Comprehensive Evaluation of Healthcare Benefits of Different Forest Types: A Case Study in Shimen National Forest Park, China
Abstract
1. Introduction
2. Materials and Methods
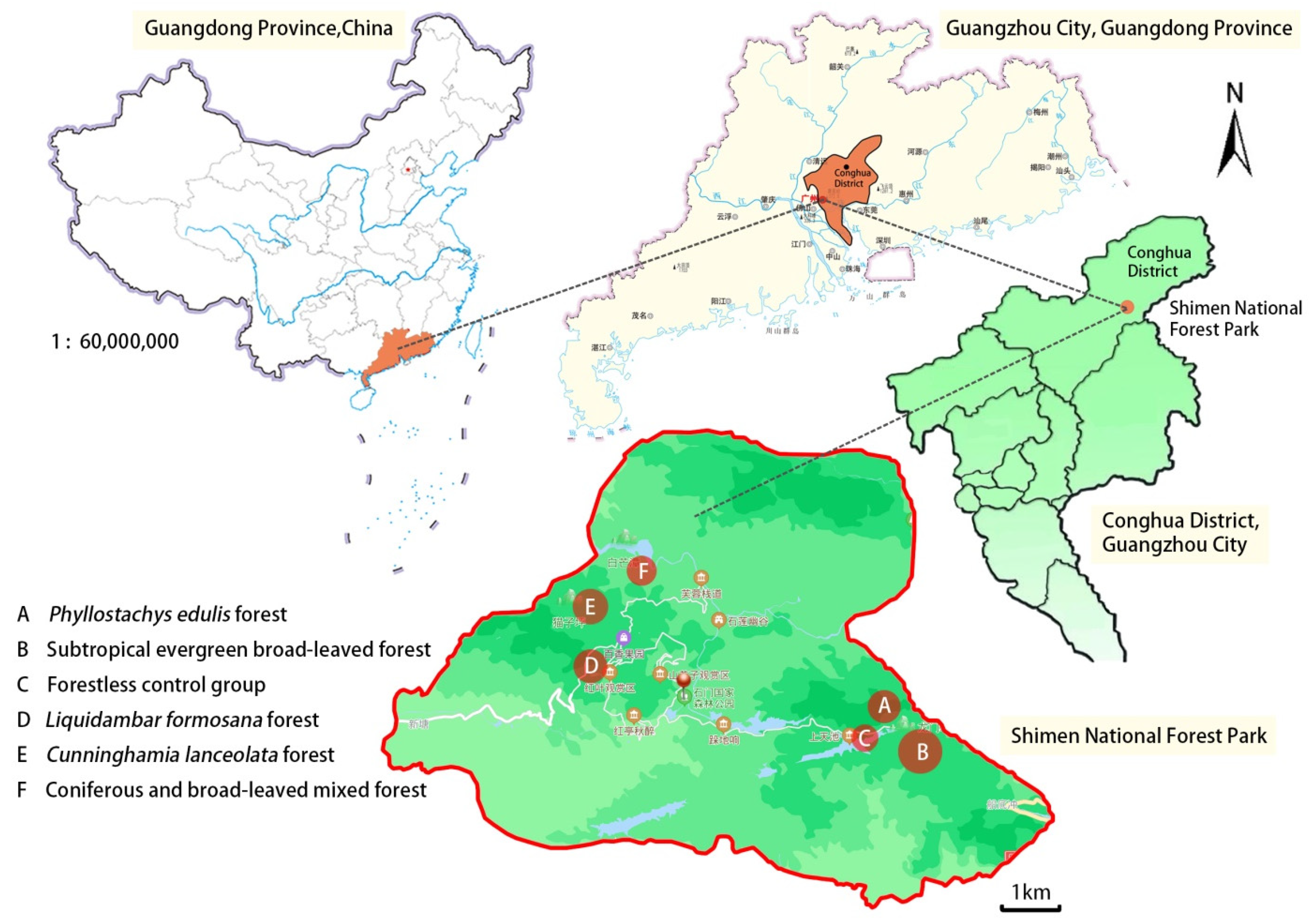
2.1. Study Area
2.2. Plot Settings
2.3. Methods
2.3.1. Observation Methods
2.3.2. Specific Calculation Methods
2.3.3. Multiple Indicators Comprehensive Evaluation Methods
F2 = 0.754X1 − 0.299X2 − 0.016X3 + 0.379X4
2.3.4. Data Processing and Analysis
3. Results
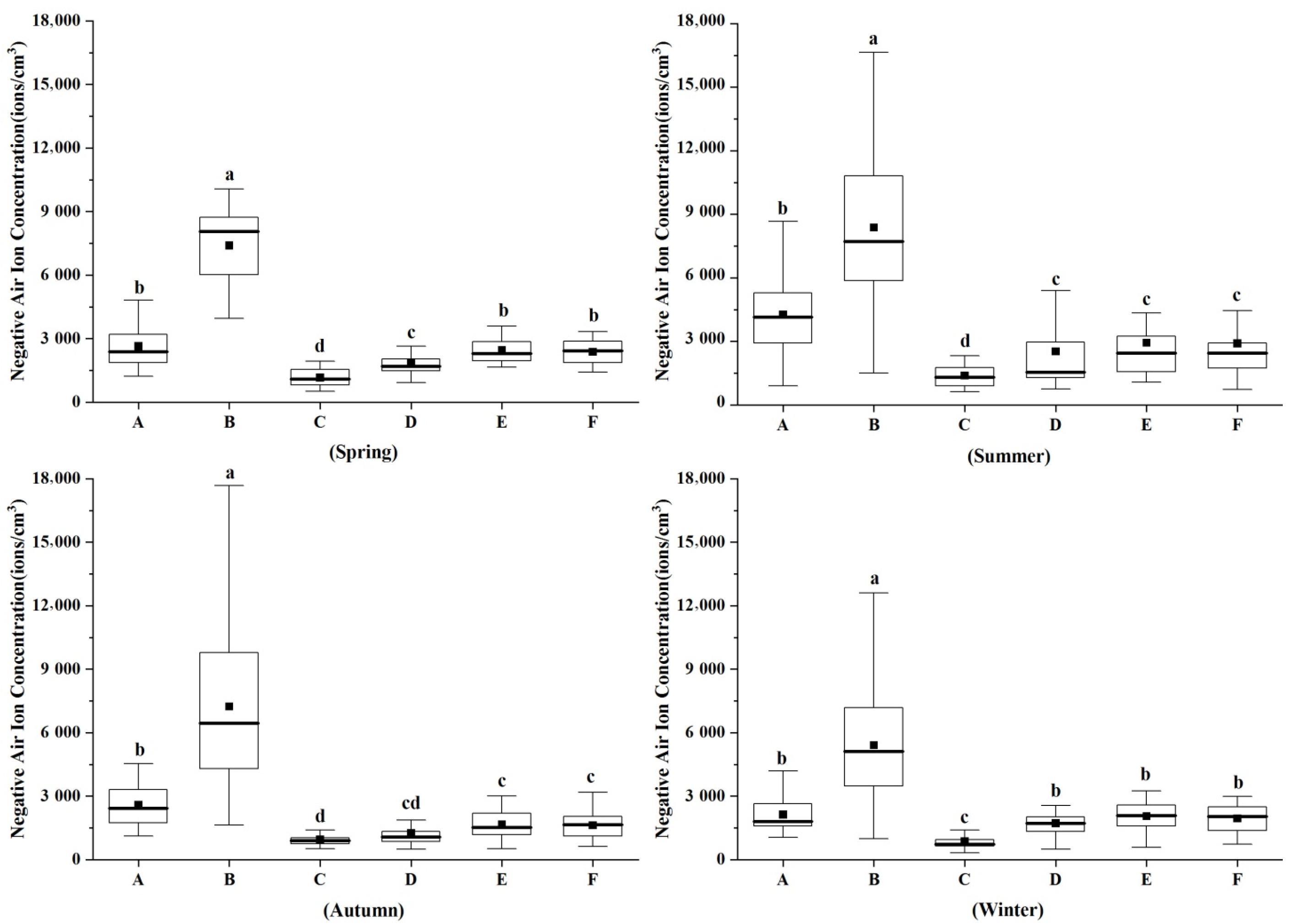
3.1. Characteristics of Negative Air Ion Concentration in Different Forest Types in Different Seasons
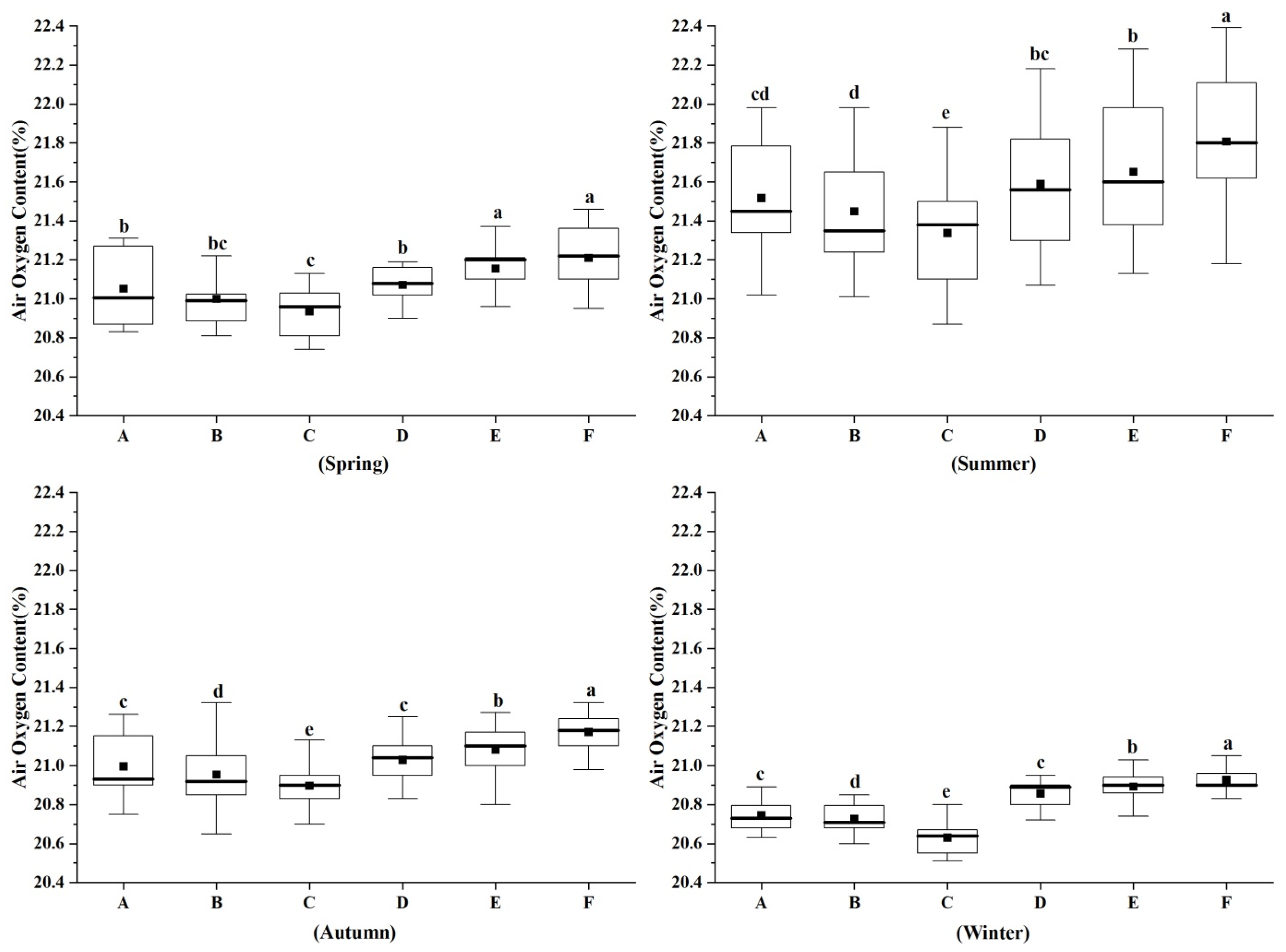
3.2. Characteristics of Air Oxygen Content in Different Forest Types in Different Seasons
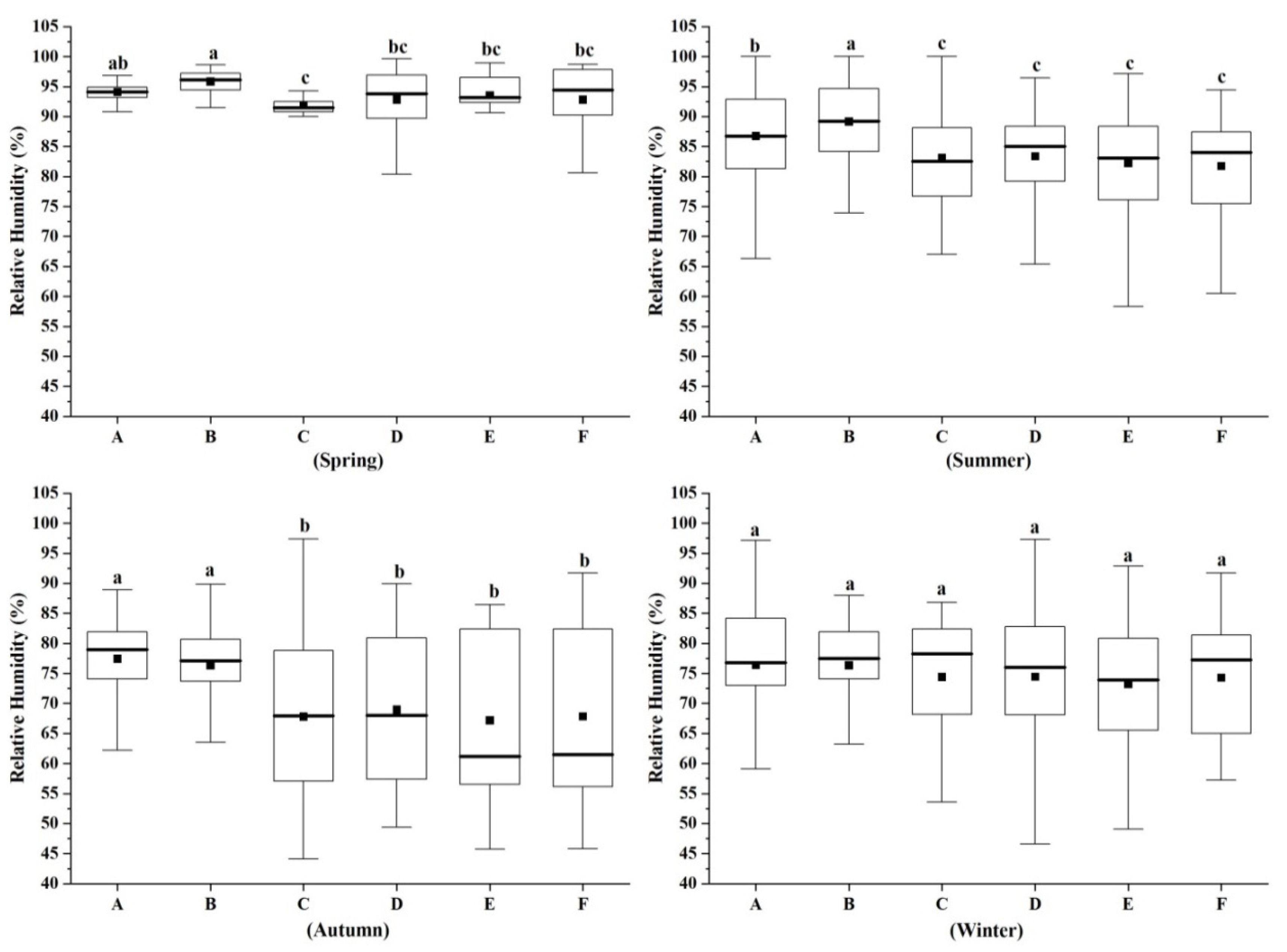
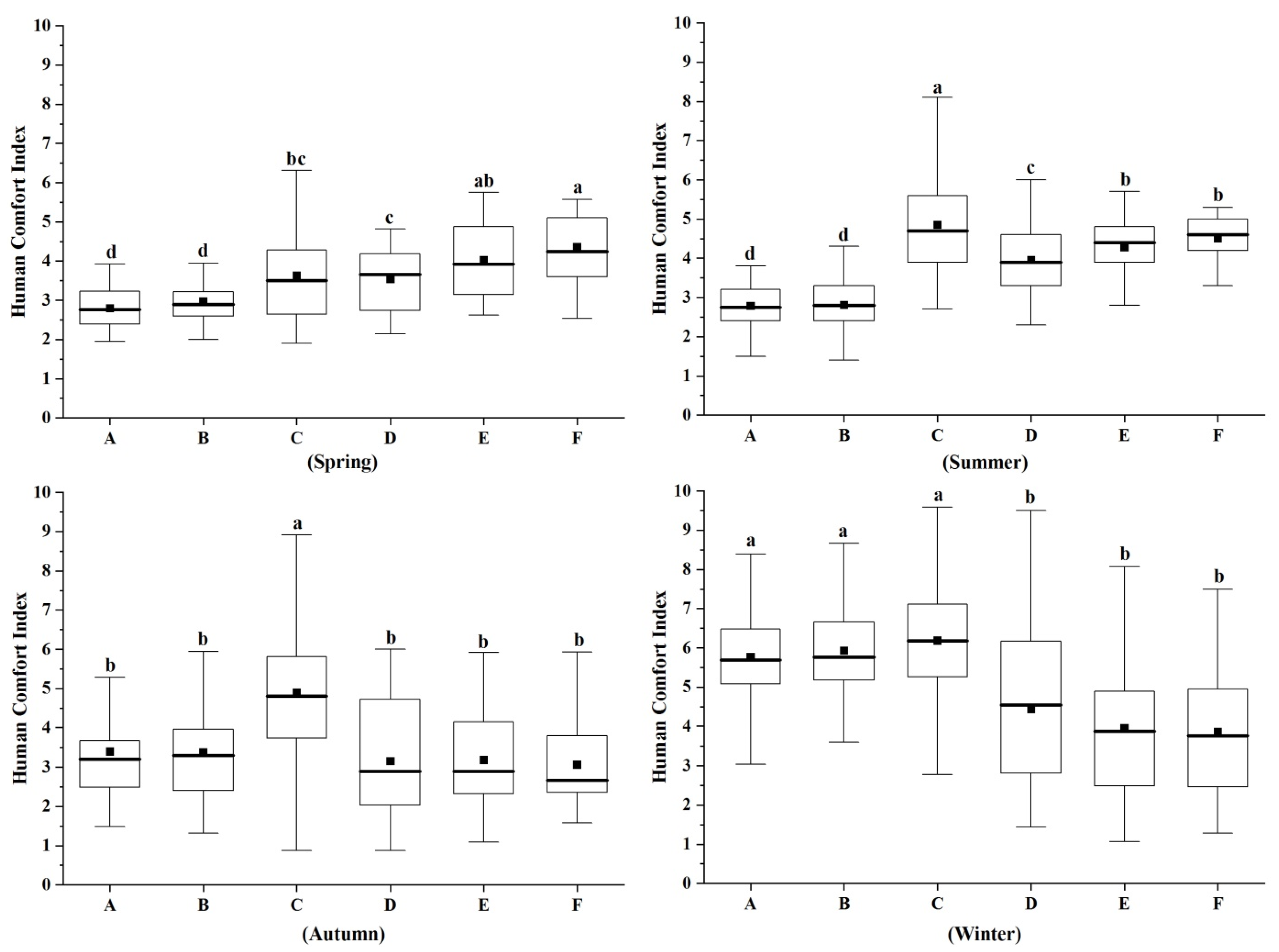
3.3. Characteristics of Human Comfort Index in Different Forest Types in Different Seasons
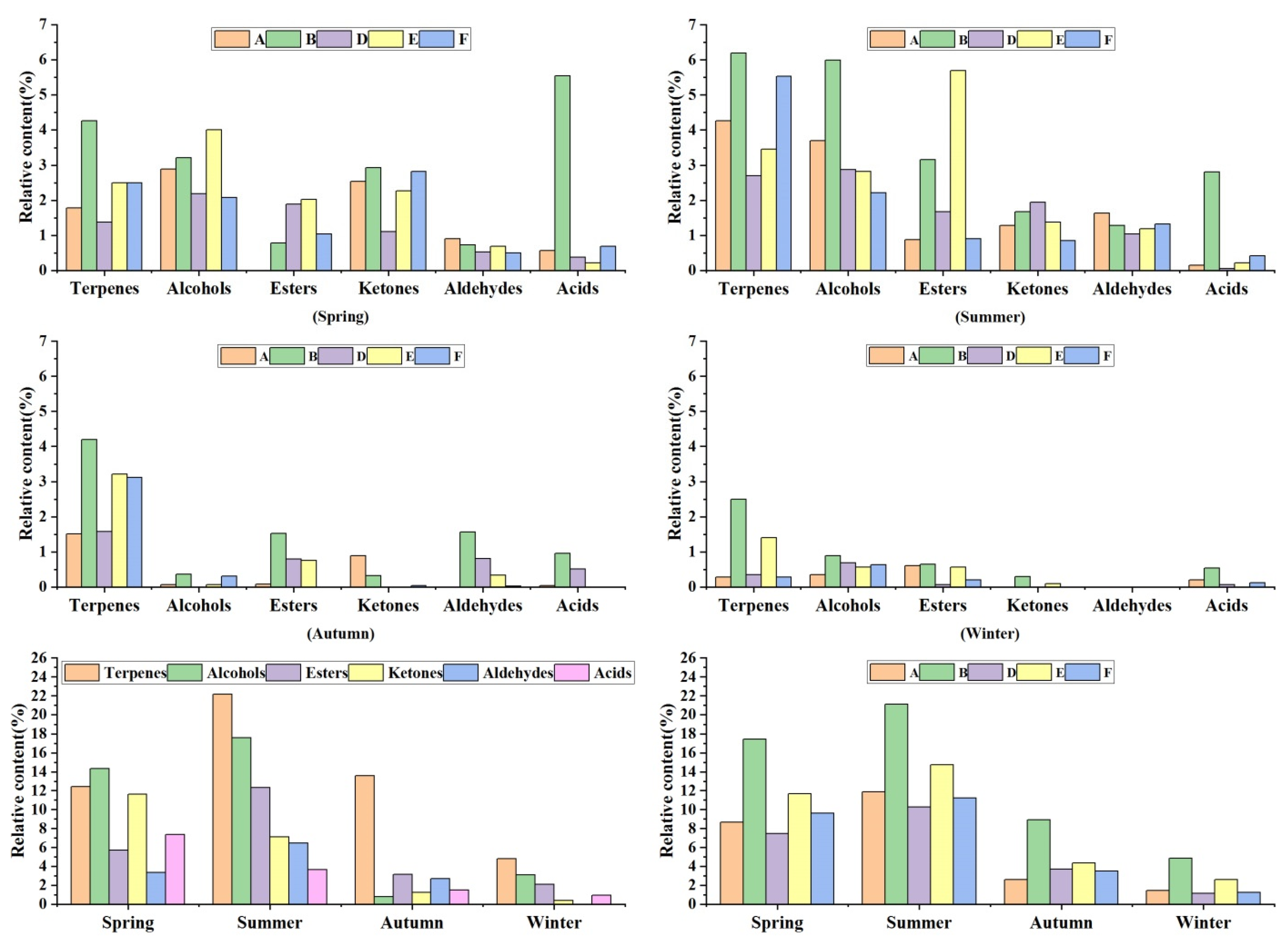
3.4. Characteristics of Phytoncide Relative Content in Different Forest Types in Different Seasons
3.5. Evaluation of Comprehensive Healthcare Benefits of Different Forest Types
4. Discussion
4.1. Characteristics of Negative Air Ion Concentration (NAIC) Differences in Different Forest Types
4.1.1. Seasonal Variation
4.1.2. Stands Difference
4.2. Characteristics of Air Oxygen Content Differences in Different Forest Types
4.3. Characteristics of Human Comfort Index Differences in Different Forest Types
4.4. Characteristics of Phytoncide Relative Content Differences in Different Forest Types
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, W.; Zheng, R.; Zhang, S.; Zeng, H.; Zuo, T.; Xia, C.; Yang, Z.; He, J. Cancer incidence and mortality in China in 2013: An analysis based on urbanization level. Chin. J. Cancer Res. 2017, 29, 1–10. [Google Scholar] [CrossRef]
- Huang, H.; Shi, J.; Guo, L.; Zhu, X.; Wang, L.; Liao, X.; Liu, G.; Bai, Y.; Mao, A.; Ren, J.; et al. Expenditure and financial burden for common cancers in China: A hospital-based multicentre cross-sectional study. Lancet 2016, 388, S10. [Google Scholar] [CrossRef]
- UNWTO; GTERC. Asia Tourism Trends—2019 Edition, Executive Summary; World Tourism Organization and Global Tourism Economy Research Centre: Macau, China, 2019; Volume 10. [Google Scholar]
- Pu, B.; Zhang, L.; Wei, J. Chinese Consumers’ Perception on Forest Therapy. In Proceedings of the 2019 International Conference on Global Economy and Business Management (GEBM 2019), Chongqing, China, 29–30 November 2019. [Google Scholar]
- Park, B.; Tsunetsugu, Y.; Kasetani, T.; Hirano, H.; Kagawa, T.; Sato, M.; Miyazaki, Y.; Chiba, P.F.R.C.; Nagasaki, J.C.; Ministry, O.T.E.; et al. Physiological Effects of Shinrin-yoku (Taking in the Atmosphere of the Forest)—Using Salivary Cortisol and Cerebral Activity as Indicators. J. Physiol. Anthropol. 2007, 26, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Grilli, G.; Sacchelli, S. Health Benefits Derived from Forest: A Review. Int. J. Envirion. Res. Pub. Health 2020, 17, 6125. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.M.; Jones, R.; Tocchini, K. Shinrin-Yoku (Forest Bathing) and Nature Therapy: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2017, 14, 851. [Google Scholar] [CrossRef]
- Li, Q. Effect of forest bathing (shinrin-yoku) on human health: A review of the literature. Santé Publique (Vandoeuvre-lès-Nancy, France) 2019, S1, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.D.; Wu, Q.; Mao, G.X.; Wang, S.Y.; Jia, B.B.; Wang, G.F. Current Status of Forest Medicine Research in China. Biomed. Environ. Sci. 2018, 31, 551–554. [Google Scholar]
- Pino, O.; La Ragione, F. There’s Something in the Air: Empirical Evidence for the Effects of Negative Air Ions (NAI) on Psychophysiological State and Performance. Res. Psychol. Behav. Sci. 2013, 1, 48–53. [Google Scholar]
- Bowers, B.; Flory, R.; Ametepe, J.; Staley, L.; Patrick, A.; Carrington, H. Controlled trial evaluation of exposure duration to negative air ions for the treatment of seasonal affective disorder. Psychiatry Res. 2018, 259, 7–14. [Google Scholar] [CrossRef]
- Liu, R.; Lian, Z.; Lan, L.; Qian, X.; Chen, K.; Hou, K.; Li, X. Effects of negative oxygen ions on sleep quality. Proc. Eng. 2017, 205, 2980–2986. [Google Scholar] [CrossRef]
- Woo, J.; Lee, C.J. Sleep-enhancing Effects of Phytoncide via Behavioral, Electrophysiological, and Molecular Modeling Approaches. Exp. Neurobiol. 2020, 29, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review. Int. J. Environ. Res. Pub. Health 2020, 17, 6506. [Google Scholar] [CrossRef] [PubMed]
- Hmamouchi, M.; Hamamouchi, J.; Zouhdi, M.; Bessiere, J.M. Chemical and Antimicrobial Properties of Essential Oils of Five Moroccan Pinaceae. J. Essent. Oil. Res. 2001, 13, 298–302. [Google Scholar] [CrossRef]
- Kim, C.; Cho, M.; Kim, J. Effects of Phytoncide Aromatherapy on Stress, Symptoms of Stress and Heart Rate Variability among Nursing Students. J. Korean Biol. Nurs. Sci. 2012, 14, 249–257. [Google Scholar] [CrossRef]
- Süntar, I.; Tumen, I.; Ustün, O.; Keleş, H.; Küpeli Akkol, E. Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J. Ethnopharmacol. 2012, 139, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Liu, Z.; Li, Z. Chemical Composition and Antioxidant Activity of Essential Oil of Six Pinus Taxa Native to China. Molecules 2015, 20, 9380–9392. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Hisama, M.; Tanimoto, S.; Shibayama, H.; Mihara, Y.; Nomura, M. Antioxidant Effects and Antimicrobial Activites of Phytoncide. Biocontrol. Sci. 2008, 13, 23–27. [Google Scholar] [CrossRef]
- Li, Q.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Hirata, K.; Shimizu, T.; Kawada, T.; Park, B.J.; et al. Effect of phytoncide from trees on human natural killer cell function. Int. J. Immunopathol. Pharmacol. 2009, 22, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Yang, H.; Yoon, M.; Gadhe, C.G.; Pae, A.N.; Cho, S.; Lee, C.J. 3-Carene, a Phytoncide from Pine Tree Has a Sleep-enhancing Effect by Targeting the GABAA-benzodiazepine Receptors. Exp. Neurobiol. 2019, 28, 593–601. [Google Scholar] [CrossRef]
- Santhosh, K.V.; Roy, B.K.; Bhowmik, P.K. Oxygen level monitoring in an oxygen cylinder. In Proceedings of the IEEE-International Conference on Advances in Engineering, Science and Management, ICAESM Nagapattinam, India, 30–31 March 2012; pp. 592–595. [Google Scholar]
- Rahman, M.A.; Hartmann, C.; Moser-Reischl, A.; von Strachwitz, M.F.; Paeth, H.; Pretzsch, H.; Pauleit, S.; Rötzer, T. Tree cooling effects and human thermal comfort under contrasting species and sites. Agric. For. Meteorol. 2020, 287, 107947. [Google Scholar] [CrossRef]
- Teshnehdel, S.; Akbari, H.; Di Giuseppe, E.; Brown, R.D. Effect of tree cover and tree species on microclimate and pedestrian comfort in a residential district in Iran. Build. Environ. 2020, 178, 106899. [Google Scholar] [CrossRef]
- Zhao, J.J.; Li, Y.M.; Tian, H.L.; Liu, Y.S.; Xiang, S.M. Analysis of oxygen content in forest environment in Qiannan Guizhou province. Shandong For. Sci. Technol. 2012, 42, 24–26. [Google Scholar]
- Lu, D.H.; Cui, S.; Li, C.H. The influence of Beijing urban greening and summer microelimatic conditions on human fitness. In Forestry and Metrology Papers; Meteorological Press: Beijing, China, 1984; pp. 144–152. [Google Scholar]
- Hong, L.X.C.J. The spatial-temporal pattern and influencing factors of negative air ions in urban forests, Shanghai, China. J. For. Res. 2014, 25, 847–856. [Google Scholar]
- Lin, H.; Lin, J. Generation and Determination of Negative Air Ions. J. Anal. Testing 2017, 1, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Niu, X.; Song, Q.; Li, M.; Luo, Y.; Liang, L.; Du, P.; Peng, W. Study on the change of negative air ion concentration and its influencing factors at different spatio-temporal scales. Glob. Ecol. Conserv. 2020, 23, e1008. [Google Scholar] [CrossRef]
- Xing, G.W.; Li, Y.; Lin, J.M. Recent advances in generation and detection of gaseous negative ions. Chin. J. Anal. Labor. 2019, 38, 112–118. [Google Scholar]
- Li, S.N.; Han, S.W.; Shang, T.Y.; Lu, R.Q.; Zhang, Y.P.; Pan, Q.H.; Jin, W.M. Research progresson aero-anion’s monitoring and evaluation at home and abroad. J. Anhui Agric. Sci. 2009, 37, 3736–3738. [Google Scholar]
- Wang, J.M.; Yi, Z.J.; Sui, T.B.; Wang, J.; Jin, Z.Z. Influence of temperature and humidity on negative ion concentration. Res. Environ. Sci. 2004, 17, 68–70. [Google Scholar]
- Yu, H.; Xin, X.B.; Pei, S.X.; Wu, D.; Wu, S.; Fa, L.; Ma, S.M.; Guo, H. Characteristics of air anion change and its relationship with meteorological factors in forest margin area of Jiulong Mountain. Ecol. Sci. 2018, 37, 191–198. [Google Scholar]
- Wei, C.L.; Wang, J.T.; Jiang, Y.L.; Zhang, Q.G. Air negative charge ion concentration and its relationships with meteorological factors in different ecological functional zones of Hefei City. Chin. J. Appl. Ecol. 2006, 17, 2158–2162. [Google Scholar]
- Miao, S.; Zhang, X.; Han, Y.; Sun, W.; Liu, C.; Yin, S. Random Forest Algorithm for the Relationship between Negative Air Ions and Environmental Factors in an Urban Park. Atmosphere (Basel) 2018, 9, 463. [Google Scholar] [CrossRef]
- Luo, L.; Sun, W.; Han, Y.; Zhang, W.; Liu, C.; Yin, S. Importance Evaluation Based on Random Forest Algorithms: Insights into the Relationship between Negative Air Ions Variability and Environmental Factors in Urban Green Spaces. Atmosphere (Basel) 2020, 11, 706. [Google Scholar] [CrossRef]
- Feng, P.F.; Yu, X.W.; Zhang, X. Variations in Negative Air Ion Concentrations Associated with Different Vegetation Types and Influencing Factors in Beijing. Ecol. Environ. Sci. 2015, 24, 818–824. [Google Scholar]
- Liang, H.; Chen, X.S.; Da, L.J. Aero-Anion Dynamics and Its Influence Factors in Sheshan National Forest Park, Shanghai. Urb. Environ. Econ. 2014, 27, 7–11. [Google Scholar]
- Wu, C.C.; Zhong, L.S.; Liu, X.M. The influence of stand factors on aero-anion concentration in masson’ 5 pine pure forest. J. Cent. South For. Univ. 1998, 18, 70–73. [Google Scholar]
- Liu, X.; Wu, L.H.; Zhang, H.; Wang, X.R. Study on the concentration of negative air ions and the influential factors in different urban plant communities. J. Fudan Univ. (Nat. Sci.) 2011, 50, 206–212. [Google Scholar]
- Wang, L.J.; Han, Z.R.G.T.; Bai, Y.R.; Li, X.F.; Mou, Y.N.; Sun, G.C. Study on Spatial and Temporal Variation Characteristics of Negative Air Ion Concentration in Honghuaerji Forest Park. For. Sci. Technol. 2018, 23, 46–48. [Google Scholar]
- Liu, X.X.; Hua, C.; Zhang, M.R.; Zhang, J.G.; Liu, D. Aero-anion concentration in different forest communities of Laoshan Forest Farm, Chun’an County. J. Zhejiang A F Univ. 2012, 29, 366–373. [Google Scholar]
- Tan, Y.J.; Wang, E.; Zhang, P.C.; Zhang, Y.K. Research progress of spatio-temporal changes and health effects of negative air ions. North. Horticul. 2013, 9, 208–211. [Google Scholar]
- Qin, j.; Wang, L.M.; Gao, K.; Hu, Y.H.; Wang, Y.Q.; You, W.H. Improvement of Negative Air Ions Concentration by Plant Communities. J. Huazhong Agric. Univ. 2008, 27, 303–308. [Google Scholar]
- Shi, Y.L.; Yu, S.Q.; Zheng, Q.L. Aero-anion ecological efficacy in six types of plant communities. J. Zhejiang For. Coll. 2010, 27, 185–189. [Google Scholar]
- Pan, J.B.; Dong, L.; Liao, S.X.; Qiao, L.; Yan, H. Negative air ion concentration and affecting factors in Beijing Olympic Forest Park. J. Beijing For. Univ. 2011, 33, 59–64. [Google Scholar]
- Li, C.H.; Liu, F.G.; Chen, R.; Luo, Z.X.; Wu, C.Y. Quantitative study on tourism climate comfort in Lhasa. J. Arid Land Res. Environ. 2014, 28, 203–208. [Google Scholar]
- Wang, Y.Y.; Wang, C.; Dong, J.W.; Wang, Q.; Lin, S.Y.; Fu, W.C.; Zhu, L.X. Diurnal Oxygen Concentration Changes in Mixed Evergreen Broad-Leaved Forest at Qishan, Fuzhou. J. Chin. Urb. For. 2014, 12, 6–9. [Google Scholar]
- Chen, T.; Wang, W.; La, B. Study on Oxygen Content Change in Lhasa. Plat. Mount. Meteorol. Res. 2010, 30, 65–67. [Google Scholar]
- Hong, H.Y. Research progress of medical oxygen. World Latest Med. Infor. (Electronic Version) 2015, 15, 55–57. [Google Scholar]
- Gu, L.; Wang, C.; Wang, Y.Y.; Wang, X.L.; Sun, Z.K.; Wang, Q.; Sun, R.L. Patterns of temporal variation of microclimate and extent of human comfort in the recreation forests in Huishan National Forest Park. Sci. Silvae Sin. 2019, 55, 150–159. [Google Scholar]
- Dong, J.H.; He, Q.J.; Gao, Y.; Wang, Y.Y.; Ye, B.; Zhang, Z.Y.; Hua, J.X. The Microclimate Effects of Typical Urban Forests and Wetland and Its Effects on Human Comfort Degree: Taking Hangzhou as an Example. Environ. Protect. Sci. 2019, 45, 101–107. [Google Scholar]
- Ruiz, M.A.; Correa, E.N. Adaptive model for outdoor thermal comfort assessment in an Oasis city of arid climate. Build. Environ. 2015, 85, 40–51. [Google Scholar] [CrossRef]
- Liu, C.; Xu, N.; Song, J.D.; Hu, S.C. Research on visitors’ thermal sensation and space choices in an urban forest park. Acta Ecol. Sin. 2017, 37, 3561–3569. [Google Scholar]
- Kesselmeier, J.; Staudt, M. Biogenic Volatile Organic Compounds (VOC): An Overview on Emission, Physiology and Ecology. J. Atmos Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Harley, P.; Eller, A.; Guenther, A.; Monson, R.K. Observations and models of emissions of volatile terpenoid compounds from needles of ponderosa pine trees growing in situ: Control by light, temperature and stomatal conductance. Oecologia 2014, 176, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Faubert, P.; Tiiva, P.; Michelsen, A.; Rinnan, Å.; Ro-Poulsen, H.; Rinnan, R. The shift in plant species composition in a subarctic mountain birch forest floor due to climate change would modify the biogenic volatile organic compound emission profile. Plant. Soil 2012, 352, 199–215. [Google Scholar] [CrossRef]
- Tingey, D.T.; Manning, M.; Grothaus, L.C.; Burns, W.F. Influence of light and temperature on monoterpene emission rates from slash pine. Plant. Physiol. 1980, 65, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Vallat, A.; Gu, H.; Dorn, S. How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochemistry 2005, 66, 1540–1550. [Google Scholar] [CrossRef]
- Guenther, A.B.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Observations with eucalyptus and emission rate algorithm development. J. Geophys. Res. 1991, 96, 10799. [Google Scholar] [CrossRef]
- Guenther, A.B.; Zimmerman, P.R.; Harley, P.C.; Monson, R.K.; Fall, R. Isoprene and monoterpene emission rate variability: Model evaluations and sensitivity analyses. J. Geophys. Res. 1993, 98, 12609. [Google Scholar] [CrossRef]
- Gouinguené, S.P.; Turlings, T.C.J. The Effects of Abiotic Factors on Induced Volatile Emissions in Corn Plants. Plant. Physiol. 2002, 129, 1296–1307. [Google Scholar] [CrossRef]
- Meeningen, Y.V.; Schurgers, G.; Rinnan, R.; Holst, T. Isoprenoid emission response to changing light conditions of English oak, European beech and Norway spruce. Biogeosciences 2017, 14, 4045–4060. [Google Scholar] [CrossRef]
- Xu, J.H.; Wen, S.W.; Wu, D.P. Influence of temperature on pythoncidere of Lavandula angustifolia Mill.flower. Acta Ecol. Sin. 2010, 30, 645–651. [Google Scholar]
- Wu, L.; Sun, Q.; Zhao, J.; Zhang, Y. Current Status and Prospects of Research on Plant Pythoncidere. Int. J. Ecol. 2019, 8, 9–14. [Google Scholar] [CrossRef]
- Lun, X.; Lin, Y.; Chai, F.; Fan, C.; Li, H.; Liu, J. Reviews of emission of biogenic volatile organic compounds (BVOCs) in Asia. J. Environ. Sci. 2020, 95, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.; Kim, D.; Han, J. Seasonal variations of monoterpene emissions from coniferous trees of different ages in Korea. Chemosphere 2005, 59, 1685–1696. [Google Scholar] [CrossRef] [PubMed]



| Forest Types | Test Site | Longitude (°E) | Latitude (°N) | Altitude (m) | Area (hm2) | Stand Age (year) | Stand Density (N·hm−2) | Tree’s Height (m) | Tree’s Average Diameter (cm) | Canopy Density | Distance from Water Source (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phyllostachys edulis forest | A | 113.824 | 23.628 | 810 | 19.3 | ≥30 | 1143 | 20.54 ± 2.36 | 11.48 ± 1.59 | 0.8 | ≈300 |
| Subtropical evergreen broad-leaved forest | B | 113.829 | 23.626 | 820 | 65 | ≥100 | 1045 | 36.69 ± 6.34 | 35.45 ± 5.69 | 0.9 | ≈400 |
| Liquidambar formosana forest | D | 113.776 | 23.636 | 450 | 15.6 | ≥20 | 637 | 24.55 ± 3.04 | 20.36 ± 2.41 | 0.6 | - |
| Cunninghamia lanceolata forest | E | 113.774 | 23.649 | 350 | 17.9 | ≥30 | 861 | 33.10 ± 4.83 | 25.04 ± 3.17 | 0.7 | - |
| Coniferous and broad-leaved mixed forest | F | 113.782 | 23.652 | 290 | 25.6 | ≥20 | 608 | 26.38 ± 5.44 | 19.52 ± 3.78 | 0.5 | ≈150 |
| Forestless control group | C | 113.818 | 23.625 | 790 | 1.7 | - | - | - | - | - | ≈30 |
| Indicators | Principal Component | Component Matrix | Weight Value | ||
|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | ||
| Negative air ion concentration/NAIC (X1) | −0.302 | 0.754 | 0.750 | −0.635 | 0.10 |
| Air oxygen content/AOC (X2) | 0.710 | −0.299 | 0.690 | 0.606 | 0.35 |
| Human comfort index/HCI (X3) | 0.411 | −0.016 | 0.661 | 0.256 | 0.27 |
| Phytoncide relative content/PRC (X4) | 0.185 | 0.379 | 0.941 | −0.117 | 0.28 |
| Eigenvalues | 2.359 | 1.050 | |||
| Variance contribution rate (%) | 58.983 | 26.254 | |||
| Cumulated contribution rate (%) | 58.983 | 85.237 | |||
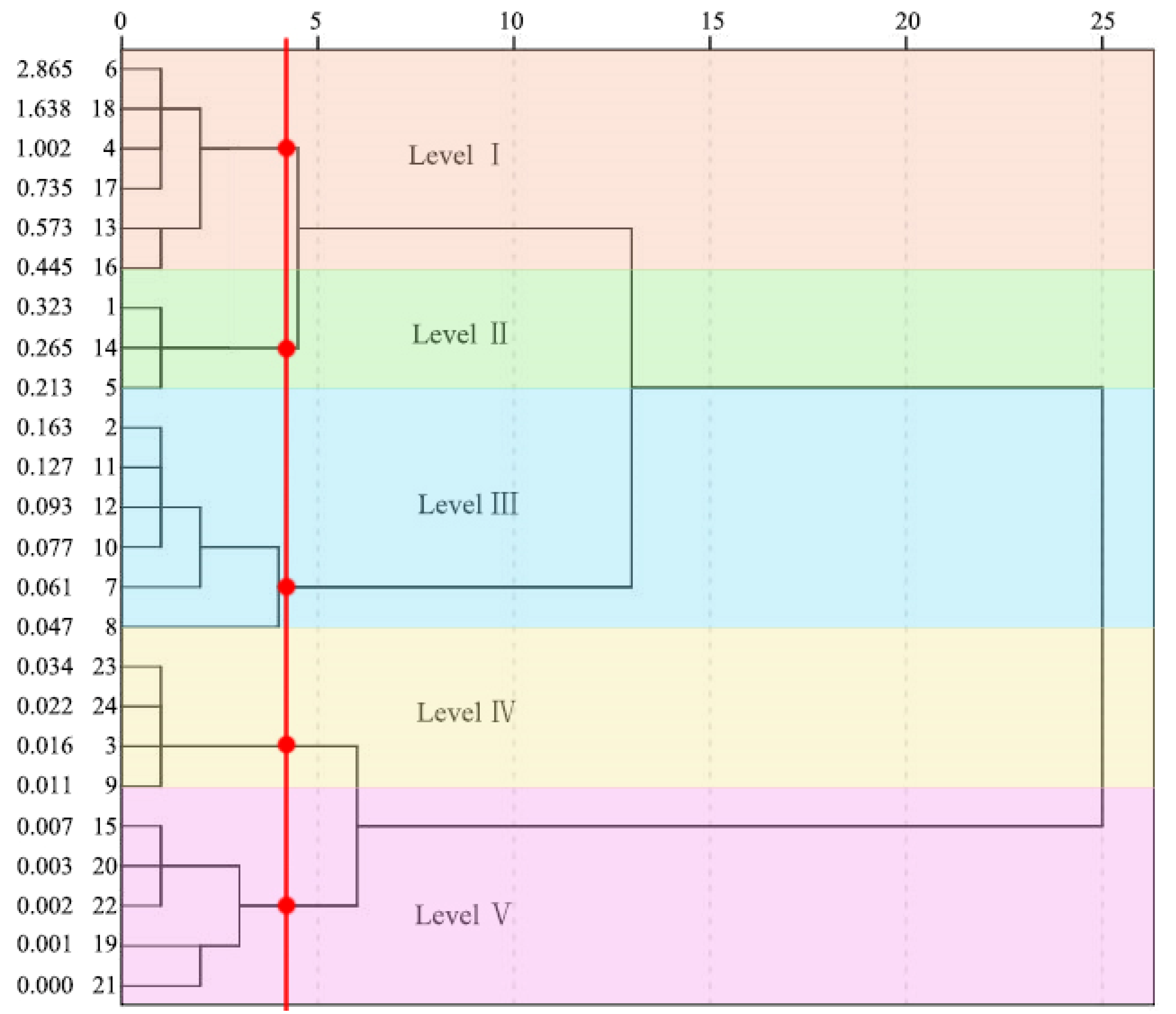
| Grades | Index Range | Degree of Comprehensive Healthcare Benefits |
|---|---|---|
| Level I | FCHI ≥ 0.445 | Very strong |
| Level II | 0. 445 > FCHI ≥ 0.213 | Strong |
| Level III | 0.213 > FCHI ≥ 0.047 | Weak |
| Level IV | 0.047 > FCHI ≥ 0.011 | Very weak |
| Level V | FCHI < 0.011 | Null |
| Serial Number | Species | Chemical Compound | Chemical Formula | Relative Contents of Phytoncide in Spring (%) | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | D | E | F | ||||
| 1 | Terpenes | 1-Heptene,2,4-dimethyl- | C9H18 | 0.51 | 0.08 | 1.63 | ||
| 2 | 1-Undecene | C11H22 | 0.67 | 0.3 | 0.21 | 1.47 | ||
| 3 | (+)-Limonene | C10H16 | 0.75 | 2.25 | 0.23 | 1.02 | 0.56 | |
| 4 | cis-3-Methyl-2-pentene | C6H12 | 0.36 | 1.2 | 0.86 | 0.31 | ||
| 5 | Alcohols | 2-Ethylhexanol | C8H18O | 1.92 | 1.32 | 0.98 | 1.07 | 0.69 |
| 6 | Linalool | C10H18O | 0.65 | 1.2 | 1.81 | 0.52 | ||
| 7 | 2-Ethyl-4-(2,2,3-trimethylcyclopent-3-en-yl)-but-2-en-1-ol | C14H24O | 0.96 | 1.24 | 1.12 | 0.86 | ||
| 8 | Esters | Bornyl acetate | C12H20O2 | 0.78 | 1.88 | 2.02 | 1.03 | |
| 9 | Ketones | 2-Camphanone | C10H16O | 1.67 | 2.36 | 0.87 | 1.75 | 2.61 |
| 10 | 6-Methyl-5-hepten-2-one | C8H14O | 0.56 | 0.21 | ||||
| 11 | Cyclopentyl methyl ketone | C7H12O | 0.87 | 0.23 | 0.51 | |||
| 12 | Aldehydes | Undecanal | C11H22O | 0.73 | 0.5 | |||
| 13 | Decyl aldehyde | C10H20O | 0.9 | 0.52 | 0.69 | |||
| 14 | Acids | Decanoic acid | C10H20O2 | 0.86 | 0.21 | |||
| 15 | Palmitic acid | C16H32O2 | 0.56 | 2.36 | 0.15 | 0.69 | ||
| 16 | Tridecanoic acid | C13H26O2 | 2.32 | 0.23 | ||||
| Total | 8.66 | 17.44 | 7.44 | 11.67 | 9.61 | |||
| Serial Number | Species | Chemical Compound | Chemical Formula | Relative Contents of Phytoncide in Summer (%) | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | D | E | F | ||||
| 1 | Terpenes | (E)-(β)-Farnesene | C15H24 | 0.23 | 0.54 | 0.52 | ||
| 2 | α-Cedrene | C15H24 | 0.06 | 0.17 | 0.03 | 0.54 | ||
| 3 | Longifolene | C15H24 | 0.91 | 2.22 | 0.24 | 0.67 | 1.86 | |
| 4 | Camphene | C10H16 | 1.29 | 1.25 | 0.14 | 0.98 | 0.12 | |
| 5 | Pinene | C10H16 | 0.55 | 0.86 | 1.63 | 0.6 | 1.21 | |
| 6 | Sabinene | C10H16 | 0.67 | 1.54 | 0.75 | |||
| 7 | Terpinolene | C10H16 | 0.69 | 0.03 | ||||
| 8 | β-Myrcene | C10H16 | 1.48 | 1.36 | 1.33 | 1.45 | 0.66 | |
| 9 | cis-2-Octene | C8H16 | 0.08 | 0.02 | 0.37 | |||
| 10 | α-Longipinene | C10H16 | 0.14 | 0.07 | ||||
| 11 | (+)-Limonene | C10H16 | 0.24 | 0.47 | 0.75 | 0.37 | 0.14 | |
| 12 | 1,5-dimethyl-1,5-cyclooctadiene | C10H16 | 0.28 | 0.71 | ||||
| 13 | Dipentene | C10H16 | 0.19 | 0.11 | ||||
| 14 | 1-Undecene | C11H22 | 0.18 | 1.16 | 0.02 | 0.38 | 0.5 | |
| 15 | 6,6-Dimethylfulvene | C8H10 | 0.23 | 0.76 | 0.27 | 1.25 | ||
| 16 | cis-3-Methyl-2-pentene | C6H12 | 0.2 | 3.36 | 1.1 | 4.18 | ||
| 17 | Tricyclene | C15H24 | 0.21 | 0.06 | ||||
| 18 | 1-Tridecene | C13H26 | 0.14 | 0.02 | 0.06 | |||
| 19 | Terpinen-4-ol | C10H16 | 0.07 | 0.24 | 0.08 | |||
| 20 | 3-Carene | C10H16 | 0.12 | 0.58 | 0.3 | |||
| 21 | α-Pinene | C10H16 | 0.56 | 0.12 | 0.86 | 1.23 | ||
| 22 | β-Pinene | C10H16 | 2.35 | 1.44 | 1.19 | 0.89 | ||
| 23 | Limonene | C10H16 | 3.17 | 1.23 | 0.89 | 0.56 | ||
| 24 | 2,3-dimethylpent-1-ene | C7H14 | 0.15 | 0.76 | ||||
| 25 | tetramethylethylene | C6H12 | 0.25 | 1.62 | ||||
| 26 | 2-methylhept-2-ene | C8H16 | 0.08 | 0.42 | 1.62 | |||
| 27 | trans-3-methyl-2-pentene | C6H12 | 0.23 | 0.81 | ||||
| 28 | Alcohols | Linalool | C10H18O | 1.16 | 2.69 | 0.92 | 1.75 | 0.47 |
| 29 | Geranyllinalool | C20H34O | 0.42 | 0.05 | ||||
| 30 | 2-Ethylhexanol | C8H18O | 4.53 | 5.67 | 2.68 | 2.69 | 3.84 | |
| 31 | Cedrol | C15H26O | 0.75 | 0.32 | ||||
| 32 | Geraniol | C10H18O | 0.85 | 0.64 | 0.24 | 1.02 | 0.22 | |
| 33 | α-Terpineol | C10H18O | 1.07 | 0.47 | 0.77 | 0.16 | ||
| 34 | Isooctyl alcohol | C8H18O | 0.5 | 0.21 | ||||
| 35 | Tridecanol | C13H28O | 0.14 | 0.21 | 0.87 | 0.72 | ||
| 36 | 2-propylpentan-1-ol | C8H18O | 2.1 | 1.04 | 1.46 | 0.56 | ||
| 37 | Spathulenol | C15H24O | 0.8 | 1.41 | 2.34 | |||
| 38 | Borneol | C10H18O | 0.05 | 0.81 | ||||
| 39 | Citronellol-dextro | C10H18O | 0.21 | 0.14 | ||||
| 40 | 1-Hexadecanol | C16H34O | 0.56 | |||||
| 41 | Isophytol | C20H40O | 1.54 | 0.84 | ||||
| 42 | Phytol | C20H40O | 0.54 | 1.32 | ||||
| 43 | (E)-3-Methylpent-2-en-4-yn-1-ol | C6H8O | 0.58 | 0.47 | ||||
| 44 | Esters | Isoamyl nonanoate | C14H28O2 | 0.56 | 1.98 | 1.2 | 2.54 | 0.53 |
| 45 | 2-Ethylhexyl acetate | C10H20O2 | 0.53 | 0.43 | 0.12 | |||
| 46 | Bornyl acetate | C12H20O2 | 0.98 | 4.89 | 2.6 | 8.7 | 0.89 | |
| 47 | Triethyl citrate | C12H20O7 | 0.32 | 0.03 | ||||
| 48 | Isobornyl acetate | C12H20O2 | 0.68 | 0.79 | ||||
| 49 | L-Bornyl acetate | C12H20O2 | 0.55 | 1.18 | 5.73 | |||
| 50 | Linalyl acetate | C12H20O2 | 1.18 | 0.51 | ||||
| 51 | Ketones | 1-Menthone | C10H18O | 1.21 | 1.14 | 1.33 | 1.3 | 0.78 |
| 52 | Pulegone | C10H18O | 1.02 | 1.89 | 1.9 | 0.52 | ||
| 53 | L (-)-Carvone | C9H12O2 | 0.85 | 0.41 | ||||
| 54 | 6-Methyl-5-hepten-2-one | C8H14O | 0.21 | 0.54 | 0.26 | 0.21 | 0.56 | |
| 55 | 6-Methyl-2-heptanone | C8H16O | 0.81 | 0.11 | ||||
| 56 | 2-Camphanone | C10H16O | 1.02 | 0.23 | 0.15 | |||
| 57 | 2-Methyl-5-(1-methylethenyl)-2-Cyclohexen-1-one | C10H14O | 0.4 | 0.03 | ||||
| 58 | 2-Acetyl-cyclopentanone | C7H10O2 | 0.5 | 0.58 | 1.12 | 0.13 | ||
| 59 | 3-Octanone | C8H16O | 0.89 | 0.24 | 0.57 | |||
| 60 | 3-Ene-nonanone-2 | C9H16O | 0.42 | |||||
| 61 | Aldehydes | α-Citral | C10H16O | 0.26 | 0.84 | 0.42 | 0.89 | 1.2 |
| 62 | Nonanal | C9H18O | 0.5 | 0.82 | 0.51 | 0.08 | ||
| 63 | Undecanal | C11H22O | 0.64 | 0.24 | 0.22 | 0.44 | ||
| 64 | Decyl aldehyde | C10H20O | 0.12 | 0.52 | ||||
| 65 | Heptaldehyde | C7H14O | 0.57 | 0.4 | ||||
| 66 | Octanal | C8H18 | 0.32 | 0.21 | 0.18 | |||
| 67 | Undecan-4-olide | C14H28O | 0.43 | 0.47 | 0.84 | 0.8 | ||
| 68 | Tridecanal | C13H26O | 0.18 | |||||
| 69 | α-Longine alkenal | C10H16O | 0.39 | 0.51 | 0.41 | 0.27 | ||
| 70 | β-Cyclocitral | C10H16O | 0.25 | 0.57 | 0.49 | |||
| 71 | trans-Cinnamaldehyde | C9H8O | 0.27 | 0.5 | ||||
| 72 | trans-2-Decenal | C10H18O | 2.1 | |||||
| 73 | 2-Nonenal | C9H16O | 1.51 | |||||
| 74 | Acids | Lauric acid | C12H24O2 | 0.42 | 0.75 | 0.45 | ||
| 75 | Myristic acid | C14H28O2 | 0.36 | 0.17 | 0.02 | |||
| 76 | Palmitic acid | C16H32O2 | 1.18 | 0.12 | 0.8 | |||
| 77 | Neoabietic acid | C20H30O2 | 5.62 | |||||
| 78 | Neodecanoic acid | C10H20O2 | 0.5 | 0.49 | ||||
| Total | 35.64 | 63.32 | 30.81 | 44.26 | 33.69 | |||
| Serial Number | Species | Chemical Compound | Chemical Formula | Relative Contents of Phytoncide in Autumn (%) | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | D | E | F | ||||
| 1 | Terpenes | α-Pinene | C10H16 | 0.7 | 0.8 | 0.71 | 0.7 | 0.03 |
| 2 | (+)-Limonene | C10H16 | 0.7 | 2.47 | 1.72 | 1.78 | 3.61 | |
| 3 | Artemisia triene | C10H16 | 0.62 | 1.55 | 0.5 | |||
| 4 | 6,6-Dimethylfulvene | C8H10 | 0.12 | 0.06 | 0.26 | |||
| 5 | Camphene | C10H16 | 0.38 | 0.52 | 0.34 | 1.12 | 0.02 | |
| 6 | 1-Undecene | C11H22 | 0.04 | 0.11 | ||||
| 7 | 1-Tridecene | C13H26 | 0.25 | |||||
| 8 | Terpinen-4-ol | C10H16 | 0.5 | 0.4 | 0.3 | 0.12 | ||
| 9 | 3-carene | C10H16 | 0.44 | 0.17 | 0.7 | |||
| 10 | trans-2-Heptene | C7H14 | 0.08 | 0.06 | ||||
| 11 | 1,5-Dimethyl-1,5-cyclooctadiene | C10H16 | 0.72 | |||||
| 12 | trans-2,2-Dimethyl-3-heptene | C9H18 | 0.11 | |||||
| 13 | 2-Ethyl-1-butene | C6H12 | 0.11 | |||||
| 14 | trans-3-Methyl-2-pentene | C6H12 | 0.11 | |||||
| 15 | Dipentene | C10H16 | 0.25 | 1.73 | 0.2 | 1.5 | 4.22 | |
| 16 | (+)-α-Longipinene | C15H24 | 0.2 | 0.22 | ||||
| 17 | Longifolene | C15H24 | 0.94 | 1.05 | 0.03 | 0.7 | 0.63 | |
| 18 | (-)-Thujopsene | C15H24 | 0.35 | 0.05 | 0.25 | |||
| 19 | Cembrene | C20H32 | 0.21 | 0.06 | ||||
| 20 | Octadec-1-ene | C18H36 | 0.06 | |||||
| 21 | trans-Squalene | C30H50 | 0.58 | 0.22 | ||||
| 22 | Squalene | C30H50 | 0.14 | |||||
| 23 | Pinene | C10H16 | 0.08 | 0.96 | 0.05 | 0.18 | ||
| 24 | Valencene | C15H24 | 0.47 | |||||
| 25 | α-Cedrene | C15H24 | 0.62 | 1.92 | ||||
| 26 | Alcohols | Isooctyl alcohol | C8H18O | 0.21 | 0.05 | 0.2 | ||
| 27 | Tridecanol | C13H28O | 0.01 | |||||
| 28 | 2-Hexyl-1-decanol | C16H34O | 0.04 | |||||
| 29 | Hexadecanol | C16H34O | 0.02 | |||||
| 30 | 2-Octyldodecyl alcohol | C20H42O | 0.06 | |||||
| 31 | Tetrahydrolavandulol | C10H20O | 0.4 | |||||
| 32 | Menthol | C10H20O | 0.54 | 0.3 | ||||
| 33 | (3S)-3,7-Dimethyloct-7-en-1-ol | C10H20O | 0.6 | |||||
| 34 | Esters | Isoamyl nonanoate | C14H28O2 | 0.25 | 0.1 | |||
| 35 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | C16H30O4 | 0.17 | 0.11 | 0.08 | |||
| 36 | octyl P-Methoxycinnamate | C18H26O3 | 0.34 | 0.18 | ||||
| 37 | tributyl O-acetylcitrate | C20H34O8 | 2.52 | 0.97 | 0.72 | |||
| 38 | Tributyl citrate | C18H32O7 | 1.52 | 1 | 1.44 | |||
| 39 | Ketones | 3,5,5-Trimethylcyclohex-3-en-1-one | C9H14O | 0.14 | 0.18 | |||
| 40 | Cyclohexane-1,2-dione | C6H8O2 | 0.03 | |||||
| 41 | 6-Methyl-5-hepten-2-one | C8H14O | 0.2 | |||||
| 42 | Cyclopentyl methyl ketone | C7H12O | 2.54 | 0.56 | 0.12 | |||
| 43 | Aldehydes | Decyl aldehyde | C10H20O | 1.56 | 1.23 | 0.05 | ||
| 44 | Nonanal | C9H18O | 0.6 | 0.4 | ||||
| 45 | Octanal | C8H18 | 0.2 | |||||
| 46 | Heptaldehyde | C7H14O | 0.2 | 0.45 | ||||
| 47 | Dodecanal | C12H24O | 0.29 | 0.1 | ||||
| 48 | Tridecanal | C13H26O | 0.7 | |||||
| 49 | Undecan-4-olide | C14H28O | 0.7 | |||||
| 50 | L-Perillylaldehyde | C10H14O | 0.72 | |||||
| 51 | trans-2-Dodecen-1-al | C12H22O | 0.17 | |||||
| 52 | Isocyclocitral | C10H16O | 0.07 | |||||
| 53 | trans-2-Pentenal | C5H8O | 0.65 | 0.06 | ||||
| 54 | Acids | (5-Methyl-2-propan-2-ylhexyl) acetate | C12H24O2 | 0.06 | ||||
| 55 | 8-Dodecen-1-ol,1-acetate, (8E)- | C14H26O2 | 0.04 | |||||
| 56 | 2-Propenoic acid,3-(4-methoxyphenyl)-, 2-ethylhexyl ester, (2E)- | C18H26O3 | 0.23 | |||||
| 57 | Palmitic acid | C16H32O2 | 2.12 | 1.26 | ||||
| 58 | trans-13-Octadecenoic acid | C18H34O2 | 0.06 | |||||
| 59 | Stearic acid | C18H36O2 | 0.45 | 0.27 | ||||
| Total | 7.77 | 26.74 | 11.02 | 13.06 | 10.45 | |||
| Serial Number | Species | Chemical Compound | Chemical Formula | Relative Contents of Phytoncide in Winter (%) | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | D | E | F | ||||
| 1 | Terpenes | 1-Octadecene | C18H36 | 0.18 | 1.43 | 0.84 | 0.21 | |
| 2 | 1-Heptadecene | C17H34 | 0.2 | 1.5 | 0.7 | 0.73 | 0.36 | |
| 3 | 3,3-Dimethyl-1-hexene | C8H16 | 0.18 | |||||
| 4 | 1,5-Dimethyl-1,5-cyclooctadiene | C10H16 | 0.79 | |||||
| 5 | Dipentene | C10H16 | 0.18 | 1.09 | 1.23 | |||
| 6 | Alcohols | 2-Ethylhexanol | C8H18O | 0.7 | 0.63 | 1.03 | 0.56 | 0.95 |
| 7 | Linalool | C10H18O | 1.14 | 0.34 | 0.56 | 0.32 | ||
| 8 | Esters | Isoamyl nonanoate | C14H28O2 | 0.67 | 0.57 | 0.49 | ||
| 9 | 2-Ethylhexyl acetate | C10H20O2 | 0.59 | 0.63 | ||||
| 10 | Bornyl acetate | C12H20O2 | 0.54 | 0.12 | 0.13 | 0.4 | ||
| 11 | Ketones | 2-Camphanone | C10H16O | 0.58 | 0.18 | |||
| 12 | Acids | Lauric acid | C12H24O2 | 0.4 | 0.51 | 0.12 | ||
| 13 | Palmitic acid | C16H32O2 | 0.57 | 0.23 | ||||
| Total | 2.87 | 9.7 | 2.32 | 5.22 | 2.47 | |||
| Season | Forest Types | NAIC | AOC | HCI | PRC | FCHI | Grades |
|---|---|---|---|---|---|---|---|
| Spring | Phyllostachys edulis forest | 0.237 | 0.359 | 0.995 | 0.41 | 0.533 | Level I |
| Subtropical evergreen broad-leaved forest | 0.869 | 0.314 | 0.944 | 0.826 | 0.683 | Level I | |
| Forestless control group | 0.039 | 0.259 | 0.752 | 0 | 0.298 | Level II | |
| Liquidambar formosana forest | 0.128 | 0.375 | 0.777 | 0.352 | 0.452 | Level I | |
| Cunninghamia lanceolata forest | 0.213 | 0.445 | 0.636 | 0.553 | 0.504 | Level I | |
| Coniferous and broad-leaved mixed forest | 0.201 | 0.493 | 0.538 | 0.455 | 0.465 | Level I | |
| Summer | Phyllostachys edulis forest | 0.452 | 0.753 | 1 | 0.563 | 0.736 | Level I |
| Subtropical evergreen broad-leaved forest | 1 | 0.695 | 0.991 | 1 | 0.891 | Level I | |
| Forestless control group | 0.07 | 0.601 | 0.395 | 0 | 0.324 | Level II | |
| Liquidambar formosana forest | 0.222 | 0.814 | 0.656 | 0.486 | 0.620 | Level I | |
| Cunninghamia lanceolata forest | 0.275 | 0.868 | 0.559 | 0.699 | 0.678 | Level I | |
| Coniferous and broad-leaved mixed forest | 0.272 | 1 | 0.495 | 0.532 | 0.660 | Level I | |
| Autumn | Phyllostachys edulis forest | 0.23 | 0.311 | 0.818 | 0.123 | 0.387 | Level II |
| Subtropical evergreen broad-leaved forest | 0.848 | 0.275 | 0.826 | 0.422 | 0.522 | Level I | |
| Forestless control group | 0.01 | 0.227 | 0.381 | 0 | 0.183 | Level III | |
| Liquidambar formosana forest | 0.051 | 0.339 | 0.89 | 0.174 | 0.413 | Level II | |
| Cunninghamia lanceolata forest | 0.108 | 0.384 | 0.881 | 0.206 | 0.441 | Level II | |
| Coniferous and broad-leaved mixed forest | 0.101 | 0.459 | 0.917 | 0.165 | 0.465 | Level I | |
| Winter | Phyllostachys edulis forest | 0.169 | 0.097 | 0.124 | 0.068 | 0.103 | Level III |
| Subtropical evergreen broad-leaved forest | 0.605 | 0.081 | 0.079 | 0.23 | 0.175 | Level III | |
| Forestless control group | 0 | 0 | 0 | 0 | 0 | Level V | |
| Liquidambar formosana forest | 0.115 | 0.193 | 0.511 | 0.055 | 0.232 | Level II | |
| Cunninghamia lanceolata forest | 0.159 | 0.223 | 0.649 | 0.124 | 0.304 | Level II | |
| Coniferous and broad-leaved mixed forest | 0.144 | 0.252 | 0.681 | 0.059 | 0.303 | Level II |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.-x.; Hu, F.-f.; He, S.-y.; Qiu, Q.; Su, Y.; He, Q.; Li, J.-y. Comprehensive Evaluation of Healthcare Benefits of Different Forest Types: A Case Study in Shimen National Forest Park, China. Forests 2021, 12, 207. https://doi.org/10.3390/f12020207
Zhu S-x, Hu F-f, He S-y, Qiu Q, Su Y, He Q, Li J-y. Comprehensive Evaluation of Healthcare Benefits of Different Forest Types: A Case Study in Shimen National Forest Park, China. Forests. 2021; 12(2):207. https://doi.org/10.3390/f12020207
Chicago/Turabian StyleZhu, Shu-xin, Fei-fei Hu, Shuang-yu He, Quan Qiu, Yan Su, Qian He, and Ji-yue Li. 2021. "Comprehensive Evaluation of Healthcare Benefits of Different Forest Types: A Case Study in Shimen National Forest Park, China" Forests 12, no. 2: 207. https://doi.org/10.3390/f12020207
APA StyleZhu, S.-x., Hu, F.-f., He, S.-y., Qiu, Q., Su, Y., He, Q., & Li, J.-y. (2021). Comprehensive Evaluation of Healthcare Benefits of Different Forest Types: A Case Study in Shimen National Forest Park, China. Forests, 12(2), 207. https://doi.org/10.3390/f12020207







