Enhanced Cementation of Co2+ and Ni2+ from Sulfate and Chloride Solutions Using Aluminum as an Electron Donor and Conductive Particles as an Electron Pathway
Abstract
:1. Introduction
2. Materials and Methods
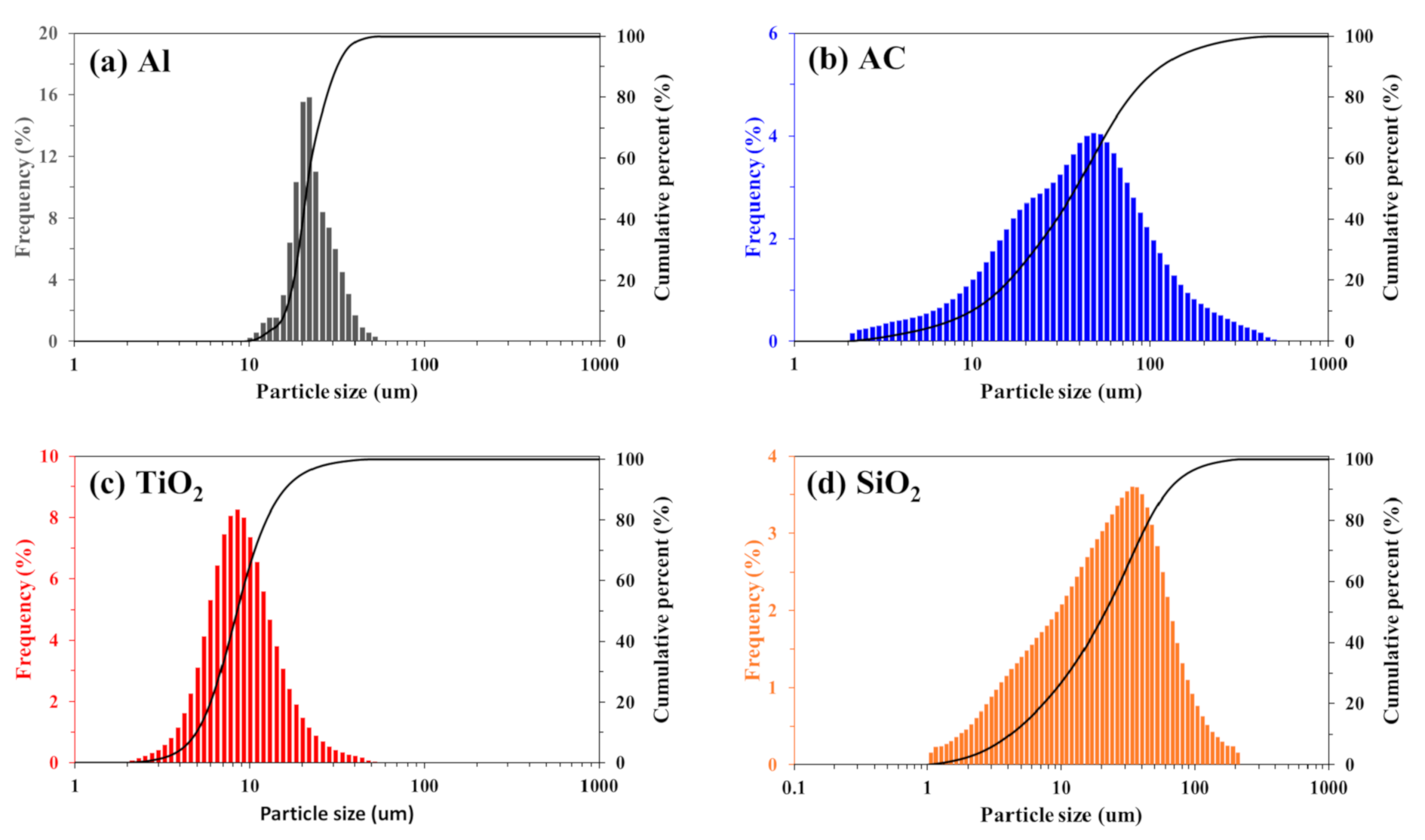
2.1. Materials
2.2. Recovery of Co2+ and Ni2+ from Sulfate and Chloride Solutions
2.2.1. Preparation of Co2+ and Ni2+ Solutions
2.2.2. Cementation Tests
2.2.3. Surface Analysis
3. Results and Discussion
3.1. Recovery of Co2+ and Ni2+
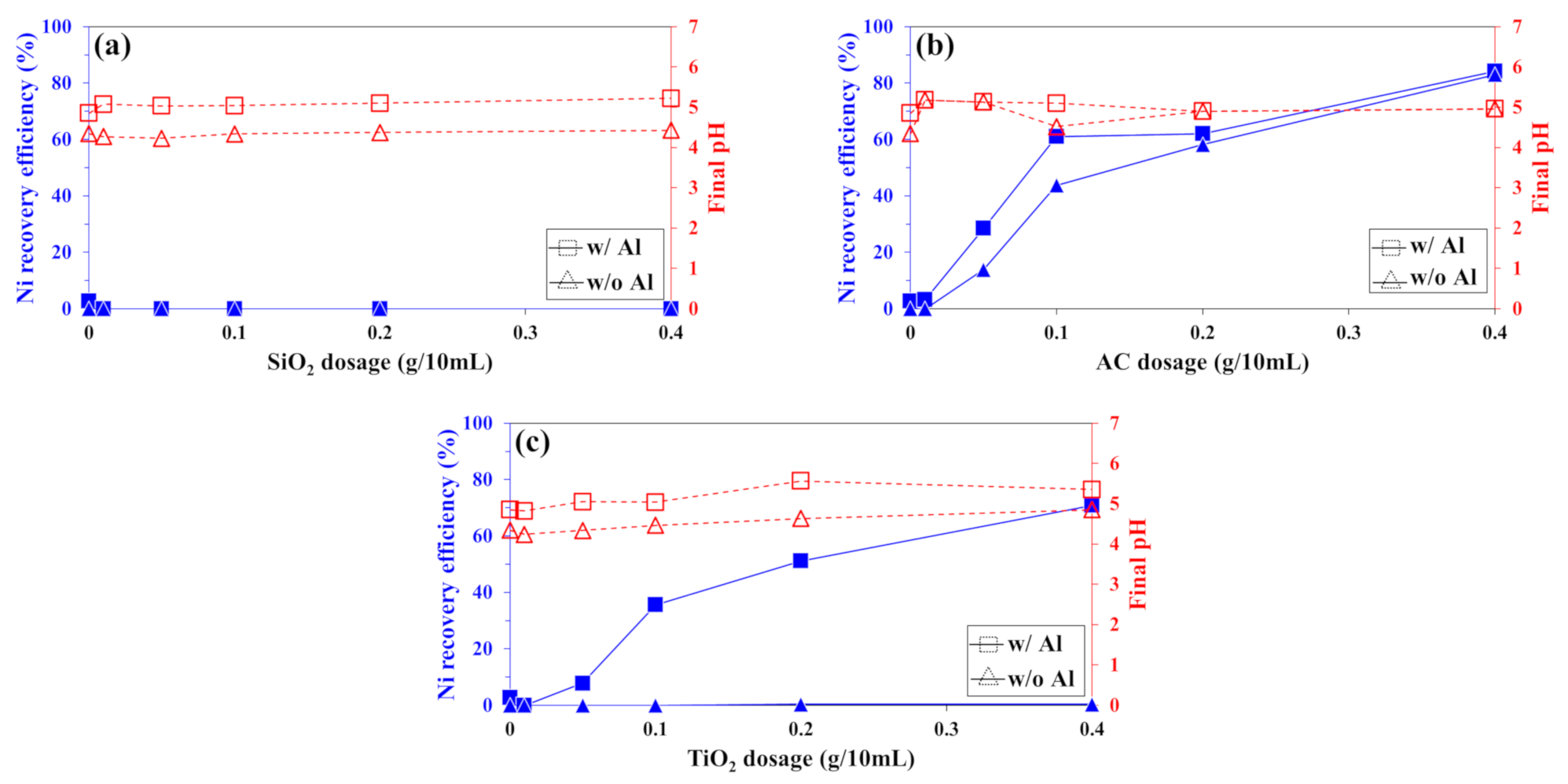
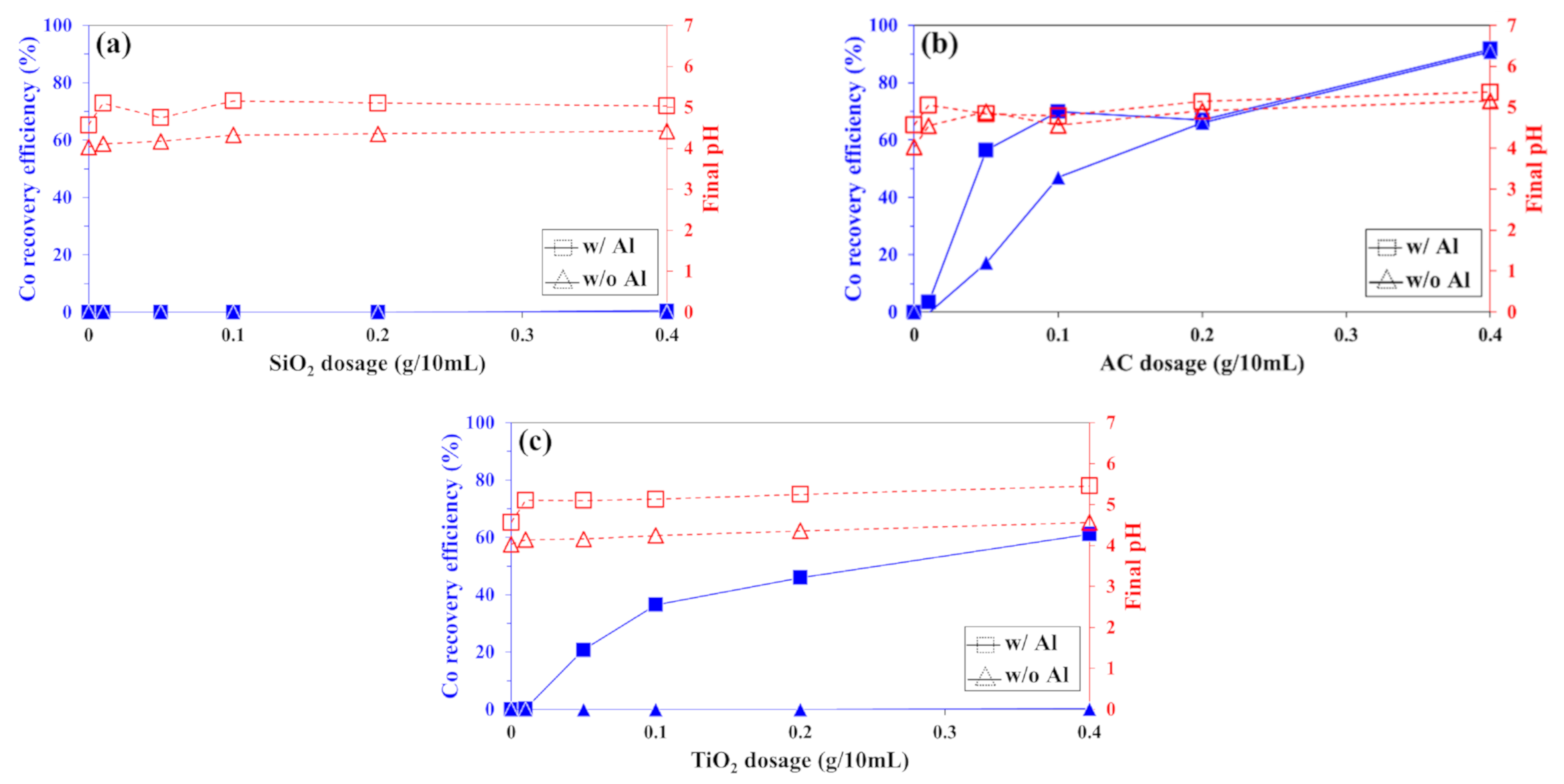
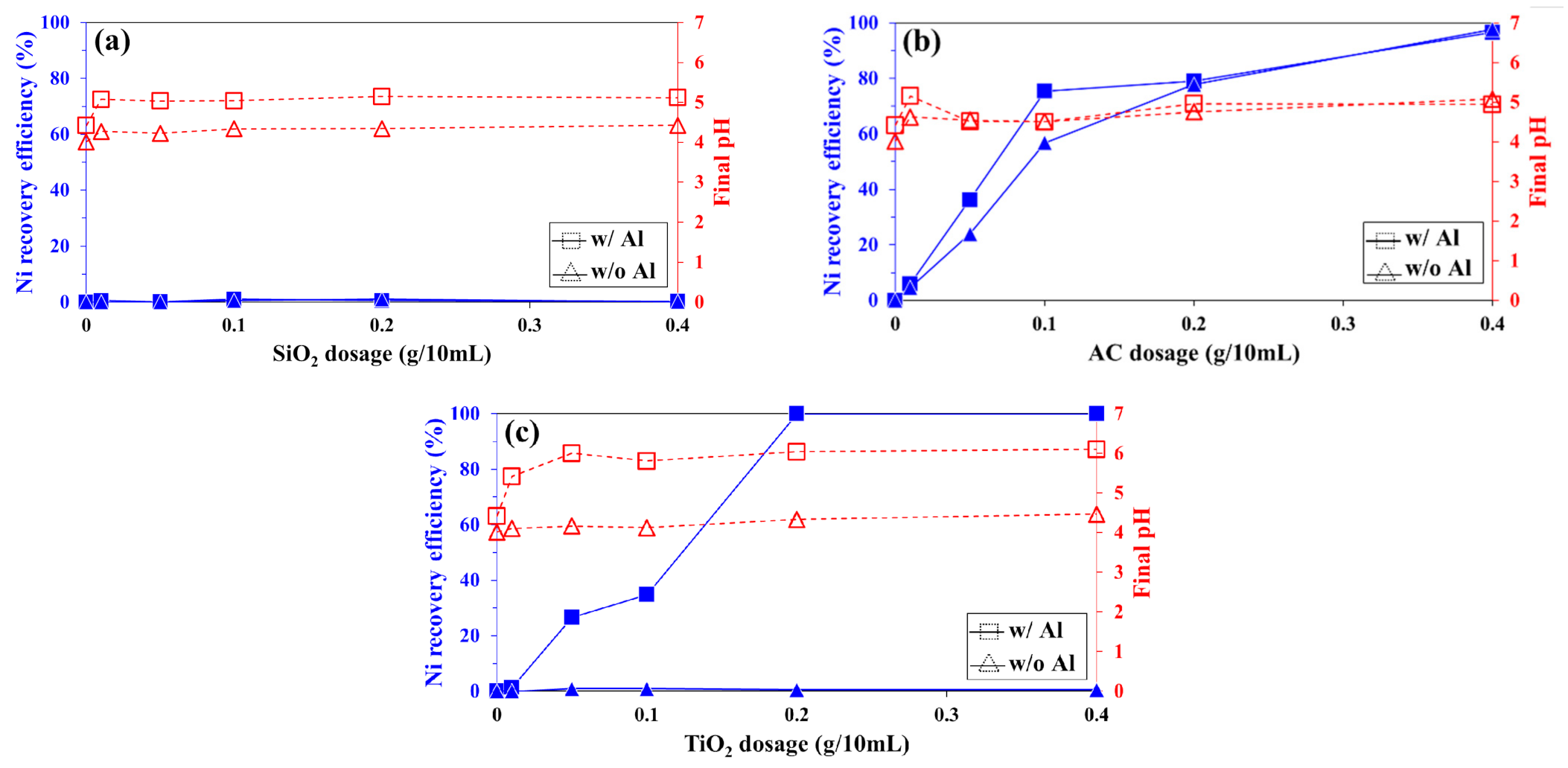
3.1.1. Recovery of Co2+ and Ni2+ from Sulfate Solution
3.1.2. Recovery of Co2+ and Ni2+ from Chloride Solution
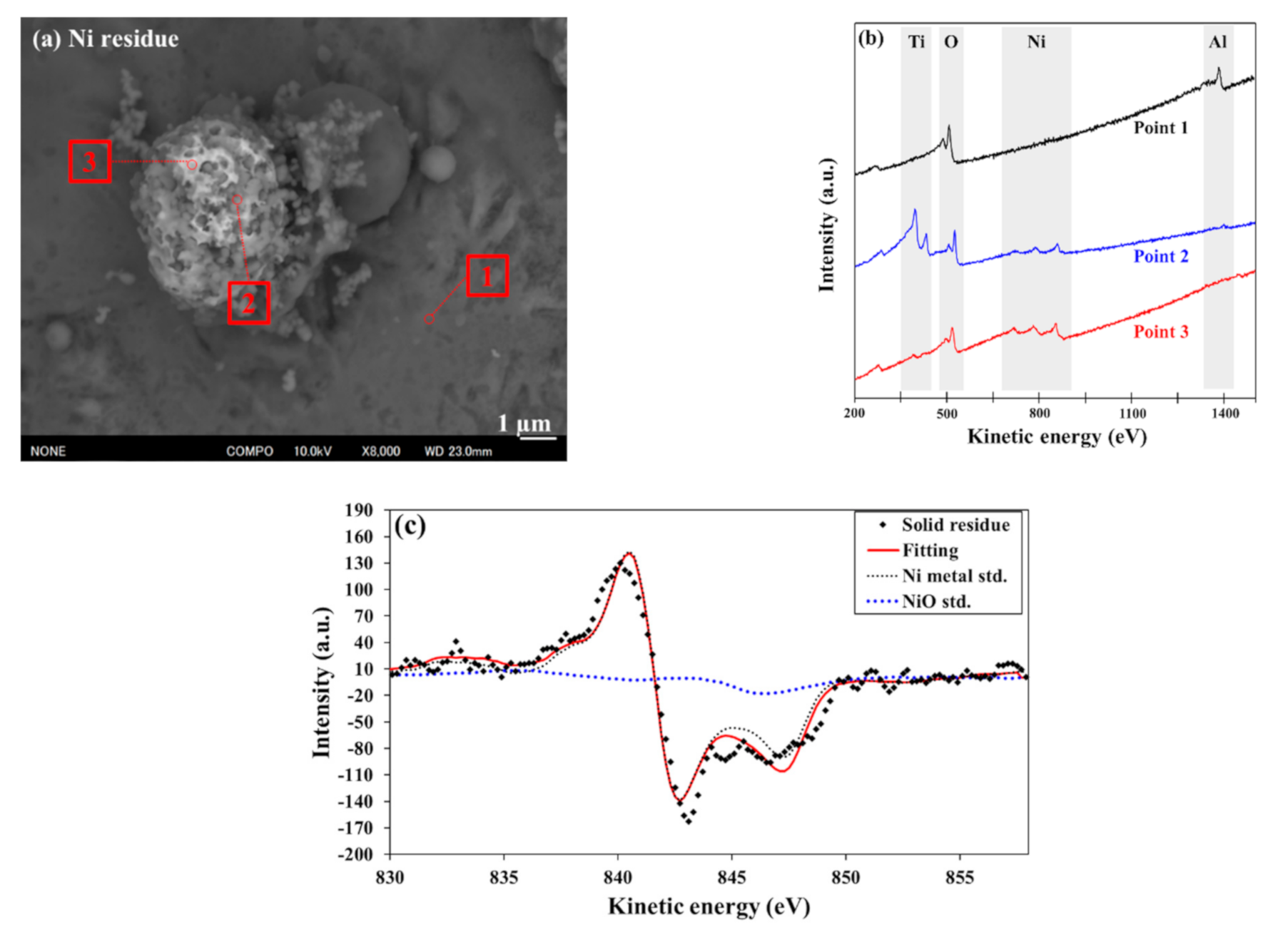
3.2. Surface Analysis of Deposited Co and Ni
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nelson, A.; Wang, W.; Demopoulos, G.P.; Houlachi, G. Removal of cobalt from zinc electrolyte by cementation: A critical review. Miner. Process. Extr. Metall. Rev. 2000, 20, 325–356. [Google Scholar] [CrossRef]
- Demirkran, N.; Künkül, A. Recovering of copper with metallic aluminum. Trans. Nonferrous Met. Soc. China 2011, 21, 2778–2782. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B. Recovery of Lead and Zinc from Zinc Plant Leach Residues by Concurrent Dissolution-Cementation. Metals 2020, 10, 531. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Yoo, K.; Alorro, R.D.; Tabelin, C.B. Cementation of Co ion in leach solution using Zn powder followed by magnetic separation of cementation-precipitate for recovery of unreacted Zn powder. Miner. Eng. 2020, 145. [Google Scholar] [CrossRef]
- Farahmand, F.; Moradkhani, D.; Sadegh Safarzadeh, M.; Rashchi, F. Optimization and kinetics of the cementation of lead with aluminum powder. Hydrometallurgy 2009, 98, 81–85. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.H.; El-Ashtoukhy, E.S.Z.; Bassyouni, M. Recovery of Copper from Effluents by Cementation on Aluminum in a Multirotating Cylinder-Agitated Vessel. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2016, 47, 657–665. [Google Scholar] [CrossRef]
- Abdollahi, P.; Yoozbashizadeh, H.; Moradkhani, D.; Behnian, D. A Study on Cementation Process of Lead from Brine Leaching Solution by Aluminum Powder. OALib 2015, 2, 1–6. [Google Scholar] [CrossRef]
- Boisvert, L.; Turgeon, K.; Boulanger, J.; Bazin, C.; Houlachi, G. Recovery of Cobalt from the Residues of an Industrial Zinc Refinery. Metals 2020, 10, 1553. [Google Scholar] [CrossRef]
- Li, W.; Cochell, T.; Manthiram, A. Activation of aluminum as an effective reducing agent by pitting corrosion for wet-chemical synthesis. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Park, I.; Tabelin, C.B.; Seno, K.; Jeon, S.; Ito, M.; Hiroyoshi, N. Simultaneous suppression of acid mine drainage formation and arsenic release by Carrier-microencapsulation using aluminum-catecholate complexes. Chemosphere 2018, 205, 414–425. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; Nyambe, I.; et al. Detoxification of lead-bearing zinc plant leach residues from Kabwe, Zambia by coupled extraction-cementation method. J. Environ. Chem. Eng. 2020, 8, 104197. [Google Scholar] [CrossRef]
- Annamalai, V.; Murr, L.E. Effects of the source of chloride ion and surface corrosion patterns on the kinetics of the copper-aluminum cementation system. Hydrometallurgy 1978, 3, 249–263. [Google Scholar] [CrossRef]
- Artamonov, V.V.; Moroz, D.R.; Bykov, A.O.; Artamonov, V.P. Experimental studies of cementation of tin in a dispersed form. Russ. J. Non-Ferrous Met. 2013, 54, 128–131. [Google Scholar] [CrossRef]
- Ekmekyapar, A.; Tanaydin, M.; Demirkiran, N. Investigation of copper cementation kinetics by rotating aluminum disc from the leach solutions containing copperions. Physicochem. Probl. Miner. Process. 2012, 48, 355–367. [Google Scholar] [CrossRef]
- Djokić, S.S. Cementation of Copper on Aluminum in Alkaline Solutions. J. Electrochem. Soc. 1996, 143, 1300–1305. [Google Scholar] [CrossRef]
- Jeon, S.; Tabelin, C.B.; Park, I.; Nagata, Y.; Ito, M.; Hiroyoshi, N. Ammonium thiosulfate extraction of gold from printed circuit boards (PCBs) of end-of-life mobile phones and its recovery from pregnant leach solution by cementation. Hydrometallurgy 2020, 191, 105214. [Google Scholar] [CrossRef]
- Jeon, S.; Tabelin, C.B.; Takahashi, H.; Park, I.; Ito, M.; Hiroyoshi, N. Enhanced cementation of gold via galvanic interactions using activated carbon and zero-valent aluminum: A novel approach to recover gold ions from ammonium thiosulfate medium. Hydrometallurgy 2020, 191, 105165. [Google Scholar] [CrossRef]
- Sulyman, M.; Namiesnik, J.; Gierak, A. Low-cost adsorbents derived from agricultural by-products/wastes for enhancing contaminant uptakes from wastewater: A review. Polish J. Environ. Stud. 2017, 26, 479–510. [Google Scholar] [CrossRef]
- Negem, M.; Nady, H.; El-Rabiei, M.M. Nanocrystalline nickel–cobalt electrocatalysts to generate hydrogen using alkaline solutions as storage fuel for the renewable energy. Int. J. Hydrog. Energy 2019, 44, 11411–11420. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, B.; Li, B.; Lai, Y.; Zheng, W.; Wang, H.; Wang, W.; Wang, M. Study on the characteristics of a high capacity nickel manganese cobalt oxide (NMC) lithium-ion battery-an experimental investigation. Energies 2018, 11, 2275. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.-R.; Noh, H.-J.; Myung, S.-T.; Amine, K.; Sun, Y.-K. High-Voltage Performance of Li[Ni[sub 0.55]Co[sub 0.15]Mn[sub 0.30]]O[sub 2] Positive Electrode Material for Rechargeable Li-Ion Batteries. J. Electrochem. Soc. 2011, 158, A180. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, L.; He, C. Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode. J. Power Sources 2014, 261, 285–291. [Google Scholar] [CrossRef]
- IEA. Global EV Outlook 2020; OECD: Paris, France, 2020; ISBN 9789264616226. [Google Scholar]
- Zeng, X.; Li, J.; Singh, N. Recycling of spent lithium-ion battery: A critical review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Hext, P.M.; Tomenson, J.A.; Thompson, P. Titanium dioxide: Inhalation toxicology and epidemiology. Ann. Occup. Hyg. 2005, 49, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Nesbitt, H.W.; Bancroft, G.M.; Davidson, R.; McIntyre, N.S.; Pratt, A.R. Minimum XPS core-level line widths of insulators, including silicate minerals. Am. Mineral. 2004, 89, 878–882. [Google Scholar] [CrossRef]
- Chen, H.J.; Lee, C. Effects of the Type of Chelating Agent and Deposit Morphology on the Kinetics of the Copper-Aluminum Cementation System. Langmuir 1994, 10, 3880–3886. [Google Scholar] [CrossRef]
- Gao, X.; Wu, L.; Xu, Q.; Tian, W.; Li, Z.; Kobayashi, N. Adsorption kinetics and mechanisms of copper ions on activated carbons derived from pinewood sawdust by fast H3PO4 activation. Environ. Sci. Pollut. Res. 2018, 25, 7907–7915. [Google Scholar] [CrossRef]
- Karnib, M.; Kabbani, A.; Holail, H.; Olama, Z. Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia 2014, 50, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Dil, E.A.; Ghaedi, M.; Ghaedi, A.M.; Asfaram, A.; Goudarzi, A.; Hajati, S.; Soylak, M.; Agarwal, S.; Gupta, V.K. Modeling of quaternary dyes adsorption onto ZnO-NR-AC artificial neural network: Analysis by derivative spectrophotometry. J. Ind. Eng. Chem. 2016, 34, 186–197. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Park, I.; Tabelin, C.B.; Seno, K.; Jeon, S.; Inano, H.; Ito, M.; Hiroyoshi, N. Carrier-microencapsulation of arsenopyrite using Al-catecholate complex: Nature of oxidation products, effects on anodic and cathodic reactions, and coating stability under simulated weathering conditions. Heliyon 2020, 6, e03189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murr, L.E.; Annamalai, V. An Electron Microscopic Study of Nucleation and Growth in Electrochemical Displacement Reactions: A Comparison of the Cu/Fe and Cu/AI Cementation Systems. Metall. Trans. B 1978, 9, 515–525. [Google Scholar] [CrossRef]
- Murr, L.E.; Annamalai, V. Characterization of copper nucleation and growth from aqueous solution on aluminum: A transmission electron microscopy study of copper cementation. Thin Solid Films 1978, 54, 189–195. [Google Scholar] [CrossRef]
- Reboul, M.C.; Warner, T.J.; Mayer, H.; Barouk, B. A Ten Step Mechanism for the Pitting Corrosion of Aluminium Alloys. Corros. Rev. 1997, 15, 471–496. [Google Scholar] [CrossRef]
- Andersson, M.; Kiselev, A.; Österlund, L.; Palmqvist, A.E.C. Microemulsion-mediated room-temperature synthesis of high-surface-area rutile and its photocatalytic performance. J. Phys. Chem. C 2007, 111, 6789–6797. [Google Scholar] [CrossRef]
- Inada, M.; Mizue, K.; Enomoto, N.; Hojo, J. Synthesis of rutile TiO2 with high specific surface area by self-hydrolysis of TiOCl2 in the presence of SDS. J. Ceram. Soc. Jpn. 2009, 117, 819–822. [Google Scholar] [CrossRef] [Green Version]
- Scherer, J. Auger Electron Spectroscopy (AES): A Versatile Microanalysis Technique in the Analyst’s Toolbox. Microsc. Microanal. 2020, 26, 1564–1565. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Jeon, S.; Park, I.; Ito, M.; Hiroyoshi, N. Enhanced Cementation of Co2+ and Ni2+ from Sulfate and Chloride Solutions Using Aluminum as an Electron Donor and Conductive Particles as an Electron Pathway. Metals 2021, 11, 248. https://doi.org/10.3390/met11020248
Choi S, Jeon S, Park I, Ito M, Hiroyoshi N. Enhanced Cementation of Co2+ and Ni2+ from Sulfate and Chloride Solutions Using Aluminum as an Electron Donor and Conductive Particles as an Electron Pathway. Metals. 2021; 11(2):248. https://doi.org/10.3390/met11020248
Chicago/Turabian StyleChoi, Sanghyeon, Sanghee Jeon, Ilhwan Park, Mayumi Ito, and Naoki Hiroyoshi. 2021. "Enhanced Cementation of Co2+ and Ni2+ from Sulfate and Chloride Solutions Using Aluminum as an Electron Donor and Conductive Particles as an Electron Pathway" Metals 11, no. 2: 248. https://doi.org/10.3390/met11020248





