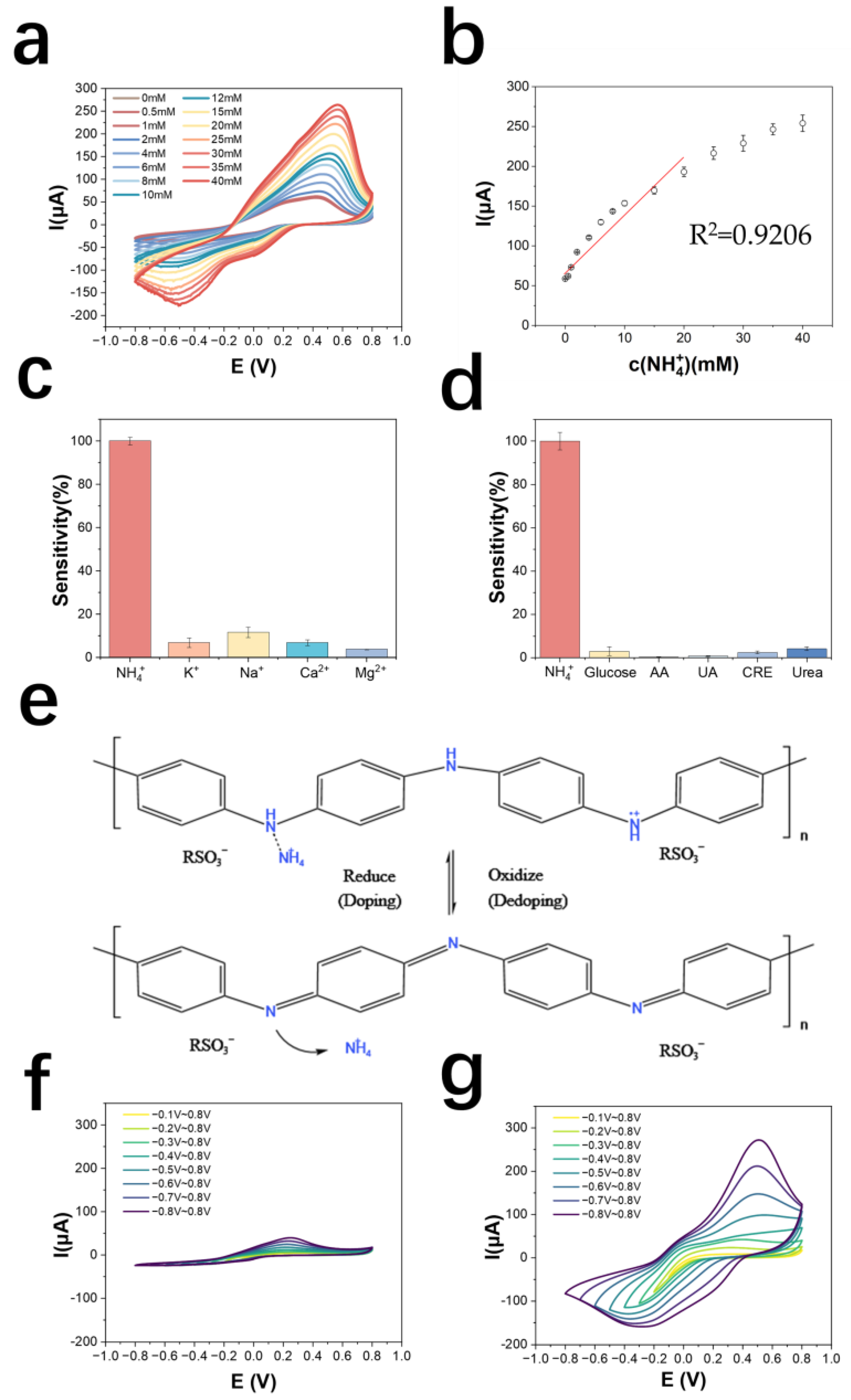
3.2. Performance Testing and Mechanism Investigation of NH4+-Selective Electrode Based on PANI: PSS
3.2.1. Performance Test
3.2.2. Exploration of the Mechanism
3.3. Detection Principle and Performance Test of Urea Electrode Based on NH4+ Detection
3.4. Detection Principle and Performance Test of Creatinine Electrode Based on NH4+ Detection
3.4.1. Performance Testing of Creatinine Electrodes
3.4.2. Optimization of Creatinine Electrodes
3.5. Real Sample Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ecder, T. Early Diagnosis Saves Lives: Focus on Patients with Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Bagalad, B.S.; Mohankumar, K.P.; Madhushankari, G.S.; Donoghue, M.; Kuberappa, P.H. Diagnostic Accuracy of Salivary Creatinine, Urea, and Potassium Levels to Assess Dialysis Need in Renal Failure Patients. Dent. Res. J. 2017, 14, 13–18. [Google Scholar] [CrossRef]
- Schelling, J.R. Tubular Atrophy in the Pathogenesis of Chronic Kidney Disease Progression. Pediatr. Nephrol. 2016, 31, 693–706. [Google Scholar] [CrossRef]
- Dennis, A.M.; Bao, G. Quantum Dot-Fluorescent Protein Pair as Ratiometric PH Sensor. Colloid. Quantum Dots Biomed. Appl. V 2010, 7575, 75750C. [Google Scholar] [CrossRef]
- Lad, U.; Khokhar, S.; Kale, G.M. Electrochemical Creatinine Biosensors. Anal. Chem. 2008, 80, 7910–7917. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. Analysis of the Clinical Significance of Urine and Renal Function Examination in the Screening of Renal Diseases. Chin. Community Dr. 2020, 36, 133–134. [Google Scholar] [CrossRef]
- Lv, Q. Performance Evaluation of Urine Clear Protein Assay and Significance of Urine Clear Protein/Creatinine Ratio Combined with Endogenous Creatinine Clearance in Detecting Renal Injury in Type 2 Diabetic Patients. Clin. Med. 2016, 36, 54–56. [Google Scholar]
- Tziakas, D.; Chalikias, G.; Kareli, D.; Tsigalou, C.; Risgits, A.; Kikas, P.; Makrygiannis, D.; Chatzikyriakou, S.; Kampouromiti, G.; Symeonidis, D.; et al. Spot Urine Albumin to Creatinine Ratio Outperforms Novel Acute Kidney Injury Biomarkers in Patients with Acute Myocardial Infarction. Int. J. Cardiol. 2015, 197, 48–55. [Google Scholar] [CrossRef]
- Coresh, J.; Astor, B.C.; Greene, T.; Eknoyan, G.; Levey, A.S. Prevalence of Chronic Kidney Disease and Decreased Kidney Function in the Adult US Population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 2003, 41, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L. Metabolic Acidosis: An Unrecognized Cause of Morbidity in the Patient with Chronic Kidney Disease. Kidney Int. 2005, 68, S15–S23. [Google Scholar] [CrossRef]
- Garibotto, G.; Sofia, A.; Robaudo, C.; Saffioti, S.; Sala, M.R.; Verzola, D.; Vettore, M.; Russo, R.; Procopio, V.; Deferrari, G.; et al. Kidney Protein Dynamics and Ammoniagenesis in Humans with Chronic Metabolic Acidosis. J. Am. Soc. Nephrol. 2004, 15, 1606–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamm, L.L.; Nakhoul, N.; Hering-Smith, K.S. Acid-Base Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Vallet, M.; Metzger, M.; Haymann, J.P.; Flamant, M.; Gauci, C.; Thervet, E.; Boffa, J.J.; Vrtovsnik, F.; Froissart, M.; Stengel, B.; et al. Urinary Ammonia and Long-Term Outcomes in Chronic Kidney Disease. Kidney Int. 2015, 88, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Moranne, O.; Froissart, M.; Rossert, J.; Gauci, C.; Boffa, J.J.; Haymann, J.P.; M’rad, M.B.; Jacquot, C.; Houillier, P.; Stengel, B.; et al. Timing of Onset of CKD-Related Metabolic Complications. J. Am. Soc. Nephrol. 2009, 20, 164–171. [Google Scholar] [CrossRef]
- Raphael, K.L.; Zhang, Y.; Ying, J.; Greene, T. Prevalence of and Risk Factors for Reduced Serum Bicarbonate in Chronic Kidney Disease. Nephrology 2014, 19, 648–654. [Google Scholar] [CrossRef]
- Raphael, K.L.; Carroll, D.J.; Murray, J.; Greene, T.; Beddhu, S. Urine Ammonium Predicts Clinical Outcomes in Hypertensive Kidney Disease. J. Am. Soc. Nephrol. 2017, 28, 2483–2490. [Google Scholar] [CrossRef]
- Hashihama, F.; Kanda, J.; Tauchi, A.; Kodama, T.; Saito, H.; Furuya, K. Liquid Waveguide Spectrophotometric Measurement of Nanomolar Ammonium in Seawater Based on the Indophenol Reaction with O-Phenylphenol (OPP). Talanta 2015, 143, 374–380. [Google Scholar] [CrossRef]
- He, Z.K.; Fuhrmann, B.; Spohn, U. Coulometric Microflow Titrations with Chemiluminescent and Amperometric Equivalence Point DetectionBromimetric Titration of Low Concentrations of Hydrazine and Ammonium. Anal. Chim. Acta 2000, 409, 83–91. [Google Scholar] [CrossRef]
- Xu, J.; Liang, W.; Xu, H. Ion Chromatography in Determination of Ammonium Ion in PM2. 5 XU. Shanghai J. Prev. Med. 2019, 31, 4–7. [Google Scholar] [CrossRef]
- Pundir, C.S.; Jakhar, S.; Narwal, V. Determination of Urea with Special Emphasis on Biosensors: A Review. Biosens. Bioelectron. 2019, 123, 36–50. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and Creatinine Metabolism. Physiol. Rev. 2000, 80, 1107. [Google Scholar] [CrossRef]
- Guinovart, T.; Bandodkar, A.J.; Windmiller, J.R.; Andrade, F.J.; Wang, J. A Potentiometric Tattoo Sensor for Monitoring Ammonium in Sweat. Analyst 2013, 138, 7031–7038. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, M.; Colozza, N.; Fernández-Pérez, B.M.; Crespo, G.A. Why Ammonium Detection Is Particularly Challenging but Insightful with Ionophore-Based Potentiometric Sensors-an Overview of the Progress in the Last 20 Years. Analyst 2020, 145, 3188–3210. [Google Scholar] [CrossRef] [PubMed]
- Cosio, M.S.; Scampicchio, M.; Benedetti, S. Electronic Noses and Tongues. In Chemical Analysis of Food: Techniques and Applications; Academic Press: Boston, MA, USA, 2012; Volume 8, pp. 219–247. [Google Scholar] [CrossRef]
- Uzunçar, S.; Meng, L.; Turner, A.P.F.; Mak, W.C. Processable and Nanofibrous Polyaniline:Polystyrene-Sulphonate (Nano-PANI: PSS) for the Fabrication of Catalyst-Free Ammonium Sensors and Enzyme-Coupled Urea Biosensors. Biosens. Bioelectron. 2021, 171, 112725. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, B.; Abidian, R.; Kim, D.; Martin, D.C. Conducting-Polymer Nanotubes for Controlled Drug Release. Adv. Mater. 2006, 18, 405–409. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, P.; Park, D.; Shim, Y. Electrochemical Sensors Based on Organic Conjugated Polymers. Sensors 2008, 8, 118–141. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Su, L.; Lei, Y. Pt Nanoflower/Polyaniline Composite Nanofibers Based Urea Biosensor. Biosens. Bioelectron. 2011, 30, 158–164. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, N.; Singh, A.K. Development of Cadmium Specific Recombinant Biosensor and Its Application in Milk Samples. Sens. Actuators B Chem. 2017, 240, 248–254. [Google Scholar] [CrossRef]
- Lorestani, F.; Shahnavaz, Z.; Nia, P.M.; Alias, Y.; Manan, N.S.A. One-Step Preparation of Silver–Polyaniline Nanotube Composite for Non-Enzymatic Hydrogen Peroxide Detection. Appl. Surf. Sci. 2015, 347, 816–823. [Google Scholar] [CrossRef]
- Singh, P.; Singh, K.R.; Verma, R.; Prasad, P.; Verma, R.; Das, S.N.; Singh, J.; Singh, R.P. Preparation, Antibacterial Activity, and Electrocatalytic Detection of Hydrazine Based on Biogenic CuFeO2/PANI Nanocomposites Synthesized Using Aloe Barbadensis Miller. New J. Chem. 2022, 46, 8805–8816. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Adhikari, B. Influence of Polymerization Condition on the Electrical Conductivity and Gas Sensing Properties of Polyaniline. Mater. Sci. Eng. A 2007, 459, 278–285. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Hasani, A.; Nouri, M.; Mahyari, M.; Salehi, A. Highly Sensitive and Flexible Ammonia Sensor Based on S and N Co-Doped Graphene Quantum Dots/Polyaniline Hybrid at Room Temperature. Sens. Actuators B Chem. 2016, 229, 239–248. [Google Scholar] [CrossRef]
- Tanguy, N.R.; Thompson, M.; Yan, N. A Review on Advances in Application of Polyaniline for Ammonia Detection. Sens. Actuators B Chem. 2018, 257, 1044–1064. [Google Scholar] [CrossRef]
- Luo, Y.C.; Do, J.S. Urea Biosensor Based on PANi(Urease)-Nafion®/Au Composite Electrode. Biosens. Bioelectron. 2004, 20, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Zhybak, M.T.; Vagin, M.Y.; Beni, V.; Liu, X.; Dempsey, E.; Turner, A.P.F.; Korpan, Y.I. Direct Detection of Ammonium Ion by Means of Oxygen Electrocatalysis at a Copper-Polyaniline Composite on a Screen-Printed Electrode. Microchim. Acta 2016, 183, 1981–1987. [Google Scholar] [CrossRef]
- Zhybak, M.; Beni, V.; Vagin, M.Y.; Dempsey, E.; Turner, A.P.F.; Korpan, Y. Creatinine and Urea Biosensors Based on a Novel Ammonium Ion-Selective Copper-Polyaniline Nano-Composite. Biosens. Bioelectron. 2016, 77, 505–511. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef]
- Fang, X.; Bai, L.; Han, X.; Wang, J.; Shi, A.; Zhang, Y. Ultra-Sensitive Biosensor for K-Ras Gene Detection Using Enzyme Capped Gold Nanoparticles Conjugates for Signal Amplification. Anal. Biochem. 2014, 460, 47–53. [Google Scholar] [CrossRef]
- Fang, L.; Liang, B.; Yang, G.; Hu, Y.; Zhu, Q.; Ye, X. A Needle-Type Glucose Biosensor Based on PANI Nanofibers and PU/E-PU Membrane for Long-Term Invasive Continuous Monitoring. Biosens. Bioelectron. 2017, 97, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, K.; Matysiak, W.; Tański, T.; Janeczek, H.; Schab-Balcerzak, E.; Libera, M. Effect of Polyaniline Content and Protonating Dopants on Electroconductive Composites. Sci. Rep. 2021, 11, 7487. [Google Scholar] [CrossRef] [PubMed]
- Strehlitz, B.; Gründig, B.; Kopinke, H. Sensor for Amperometric Determination of Ammonia and Ammonia-Forming Enzyme Reactions. Anal. Chim. Acta 2000, 403, 11–23. [Google Scholar] [CrossRef]
- Stasyuk, N.; Smutok, O.; Gayda, G.; Vus, B.; Koval’chuk, Y.; Gonchar, M. Bi-Enzyme l-Arginine-Selective Amperometric Biosensor Based on Ammonium-Sensing Polyaniline-Modified Electrode. Biosens. Bioelectron. 2012, 37, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Do, J.-S.; Chang, Y.-H.; Tsai, M.-L. Highly Sensitive Amperometric Creatinine Biosensor Based on Creatinine Deiminase/Nafion®-Nanostructured Polyaniline Composite Sensing Film Prepared with Cyclic Voltammetry. Mater. Chem. Phys. 2018, 219, 1–12. [Google Scholar] [CrossRef]
- Luo, Y.-C.; Do, J.-S. Amperometric Ammonium Ion Sensor Based on Polyaniline-Poly(Styrene Sulfonate-Co-Maleic Acid) Composite Conducting Polymeric Electrode. Sens. Actuators B Chem. 2006, 115, 102–108. [Google Scholar] [CrossRef]
- Kanungo, M.; Kumar, A.; Contractor, A.Q. Microtubule Sensors and Sensor Array Based on Polyaniline Synthesized in the Presence of Poly(Styrene Sulfonate). Anal. Chem. 2003, 75, 5673–5679. [Google Scholar] [CrossRef]
- Kumar, V.; Mahajan, R.; Kaur, I.; Kim, K.-H. Simple and Mediator-Free Urea Sensing Based on Engineered Nanodiamonds with Polyaniline Nanofibers Synthesized in Situ. ACS Appl. Mater. Interfaces 2017, 9, 16813–16823. [Google Scholar] [CrossRef]
- Singh, J.; Singh, K.R.; Kumar, M.; Verma, R.; Verma, R.; Malik, P.; Srivastava, S.; Singh, R.P.; Kumar, D. Melt-Quenched Vanadium Pentoxide-Stabilized Chitosan Nanohybrids for Efficient Hydrazine Detection. Mater. Adv. 2021, 2, 6665–6675. [Google Scholar] [CrossRef]
- Kumar, P.; Jaiwal, R.; Pundir, C.S. An Improved Amperometric Creatinine Biosensor Based on Nanoparticles of Creatininase, Creatinase and Sarcosine Oxidase. Anal. Biochem. 2017, 537, 41–49. [Google Scholar] [CrossRef]




| Electrode Materials | LOD (μM) | Sensitivity (mA M−1 cm−2) | Linear Range (mM) | Reference |
|---|---|---|---|---|
| SPE/PANI-Nafion/Cu2O/Urease | 0.5 | 250 ± 10 | 0.001~0.15 | [36] |
| Pt-C/PANI | 5 | 40 ± 20 | 0.005~1 | [42] |
| PANI-Nafion/Pt | 5.35 | 15.9 ± 0.12 | - | [43] |
| GCE/Nano-PANI: PSS/CPM-Urease | 26.9 | 106 ± 1.8 | 0.1~11.7 | [25] |
| CD/Nafion®-ns PANI/Au/Al2O3 | - | 1647 a 376 b | 0.005~1 a 0.1~0.4 b | [44] |
| PANI-PSSMA/Au/Al2O3 | - | 0.57 | 1~10 | [45] |
| Nafion®(urease)/PANI-Nafion® | 83 | 155 ± 6 | 0.083~1.68 | [35] |
| AuNP/PANI: PSS | 290.1 | 192.6 | 0.5~20 | This work |
| Electrode Materials | LOD (μM) | Sensitivity (mA M−1 cm2) | Linear Range (mM) | Reference |
|---|---|---|---|---|
| SPCE/PANI-Nafion-Cu/Urease | 0.5 | 112 ± 3.36 | 0.001~0.1 | [37] |
| Nafion®(urease)/PANI-Nafion® | 1 × 104 | 4.2 | - | [35] |
| GCE/Nano/PANI: PSS/CPM-Urease | 51.8 | 41 ± 5 | 0.2~0.9 | [25] |
| CNT-SPE/PANI-GND/urease | 832.5 | 22.9 | - | [47] |
| AuNP/PANI: PSS/Urease/FAA | 500 | 106.8 | 0.5~15 | This work |
| Electrode Materials | LOD (μM) | Sensitivity (mA M−1 cm−2) | Linear Range (mM) | Reference |
|---|---|---|---|---|
| SPCE/PANI-Nafion-Cu/CD | 0.5 | 85 ± 3.4 | 0.001~0.1 | [37] |
| CANPs/CINPs/SOxNPs/GC | 0.01 | - | 0.00001~0.012 | [49] |
| CD/Nafion®-nsPANi/Au/Al2O3 | 5 | 1298.5 | 0.005~0.4 | [44] |
| AuNP/PANI: PSS/CD/FAA | 500 | 139.53 | 0.5~4 | This work |
| AuNP/PANI: PSS/CD/Chitosan | 562.5 | 62.34 | 2~16 | This work |
| Sample Number | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| Test Object | NH4+ (mM) | Urea (mM) | Creatinine (mM) | NH4+ (mM) | Urea (mM) | Creatinine (mM) |
| Reference value | 26.3 | 204.7 | 8.6 | 31.9 | 236.7 | 11.7 |
| Test value | 29.7 | 226.8 | 9.1 | 35.8 | 256.2 | 12.4 |
| Relative Error | 12.9% | 10.8% | 5.8% | 12.2% | 8.2% | 5.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Mao, X.; Liang, Y.; Cai, Y.; Tu, T.; Zhang, S.; Li, T.; Fang, L.; Zhou, Y.; Wang, Z.; et al. Multi-Parameter Detection of Urine Based on Electropolymerized PANI: PSS/AuNPs/SPCE. Biosensors 2023, 13, 272. https://doi.org/10.3390/bios13020272
Wang D, Mao X, Liang Y, Cai Y, Tu T, Zhang S, Li T, Fang L, Zhou Y, Wang Z, et al. Multi-Parameter Detection of Urine Based on Electropolymerized PANI: PSS/AuNPs/SPCE. Biosensors. 2023; 13(2):272. https://doi.org/10.3390/bios13020272
Chicago/Turabian StyleWang, Dong, Xiyu Mao, Yitao Liang, Yu Cai, Tingting Tu, Shanshan Zhang, Tianyu Li, Lu Fang, Yue Zhou, Zhaoyang Wang, and et al. 2023. "Multi-Parameter Detection of Urine Based on Electropolymerized PANI: PSS/AuNPs/SPCE" Biosensors 13, no. 2: 272. https://doi.org/10.3390/bios13020272
APA StyleWang, D., Mao, X., Liang, Y., Cai, Y., Tu, T., Zhang, S., Li, T., Fang, L., Zhou, Y., Wang, Z., Jiang, Y., Ye, X., & Liang, B. (2023). Multi-Parameter Detection of Urine Based on Electropolymerized PANI: PSS/AuNPs/SPCE. Biosensors, 13(2), 272. https://doi.org/10.3390/bios13020272







