Ameliorative and Antioxidative Potential of Lactobacillus plantarum-Fermented Oat (Avena sativa) and Fermented Oat Supplemented with Sidr Honey against Streptozotocin-Induced Type 2 Diabetes in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ingredients, Chemicals, and Strain
2.2. Preparation of Fermented Oat (FOE) and Honey-Supplemented Fermented Oat (HFOE) Extracts
2.3. Enumeration of L. plantarum B-59151 Viable Count
2.4. Determination of Total Phenolic Content (TPC) in FOE and HFOE
2.5. Determination of Total Antioxidant Capacity (TAC) of FOE and HFOE
2.6. Determination of the γ-Aminobutyric Acid (GABA) Content
2.7. Determination of the β-Glucan Content
2.8. Animals and Experimental Design
2.8.1. Determination of Fasting Blood Glucose Level (FBG), Lipid Profile, and Liver and Kidney Functions
2.8.2. Oxidative Stress Biomarkers
2.9. Statistical Analysis
3. Results
3.1. Survival of Lactobacillus plantarum and Related pH Value in FOE and HFOE
3.2. Phytochemicals and Antioxidant Capacity of FOE and HFOE
3.3. Gamma-Aminobutyric Acid and β-Glucan Contents during Fermentation
3.4. The Hypoglycemic Efficiency of FOE and HFOE
3.5. The Hypolipidemic Efficiency of FOE and HFOE
3.6. The Liver Functions
3.7. The Kidney Functions
3.8. Antioxidant Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wehrli, F.; Taneri, P.E.; Bano, A.; Bally, L.; Blekkenhorst, L.C.; Bussler, W.; Metzger, B.; Minder, B.; Glisic, M.; Muka, T.; et al. Oat Intake and Risk of Type 2 Diabetes, Cardiovascular Disease and All-Cause Mortality: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2560. [Google Scholar] [CrossRef]
- Sangwan, S.; Singh, R.; Tomar, S.K. Nutritional and Functional Properties of Oats: An Update. J. Innov. Biol. 2014, 1, 3–14. [Google Scholar]
- Ahmad, M.; Dar, Z.A.; Habib, M. A Review on Oat (Avena sativa L.) as A Dual-Purpose Crop. Sci. Res. Essays 2014, 9, 52–59. [Google Scholar]
- Zhu, Y.; Dong, L.; Huang, L.; Shi, Z.; Dong, J.; Yao, Y.; Shen, R. Effects of Oat β-glucan, Oat Resistant Starch, and the Whole Oat Flour on Insulin Resistance, Inflammation, and Gut Microbiota in High-Fat-Diet-Induced Type 2 Diabetic Rats. J. Funct. Foods 2020, 69, 103939. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, X.; Dong, Y.; Shi, L.; Xu, T.; Wu, F. The Anti-obesity Effect of Fermented Barley Extracts with Lactobacillus plantarum dy-1 and Saccharomyces cerevisiae in Diet-Induced Obese Rats. Food Funct. 2017, 8, 1132–1143. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Prasanth, M.I.; Chaiyasut, C. A Mini Review on Antidiabetic Properties of Fermented Foods. Nutrients 2018, 10, 1973. [Google Scholar] [CrossRef] [Green Version]
- Boz, H. Phenolic Amides (Avenanthramides) in Oats—A review. Czech J. Food Sci. 2015, 33, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Sang, S.; Chu, Y. Whole Grain Oats, More than Just A Fiber: Role of Unique Phytochemicals. Mol. Nutr. Food Res. 2017, 61, 1600715. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and Germination Improve Nutritional Value of Cereals and Legumes Through Activation of Endogenous Enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health Benefits of Fermented Foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, D.; Schlundt, J.; Conway, P.L. Development of a Dairy-Free Fermented Oat-Based Beverage With Enhanced Probiotic and Bioactive Properties. Front. Microbiol. 2020, 11, 609734. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Xue, J.; Yang, J.; Chen, X.; Shao, Y.; Kwok, L.-Y.; Bilige, M.; Mang, L.; Zhang, H. Angiotensin-Converting Enzyme Inhibitory Activity of Lactobacillus helveticus Strains from Traditional Fermented Dairy Foods and Antihypertensive Effect of Fermented Milk of Strain H9. J. Dairy Sci. 2014, 97, 6680–6692. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Naramoto, K.; Koyama, M. Blood-Pressure-Lowering Effect of Fermented Buckwheat Sprouts in Spontaneously Hypertensive Rats. J. Funct. Foods 2013, 5, 406–415. [Google Scholar] [CrossRef]
- Pouliot-Mathieu, K.; Gardner-Fortier, C.; Lemieux, S.; St-Gelais, D.; Champagne, C.P.; Vuillemard, J.-C. Effect of Cheese Containing Gamma-aminobutyric Acid-producing Lactic Acid Bacteria on Blood Pressure in Men. PharmaNutrition 2013, 1, 141–148. [Google Scholar] [CrossRef]
- Yoshinaga, M.; Toda, N.; Tamura, Y.; Terakado, S.; Ueno, M.; Otsuka, K.; Numabe, A.; Kawabata, Y.; Uehara, Y. Japanese Traditional Miso Soup Attenuates Salt-Induced Hypertension and its Organ Damage in Dahl Salt-Sensitive Rats. Nutrition 2012, 28, 924–931. [Google Scholar] [CrossRef]
- Guan, Q.; Ding, X.-W.; Zhong, L.-Y.; Zhu, C.; Nie, P.; Song, L.-H. Beneficial Efects of Lactobacillus-fermented Black Barley on High Fat Diet-Induced Fatty Liver in Rats. Food Funct. 2021, 12, 6526–6539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, X.; Dong, Y.; Zhou, X. Fermented Barley Extracts with Lactobacillus plantarum dy-1 Changes Serum Metabolomic Profiles in Rats with High-Fat Diet-Induced Obesity. Int. J. Food Sci. Nutr. 2019, 70, 303–310. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, B.K.; Lee, I.O.; Park, N.H.; Kim, S.H. Prevention of Done Loss by Using Lactobacillus-Fermented Milk Products in A Rat Model of Glucocorticoid-Induced Secondary Osteoporosis. Int. Dairy J. 2020, 109, 104788. [Google Scholar] [CrossRef]
- Robert, A.A.; Al Dawish, A.M. The Worrying Trend of Diabetes Mellitus in Saudi Arabia: An Urgent Call to Action. Curr. Diabetes Rev. 2020, 16, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Vries, M.; Vaughan, E.; Kleerebezem, M.; De Vos, W. Lactobacillus plantarum-Survival, Functional and Potential Probiotic Properties in the Human Intestinal Tract. Int. Dairy J. 2006, 16, 1018–1028. [Google Scholar] [CrossRef]
- Rathore, S.; Salmerón, I.; Pandiella, S.S. Production of Potentially Probiotic Beverages Using Single and Mixed Cereal Substrates Fermented with Lactic acid Bacteria Cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef]
- Hole, A.S.; Rud, I.; Grimmer, S.; Sigl, S.; Narvhus, J.; Sahlstrøm, S. Improved Bioavailability of Dietary Phenolic Acids in Whole Grain Barley and Oat Groat following Fermentation with Probiotic Lactobacillus acidophilus, Lactobacillus johnsonii, and Lactobacillus reuteri. J. Agric. Food Chem. 2012, 60, 6369–6375. [Google Scholar] [CrossRef]
- Dordević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of Fermentation on Antioxidant Properties of some Cereals and Pseudo Cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021; Volume 102, pp. 147–148. [Google Scholar]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.-F. Global, Regional, and National Durden and Trend of Diabetes in 195 Countries and Territories: An Analysis from 1990 to 2025. Sci. Rep. 2020, 10, 1–11. [Google Scholar]
- CDC. United States Department of Health and Human Services. 2019. Available online: https://www.cdc.gov/ (accessed on 16 May 2022).
- Aldossari, K.K.; Aldiab, A.; Al-Zahrani, J.M.; Al-Ghamdi, S.H.; Abdelrazik, M.; Batais, M.A.; Javad, S.; Nooruddin, S.; Razzak, H.A.; El-Metwally, A. Prevalence of Prediabetes, Diabetes, and Its Associated Risk Factors among Males in Saudi Arabia: A Population-Based Survey. J. Diabetes Res. 2018, 2018, 2194604. [Google Scholar] [CrossRef]
- Vinderola, C.G.; Reinheimer, J.A. Culture media for the Enumeration of Bifidobacterium bifidum and Lactobacillus acidophilus in the Presence of Yoghurt Bacteria. Int. Dairy J. 1999, 9, 497–505. [Google Scholar] [CrossRef]
- Yawadio Nsimba, R.; Kikuzaki, H.; Konishi, Y. Antioxidant activity of Various Extracts and Fractions of Chenopodium quinoa and Amaranthus spp. Seeds. Food Chem. 2008, 106, 760–766. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, H.; Chen, J.; Fan, W.; Dong, J.; Kong, W.; Sun, J.; Cao, Y.; Cai, G. Evolution of Phenolic Compounds and Antioxidant Activity During Malting. J. Agric. Food Chem. 2007, 55, 10994–11001. [Google Scholar] [CrossRef]
- Yeap, S.K.; Mohd Ali, N.; Mohd Yusof, H.; Alitheen, N.B.; Beh, B.K.; Ho, W.Y.; Koh, S.P.; Long, K. Antihyperglycemic Effects of Fermented and Nonfermented Mung Bean Extracts on Alloxan-Induced-Diabetic Mice. J. Biotechnol. Biomed. 2012, 2012, 285430. [Google Scholar] [CrossRef] [Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition ad hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Jia, Y.; Lao, Y.; Zhu, H.; Li, N.; Leung, S.W. Is Metformin Still the Most Efficacious First-Line Oral Hypoglycaemic Drug in Treating Type 2 Diabetes? A Network Meta-Analysis of Randomized Controlled Trials. Obes. Rev. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-density Lipoprotein Cholesterol in Plasma, without use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Nwagha, U.; Ikekpeazu, E.; Ejezie, F.; Neboh, E.; Maduka, I. Atherogenic Index of Plasma as Useful Predictor of Cardiovascular Risk Among Postmenopausal Women in Enugu, Nigeria. Afr. Health Sci. 2010, 10, 248–252. [Google Scholar] [PubMed]
- Beutler, E. Improved Method for the Determination of Blood Glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Steel, R.G. Pinciples and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw-Hill: Boston, MA, USA, 1997. [Google Scholar]
- Mubarak, A.; Hodgson, J.M.; Considine, M.J.; Croft, K.D.; Matthews, V.B. Supplementation of a High-Fat Diet with Chlorogenic Acid Is Associated with Insulin Resistance and Hepatic Lipid Accumulation in Mice. J. Agric. Food Chem. 2013, 61, 4371–4378. [Google Scholar] [CrossRef] [PubMed]
- Balli, D.; Bellumori, M.; Pucci, L.; Gabriele, M.; Longo, V.; Paoli, P.; Melani, F.; Mulinacci, N.; Innocenti, M. Does Fermentation Really Increase the Phenolic Content in Cereals? A Study on Millet. Foods 2020, 9, 303. [Google Scholar] [CrossRef] [Green Version]
- Al-Qabba, M.M.; El-Mowafy, M.A.; Althwab, S.A.; Alfheeaid, H.A.; Aljutaily, T.; Barakat, H. Phenolic Profile, Antioxidant Activity, and Ameliorating Efficacy of Chenopodium quinoa Sprouts against CCl4-Induced Oxidative Stress in Rats. Nutrients 2020, 12, 2904. [Google Scholar] [CrossRef]
- Hassan, B.; Tariq, I.A. Phenolic Compounds and Hepatoprotective Potential of Anastatica hierochuntica Ethanolic and Aqueous Extracts Against CCl4-induced Hepatotoxicity in Rats. J. Tradit. Chin. Med. 2020, 40, 947–955. [Google Scholar] [CrossRef]
- Almundarij, T.I.; Alharbi, Y.M.; Abdel-Rahman, H.A.; Barakat, H. Antioxidant Activity, Phenolic Profile, and Nephroprotective Potential of Anastatica hierochuntica Ethanolic and Aqueous Extracts against CCl4-Induced Nephrotoxicity in Rats. Nutrients 2021, 13, 2973. [Google Scholar] [CrossRef]
- Alharbi, Y.M.; Sakr, S.S.; Albarrak, S.M.; Almundarij, T.I.; Barakat, H.; Hassan, M.F.Y. Antioxidative, Antidiabetic, and Hypolipidemic Properties of Probiotic-Enriched Fermented Camel Milk Combined with Salvia officinalis Leaves Hydroalcoholic Extract in Streptozotocin-Induced Diabetes in Rats. Antioxidants 2022, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, R.; Bajpai, V.K.; Baek, K.-H. Production of GABA (γ-aminobutyric acid) by Microorganisms: A Review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef] [Green Version]
- Mohd Ali, N.; Mohd Yusof, H.; Long, K.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Koh, S.P.; Abdullah, M.P.; Alitheen, N.B. Antioxidant and Hepatoprotective Effect of Aqueous Extract of Germinated and Fermented Mung Bean on Ethanol-Mediated Liver Damage. Biomed. Res. Int. 2013, 2013, 693613. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T. GABA Exerts Protective and Regenerative Effects on Islet Beta Cells and Reverses Diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef] [Green Version]
- Coda, R.; Rizzello, C.G.; Gobbetti, M. Use of Sourdough Fermentation and Pseudo-Cereals and Leguminous Flours for the Making of A Functional Bread Enriched of γ-aminobutyric Acid (GABA). Int. J. Food Microbiol. 2010, 137, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Gao, F.; Zhang, X.; Wang, O.; Wu, W.; Zhu, S.; Zhang, D.; Zhou, F.; Ji, B. Evaluation of γ-Aminobutyric Acid, Phytate and Antioxidant Activity of Tempeh-Like Fermented Oats (Avena sativa L.) Prepared with Different Filamentous Fungi. J. Food Sci. Technol. 2014, 51, 2544–2551. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.; Antunes, M.D.; Faleiro, M.L. Honey As A Complementary Medicine. Integr. Med. Insights 2017, 12, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, E.F.; Lobato, R.V.; de Araújo, T.V.; Zangerônimo, M.G.; de Sousa, R.V.; Pereira, L.J. Effect of Beta-Glucans in the Control of Blood Glucose Levels of Diabetic Patients: A Systematic Review. Nutr. Hosp. 2015, 31, 170–177. [Google Scholar]
- Bozbulut, R.; Sanlier, N. Promising Effects of β-glucans on Glyceamic Control in Diabetes. Trends Food Sci. Technol. 2019, 83, 159–166. [Google Scholar] [CrossRef]
- Chen, J.; Raymond, K. Beta-Glucans in the Treatment of Diabetes and Associated Cardiovascular Risks. Vasc. Health Risk Manag. 2008, 4, 1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.; Du, B.; Xu, B. A Critical Review on Production and Industrial Applications of Beta-Glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial Roles of Honey Polyphenols Against some Human Degenerative Diseases: A Review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P. Phenolic Compounds in Honey and their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [Green Version]
- Al-Waili, N.S.; Salom, K.; Butler, G.; Al Ghamdi, A.A. Honey and Microbial Infections: A Review Supporting the Use of Honey for Microbial Control. J. Med. Food 2011, 14, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Datta, S.; Mukherjee, S.; Bose, S.; Ghosh, S.; Dhar, P. Evaluation of Antioxidative, Antibacterial and Probiotic Growth Stimulatory Activities of Sesamum indicum Honey Containing Phenolic Compounds and Lignans. LWT Food Sci. Technol. 2015, 61, 244–250. [Google Scholar] [CrossRef]
- Hou, Q.; Li, Y.; Li, L.; Cheng, G.; Sun, X.; Li, S.; Tian, H. The Metabolic Effects of Oats Intake in Patients with Type 2 Diabetes: A Systematic Review and Meta-analysis. Nutrients 2015, 7, 10369–10387. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.-C.; Huang, C.-N.; Yeh, D.-M.; Wang, S.-J.; Peng, C.-H.; Wang, C.-J. Oat Prevents Obesity and Abdominal Fat Distribution, and Improves Liver Function in Humans. Plant Foods Hum. Nutr. 2013, 68, 18–23. [Google Scholar] [CrossRef]
- Li, C.; Nie, S.-P.; Zhu, K.-X.; Ding, Q.; Li, C.; Xiong, T.; Xie, M.-Y. Lactobacillus plantarum NCU116 Improves Liver Function, Oxidative Stress and Lipid Metabolism in Rats with High Fat Diet Induced Non-Alcoholic Fatty Liver Disease. Food Funct. 2014, 5, 3216–3223. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, Z.; Duan, C.; Wang, C.; Zhao, Y.; Yang, G.; Gao, L.; Niu, C.; Xu, J.; Li, S. Lactobacillus plantarum C88 Protects Against Aflatoxin B(1)-induced Liver Injury in Mice via Inhibition of NF-κB-Mediated Inflammatory Responses and Excessive Apoptosis. BMC Microbiol. 2019, 19, 170. [Google Scholar] [CrossRef] [Green Version]
- Al-Waili, N.; Al-Waili, H.; Al-Waili, T.; Salom, K. Natural Antioxidants in the Treatment and Prevention of Diabetic Nephropathy; A Potential Approach that Warrants Clinical Trials. Redox Rep. 2017, 22, 99–118. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Abensur, H.; Betônico, C.C.R.; Machado, A.D.; Parente, E.B.; Queiroz, M.; Salles, J.E.N.; Titan, S.; Vencio, S. Interactions Between Kidney Disease and Diabetes: Dangerous Liaisons. Diabetol. Metab. Syndr. 2016, 8, 50. [Google Scholar] [CrossRef]
- Liu, S.; Yin, X.; Hou, C.; Liu, X.; Ma, H.; Zhang, X.; Xu, M.; Xie, Y.; Li, Y.; Wang, J. As a Staple Food Substitute, Oat and Buckwheat Compound Has Health-Promoting Effects for Diabetic Rats. Front. Nutr. 2021, 8, 762277. [Google Scholar] [CrossRef]
- Bokhary, K.; Aljaser, F.; Abudawood, M.; Tabassum, H.; Bakhsh, A.; Alhammad, S.; Aleyadhi, R.; Almajed, F.; Alsubki, R. Role of Oxidative Stress and Severity of Diabetic Retinopathy in Type 1 and Type 2 Diabetes. Ophthalmic Res. 2021, 64, 613–621. [Google Scholar] [CrossRef]
- Das, R.R.; Rahman, M.A.; Al-Araby, S.Q.; Islam, M.S.; Rashid, M.M.; Babteen, N.A.; Alnajeebi, A.M.; Alharbi, H.F.H.; Jeandet, P.; Rafi, M.K.J.; et al. The Antioxidative Role of Natural Compounds from a Green Coconut Mesocarp Undeniably Contributes to Control Diabetic Complications as Evidenced by the Associated Genes and Biochemical Indexes. Oxidative Med. Cell. Longev. 2021, 2021, 060803. [Google Scholar] [CrossRef]
- Al-Malki, A.L. Oat Protects Against Diabetic Nephropathy in Rats via Attenuating Advanced Glycation End Products and Nuclear Factor Kappa B. Evid. Based Complement. Altern. Med. 2013, 2013, 609745. [Google Scholar] [CrossRef] [Green Version]

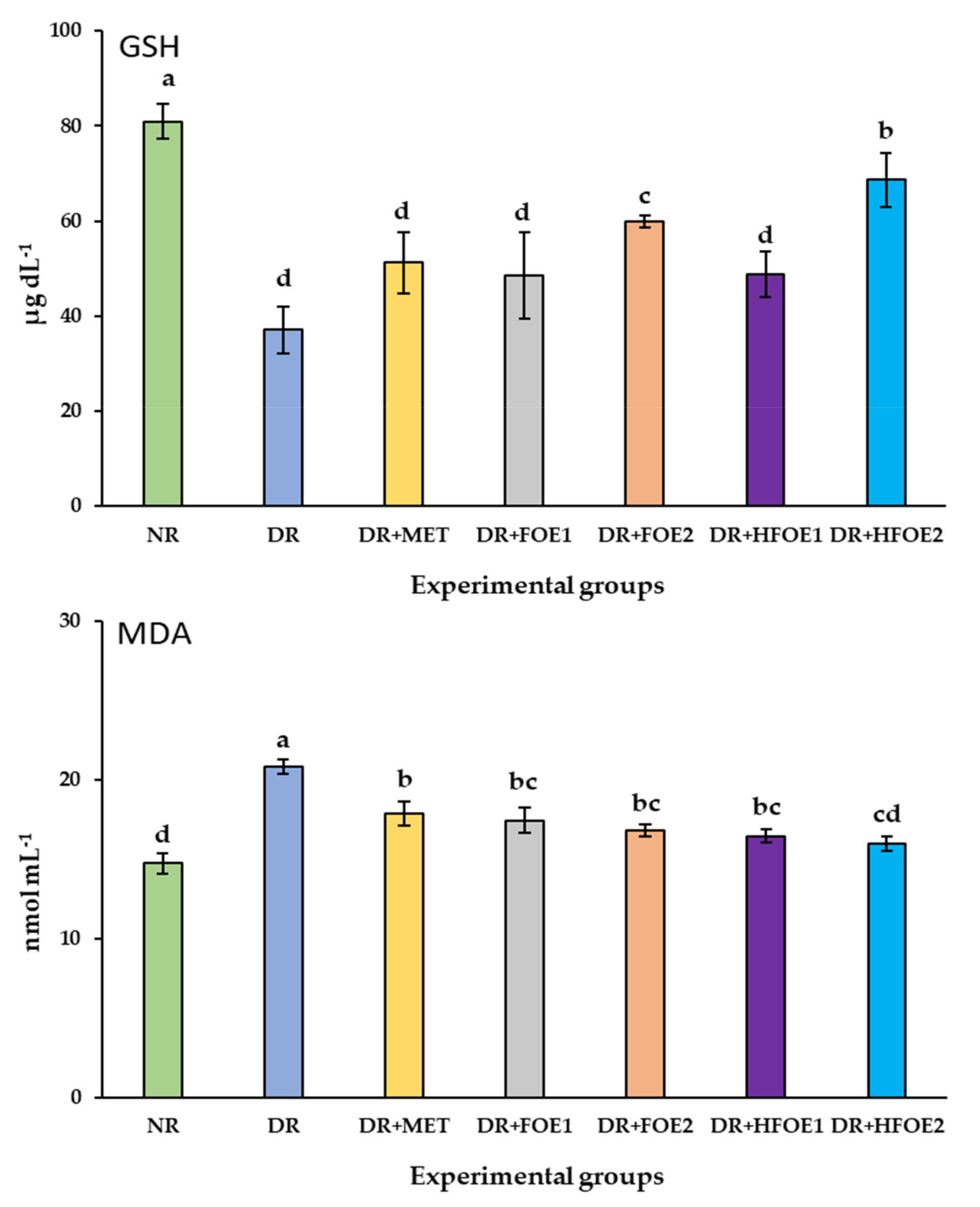
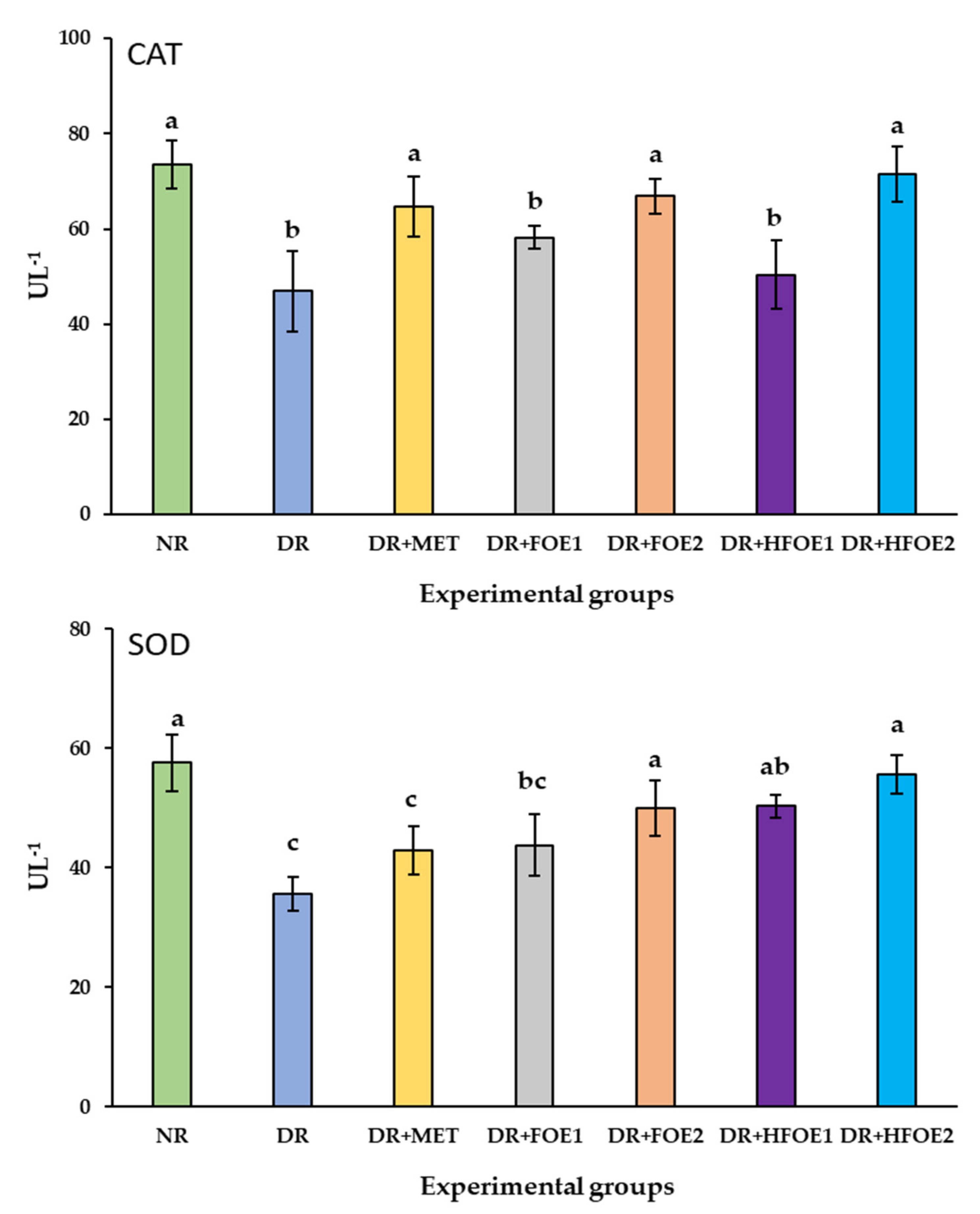
| Group | Experimental Treatment |
|---|---|
| NR | Normal rats |
| DR | Untreated diabetic rats + distilled water (7 mL) |
| DR + MET | Diabetic rats + Metformin (50 mg kg−1) |
| DR + FOE1 | Diabetic rats + FOE (3.5 mL) |
| DR + FOE2 | Diabetic rats + FOE (7 mL) |
| DR + HFOE1 | Diabetic rats + HFOE (3.5 mL) |
| DR + HFOE2 | Diabetic rats + HFOE (7 mL) |
| Fermentation Time | Item | |||
|---|---|---|---|---|
| TPC (mg GAE g−1) | DPPH (µmol of TE g−1) | ABTS (µmol of TE g−1) | ||
| FOE | 0 h | 0.70 ± 0.08 b | 2.14 ± 0.09 b | 3.47 ± 0.29 c |
| 24 h | 0.74 ± 0.07 b | 2.22 ± 0.11 b | 3.89 ± 0.13 c | |
| 48 h | 0.94 ± 0.15 ab | 3.19 ± 0.12 ab | 4.58 ± 0.31 b | |
| 72 h | 1.04 ± 0.08 a | 3.56 ± 0.21 a | 5.27 ± 0.14 a | |
| HFOE | 0 h | 1.41 ± 0.13 b | 5.12 ± 0.15 c | 7.68 ± 0.31 d |
| 24 h | 1.48 ± 0.12 ab | 6.01 ± 0.22 b | 9.14 ± 0.47 c | |
| 48 h | 1.52 ± 0.06 a | 6.47 ± 0.41 b | 9.71 ± 0.27 b | |
| 72 h | 1.64 ± 0.14 a | 7.15 ± 0.24 a | 11.73 ± 0.16 a | |
| Fermentation Time | Item | ||
|---|---|---|---|
| GABA * (mg 100 g−1) | β-Glucan (g 100 g−1) | ||
| FOE | 0 h | 4.12 ± 0.14 b | 2.62 ± 0.02 a |
| 24 h | 4.77 ± 0.17 b | 2.60 ± 0.01 a | |
| 48 h | 6.10 ± 0.52 ab | 2.56 ± 0.03 a | |
| 72 h | 7.35 ± 0.40 a | 2.45 ± 0.06 b | |
| HFOE | 0 h | 4.42 ± 1.02 c | 2.72 ± 0.14 a |
| 24 h | 4.78 ± 0.49 c | 2.69 ± 0.04 a | |
| 48 h | 6.54 ± 0.21 b | 2.67 ± 0.01 a | |
| 72 h | 8.49 ± 1.13 a | 2.63 ± 0.03 a | |
| Groups * | RBG | FBG | ||
|---|---|---|---|---|
| Week 0 | Week 3 | Week 6 | ||
| NR | 113.83 ± 3.82 dA | 114.33 ± 3.06 dA | 110.67 ± 2.55 dA | 87.16 ± 4.44 b |
| DR | 254.17 ± 33.65 aA | 286.33 ± 36.6 aA | 299.50 ± 29.11 aA | 156.27 ± 13.34 a |
| DR + MET | 284.33 ± 24.90 abA | 238.67 ± 40.01 bcB | 201.50 ± 28.49 bcC | 89.39 ± 3.25 b |
| DR + FOE1 | 252.5 ± 36.93 bcA | 237.5 ± 20.92 bcA | 218.67 ± 4.66 bcB | 102.77 ± 10.17 b |
| DR + FOE2 | 308.5 ± 22.13 abA | 244.83 ± 28.82 abB | 232.50 ± 15.24 bB | 82.03 ± 7.55 b |
| DR + HFOE1 | 276.67 ± 37.38 bcA | 254.33 ± 29.58 abB | 241.50 ± 25.39 bB | 94.04 ± 10.36 b |
| DR + HFOE2 | 320.83 ± 39.36 aA | 225.17 ± 18.35 bcB | 211.50 ± 32.66 bcC | 86.48 ± 11.13 b |
| Groups * | Lipid Profile Parameters | |||||
|---|---|---|---|---|---|---|
| TG | CHO | HDL-CHO | LDL-CHO | VLDL-CHO | AI | |
| NR | 72.88 ± 3.43 c | 89.73 ± 9.99 b | 33.33 ± 2.28 a | 43.49 ± 12.37 cd | 14.58 ± 0.69 c | 0.37 ± 0.09 c |
| DR | 119.07 ± 3.82 a | 138.61 ± 10.34 a | 26.52 ± 2.43 b | 88.28 ± 13.70 a | 23.81 ± 0.77 a | 0.70 ± 0.10 a |
| DR + MET | 101.74 ± 11.16 ab | 107.58 ± 12.63 b | 36.36 ± 4.55 a | 55.41 ± 2.55 bc | 20.35 ± 2.23 a | 0.45 ± 0.08 b |
| DR + FOE1 | 92.29 ± 6.05 b | 111.2 ± 7.10 ab | 28.79 ± 2.20 b | 67.74 ± 6.77 b | 18.46 ± 1.21 b | 0.54 ± 0.7 b |
| DR + FOE2 | 84.33 ± 4.93 bc | 107.84 ± 13.39 b | 38.64 ± 3.08 a | 58.98 ± 7.48 bc | 20.35 ± 2.23 ab | 0.36 ± 0.05 c |
| DR + HFOE1 | 99.25 ± 7.96 b | 104.99 ± 3.44 b | 34.09 ± 4.02 a | 54.50 ± 4.48 bc | 19.85 ± 1.59 ab | 0.47 ± 0.07 b |
| DR + HFOE2 | 87.07 ± 3.73 bc | 99.82 ± 11.96 b | 37.88 ± 1.92 a | 44.53 ± 7.70 cd | 17.81 ± 0.57 bc | 0.35 ± 0.02 c |
| Groups * | Liver Function Biomarkers | |||
|---|---|---|---|---|
| ALT (U L−1) | AST(U L−1) | ALP(U L−1) | T. Bili (mg dL−1) | |
| NR | 44.50 ± 3.46 b | 57.66 ± 5.37 b | 60.88 ± 12.33 c | 0.69 ± 0.2 c |
| DR | 34.50 ± 3.46 c | 87.66 ± 9.26 a | 157.38 ± 7.41 a | 1.06 ± 0.23 a |
| DR + MET | 46.43 ± 2.12 b | 68.84 ± 7.50 ab | 105.11 ± 11.60 b | 0.75 ± 0.10 b |
| DR + FOE1 | 57.19 ± 1.96 a | 68.84 ± 7.50 ab | 97.76 ± 11.37 b | 0.75 ± 0.05 b |
| DR + FOE2 | 49.84 ± 4.75 b | 69.90 ± 2.01 ab | 76.85 ± 9.46 c | 0.66 ± 0.09 c |
| DR + HFOE1 | 54.29 ± 2.78 a | 76.49 ± 5.95 ab | 91.72 ± 13.51 b | 0.73 ± 0.06 b |
| DR + HFOE2 | 47.62 ± 3.05 b | 64.78 ± 8.28 b | 77.08 ± 5.94 c | 0.67 ± 0.08 c |
| Group * | Kidney Function Biomarkers | |||||
|---|---|---|---|---|---|---|
| T. Protein (g dL−1) | Albumin (g dL−1) | Globulin (g dL−1) | Creatinine (mg dL−1) | Urea (mg dL−1) | BUN (mg dL−1) | |
| NR | 8.91 ± 0.19 b | 4.14 ± 0.29 a | 4.77 ± 0.23 a | 0.79 ± 0.04 b | 31.44 ± 4.90 b | 14.78 ± 4.08 b |
| DR | 6.96 ± 0.32 d | 3.58 ± 0.09 b | 3.37 ± 0.32 b | 1.29 ± 0.05 a | 74.63 ± 3.62 a | 35.08 ± 6.26 a |
| DR + MET | 7.57 ± 0.36 cd | 4.08 ± 0.40 a | 3.49 ± 0.61 ab | 0.95 ± 0.09 b | 44.58 ± 5.88 b | 20.95 ± 2.76 b |
| DR + FOE1 | 7.31 ± 0.28 d | 3.66 ± 0.26 ab | 3.66 ± 0.45 ab | 0.91 ± 0.09 b | 44.58 ± 5.88 b | 20.11 ± 1.49 b |
| DR + FOE2 | 7.96 ± 0.48 cd | 3.86 ± 0.38 ab | 4.11 ± 0.56 a | 0.83 ± 0.07 b | 44.43 ± 7.35 b | 20.88 ± 3.45 b |
| DR + HFOE1 | 8.40 ± 0.33 bc | 3.55 ± 0.29 ab | 4.85 ± 0.47 a | 0.97 ± 0.12 b | 46.92 ± 4.29 b | 21.21 ± 5.98 b |
| DR + HFOE2 | 10.25 ± 0.34 a | 4.26 ± 0.22 a | 4.66 ± 0.42 a | 0.82 ± 0.05 b | 38.21 ± 7.46 b | 17.96 ± 3.50 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, H.F.; Algonaiman, R.; Barakat, H. Ameliorative and Antioxidative Potential of Lactobacillus plantarum-Fermented Oat (Avena sativa) and Fermented Oat Supplemented with Sidr Honey against Streptozotocin-Induced Type 2 Diabetes in Rats. Antioxidants 2022, 11, 1122. https://doi.org/10.3390/antiox11061122
Alharbi HF, Algonaiman R, Barakat H. Ameliorative and Antioxidative Potential of Lactobacillus plantarum-Fermented Oat (Avena sativa) and Fermented Oat Supplemented with Sidr Honey against Streptozotocin-Induced Type 2 Diabetes in Rats. Antioxidants. 2022; 11(6):1122. https://doi.org/10.3390/antiox11061122
Chicago/Turabian StyleAlharbi, Hend F., Raya Algonaiman, and Hassan Barakat. 2022. "Ameliorative and Antioxidative Potential of Lactobacillus plantarum-Fermented Oat (Avena sativa) and Fermented Oat Supplemented with Sidr Honey against Streptozotocin-Induced Type 2 Diabetes in Rats" Antioxidants 11, no. 6: 1122. https://doi.org/10.3390/antiox11061122
APA StyleAlharbi, H. F., Algonaiman, R., & Barakat, H. (2022). Ameliorative and Antioxidative Potential of Lactobacillus plantarum-Fermented Oat (Avena sativa) and Fermented Oat Supplemented with Sidr Honey against Streptozotocin-Induced Type 2 Diabetes in Rats. Antioxidants, 11(6), 1122. https://doi.org/10.3390/antiox11061122







