Elderly Subjects Supplemented with L-Glutamine Shows an Improvement of Mucosal Immunity in the Upper Airways in Response to Influenza Virus Vaccination
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Anthropometric Characteristics and Nutritional and Physical Activity Evaluations
2.3. L-Glutamine Supplementation
2.4. Influenza Virus Vaccination
2.5. Detection of the Presence of Influenza Virus in the Upper Respiratory Tract by qRT-PCR
2.6. Collection of Saliva Samples
2.7. Determination of Total and Specific Secretory Immunoglobulin A (SIgA) for the Influenza Virus Vaccine
2.8. Determination of Salivary Cytokines
2.9. Statistical Analysis
3. Results
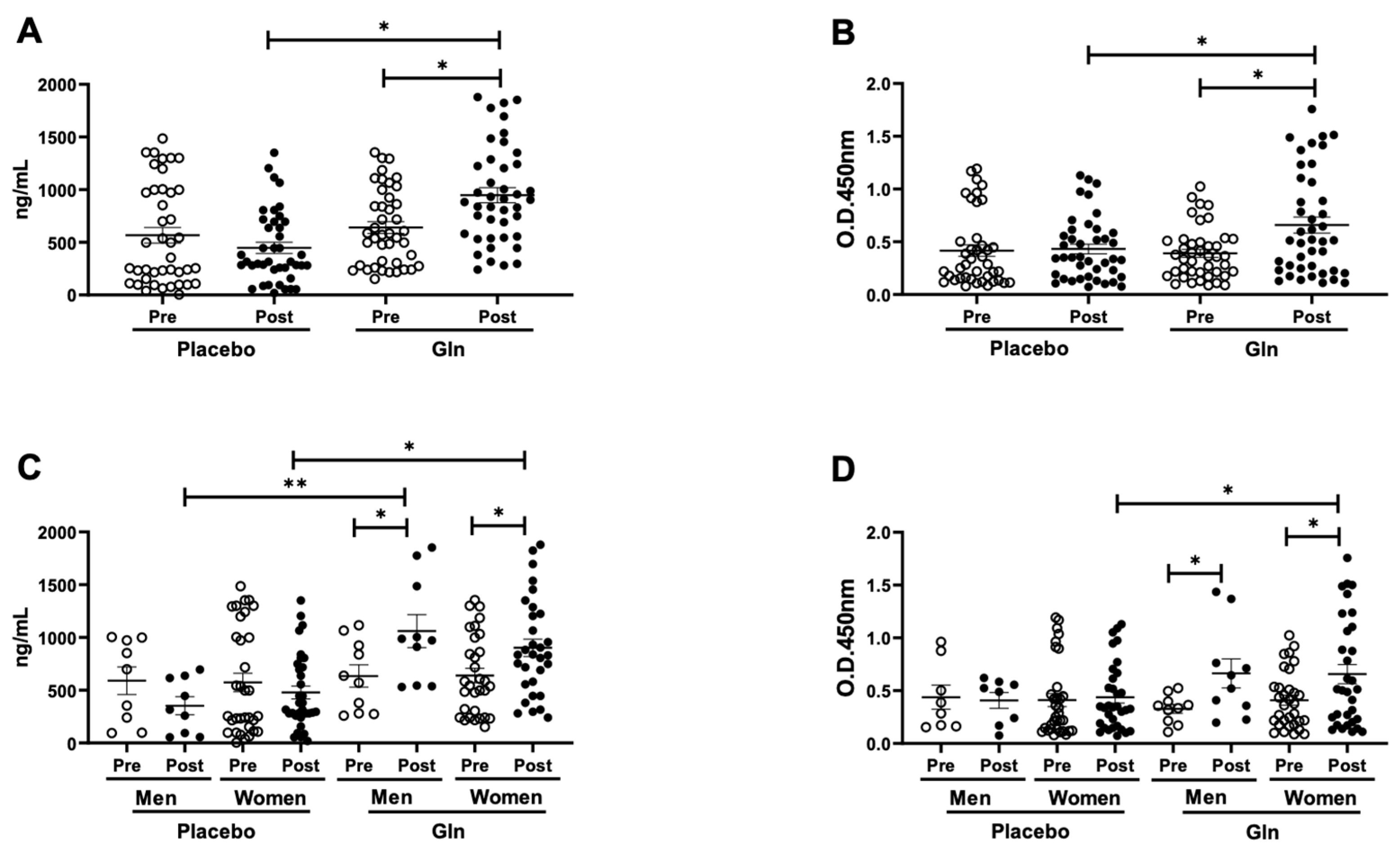
3.1. Gln Supplementation Enhances the Salivary SIgA Levels after the Vaccination
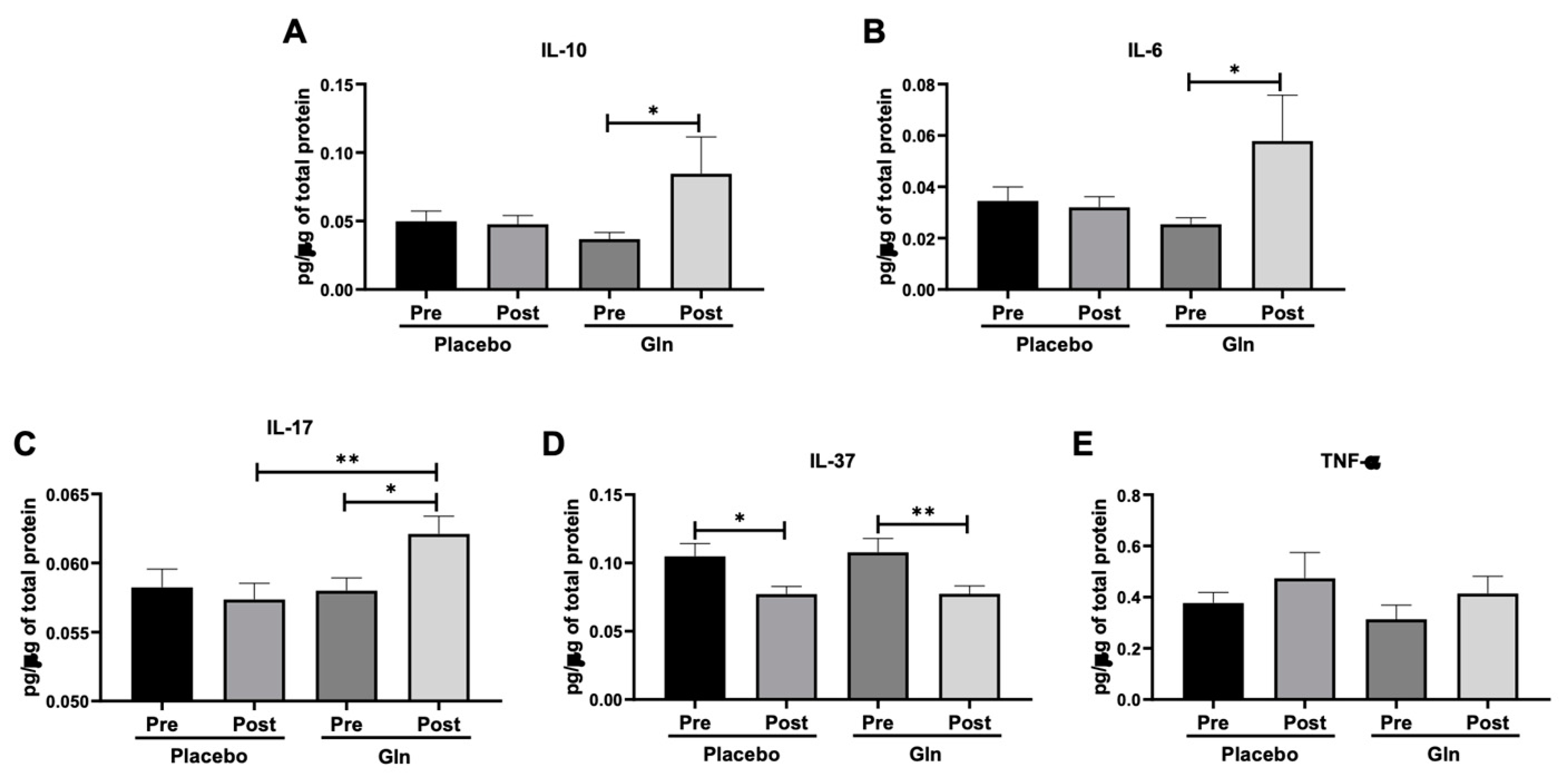
3.2. Gln Supplementation Modulates the Salivary Cytokines Levels
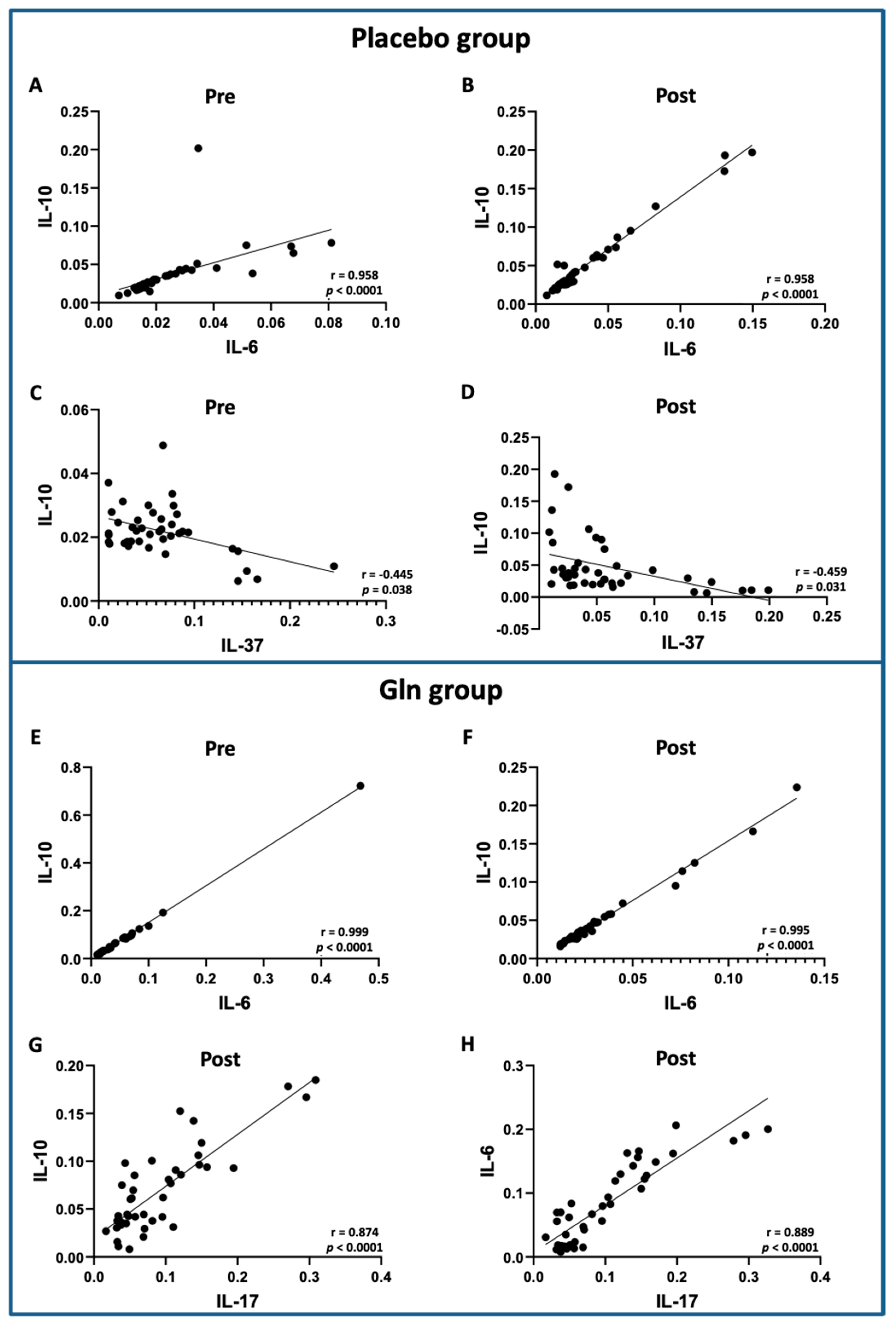
3.3. Correlation Analysis between the Salivary Cytokine Levels Showed Significant Differences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bahadoran, A.; Lee, S.H.; Wang, S.M.; Manikam, R.; Rajarajeswaran, J.; Raju, C.S.; Sekaran, S.D. Immune Responses to Influenza Virus and Its Correlation to Age and Inherited Factors. Front. Microbiol. 2016, 7, 1841. [Google Scholar] [CrossRef] [PubMed]
- Loerbroks, A.; Stock, C.; Bosch, J.A.; Litaker, D.G.; Apfelbacher, C.J. Influenza vaccination coverage among high-risk groups in 11 European countries. Eur. J. Public Health 2012, 22, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Cox, N.; Anderson, L.J.; Fukuda, K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003, 289, 179–186. [Google Scholar] [CrossRef] [PubMed]
- CDC; NCIRD. Vaccine Effectiveness: How Well Do the Flu Vaccines Work? U.S. Department of Health & Human Services: Washington, DC, USA, 2020.
- Goodwin, K.; Viboud, C.; Simonsen, L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine 2006, 24, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Moro-García, M.A.; Alonso-Arias, R.; López-Larrea, C. Molecular mechanisms involved in the aging of the T-cell immune response. Curr. Genom. 2012, 13, 589–602. [Google Scholar] [CrossRef]
- Vasto, S.; Candore, G.; Balistreri, C.R.; Caruso, M.; Colonna-Romano, G.; Grimaldi, M.P.; Listi, F.; Nuzzo, D.; Lio, D.; Caruso, C. Inflammatory networks in ageing, age-related diseases and longevity. Mech. Ageing Dev. 2007, 128, 83–91. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef]
- Richter, V.; Rassoul, F.; Purschwitz, K.; Hentschel, B.; Reuter, W.; Kuntze, T. Circulating vascular cell adhesion molecules VCAM-1, ICAM-1, and E-selectin in dependence on aging. Gerontology 2003, 49, 293–300. [Google Scholar] [CrossRef]
- Bandaranayake, T.; Shaw, A.C. Host Resistance and Immune Aging. Clin. Geriatr. Med. 2016, 32, 415–432. [Google Scholar] [CrossRef]
- Bachi, A.L.; Suguri, V.M.; Ramos, L.R.; Mariano, M.; Vaisberg, M.; Lopes, J.D. Increased production of autoantibodies and specific antibodies in response to influenza virus vaccination in physically active older individuals. Results Immunol. 2013, 3, 10–16. [Google Scholar] [CrossRef]
- Miyagawa, K.; Hayashi, Y.; Kurihara, S.; Maeda, A. Co-administration of l-cystine and l-theanine enhances efficacy of influenza vaccination in elderly persons: Nutritional status-dependent immunogenicity. Geriatr. Gerontol. Int. 2008, 8, 243–250. [Google Scholar] [CrossRef] [PubMed]
- De Flora, S.; Grassi, C.; Carati, L. Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur. Respir J. 1997, 10, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.R.; Roseira, T.; Amaral, J.B.; Paixão, V.; Almeida, E.B.; Foster, R.; Sperandio, A.; Rossi, M.; Amirato, G.R.; Apostólico, J.S.; et al. Combined Exercise Training and l-Glutamine Supplementation Enhances Both Humoral and Cellular Immune Responses after Influenza Virus Vaccination in Elderly Subjects. Vaccines 2020, 8, 685. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.B.; Santos, J.M.B.; Paixão, V.; Amaral, J.B.; Foster, R.; Sperandio, A.; Roseira, T.; Rossi, M.; Cordeiro, T.G.; Monteiro, F.R.; et al. L-Glutamine Supplementation Improves the Benefits of Combined-Exercise Training on Oral Redox Balance and Inflammatory Status in Elderly Individuals. Oxid. Med. Cell. Longev. 2020, 2020, 2852181. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Wang, Z.; Ma, J. Glutamine Metabolism and Its Role in Immunity, a Comprehensive Review. Animals 2020, 10, 326. [Google Scholar] [CrossRef]
- Ardawi, M.S.; Newsholme, E.A. Maximum activities of some enzymes of glycolysis, the tricarboxylic acid cycle and ketone-body and glutamine utilization pathways in lymphocytes of the rat. Biochem. J. 1982, 208, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Curi, R.; Gordon, S.; Newsholme, E.A. Metabolism of glucose, glutamine, long-chain fatty acids and ketone bodies by murine macrophages. Biochem. J. 1986, 239, 121–125. [Google Scholar] [CrossRef]
- Curi, T.C.; De Melo, M.P.; De Azevedo, R.B.; Zorn, T.M.; Curi, R. Glutamine utilization by rat neutrophils: Presence of phosphate-dependent glutaminase. Am. J. Physiol. 1997, 273, C1124–C1129. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.; Wei, Y.; Szwajcer, A.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M.; Mahmud, S.M. Efficacy and safety of high-dose influenza vaccine in elderly adults: A systematic review and meta-analysis. Vaccine 2017, 35, 2775–2780. [Google Scholar] [CrossRef]
- Fernández-Garrido, J.; Ruiz-Ros, V.; Buigues, C.; Navarro-Martinez, R.; Cauli, O. Clinical features of prefrail older individuals and emerging peripheral biomarkers: A systematic review. Arch. Gerontol. Geriatr. 2014, 59, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.; Moldoveanu, Z.; Ogra, P.; Mestecky, J. Mucosal immunity in COVID-19: A neglected but critical aspect of SARS-CoV-2 infection. Front. Immunol. 2020, 11, 611337. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Matsudo, S.M.M. Envelhecimento, Atividade Física e Saúde; BIS. Boletim do Instituto de Saúde (Impresso): São Paulo, Brazil, 2009; pp. 76–79. [Google Scholar]
- Gleeson, M. Dosing and efficacy of glutamine supplementation in human exercise and sport training. J. Nutr. 2008, 138, 2045S–2049S. [Google Scholar] [CrossRef] [PubMed]
- Sayles, C.; Hickerson, S.C.; Bhat, R.R.; Hall, J.; Garey, K.W.; Trivedi, M.V. Oral Glutamine in Preventing Treatment-Related Mucositis in Adult Patients With Cancer: A Systematic Review. Nutr. Clin. Pract. 2016, 31, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Qorbani, M.; Heshmat, R.; Larijani, B.; Hosseini, S. Effect of glutamine supplementation on cardiovascular risk factors in patients with type 2 diabetes. Nutrition 2015, 31, 119–126. [Google Scholar] [CrossRef]
- Legault, Z.; Bagnall, N.; Kimmerly, D.S. The Influence of Oral L-Glutamine Supplementation on Muscle Strength Recovery and Soreness Following Unilateral Knee Extension Eccentric Exercise. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 417–426. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Smetana, J.; Chlibek, R.; Shaw, J.; Splino, M.; Prymula, R. Influenza vaccination in the elderly. Hum. Vaccin Immunother 2018, 14, 540–549. [Google Scholar] [CrossRef]
- Freitas, E.V.; Py, L.; Neri, A.L. Tratado de Geriatria e Gerontologia, 2nd ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2006. [Google Scholar]
- Webster, R.G. Immunity to influenza in the elderly. Vaccine 2000, 18, 1686–1689. [Google Scholar] [CrossRef]
- Ivanov, A.; Dragunsky, E.; Ivanova, O.; Rezapkin, G.; Potapova, S.; Chumakov, K. Determination of poliovirus-specific IgA in saliva by ELISA tests. J. Virol. Methods 2005, 126, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Pfaffe, T.; Cooper-White, J.; Beyerlein, P.; Kostner, K.; Punyadeera, C. Diagnostic potential of saliva: Current state and future applications. Clin. Chem. 2011, 57, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Campbell, J.L.; Cooper-White, J.; Dimeski, G.; Punyadeera, C. The impact of saliva collection and processing methods on CRP, IgE, and Myoglobin immunoassays. Clin. Transl. Med. 2012, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Punyadeera, C.; Dimeski, G.; Kostner, K.; Beyerlein, P.; Cooper-White, J. One-step homogeneous C-reactive protein assay for saliva. J. Immunol. Methods 2011, 373, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.Y.; Wan, Y.; Kostner, K.; Arivalagan, A.; Atherton, J.; Cooper-White, J.; Dimeski, G.; Punyadeera, C. NT-ProBNP levels in saliva and its clinical relevance to heart failure. PLoS ONE 2012, 7, e48452. [Google Scholar] [CrossRef]
- Vaisberg, M.; Paixão, V.; Almeida, E.B.; Santos, J.M.B.; Foster, R.; Rossi, M.; Pithon-Curi, T.C.; Gorjão, R.; Momesso, C.M.; Andrade, M.S.; et al. Daily Intake of Fermented Milk Containing. Nutrients 2019, 11, 1678. [Google Scholar] [CrossRef]
- Santos, J.M.B.D.; Foster, R.; Jonckheere, A.C.; Rossi, M.; Luna Junior, L.A.; Katekaru, C.M.; de Sá, M.C.; Pagani, L.G.; Almeida, F.M.; Amaral, J.D.B.; et al. Outdoor Endurance Training with Air Pollutant Exposure Versus Sedentary Lifestyle: A Comparison of Airway Immune Responses. Int. J. Environ. Res. Public Health 2019, 16, 4418. [Google Scholar] [CrossRef]
- Liang, B.; Hyland, L.; Hou, S. Nasal-associated lymphoid tissue is a site of long-term virus-specific antibody production following respiratory virus infection of mice. J. Virol. 2001, 75, 5416–5420. [Google Scholar] [CrossRef]
- Essaidi-Laziosi, M.; Brito, F.; Benaoudia, S.; Royston, L.; Cagno, V.; Fernandes-Rocha, M.; Piuz, I.; Zdobnov, E.; Huang, S.; Constant, S.; et al. Propagation of respiratory viruses in human airway epithelia reveals persistent virus-specific signatures. J. Allergy Clin. Immunol. 2018, 141, 2074–2084. [Google Scholar] [CrossRef]
- Zhao, S.; Yuan, L.; Li, Y.; Liu, L.; Luo, Z.; Lv, Q.; Rong, R.; Yang, Y. Secretory IgA in Mucosa of Pharynx and Larynx Plays an Important Role against Influenza A Virus Infection in Kidney Yang Deficiency Syndrome Model. Evid. Based Complement. Altern. Med. 2020, 2020, 9316763. [Google Scholar] [CrossRef]
- Johnstone, J.; Parsons, R.; Botelho, F.; Millar, J.; McNeil, S.; Fulop, T.; McElhaney, J.; Andrew, M.K.; Walter, S.D.; Devereaux, P.J.; et al. Immune biomarkers predictive of respiratory viral infection in elderly nursing home residents. PLoS ONE 2014, 9, e108481. [Google Scholar] [CrossRef] [PubMed]
- Rector, J.L.; Dowd, J.B.; Loerbroks, A.; Burns, V.E.; Moss, P.A.; Jarczok, M.N.; Stalder, T.; Hoffman, K.; Fischer, J.E.; Bosch, J.A. Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain Behav. Immun. 2014, 38, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ogra, P.L. Effect of tonsillectomy and adenoidectomy on nasopharyngeal antibody response to poliovirus. N. Engl. J. Med. 1971, 284, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Arranz, E.; O’Mahony, S.; Barton, J.R.; Ferguson, A. Immunosenescence and mucosal immunity: Significant effects of old age on secretory IgA concentrations and intraepithelial lymphocyte counts. Gut 1992, 33, 882–886. [Google Scholar] [CrossRef]
- Challacombe, S.J. Assessing mucosal humoral immunity. Clin. Exp. Immunol. 1995, 100, 181–182. [Google Scholar] [CrossRef]
- Finkelstein, M.S. Problems of managing infections in elderly persons. Compr. Ther. 1984, 10, 32–39. [Google Scholar]
- Flanagan, K.L.; Fink, A.L.; Plebanski, M.; Klein, S.L. Sex and Gender Differences in the Outcomes of Vaccination over the Life Course. Annu. Rev. Cell Dev. Biol. 2017, 33, 577–599. [Google Scholar] [CrossRef]
- Gubbels Bupp, M.R.; Potluri, T.; Fink, A.L.; Klein, S.L. The Confluence of Sex Hormones and Aging on Immunity. Front. Immunol. 2018, 9, 1269. [Google Scholar] [CrossRef]
- Evans, P.; Der, G.; Ford, G.; Hucklebridge, F.; Hunt, K.; Lambert, S. Social class, sex, and age differences in mucosal immunity in a large community sample. Brain Behav. Immun. 2000, 14, 41–48. [Google Scholar] [CrossRef][Green Version]
- Furtado, G.E.; Letieri, R.V.; Caldo, A.; Sardão, V.; Teixeira, A.M.; de Barros, M.P.; Vieira, R.P.; Bachi, A.L.L. Sustaining efficient immune functions with regular physical exercise in the COVID-19 era and beyond. Eur. J. Clin. Investig. 2021, e13485. [Google Scholar] [CrossRef]
- Murphy, B.R.; Clements, M.L. The systemic and mucosal immune response of humans to influenza A virus. Curr. Top. Microbiol. Immunol. 1989, 146, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, Z.; Clements, M.L.; Prince, S.J.; Murphy, B.R.; Mestecky, J. Human immune responses to influenza virus vaccines administered by systemic or mucosal routes. Vaccine 1995, 13, 1006–1012. [Google Scholar] [CrossRef]
- Boyaka, P.N. Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems. J. Immunol. 2017, 199, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Marc Rhoads, J.; Wu, G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 2009, 37, 111–122. [Google Scholar] [CrossRef]
- Caris, A.V.; Da Silva, E.T.; Dos Santos, S.A.; Tufik, S.; Dos Santos, R.V.T. Effects of Carbohydrate and Glutamine Supplementation on Oral Mucosa Immunity after Strenuous Exercise at High Altitude: A Double-Blind Randomized Trial. Nutrients 2017, 9, 692. [Google Scholar] [CrossRef]
- Ren, W.; Wang, K.; Yin, J.; Chen, S.; Liu, G.; Tan, B.; Wu, G.; Bazer, F.W.; Peng, Y.; Yin, Y. Glutamine-Induced Secretion of Intestinal Secretory Immunoglobulin A: A Mechanistic Perspective. Front. Immunol. 2016, 7, 503. [Google Scholar] [CrossRef]
- Ren, W.; Li, Y.; Yu, X.; Luo, W.; Liu, G.; Shao, H.; Yin, Y. Glutamine modifies immune responses of mice infected with porcine circovirus type 2. Br. J. Nutr. 2013, 110, 1053–1060. [Google Scholar] [CrossRef]
- Ren, W.; Liu, S.; Chen, S.; Zhang, F.; Li, N.; Yin, J.; Peng, Y.; Wu, L.; Liu, G.; Yin, Y.; et al. Dietary L-glutamine supplementation increases Pasteurella multocida burden and the expression of its major virulence factors in mice. Amino Acids 2013, 45, 947–955. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Bak-Jensen, K.S.; Chen, Y.; Tato, C.M.; Blumenschein, W.; McClanahan, T.; Cua, D.J. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007, 8, 1390–1397. [Google Scholar] [CrossRef]
- Noda, K.; Kodama, S.; Umemoto, S.; Nomi, N.; Hirano, T.; Suzuki, M. Th17 cells contribute to nontypeable Haemophilus influenzae-specific protective immunity induced by nasal vaccination with P6 outer membrane protein and α-galactosylceramide. Microbiol. Immunol. 2011, 55, 574–581. [Google Scholar] [CrossRef]
- Hamada, H.; Garcia-Hernandez, M.e.L.; Reome, J.B.; Misra, S.K.; Strutt, T.M.; McKinstry, K.K.; Cooper, A.M.; Swain, S.L.; Dutton, R.W. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 2009, 182, 3469–3481. [Google Scholar] [CrossRef] [PubMed]
- Trondsen, M.; Aqrawi, L.A.; Zhou, F.; Pedersen, G.; Trieu, M.C.; Zhou, P.; Cox, R.J. Induction of Local Secretory IgA and Multifunctional CD4⁺ T-helper Cells Following Intranasal Immunization with a H5N1 Whole Inactivated Influenza Virus Vaccine in BALB/c Mice. Scand. J. Immunol. 2015, 81, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.O.; Wolf, M.M.; Madden, M.Z.; Andrejeva, G.; Sugiura, A.; Contreras, D.C.; Maseda, D.; Liberti, M.V.; Paz, K.; Kishton, R.J.; et al. Distinct Regulation of Th17 and Th1 Cell Differentiation by Glutaminase-Dependent Metabolism. Cell 2018, 175, 1780–1795.e1719. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Duan, J.; Yin, J.; Liu, G.; Cao, Z.; Xiong, X.; Chen, S.; Li, T.; Yin, Y.; Hou, Y.; et al. Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids 2014, 46, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yin, J.; Wu, M.; Liu, G.; Yang, G.; Xion, Y.; Su, D.; Wu, L.; Li, T.; Chen, S.; et al. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS ONE 2014, 9, e88335. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost Agents 2020, 34, 327–331. [Google Scholar] [CrossRef]
- Cavalli, G.; Dinarello, C.A. Suppression of inflammation and acquired immunity by IL-37. Immunol. Rev. 2018, 281, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Salvi, R.; Patankar, P. Emerging pharmacotherapies for COVID-19. Biomed. Pharm. 2020, 128, 110267. [Google Scholar] [CrossRef]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef]
- Elfeky, O.A.; Abed, N.T.; Emam, S.M.; Elsayed, H.A. Assessment of Serum Level of Interleukin-37 in Asthmatic Children at Benha University Hospital. Egypt J. Immunol. 2018, 25, 53–60. [Google Scholar]
- Zhu, J.; Dong, J.; Ji, L.; Jiang, P.; Leung, T.F.; Liu, D.; Ng, L.G.; Tsang, M.S.; Jiao, D.; Lam, C.W.; et al. Anti-Allergic Inflammatory Activity of Interleukin-37 Is Mediated by Novel Signaling Cascades in Human Eosinophils. Front. Immunol. 2018, 9, 1445. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Volunteers (n = 83) | p Value | |
|---|---|---|---|
| Placebo (n = 41) | L-Glutamine (n = 42) | ||
| Age (year) | 73.3 ± 6.4 | 71.8 ± 5.7 | >0.05 |
| Women (n) | 33 | 32 | >0.05 |
| Men (n) | 8 | 10 | >0.05 |
| Sex ratio (M:W) | 1:4.125 | 1:3.2 | >0.05 |
| Height (m) | 1.56 ± 0.09 | 1.58 ± 0.09 | >0.05 |
| Weight (kg) | 63.5 ± 10.5 | 67.3 ± 14.6 | >0.05 |
| Body mass index (kg/m2) | 26.3 ± 3.8 | 26.5 ± 4.1 | >0.05 |
| Total body fat (%) | 37.3 ± 8.9 | 36.3 ± 8.2 | >0.05 |
| Fat-free mass (%) | 63.1 ± 8.5 | 63.9 ± 8.1 | >0.05 |
| Skeletal muscle mass (kg) | 19.1 ± 3.8 | 20.6 ± 4.1 | >0.05 |
| IPAQ | |||
| Physical activity (min/week) | 664 ± 144 | 696 ± 182 | >0.05 |
| Sitting (min/week) | 1453 ± 579 | 1557 ± 527 | >0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paixão, V.; Almeida, E.B.; Amaral, J.B.; Roseira, T.; Monteiro, F.R.; Foster, R.; Sperandio, A.; Rossi, M.; Amirato, G.R.; Santos, C.A.F.; et al. Elderly Subjects Supplemented with L-Glutamine Shows an Improvement of Mucosal Immunity in the Upper Airways in Response to Influenza Virus Vaccination. Vaccines 2021, 9, 107. https://doi.org/10.3390/vaccines9020107
Paixão V, Almeida EB, Amaral JB, Roseira T, Monteiro FR, Foster R, Sperandio A, Rossi M, Amirato GR, Santos CAF, et al. Elderly Subjects Supplemented with L-Glutamine Shows an Improvement of Mucosal Immunity in the Upper Airways in Response to Influenza Virus Vaccination. Vaccines. 2021; 9(2):107. https://doi.org/10.3390/vaccines9020107
Chicago/Turabian StylePaixão, Vitória, Ewin B. Almeida, Jonatas B. Amaral, Tamaris Roseira, Fernanda R. Monteiro, Roberta Foster, Adriane Sperandio, Marcelo Rossi, Gislene R. Amirato, Carlos A. F. Santos, and et al. 2021. "Elderly Subjects Supplemented with L-Glutamine Shows an Improvement of Mucosal Immunity in the Upper Airways in Response to Influenza Virus Vaccination" Vaccines 9, no. 2: 107. https://doi.org/10.3390/vaccines9020107
APA StylePaixão, V., Almeida, E. B., Amaral, J. B., Roseira, T., Monteiro, F. R., Foster, R., Sperandio, A., Rossi, M., Amirato, G. R., Santos, C. A. F., Pires, R. S., Leal, F. B., Durigon, E. L., Oliveira, D. B. L., Vieira, R. P., Vaisberg, M., Santos, J. M. B., & Bachi, A. L. L. (2021). Elderly Subjects Supplemented with L-Glutamine Shows an Improvement of Mucosal Immunity in the Upper Airways in Response to Influenza Virus Vaccination. Vaccines, 9(2), 107. https://doi.org/10.3390/vaccines9020107







