Using Isopropanol as a Capping Agent in the Hydrothermal Liquefaction of Kraft Lignin in Near-Critical Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Conditions
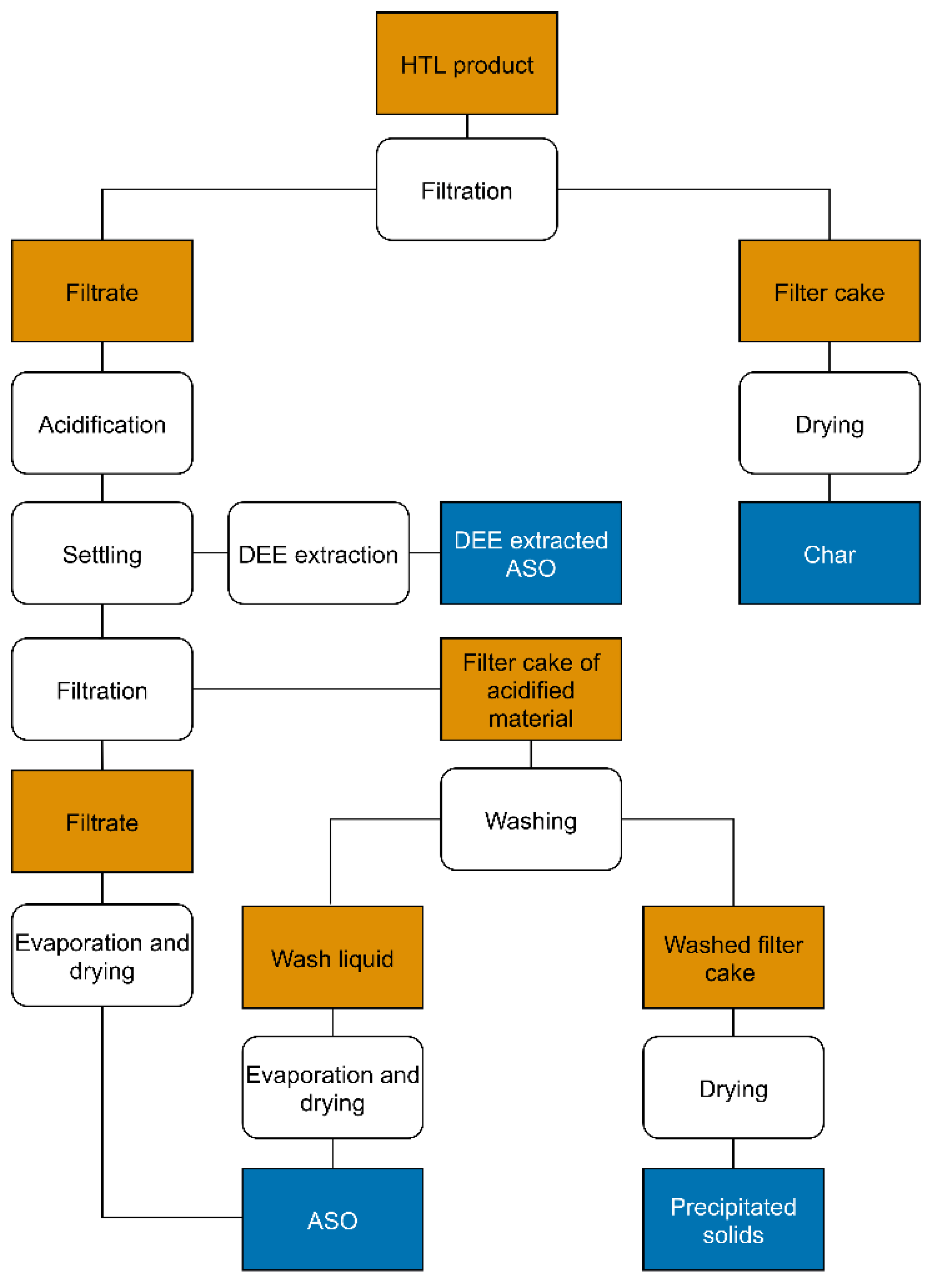
2.2.2. Fractionation of Products
2.2.3. Analytical Procedures
3. Results and Discussion
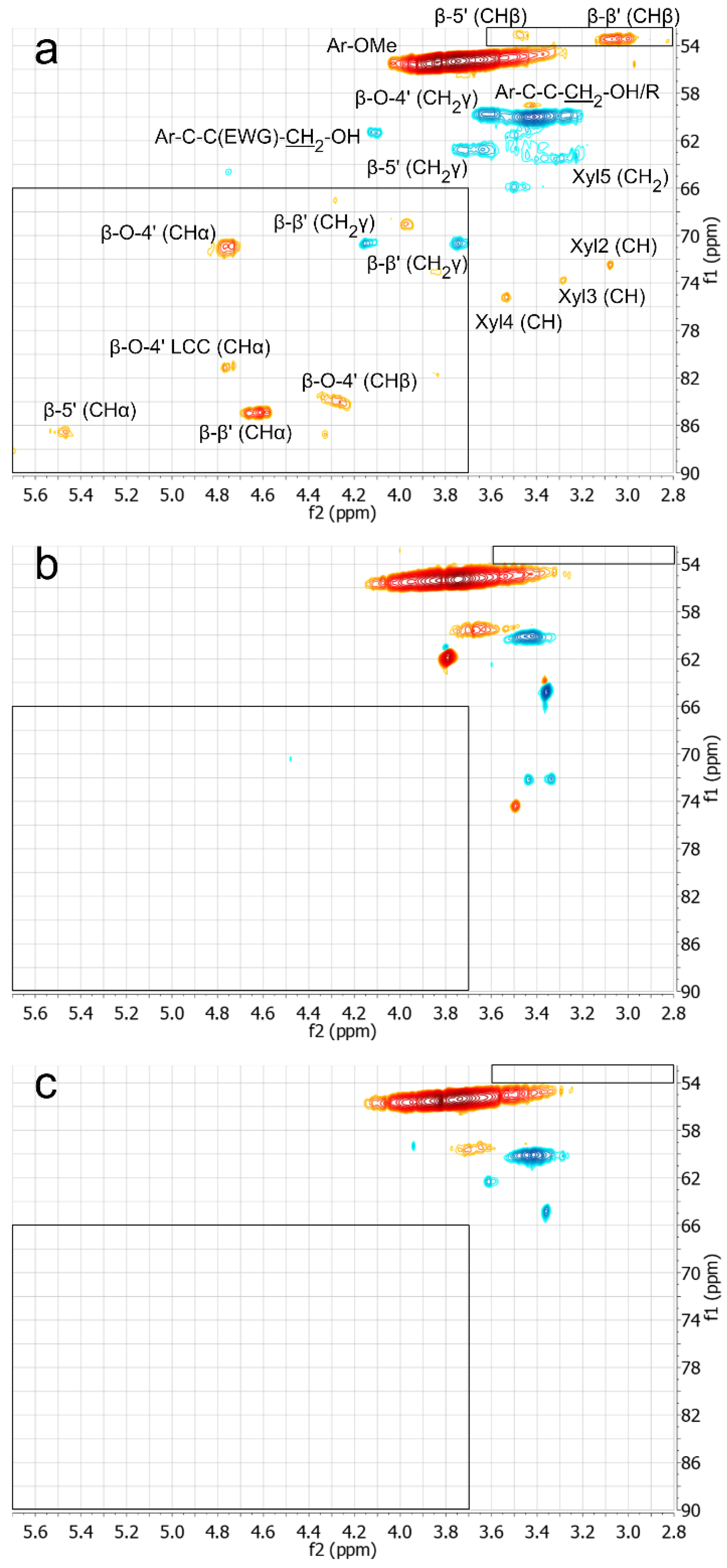
3.1. Structural Changes
3.2. Yields
3.3. Elemental Composition
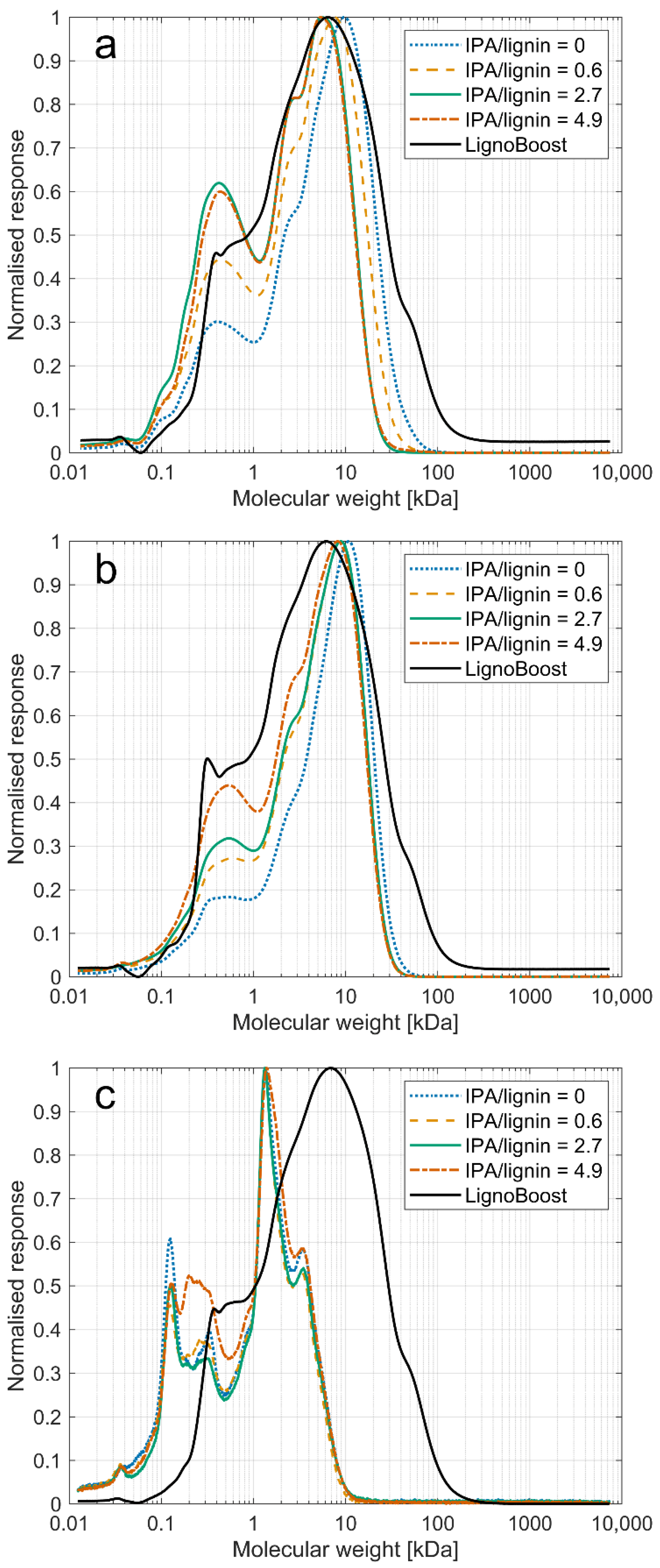
3.4. Molar Masses
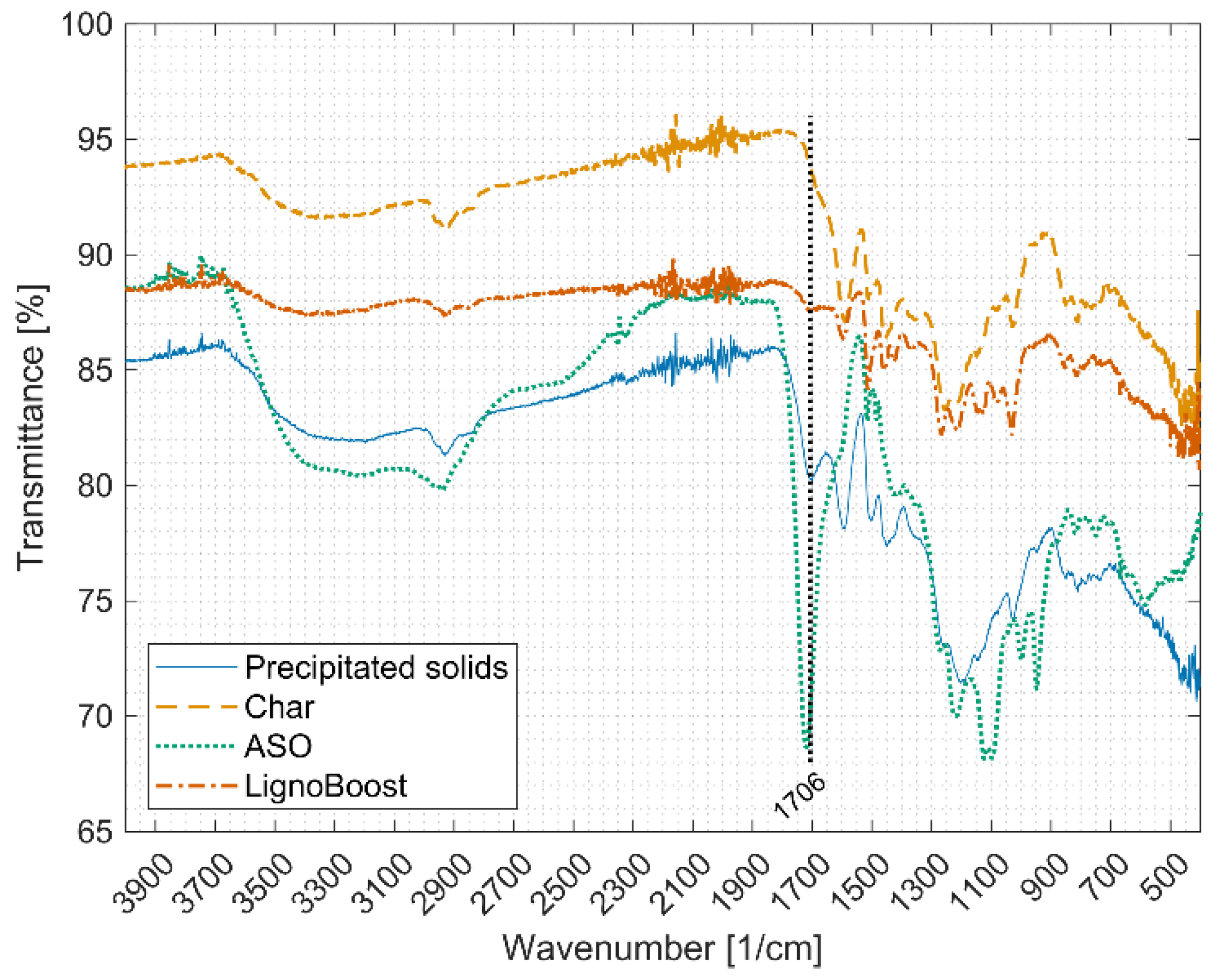
3.5. Functional Groups
3.6. Monoaromatics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAS | Atomic absorption spectroscopy |
| ASO | Acid-soluble organics |
| ATR | Attenuated total reflectance |
| BHT | Butylated hydroxytoluene |
| CHNS | Elemental analysis |
| DEE | Diethyl ether |
| DMSO | Dimethyl sulfoxide |
| DMSO-d6 | Deuterated dimethyl sulfoxide |
| EtOH | Ethanol |
| EWG | Electron withdrawing group |
| FTIR | Fourier transform infrared spectroscopy |
| GC | Gas chromatography |
| GPC | Gel permeation chromatography |
| HSQC | Heteronuclear single quantum coherence spectroscopy |
| HTL | Hydrothermal liquefaction |
| IPA | Isopropanol |
| LCC | Lignin–carbohydrate complex |
| MeOH | Methanol |
| MN MS | Number average molar mass Mass spectrometry |
| MW | Mass average molar mass |
| NMR | Nuclear magnetic resonance |
| PD | Polydispersity |
| UV | Ultraviolet light |
| Xyl | Xylan |
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef]
- Lappalainen, J.; Baudouin, D.; Hornung, U.; Schuler, J.; Melin, K.; Bjelić, S.; Vogel, F.; Konttinen, J.; Joronen, T. Sub- and Supercritical Water Liquefaction of Kraft Lignin and Black Liquor Derived Lignin. Energies 2020, 13, 3309. [Google Scholar] [CrossRef]
- Otromke, M.; White, R.J.; Sauer, J. Hydrothermal base catalyzed depolymerization and conversion of technical lignin—An introductory review. Carbon Resour. Convers. 2019, 2, 59–71. [Google Scholar] [CrossRef]
- Serrano, L.; Antonio, J.; Cristina, C.; Sancho, G. Lignin Depolymerization to BTXs. Top. Curr. Chem. 2019, 1–28. [Google Scholar] [CrossRef]
- Dellon, L.D.; Yanez, A.J.; Li, W.; Mabon, R.; Broadbelt, L.J. Computational Generation of Lignin Libraries from Diverse Biomass Sources. Energy Fuels 2017, 31, 8263–8274. [Google Scholar] [CrossRef]
- Saisu, M.; Sato, T.; Watanabe, M.; Adschiri, T.; Arai, K. Conversion of lignin with supercritical water-phenol mixtures. Energy Fuels 2003, 17, 922–928. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Lora, J. Industrial Commercial Lignins: Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 225–241. ISBN 978-0-08-045316-3. [Google Scholar]
- Tomani, P. The lignoboost process. Cellul. Chem. Technol. 2010, 44, 53–58. [Google Scholar]
- Berlin, A.; Balakshin, M. Industrial Lignins: Analysis, Properties, and Applications. In Bioenergy Research: Advances and Applications; Gupta, V.K., Tuohy, M.G., Kubicek, C.P., Saddler, J., Xu, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 315–336. ISBN 9780444595614. [Google Scholar]
- Strassberger, Z.; Tanase, S.; Rothenberg, G. The pros and cons of lignin valorisation in an integrated biorefinery. RSC Adv. 2014, 4, 25310–25318. [Google Scholar] [CrossRef]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Kruse, A.; Dahmen, N. Water—A magic solvent for biomass conversion. J. Supercrit. Fluids 2015, 96, 36–45. [Google Scholar] [CrossRef]
- Belkheiri, T.; Vamling, L.; Nguyen, T.D.H.; Maschietti, M.; Olausson, L.; Andersson, S.-I.; Åmand, L.-E.; Theliander, H. Kraft lignin depolymerization in near-critical water: Effect of changing co-solvent. Cellul. Chem. Technol. 2014, 48, 813–818. [Google Scholar]
- Abdelaziz, O.Y.; Li, K.; Tunå, P.; Hulteberg, C.P. Continuous catalytic depolymerisation and conversion of industrial kraft lignin into low-molecular-weight aromatics. Biomass Convers. Biorefinery 2018, 8, 455–470. [Google Scholar] [CrossRef]
- Schuler, J.; Hornung, U.; Kruse, A.; Dahmen, N.; Sauer, J. Hydrothermal Liquefaction of Lignin. J. Biomater. Nanobiotechnol. 2017, 8, 96–108. [Google Scholar] [CrossRef]
- Nguyen, T.D.H.; Maschietti, M.; Belkheiri, T.; Åmand, L.E.; Theliander, H.; Vamling, L.; Olausson, L.; Andersson, S.I. Catalytic depolymerisation and conversion of Kraft lignin into liquid products using near-critical water. J. Supercrit. Fluids 2014, 86, 67–75. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.-I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal liquefaction of kraft lignin in sub-critical water: The influence of the sodium and potassium fraction. Biomass Convers. Biorefinery 2018, 8, 585–595. [Google Scholar] [CrossRef]
- Nguyen, T.D.H.; Maschietti, M.; Åmand, L.E.; Vamling, L.; Olausson, L.; Andersson, S.I.; Theliander, H. The effect of temperature on the catalytic conversion of Kraft lignin using near-critical water. Bioresour. Technol. 2014, 170, 196–203. [Google Scholar] [CrossRef]
- Mattsson, C.; Andersson, S.I.; Belkheiri, T.; Åmand, L.E.; Olausson, L.; Vamling, L.; Theliander, H. Using 2D NMR to characterize the structure of the low and high molecular weight fractions of bio-oil obtained from LignoBoostTM kraft lignin depolymerized in subcritical water. Biomass Bioenergy 2016, 95, 364–377. [Google Scholar] [CrossRef]
- Thring, R.W. Alkaline degradation of ALCELL® lignin. Biomass Bioenergy 1994, 7, 125–130. [Google Scholar] [CrossRef]
- Karagöz, S.; Bhaskar, T.; Muto, A.; Sakata, Y. Hydrothermal upgrading of biomass: Effect of K2CO3 concentration and biomass/water ratio on products distribution. Bioresour. Technol. 2006, 97, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Dunn, K.G.; Hobson, P.A. Hydrothermal liquefaction of lignin. In Sugarcane-Based Biofuels and Bioproducts; O’Hara, I., Mundree, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 165–206. ISBN 9781118719923. [Google Scholar]
- Chandler, K.; Deng, F.; Dillow, A.K.; Liotta, C.L.; Eckert, C.A. Alkylation Reactions in Near-Critical Water in the Absence of Acid Catalysts. Ind. Eng. Chem. Res. 1997, 36, 5175–5179. [Google Scholar] [CrossRef]
- Arturi, K.R.; Strandgaard, M.; Nielsen, R.P.; Søgaard, E.G.; Maschietti, M. Hydrothermal liquefaction of lignin in near-critical water in a new batch reactor: Influence of phenol and temperature. J. Supercrit. Fluids 2017, 123, 28–39. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Bobleter, O.; Concin, R. Degradation of poplar lignin by hydrothermal treatment. Cellul. Chem. Technol. 1979, 13, 583–593. [Google Scholar]
- Zhang, B.; Huang, H.J.; Ramaswamy, S. Reaction kinetics of the hydrothermal treatment of lignin. Appl. Biochem. Biotechnol. 2008, 147, 119–131. [Google Scholar] [CrossRef]
- Lee, H.-S.; Jae, J.; Ha, J.-M.; Suh, D.J. Hydro- and solvothermolysis of kraft lignin for maximizing production of monomeric aromatic chemicals. Bioresour. Technol. 2016, 203, 142–149. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, Z.; Alma, M.H.; Sun, D.; Zhang, W.; Jiang, J. Direct liquefaction of alkali lignin in methanol and water mixture for the production of oligomeric phenols and aromatic ethers. J. Biobased Mater. Bioenergy 2016, 10, 76–80. [Google Scholar] [CrossRef]
- Cheng, S.; Wilks, C.; Yuan, Z.; Leitch, M.; Xu, C. Hydrothermal degradation of alkali lignin to bio-phenolic compounds in sub/supercritical ethanol and water-ethanol co-solvent. Polym. Degrad. Stab. 2012, 97, 839–848. [Google Scholar] [CrossRef]
- Sebhat, W. Valorisation de la Lignine par Catalyse Hétérogène en Condition Sous-Critique en Milieux Aqueux et eau/alcool. Ph.D. Dissertation, Université de Lyon, Lyon, France, 2016. [Google Scholar]
- Wu, Y.; Chen, Y.; Wu, K. Role of co-solvents in biomass conversion reactions using sub/supercritical water. In Near-Critical and Supercritical Water and Their Applications for Biorefineries; Fang, Z., Xu, C., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 69–98. [Google Scholar]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Hydrothermal conversion of lignin: A review. Renew. Sustain. Energy Rev. 2013, 27, 546–558. [Google Scholar] [CrossRef]
- Deepa, A.K.; Dhepe, P.L. Solid acid catalyzed depolymerization of lignin into value added aromatic monomers. RSC Adv. 2014, 4, 12625–12629. [Google Scholar] [CrossRef]
- Zakzeski, J.; Jongerius, A.L.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Catalytic lignin valorization process for the production of aromatic chemicals and hydrogen. ChemSusChem 2012, 5, 1602–1609. [Google Scholar] [CrossRef]
- Ye, Y.; Fan, J.; Chang, J. Effect of reaction conditions on hydrothermal degradation of cornstalk lignin. J. Anal. Appl. Pyrolysis 2012, 94, 190–195. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, Y.; Fan, J.; Chang, J. Novel method for production of phenolics by combining lignin extraction with lignin depolymerization in aqueous ethanol. Ind. Eng. Chem. Res. 2012, 51, 103–110. [Google Scholar] [CrossRef]
- Yuan, Z.S.; Cheng, S.N.; Leitch, M.; Xu, C.B. Hydrolytic degradation of alkaline lignin in hot-compressed water and ethanol. Bioresour. Technol. 2010, 101, 9308–9313. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Yagi, T.; Shinohara, S.; Fukunaga, T.; Nakasaka, Y.; Tago, T.; Masuda, T. Production of phenols from lignin via depolymerization and catalytic cracking. Fuel Process. Technol. 2013, 108, 69–75. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Tang, X.; Sun, Y.; Zeng, X.; Liu, S.; Lin, L. Depolymerization of cellulolytic enzyme lignin for the production of monomeric phenols over Raney Ni and acidic zeolite catalysts. Energy & Fuels 2015, 29, 1662–1668. [Google Scholar] [CrossRef]
- Güvenatam, B.; Heeres, E.H.J.; Pidko, E.A.; Hensen, E.J.M. Lewis-acid catalyzed depolymerization of Protobind lignin in supercritical water and ethanol. Catal. Today 2016, 259, 460–466. [Google Scholar] [CrossRef]
- Mattsson, C.; Hasani, M.; Dang, B.; Mayzel, M.; Theliander, H. About structural changes of lignin during kraft cooking and the kinetics of delignification. Holzforschung 2017, 71, 545–553. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.-I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal Liquefaction of Kraft Lignin in Subcritical Water: Influence of Phenol as Capping Agent. Energy Fuels 2018, 32, 5923–5932. [Google Scholar] [CrossRef]
- Sasaki, M.; Goto, M. Thermal decomposition of guaiacol in sub- and supercritical water and its kinetic analysis. J. Mater. Cycles Waste Manag. 2011, 13, 68–79. [Google Scholar] [CrossRef]
- Sato, T.; Sekiguchi, G.; Adschiri, T.; Arai, K. Ortho-selective alkylation of phenol with 2-propanol without catalyst in supercritical water. Ind. Eng. Chem. Res. 2002, 41, 3064–3070. [Google Scholar] [CrossRef]


| IPA/Dry Lignin [g/g] | T [°C] | P [bar] | Reaction Product pH | Na2CO3 [wt %] | Water [wt %] | IPA [wt %] | Lignin (Dry) [wt %] |
|---|---|---|---|---|---|---|---|
| IPA Series | |||||||
| 0 | 320 | 250 | 8.7 | 1.6 | 92.9 | 0.0 | 5.5 |
| 0.6 | 320 | 250 | 8.7 | 1.6 | 88.9 | 3.6 | 5.9 |
| 2.7 | 320 | 250 | 9.2 | 1.6 | 80.7 | 12.9 | 4.8 |
| 4.9 | 320 | 250 | 9.0 | 1.6 | 69.3 | 24.2 | 4.9 |
| Ref. | 320 | 250 | 9.2 | 1.6 | 85.0 | 13.4 | 0.0 |
| Temperature Series | |||||||
| 2.7 | 290 | 250 | 8.9 | 1.6 | 78.0 | 14.9 | 5.5 |
| 2.7 | 320 | 250 | 9.2 | 1.6 | 80.7 | 12.9 | 4.8 |
| 2.7 | 335 | 250 | 9.2 | 1.6 | 80.6 | 13.0 | 4.8 |
| Char | Precipitated Solids | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | C [wt %] | H [wt %] | O a [wt %] | S [wt %] | C [wt %] | H [wt %] | O a [wt %] | S [wt %] |
| IPA Series | ||||||||
| LignoBoost | 68.0 ± 0.0 | 5.7 ± 0.0 | 24.4 ± 0.1 | 1.97 ± 0.2 | 68.0 ± 0.0 | 5.7 ± 0.0 | 24.4 ± 0.1 | 1.97 ± 0.2 |
| 0 | 72.3 ± 0.1 | 4.8 ± 0.0 | 22.9 ± 0.1 | <0.8 b | 71.3 ± 0.0 | 4.8 ± 0.0 | 23.9 ± 0.0 | <0.8 b |
| 0.6 | 73.5 ± 0.5 | 4.9 ± 0.0 | 21.6 ± 0.5 | <0.8 b | 70.0 ± 0.1 | 4.8 ± 0.0 | 25.3 ± 0.1 | <0.8 b |
| 2.7 | 73.8 ± 2.0 | 5.1 ± 0.1 | 21.1 ± 2.1 | <0.8 b | 72.5 ± 0.1 | 5.0 ± 0.0 | 22.5 ± 0.1 | <0.8 b |
| 4.9 | 70.6 ± 0.0 | 5.0 ± 0.0 | 24.4 ± 0.1 | <0.8 b | 72.9 ± 0.1 | 5.2 ± 0.0 | 21.9 ± 0.1 | <0.8 b |
| Temperature Series | ||||||||
| LignoBoost | 68.0 ± 0.0 | 5.7 ± 0.0 | 24.4 ± 0.1 | 1.97 ± 0.2 | 68.0 ± 0.0 | 5.7 ± 0.0 | 24.4 ± 0.1 | 1.97 ± 0.2 |
| 290 °C | 70.1 ± 0.6 | 5.1 ± 0.1 | 24.9 ± 0.7 | <0.8 b | 72.3 ± 0.2 | 5.2 ± 0.0 | 22.6 ± 0.2 | <0.8 b |
| 320 °C | 73.8 ± 2.0 | 5.1 ± 0.1 | 21.1 ± 2.1 | <0.8 b | 72.5 ± 0.1 | 5.0 ± 0.0 | 22.5 ± 0.1 | <0.8 b |
| 335 °C | 76.1 ± 0.1 | 5.2 ± 0.0 | 18.7 ± 0.0 | <0.8 b | 70.9 ± 0.4 | 4.8 ± 0.0 | 24.3 ± 0.3 | <0.8 b |
| Char | Precipitated Solids | ASO | ||||
|---|---|---|---|---|---|---|
| Sample | MW [kDa] | PD | MW [kDa] | PD | MW [kDa] | PD |
| IPA Series | ||||||
| LignoBoost | 11.38 ± 0.08 | 7.0 ± 0.0 | 11.38 ± 0.08 | 7.0 ± 0.0 | 11.38 ± 0.08 | 7.0 ± 0.0 |
| 0 | 8.21 ± 0.04 | 6.4 ± 0.0 | 8.34 ± 0.03 | 4.7 ± 0.0 | 1.66 ± 0.01 | 5.3 ± 0.1 |
| 0.6 | 5.83 ± 0.01 | 5.8 ± 0.0 | 6.44 ± 0.01 | 4.6 ± 0.0 | 1.56 ± 0.00 | 5.1 ± 0.1 |
| 2.7 | 4.03 ± 0.01 | 5.2 ± 0.0 | 6.37 ± 0.03 | 4.8 ± 0.0 | 1.69 ± 0.02 | 5.1 ± 0.0 |
| 4.9 | 4.14 ± 0.00 | 4.9 ± 0.0 | 5.65 ± 0.01 | 5.0 ± 0.0 | 1.58 ± 0.00 | 4.8 ± 0.0 |
| Temperature Series | ||||||
| LignoBoost | 11.38 ±0.08 | 7.0 ± 0.0 | 11.38 ± 0.08 | 7.0 ± 0.0 | 11.38 ± 0.08 | 7.0 ± 0.0 |
| 290 °C | 4.86 ± 0.00 | 4.7 ± 0.0 | 5.96 ± 0.00 | 4.6 ± 0.0 | 1.38 ± 0.00 | 4.7 ± 0.1 |
| 320 °C | 4.03 ± 0.01 | 5.2 ± 0.0 | 6.37 ± 0.03 | 4.8 ± 0.0 | 1.69 ± 0.02 | 5.1 ± 0.0 |
| 335 °C | 3.94 ± 0.01 | 5.4 ± 0.0 | 6.77 ± 0.01 | 5.0 ± 0.0 | 1.89 ± 0.00 | 4.9 ± 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahlbom, A.; Maschietti, M.; Nielsen, R.; Lyckeskog, H.; Hasani, M.; Theliander, H. Using Isopropanol as a Capping Agent in the Hydrothermal Liquefaction of Kraft Lignin in Near-Critical Water. Energies 2021, 14, 932. https://doi.org/10.3390/en14040932
Ahlbom A, Maschietti M, Nielsen R, Lyckeskog H, Hasani M, Theliander H. Using Isopropanol as a Capping Agent in the Hydrothermal Liquefaction of Kraft Lignin in Near-Critical Water. Energies. 2021; 14(4):932. https://doi.org/10.3390/en14040932
Chicago/Turabian StyleAhlbom, Anders, Marco Maschietti, Rudi Nielsen, Huyen Lyckeskog, Merima Hasani, and Hans Theliander. 2021. "Using Isopropanol as a Capping Agent in the Hydrothermal Liquefaction of Kraft Lignin in Near-Critical Water" Energies 14, no. 4: 932. https://doi.org/10.3390/en14040932
APA StyleAhlbom, A., Maschietti, M., Nielsen, R., Lyckeskog, H., Hasani, M., & Theliander, H. (2021). Using Isopropanol as a Capping Agent in the Hydrothermal Liquefaction of Kraft Lignin in Near-Critical Water. Energies, 14(4), 932. https://doi.org/10.3390/en14040932








