Interactive Effects of Soil Water, Nutrients and Clonal Fragmentation on Root Growth of Xerophilic Plant Stipa breviflora
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Measurements
2.3. Statistical Analyses
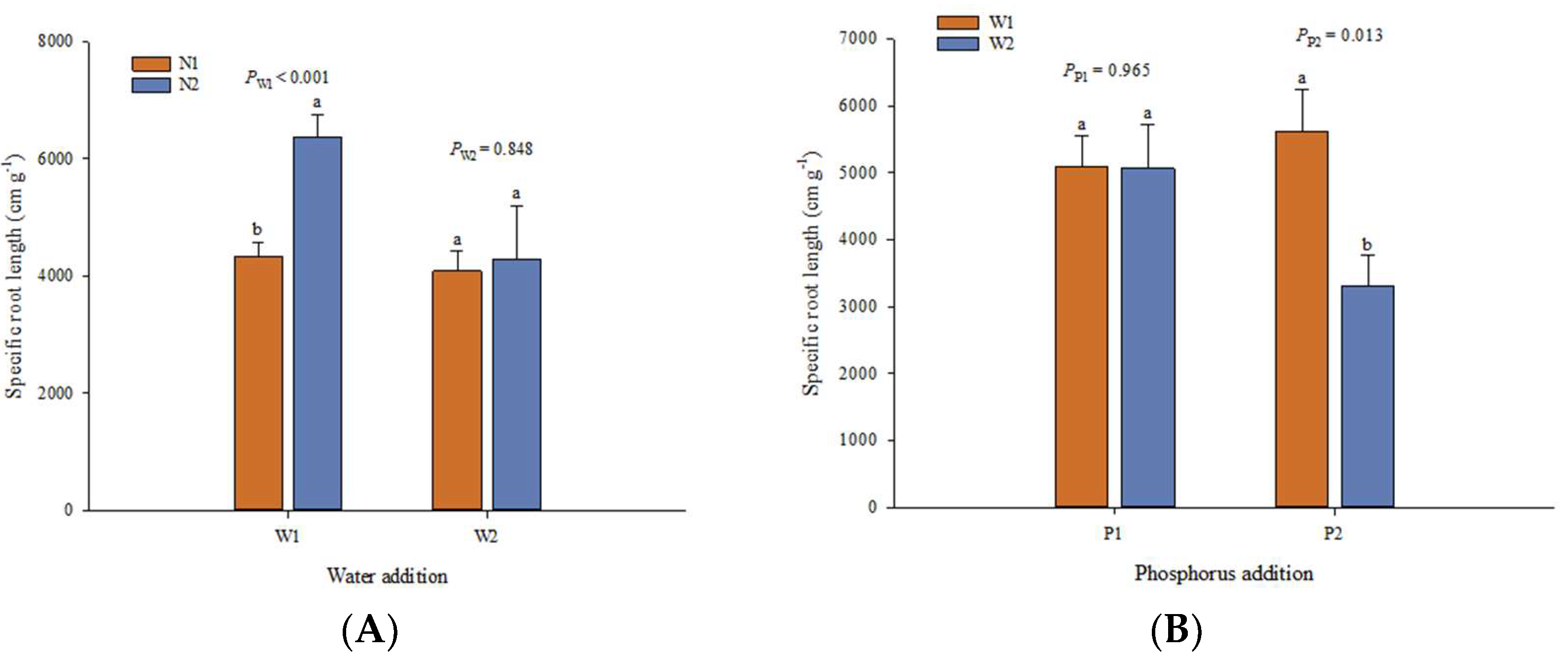
3. Results
3.1. Effects of Experimental Factors on Root Traits
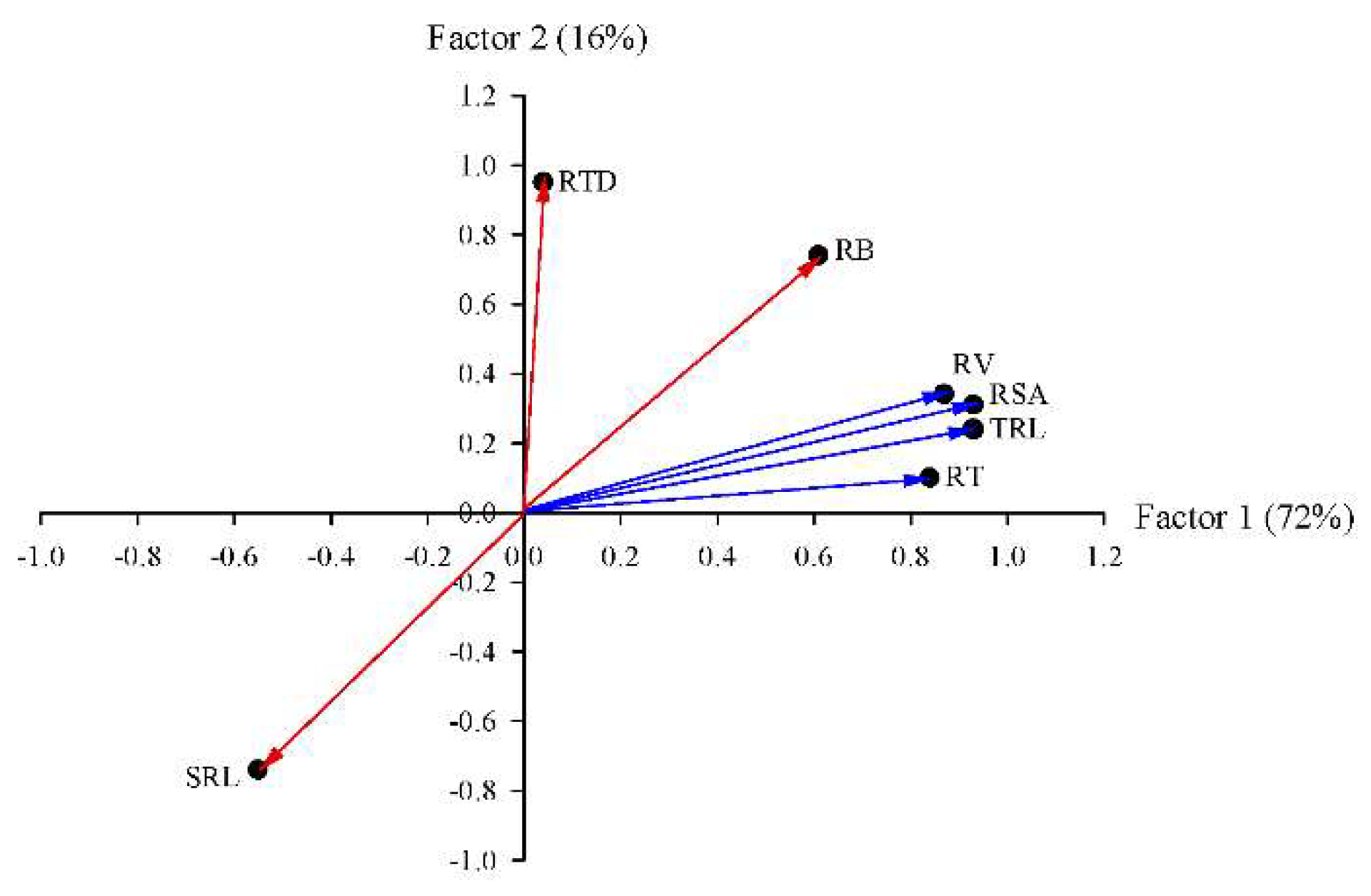
3.2. Relationships among Root Traits
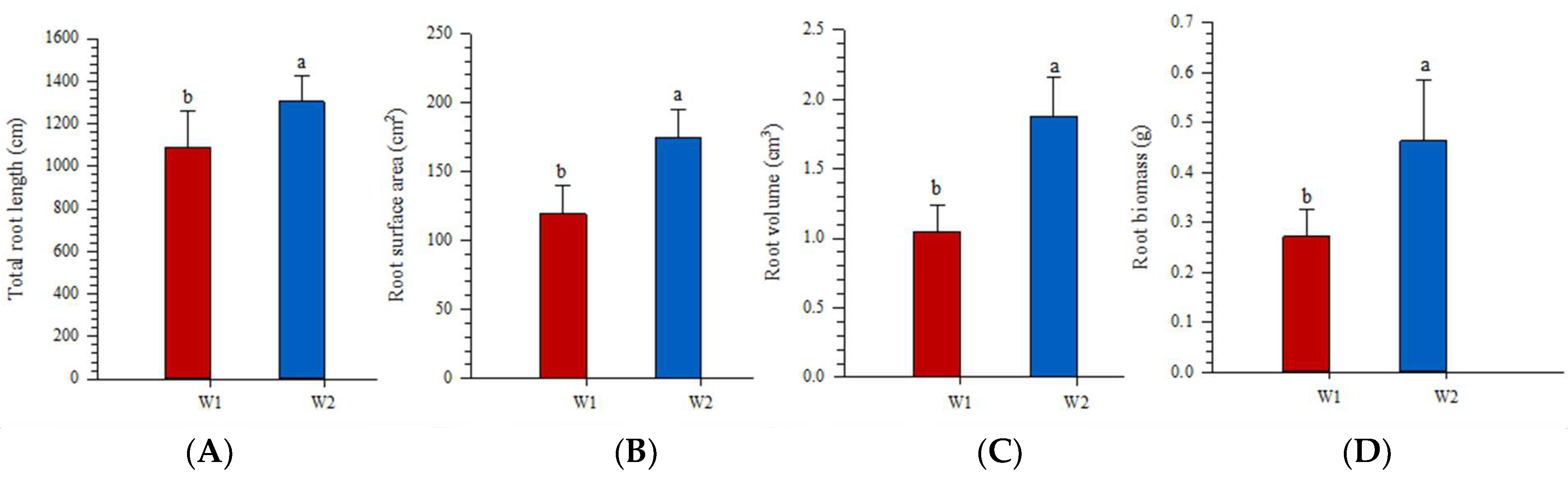
3.3. Root Morphological Traits and Root Biomass Respond to Water Addition
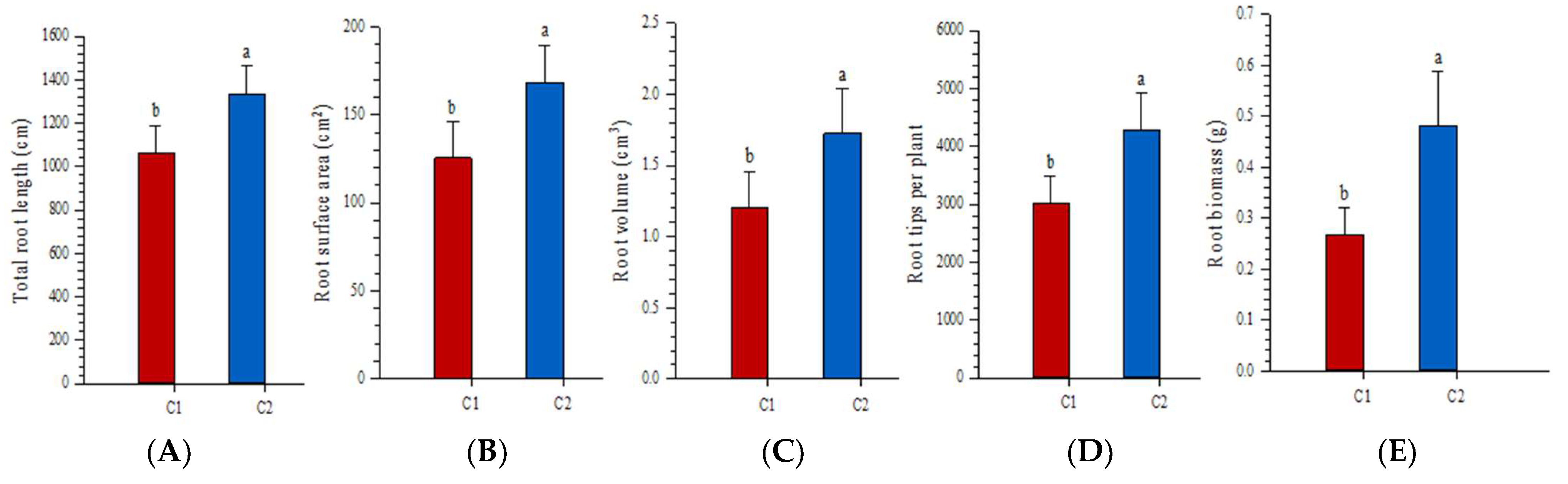
3.4. Effects of Fragment Size on Root Growth
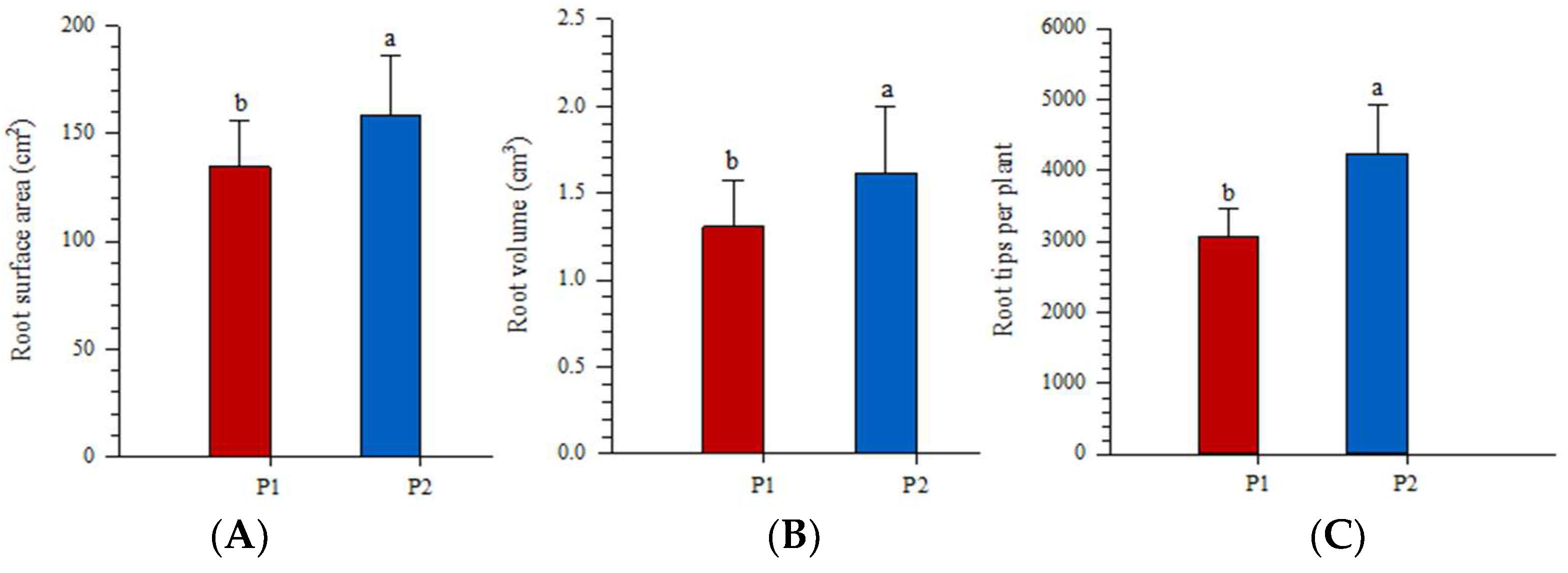
3.5. Root Morphological Traits and Root Biomass Respond to Nutrient Addition
3.6. Root Morphological Traits and Root Biomass Respond to the Interaction between Water and Nutrients
4. Discussion
4.1. Effects of Drought on Root Growth
4.2. Effects of Nutrient Additions on Root Growth
4.3. Effects of Interactions of Water and Nutrients on Root Growth
4.4. Effects of Fragment Size on Root Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kang, L.; Han, X.; Zhang, Z.; Sun, O.J. Grassland ecosystems in China: Review of current knowledge and research advancement. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 997–1008. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, G.; Wang, Y.; Song, X. Sensitive Indicators of Zonal Stipa Species to Changing Temperature and Precipitation in Inner Mongolia Grassland, China. Front. Plant Sci. 2016, 7, 73. [Google Scholar] [CrossRef]
- Distel, R.A.; Moretto, A.S.; Didone, N.G. Regrowth capacity in relation to defence strategy in Stipa clarazii and Stipa trichotoma, native to semiarid Argentina. Austral Ecol. 2007, 32, 651–655. [Google Scholar] [CrossRef]
- Hu, X.W.; Zhou, Z.Q.; Li, T.S.; Wu, Y.P.; Wang, Y.R. Environmental factors controlling seed germination and seedling recruitment of Stipa bungeana on the Loess Plateau of northwestern China. Ecol. Res. 2013, 28, 801–809. [Google Scholar] [CrossRef]
- Chen, L.; Saixi, Y.; Yi, R.; Baoyin, T. Characterization of soil microbes associated with a grazing-tolerant grass species, Stipa breviflora, in the Inner Mongolian desert steppe. Ecol. Evol. 2020, 10, 10607–10618. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, J.M.; Wu, S.; Buyantuyev, A.; Dong, J.J. Impact of climatic factors on genetic diversity of Stipa breviflora populations in Inner Mongolia. Genet. Mol. Res. 2012, 11, 2081–2093. [Google Scholar] [CrossRef]
- Wasaya, A.; Zhang, X.; Fang, Q.; Yan, Z. Root phenotyping for drought tolerance: A review. Agronomy 2018, 8, 241. [Google Scholar] [CrossRef]
- van der Bom, F.J.; Williams, A.; Bell, M.J. Root architecture for improved resource capture: Trade-offs in complex environments. J. Exp. Bot. 2020, 71, 5752–5763. [Google Scholar] [CrossRef] [PubMed]
- López-Bucio, J.; Cruz-Ramırez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Gao, Y.; Lynch, J.P. Large crown root number improves topsoil foraging and phosphorus acquisition. Plant Physiol. 2018, 177, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Buxbaum, C.; Vanderbilt, K. Soil heterogeneity and the distribution of desert and steppe plant species across a desert-grassland ecotone. J. Arid. Environ. 2007, 69, 617–632. [Google Scholar] [CrossRef]
- Knapp, A.K.; Ciais, P.; Smith, M.D. Reconciling inconsistencies in precipitation–productivity relationships: Implications for climate change. New Phytol. 2017, 214, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Autumn, K.; Pugnaire, F. Evolution of suites of traits in response to environmental stress. Am. Nat. 1993, 142, S78–S92. [Google Scholar] [CrossRef]
- de Vries, F.T.; Brown, C.; Stevens, C.J. Grassland species root response to drought: Consequences for soil carbon and nitrogen availability. Plant Soil 2016, 409, 297–312. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Yahdjian, L.; Gherardi, L.; Sala, O.E. Nitrogen limitation in arid-subhumid ecosystems: A meta-analysis of fertilization studies. J. Arid. Environ. 2011, 75, 675–680. [Google Scholar] [CrossRef]
- Gong, X.Y.; Chen, Q.; Dittert, K.; Taube, F.; Lin, S. Nitrogen, phosphorus and potassium nutritional status of semiarid steppe grassland in Inner Mongolia. Plant Soil 2011, 340, 265–278. [Google Scholar] [CrossRef]
- Stasovski, E.; Peterson, C.A. The effects of drought and subsequent rehydration on the structure and vitality of Zea mays seedling roots. Can. J. Bot. 1991, 69, 1170–1178. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Schwarz, D.; Franken, P.; Colla, G. Effects of drought on nutrient uptake and assimilation in vegetable crops. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 171–195. [Google Scholar] [CrossRef]
- Xue, R.; Shen, Y.; Marschner, P. Soil water content during and after plant growth influence nutrient availability and microbial biomass. J. Soil Sci. Plant Nutr. 2017, 17, 702–715. [Google Scholar] [CrossRef]
- Liu, F.; Liu, J.; Dong, M. Ecological consequences of clonal integration in plants. Front. Plant Sci. 2016, 7, 770. [Google Scholar] [CrossRef]
- Lv, S.; Yan, B.; Wang, Z.; Han, G.; Kang, S. Grazing intensity enhances spatial aggregation of dominant species in a desert steppe. Ecol. Evol. 2019, 9, 6138–6147. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Alpert, P.; Si, C.; Yu, F.-H. Interactive effects of fragment size, nutrients, and interspecific competition on growth of the floating, clonal plant Salvinia natans. Aquat. Bot. 2019, 153, 81–87. [Google Scholar] [CrossRef]
- Stuefer, J.F.; Gómez, S.; Mölken, T.V. Clonal integration beyond resource sharing: Implications for defence signalling and disease transmission in clonal plant networks. Evol. Ecol. 2004, 18, 647–667. [Google Scholar] [CrossRef]
- Song, Y.-B.; Yu, F.-H.; Keser, L.H.; Dawson, W.; Fischer, M.; Dong, M.; van Kleunen, M. United we stand, divided we fall: A meta-analysis of experiments on clonal integration and its relationship to invasiveness. Oecologia 2013, 171, 317. [Google Scholar] [CrossRef]
- Pennings, S.C.; Callaway, R.M. The advantages of clonal integration under different ecological conditions: A community-wide test. Ecology 2000, 81, 709–716. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Pang, X.-Y.; Wang, A.; Dong, B.-C.; Lei, J.-P.; Yu, F.-H.; Li, M.-H. Clonal integration increases tolerance of a phalanx clonal plant to defoliation. Sci. Total Environ. 2017, 593, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Truscott, A.M.; Soulsby, C.; Palmer, S.; Newell, L.; Hulme, P. The dispersal characteristics of the invasive plant Mimulus guttatus and the ecological significance of increased occurrence of high-flow events. J. Ecol. 2006, 94, 1080–1091. [Google Scholar] [CrossRef]
- Dong, B.-C.; Alpert, P.; Guo, W.; Yu, F.-H. Effects of fragmentation on the survival and growth of the invasive, clonal plant Alternanthera philoxeroides. Biol. Invasions 2012, 14, 1101–1110. [Google Scholar] [CrossRef]
- Lin, H.-F.; Alpert, P.; Yu, F.-H. Effects of fragment size and water depth on performance of stem fragments of the invasive, amphibious, clonal plant Ipomoea aquatica. Aquat. Bot. 2012, 99, 34–40. [Google Scholar] [CrossRef]
- Qin, J.; Bao, Y.-J.; Li, Z.-H.; Hu, Z.-C.; Gao, W. The response of root characteristics of Stipa grandis to nitrogen addition in degraded grassland. Acta Prataculturae Sin. 2014, 23, 40. [Google Scholar] [CrossRef]
- Li, W.; Yu, W.; Bai, L.; Liu, H.; Yang, D. Effects of nitrogen addition on the mixed litter decomposition in Stipa baicalensis steppe in Inner Mongolia. Am. J. Plant Sci. 2016, 7, 547. [Google Scholar] [CrossRef][Green Version]
- Wang, M.-B.; Zhang, Q. Issues in using the WinRHIZO system to determine physical characteristics of plant fine roots. Acta Ecol. Sin. 2009, 29, 136–138. [Google Scholar] [CrossRef]
- Song, C.; Chen, X. Performance Comparison of Machine Learning Models for Annual Precipitation Prediction Using Different Decomposition Methods. Remote Sens. 2021, 13, 1018. [Google Scholar] [CrossRef]
- Kaiser, H.F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 1958, 23, 187–200. [Google Scholar] [CrossRef]
- Ren, J.; Su, Z.-Z.; Dang, Z.-H.; Ding, Y.; Wang, P.-X.; Niu, J.-M. Development and characterization of EST-SSR markers in Stipa breviflora (Poaceae). Appl. Plant Sci. 2017, 5, 1600157. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-P.; Zhao, N.-X.; Zhang, L.-H.; Gao, Y.-B. Responses of two dominant plant species to drought stress and defoliation in the Inner Mongolia Steppe of China. Plant Ecol. 2013, 214, 221–229. [Google Scholar] [CrossRef]
- Zhang, R.; Schellenberg, M.P.; Han, G.; Wang, H.; Li, J. Drought weakens the positive effects of defoliation on native rhizomatous grasses but enhances the drought-tolerance traits of native caespitose grasses. Ecol. Evol. 2018, 8, 12126–12139. [Google Scholar] [CrossRef]
- Huang, B. Nutrient accumulation and associated root characteristics in response to drought stress in tall fescue cultivars. HortScience 2001, 36, 148–152. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; Nie, Y.; Bai, S.H.; Zhou, L.; Shao, J.; Cheng, W.; Wang, J.; Hu, F.; Fu, Y. Drought-induced changes in root biomass largely result from altered root morphological traits: Evidence from a synthesis of global field trials. Plant Cell Environ. 2018, 41, 2589–2599. [Google Scholar] [CrossRef]
- Walch-Liu, P.; Ivanov, I.I.; Filleur, S.; Gan, Y.; Remans, T.; Forde, B.G. Nitrogen regulation of root branching. Ann. Bot. 2006, 97, 875–881. [Google Scholar] [CrossRef]
- Gruber, B.D.; Giehl, R.F.; Friedel, S.; von Wirén, N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef]
- Han, Y.-Y.; Zhou, S.; Chen, Y.-H.; Kong, X.; Xu, Y.; Wang, W. The involvement of expansins in responses to phosphorus availability in wheat, and its potentials in improving phosphorus efficiency of plants. Plant Physiol. Biochem. 2014, 78, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Paez-Garcia, A.; Motes, C.M.; Scheible, W.-R.; Chen, R.; Blancaflor, E.B.; Monteros, M.J. Root traits and phenotyping strategies for plant improvement. Plants 2015, 4, 334–355. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Robinson, D.; Griffiths, B.; Fitter, A. Why plants bother: Root proliferation results in increased nitrogen capture from an organic patch when two grasses compete. Plant Cell Environ. 1999, 22, 811–820. [Google Scholar] [CrossRef]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Fort, F.; Cruz, P.; Lecloux, E.; Bittencourt de Oliveira, L.; Stroia, C.; Theau, J.P.; Jouany, C. Grassland root functional parameters vary according to a community-level resource acquisition–conservation trade-off. J. Veg. Sci. 2016, 27, 749–758. [Google Scholar] [CrossRef]
- Hernández, E.; Vilagrosa, A.; Pausas, J.; Bellot, J. Morphological traits and water use strategies in seedlings of Mediterranean coexisting species. Plant Ecol. 2010, 207, 233–244. [Google Scholar] [CrossRef]
- Craine, J.M.; Froehle, J.; Tilman, D.G.; Wedin, D.A.; Chapin, F.S., III. The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos 2001, 93, 274–285. [Google Scholar] [CrossRef]
- de Vries, F.T.; Bardgett, R.D. Plant community controls on short-term ecosystem nitrogen retention. New Phytol. 2016, 210, 861–874. [Google Scholar] [CrossRef]
- Hodge, A.; Stewart, J.; Robinson, D.; Griffiths, B.; Fitter, A. Root proliferation, soil fauna and plant nitrogen capture from nutrient-rich patches in soil. New Phytol. 1998, 139, 479–494. [Google Scholar] [CrossRef]

| Properties | |
|---|---|
| pH | 8.29 |
| Organic carbon | 3.30 g/kg |
| Total nitrogen | 0.34 g/kg |
| Total phosphorus | 0.32 g/kg |
| Total potassium | 20.21 g/kg |
| Available N | 29.25 mg/kg |
| Available P | 1.85 mg/kg |
| Available K | 73.70 mg/kg |
| Treatments | Nitrogen Addition (N) | Phosphorus Addition (P) | N × P | Water Addition (W) | N × W | P × W | Fragment Size (C) |
|---|---|---|---|---|---|---|---|
| 1 | N1 | P1 | N1P1 | W1 | N1W1 | P1W1 | C1 |
| 2 | N1 | P1 | N1P1 | W2 | N1W2 | P1W2 | C2 |
| 3 | N1 | P2 | N1P2 | W1 | N1W1 | P2W1 | C2 |
| 4 | N1 | P2 | N1P2 | W2 | N1W2 | P2W2 | C1 |
| 5 | N2 | P1 | N2P1 | W1 | N2W1 | P1W1 | C2 |
| 6 | N2 | P1 | N2P1 | W2 | N2W2 | P1W2 | C1 |
| 7 | N2 | P2 | N2P2 | W1 | N2W1 | P2W1 | C1 |
| 8 | N2 | P2 | N2P2 | W2 | N2W2 | P2W2 | C2 |
| Traits | N | P | N × P | W | N × W | P × W | C |
|---|---|---|---|---|---|---|---|
| Total root length (TRL) | ** | NS | NS | * | NS | NS | ** |
| Root surface area (RSA) | * | * | NS | *** | NS | NS | ** |
| Root volume (RV) | NS | * | NS | *** | NS | NS | ** |
| Root tips (RT) | ** | ** | NS | NS | NS | NS | *** |
| Root biomass (RB) | NS | NS | NS | ** | NS | NS | ** |
| SRL | ** | NS | NS | ** | * | ** | ** |
| RTD | NS | NS | NS | NS | NS | NS | NS |
| Traits | VCR (%) | ||||||
|---|---|---|---|---|---|---|---|
| N | P | N × P | W | N × W | P × W | C | |
| Total root length (TRL) | 22.23 | 6.46 | 0.12 | 13.68 | 1.75 | 0.83 | 21.97 |
| Root surface area (RSA) | 9.74 | 6.67 | 0.42 | 34.78 | 0.96 | 3.31 | 20.79 |
| Root volume (RV) | 3.80 | 6.38 | 1.74 | 45.83 | 0.57 | 5.08 | 17.97 |
| Root tips (RT) | 19.88 | 22.51 | 3.62 | 4.83 | 2.99 | 0.15 | 26.54 |
| Root biomass (RB) | 0.45 | 6.04 | 6.33 | 21.19 | 4.98 | 5.94 | 22.81 |
| SRL | 13.44 | 4.16 | 5.42 | 14.93 | 9.20 | 14.04 | 16.51 |
| Total | 69.54 | 52.22 | 17.65 | 135.24 | 20.45 | 29.35 | 126.59 |
| Treatments (120 Samples) | Level | TRL (cm) | RSA (cm2) | RV (cm3) | RT (Number/Plant) | RB (g) | SRL | |
|---|---|---|---|---|---|---|---|---|
| C1 | Nitrogen addition | N1 | 1223.88 a | 148.50 a | 1.45 a | 3521.03 a | 0.33 a | 4262.66 b |
| N2 | 896.76 b | 102.36 b | 0.94 a | 2524.07 a | 0.20 b | 6515.86 a | ||
| Phosphorus addition | P1 | 1024.85 a | 117.95 a | 1.09 a | 2653.97 a | 0.26 a | 5238.14 a | |
| P2 | 1095.79 a | 132.91 a | 1.31 a | 3391.13 a | 0.27 a | 5540.39 a | ||
| Water addition | W1 | 942.83 a | 100.91 b | 0.87 b | 2521.23 a | 0.27 a | 5622.80 a | |
| W2 | 1177.81 a | 149.95 a | 1.54 a | 3523.87 a | 0.41 a | 5155.72 a | ||
| C2 | Nitrogen addition | N1 | 1443.59 a | 174.11 a | 1.70 a | 4874.17 a | 0.43 a | 4166.34 a |
| N2 | 1222.25 a | 161.94 a | 1.74 a | 3690.57 a | 0.50 a | 4141.87 a | ||
| Phosphorus addition | P1 | 1220.54 a | 151.39 a | 1.52 a | 3490.77 b | 0.36 a | 4925.05 a | |
| P2 | 1445.30 a | 184.66 a | 1.92 a | 5073.97 a | 0.57 a | 3383.17 a | ||
| Water addition | W1 | 1235.29 a | 137.45 b | 1.23 b | 4246.4 a | 0.44 b | 5095.07 a | |
| W2 | 1430.55 a | 198.60 a | 2.22 a | 4318.33 a | 0.75 a | 3213.14 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, R.; Lv, S.; Ding, Y.; Li, Q. Interactive Effects of Soil Water, Nutrients and Clonal Fragmentation on Root Growth of Xerophilic Plant Stipa breviflora. Agriculture 2022, 12, 2112. https://doi.org/10.3390/agriculture12122112
Fan R, Lv S, Ding Y, Li Q. Interactive Effects of Soil Water, Nutrients and Clonal Fragmentation on Root Growth of Xerophilic Plant Stipa breviflora. Agriculture. 2022; 12(12):2112. https://doi.org/10.3390/agriculture12122112
Chicago/Turabian StyleFan, Ruyue, Shijie Lv, Yong Ding, and Qingfeng Li. 2022. "Interactive Effects of Soil Water, Nutrients and Clonal Fragmentation on Root Growth of Xerophilic Plant Stipa breviflora" Agriculture 12, no. 12: 2112. https://doi.org/10.3390/agriculture12122112
APA StyleFan, R., Lv, S., Ding, Y., & Li, Q. (2022). Interactive Effects of Soil Water, Nutrients and Clonal Fragmentation on Root Growth of Xerophilic Plant Stipa breviflora. Agriculture, 12(12), 2112. https://doi.org/10.3390/agriculture12122112





