Sirt1 Activity in the Brain: Simultaneous Effects on Energy Homeostasis and Reproduction
Abstract
:1. Introduction
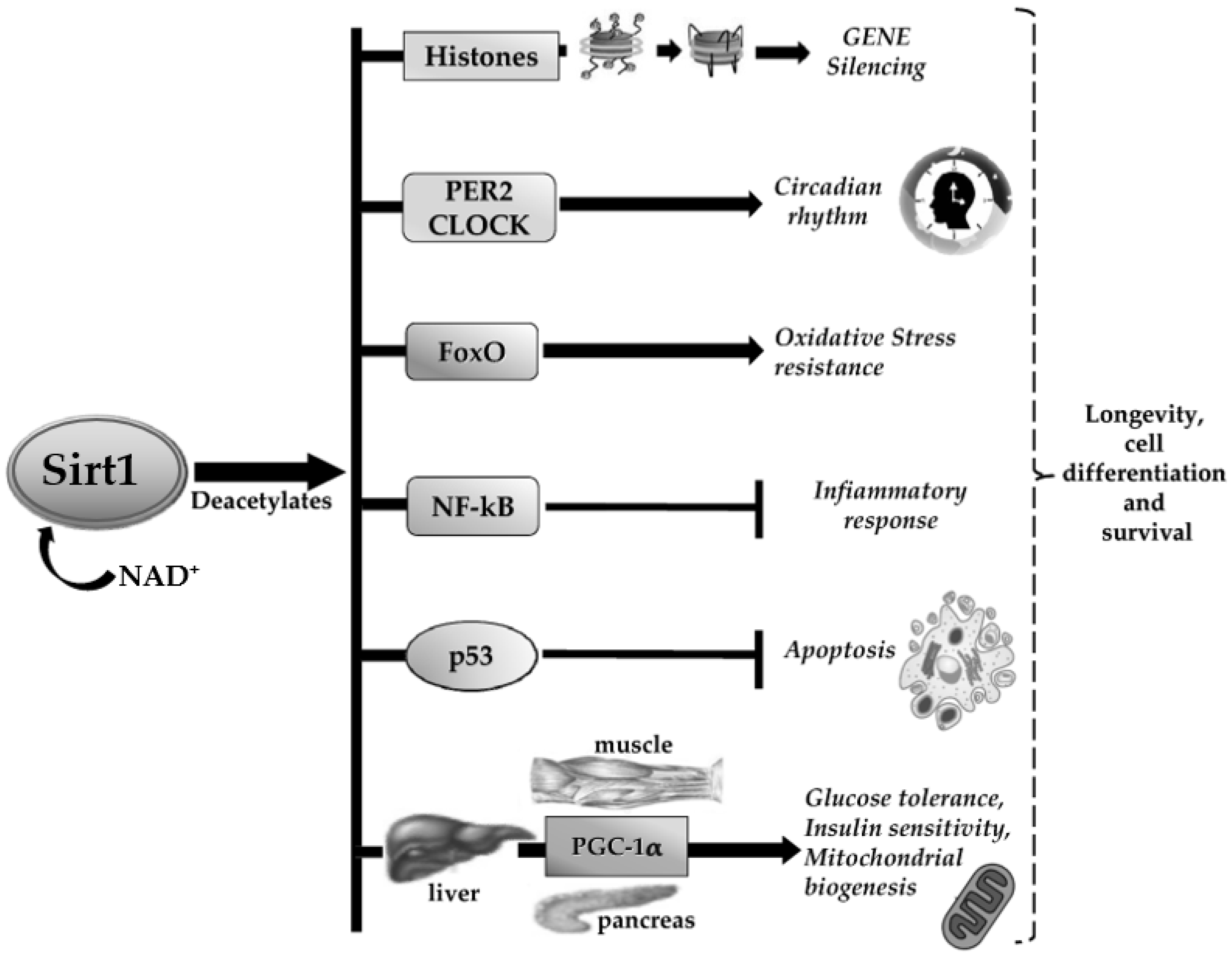
2. Sirt1
3. Sirt1 Activity in the Brain
4. Sirt1 and the Relationship between the Central Control of Energy Homeostasis and Reproduction
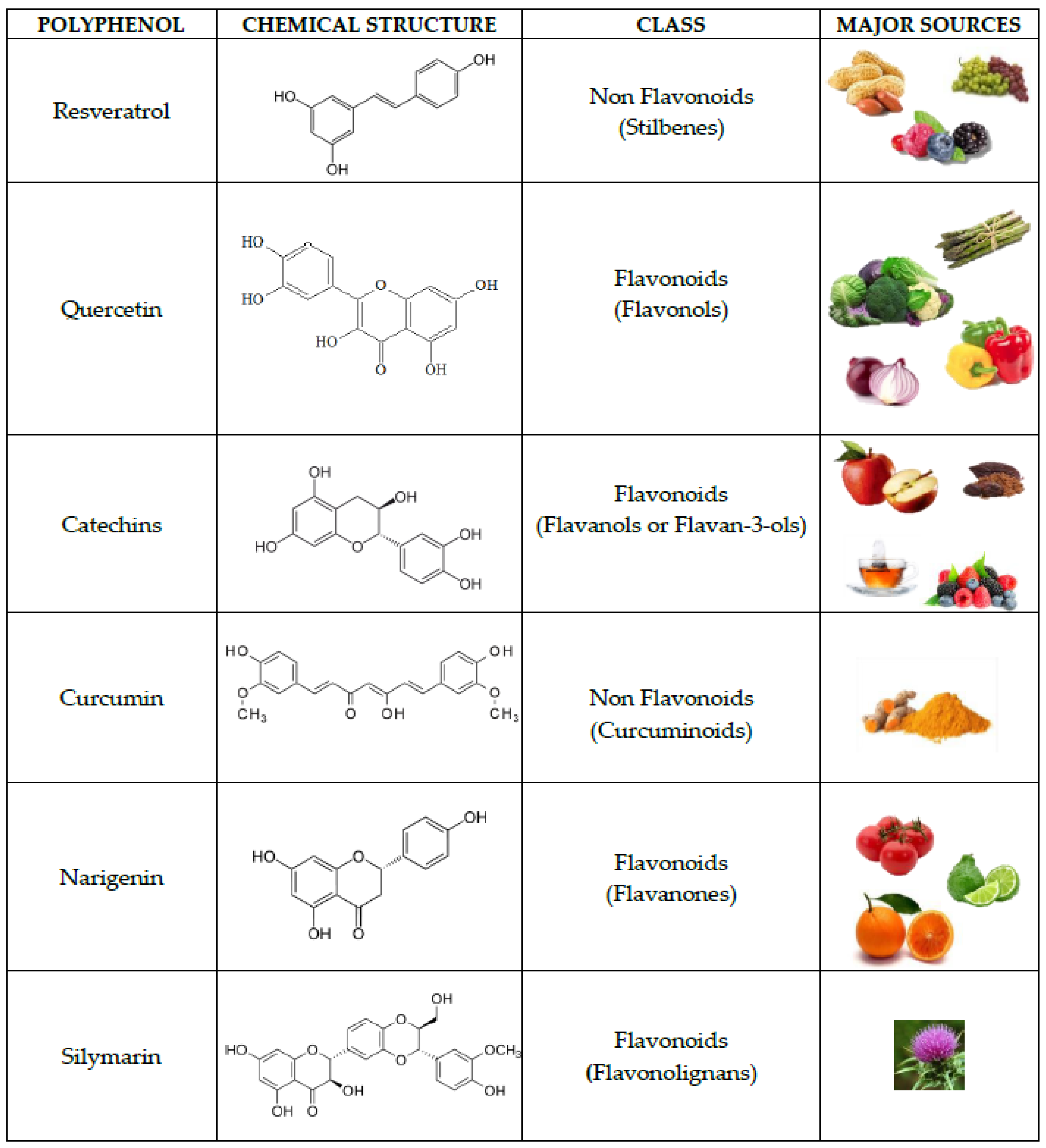
5. Dietary Preservation of Sirt1 Activity: The Role of Polyphenols
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and Trends in Obesity Among US Adults, 1999–2008. JAMA 2010, 303, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, T.; Kannel, W.B. Obesity and cardiovascular disease: The Framingham study. Clin. Endocrinol. Metab. 1976, 5, 367–375. [Google Scholar] [CrossRef]
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- World Health Organization. Infertility. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 13 December 2020).
- Tavares, R.S.; Escada-Rebelo, S.; Correia, M.; Mota, P.C.; Ramalho-Santos, J. The non-genomic effects of endocrine-disrupting chemicals on mammalian sperm. Reproduction 2016, 151, R1–R13. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, S.; Scafuro, M.; Meccariello, R. BPA and Nutraceuticals, Simultaneous Effects on Endocrine Functions. Endocr. Metab. Immune. Disord. Drug Targets 2019, 19, 594–604. [Google Scholar] [CrossRef]
- Santoro, A.; Chianese, R.; Troisi, J.; Richards, S.; Nori, S.L.; Fasano, S.; Guida, M.; Plunck, E.; Viggiano, A.; Pierantoni, R.; et al. Neuro-toxic and reproductive effects of BPA. Curr. Neuropharmacol. 2019, 17, 1109–1132. [Google Scholar] [CrossRef]
- Meccariello, R. Endocannabinoid System in Health and Disease: Current Situation and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 3549. [Google Scholar] [CrossRef]
- Santoro, A.; Mele, E.; Marino, M.; Viggiano, A.; Nori, S.L.; Meccariello, R. The complex interplay between Endocannabinoid System and the Estrogen System in Central Nervous System and periphery. Int. J. Mol. Sci. 2021, 22, 972. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Troisi, J.; Richards, S.; Scafuro, M.; Fasano, S.; Guida, M.; Pierantoni, R.; Meccariello, R. Bisphenol A in Reproduction: Epigenetic Effects. Curr. Med. Chem. 2018, 25, 748–770. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C. The agouti mouse model: An epigenetic bio- sensor for nutritional and environmental alterations on the fetal epigenome. Nutr. Rev. 2008, 66, S7–S11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seisenberger, S.; Peat, J.R.; Hore, T.A.; Santos, F.; Dean, W.; Reik, W. Reprogramming DNA methylation in the mammalian life cycle: Building and breaking epigenetic barriers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20110330. [Google Scholar] [CrossRef] [Green Version]
- Hogg, K.; Western, P.S. Refurbishing the germline epigenome: Out with the old, in with the new. Semin. Cell. Dev. Biol. 2015, 45, 104–113. [Google Scholar] [CrossRef]
- Kim, J.K.; Samaranayake, M.; Pradhan, S. Epigenetic mechanisms in mammals. Cell. Mol. Life Sci. 2009, 66, 596–612. [Google Scholar] [CrossRef] [Green Version]
- Motti, M.L.; D’Angelo, S.; Meccariello, R. MicroRNAs, Cancer and Diet: Facts and New Exciting Perspectives. Curr. Mol. Pharmacol. 2018, 11, 90–96. [Google Scholar] [CrossRef]
- Meccariello, R.; Santoro, A.; D’Angelo, S.; Morrone, R.; Fasano, S.; Viggiano, A.; Pierantoni, R. The Epigenetics of the Endocannabinoid System. Int. J. Mol. Sci. 2020, 21, 1113. [Google Scholar] [CrossRef]
- Kanherkar, R.R.; Bhatia-Dey, N.; Csoka, A.B. Epigenetics across the human lifespan. Front. Cell. Dev. Biol. 2014, 2, 49. [Google Scholar] [CrossRef] [Green Version]
- Daxinger, L.; Whitelaw, E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 2012, 13, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Derghal, A.; Djelloul, M.; Trouslard, J.; Mounien, L. An Emerging Role of micro-RNA in the Effect of the Endocrine Disruptors. Front. Neurosci. 2016, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Norouzitallab, P.; Baruah, K.; Vanrompay, D.; Bossier, P. Can epigenetics translate environmental cues into phenotypes? Sci. Total Environ. 2019, 647, 1281–1293. [Google Scholar] [CrossRef]
- Chianese, R.; Coccurello, R.; Viggiano, A.; Scafuro, M.; Fiore, M.; Coppola, G.; Operto, F.F.; Fasano, S.; Layé, S.; Pierantoni, R.; et al. Impact of Dietary Fats on Brain Functions. Curr. Neuropharmacol. 2018, 16, 1059–1085. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- Viggiano, A.; Meccariello, R.; Santoro, A.; Secondulfo, C.; Operto, F.F.; Monda, M.; Coppola, G. A Calorie-Restricted Ketogenic Diet Reduces Cerebral Cortex Vascularization in Prepubertal Rats. Nutrients 2019, 11, 2681. [Google Scholar] [CrossRef] [Green Version]
- Gomes, P.; Fleming-Outeiro, T.; Cavadas, C. Emerging role of sirtuin 2 in the regulation of mammalian metabolism. Trends Pharmacol. Sci. 2015, 36, 756–768. [Google Scholar] [CrossRef]
- Haigis, M.C.; Guarente, L.P. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006, 20, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Ding, R.B.; Bao, J.; Deng, C.X. Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 2017, 13, 852–867. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell. Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, C.; Liang, Y.; Vanhoutte, P.M. SIRT1 in metabolic syndrome: Where to target matters. Pharmacol. Ther. 2012, 136, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Viggiano, A.; Urbanek, K.; Cappetta, D.; Troisi, J.; Scafuro, M.; Guida, M.; Esposito, G.; Ciuffreda, L.P.; Rossi, F.; et al. Chronic exposure to low dose of bisphenol A impacts on the first round of spermatogenesis via SIRT1 modulation. Sci. Rep. 2018, 8, 2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemieux, M.E.; Yang, X.; Jardine, K.; He, X.; Jacobsen, K.X.; Staines, W.A.; Harper, M.E.; McBurney, M.W. The Sirt1 deacetylase modulates the insulin like growth factor signaling pathway in mammals. Mech. Ageing Dev. 2005, 126, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jackson, C.W.; Khoury, N.; Escobar, I.; Perez-Pinzon, M.A. Brain SIRT1 Mediates Metabolic Homeostasis and Neuroprotection. Front. Endocrinol. 2018, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Mazucanti, C.H.; Cabral-Costa, J.V.; Vasconcelos, A.R.; Andreotti, D.Z.; Scavone, C.; Kawamoto, E.M. Longevity Pathways (mTOR, SIRT, Insulin/IGF-1) as Key Modulatory Targets on Aging and Neurodegeneration. Curr. Top. Med. Chem. 2015, 15, 2116–2138. [Google Scholar] [CrossRef] [PubMed]
- De Mello, N.P.; Orellana, A.M.; Mazucanti, C.H.; de Morais-Lima, G.; Scavone, C.; Kawamoto, E.M. Insulin and Autophagy in Neurodegeneration. Front. Neurosci. 2019, 13, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; Machado De Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004, 429, 771–776. [Google Scholar] [CrossRef]
- Chalkiadaki, A.; Guarente, L. High-fat diet triggers inflammation induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell. Metab. 2012, 16, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Gillum, M.P.; Kotas, M.E.; Erion, D.; Kursawe, R.; Chatterjee, P.; Nead, K.T.; Muise, E.S.; Hsiao, J.J.; Frederick, D.W.; Yonemitsu, S.; et al. SirT1 regulates adipose tissue inflammation. Diabetes 2011, 60, 3235–3245. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, J.T.; Puigserver, P. Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc. Natl. Acad. Sci. USA 2007, 104, 12861–12866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell. Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutant, M.; Canto, C. SIRT1 metabolic actions: Integrating recent advances from mouse models. Mol. Metab. 2014, 3, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, K.A.; Grimm, A.A.; Plueger, M.M.; Bernal-Mizrachi, E.; Ford, E.; Cras-Méneur, C.; Permutt, M.A.; Imai, S. Increased dosage of mammalian Sir2 in pancreatic cells enhances glucose-stimulated insulin secretion in mice. Cell. Metab. 2005, 2, 105–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Koronowski, K.B.; Perez-Pinzon, M.A. Sirt1 in cerebral ischemia. Brain Circ. 2015, 1, 69–78. [Google Scholar] [PubMed] [Green Version]
- Mitchell, S.J.; Martin-Montalvo, A.; Mercken, E.M.; Palacios, H.H.; Ward, T.M.; Abulwerdi, G.; Minor, R.K.; Vlasuk, G.P.; Ellis, J.L.; Sinclair, D.A.; et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014, 6, 836–843. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Dong, W.; Wang, R.; Li, Y.; Xu, B.; Zhang, J.; Zhao, Z.; Wang, Y. Effect of caloric restriction on the SIRT1/mTOR signaling pathways in senile mice. Brain Res. Bull. 2015, 116, 67–72. [Google Scholar] [CrossRef]
- Huang, R.; Wu, F.; Zhao, J.; Li, H.B.; Ding, J.; Xiong, K.R. Electroacupuncture plus Gastrodin Improves Learning-memory Ability Possibly by Up-regulating Expression of SIRT 1 and PGC-1 ɑ in Hippocampal CA 1 Region of Alzheimer’s Disease Rats. Zhen Ci Yan Jiu 2018, 43, 140–145. [Google Scholar]
- Koo, J.H.; Kang, E.B.; Oh, Y.S.; Yang, D.S.; Cho, J.Y. Treadmill exercise decreases amyloid-β burden possibly via activation of SIRT-1 signaling in a mouse model of Alzheimer’s disease. Exp. Neurol. 2017, 288, 142–152. [Google Scholar] [CrossRef]
- Kumar, R.; Chaterjee, P.; Sharma, P.K.; Singh, A.K.; Gupta, A.; Gill, K.; Tripathi, M.; Dey, A.B.; Dey, S. Sirtuin1: A promising serum protein marker for early detection of Alzheimer’s disease. PLoS ONE 2013, 8, e61560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Nguyen, M.D.; Dobbin, M.M.; Fischer, A.; Sananbenesi, F.; Rodgers, J.T.; Delalle, I.; Baur, J.A.; Sui, G.C.; Armour, S.M.; et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007, 26, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.S.; Li, J.J.; Diao, S.Y.; Kwak, Y.D.; Liu, L.; Zhi, L.T.; Bueler, H.; Bhat, N.R.; Williams, R.W.; Park, E.A.; et al. Metabolic stress modulates Alzheimer’s beta-secretase gene transcription via SIRT1-PPAR gamma-PGC-1 in neurons. Cell. Metab. 2013, 17, 685–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.H.; Chen, J.A.; Sayed, F.; Ward, M.E.; Gao, F.Y.; Nguyen, T.A.; Krabbe, G.; Sohn, P.D.; Lo, I.; Minami, S.; et al. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1 beta. J. Neurosci. 2015, 35, 807–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.; Hanson, P.S.; Morris, C.M. SIRT1 ameliorates oxidative stress induced neural cell death and is down-regulated in Parkinson’s disease. BMC Neurosci. 2017, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Solis, R.; Ramadori, G.; Coppari, R.; Sassone-Corsi, P. SIRT1 Relays Nutritional Inputs to the Circadian Clock Through the Sf1 Neurons of the Ventromedial Hypothalamus. Endocrinology 2015, 156, 2174–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matarese, G.; Procaccini, C.; Menale, C.; Kim, J.G.; Kim, J.D.; Diano, S.; Diano, N.; De Rosa, V.; Dietrich, M.O.; Horvath, T.L. Hunger-promoting hypothalamic neurons modulate effector and regulatory T-cell responses. Proc. Natl. Acad. Sci. USA 2013, 110, 6193–6198. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, J.; Lu, T.; Zhan, Z.; Wei, W.; Lyu, X.; Jiang, Y.; Xue, X. The effect of swimming exercise and diet on the hypothalamic inflammation of ApoE-/- micebased on SIRT1-NF-κB-GnRH expression. Aging 2020, 12, 11085–11099. [Google Scholar] [CrossRef]
- Kishi, T.; Fukuo, Y.; Kitajima, T.; Okochi, T.; Yamanouchi, Y.; Kinoshita, Y.; Kawashima, K.; Inada, T.; Kunugi, H.; Kato, T.; et al. SIRT1 gene, schizophrenia and bipolar disorder in the Japanese population: An association study. Genes Brain Behav. 2011, 10, 257–263. [Google Scholar] [CrossRef]
- Libert, S.; Pointer, K.; Bell, E.L.; Das, A.; Cohen, D.E.; Asara, J.M.; Kapur, K.; Bergmann, S.; Preisig, M.; Otowa, T.; et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell 2011, 147, 1459–1472. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, D.; Koo, J.W.; Feng, J.; Heller, E.; Rabkin, J.; Heshmati, M.; Renthal, W.; Neve, R.; Liu, X.; Shao, N.; et al. Essential Role of SIRT1 Signaling in the Nucleus Accumbens in Cocaine and Morphine Action. J. Neurosci. 2013, 33, 16088–16098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, F.; Wijaya, L.; Tang, B.L. SIRT1 in the brain—Connections with aging-associated disorders and lifespan. Front. Cell. Neurosci. 2015, 9, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prozorovski, T.; Schulze-Topphoff, U.; Glumm, R.; Baumgart, J.; Schröter, F.; Ninnemann, O.; Siegert, E.; Bendix, I.; Brüstle, O.; Nitsch, R.; et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat. Cell Biol. 2008, 10, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Esbensen, Y.; Kunke, D.; Suganthan, R.; Rachek, L.; Bjørås, M.; Eide, L. Mitochondrial DNA damage level determines neural stem cell differentiation fate. J. Neurosci. 2011, 31, 9746–9751. [Google Scholar] [CrossRef] [Green Version]
- Saharan, S.; Jhaveri, D.J.; Bartlett, P.F. SIRT1 regulates the neurogenic potential of neural precursors in the adult subventricular zone and hippocampus. J. Neurosci. Res. 2013, 91, 642–659. [Google Scholar] [CrossRef] [Green Version]
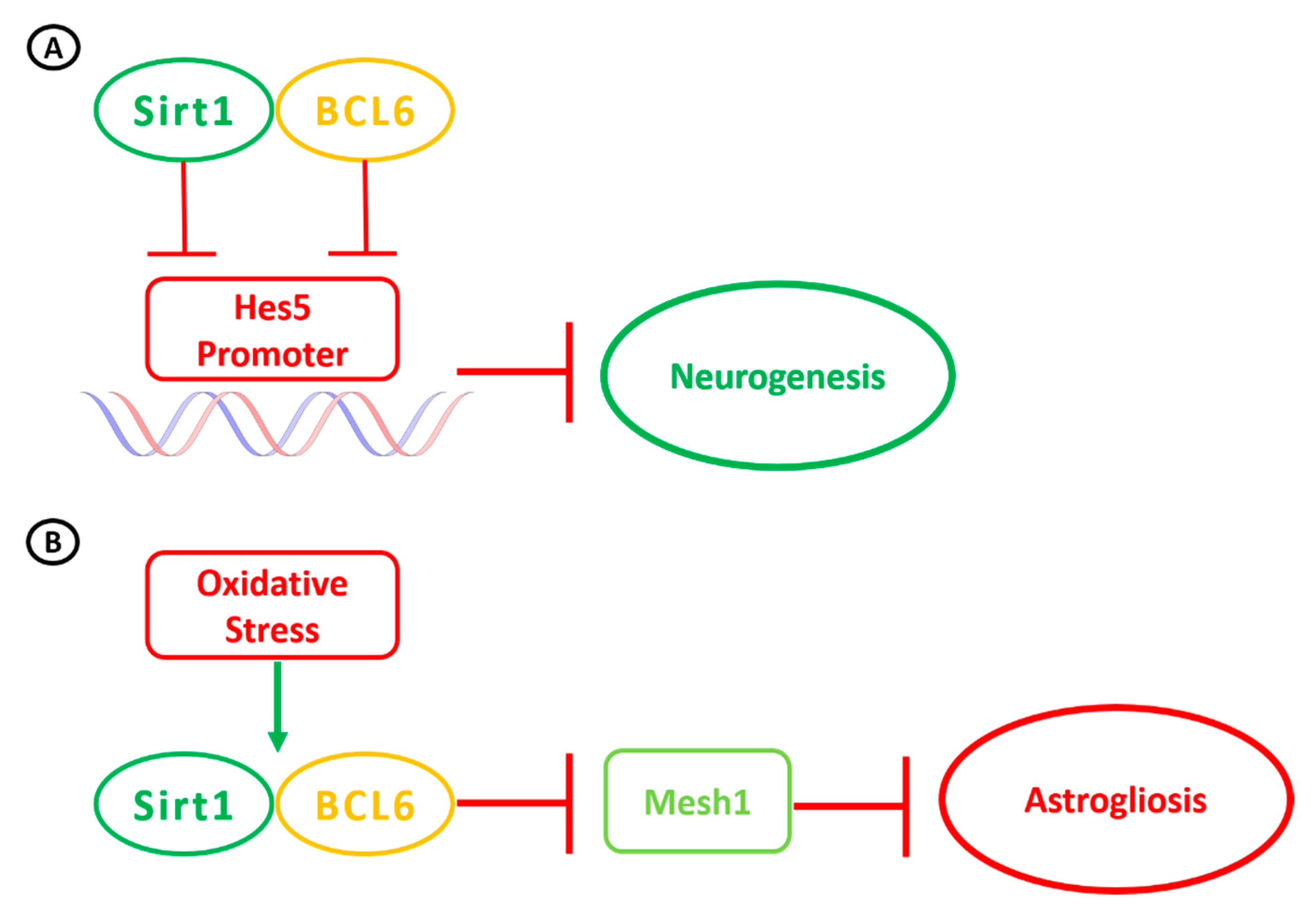
- Ichi, S.; Boshnjaku, V.; Shen, Y.W.; Mania-Farnell, B.; Ahlgren, S.; Sapru, S.; Mansukhani, N.; McLone, D.G.; Tomita, T.; Mayanil, C.S. Role of Pax3 acetylation in the regulation of Hes1 and Neurog2. Mol. Biol. Cell 2011, 22, 503–512. [Google Scholar] [CrossRef]
- Tiberi, L.; van den Ameele, J.; Dimidschstein, J.; Piccirilli, J.; Gall, D.; Herpoel, A.; Bilheu, A.; Bonnefont, J.; Iacovino, M.; Kyba, M.; et al. BCL6 controls neurogenesis through Sirt1-dependent epigenetic repression of selective Notch targets. Nat. Neurosci. 2012, 15, 1627–1635. [Google Scholar] [CrossRef]
- Sugino, T.; Maruyama, M.; Tanno, M.; Kuno, A.; Houkin, K.; Horio, Y. Protein deacetylase SIRT1 in the cytoplasm promotes nerve growth factor-induced neurite outgrowth in PC12 cells. FEBS Lett. 2010, 584, 2821–2826. [Google Scholar] [CrossRef] [Green Version]
- Codocedo, J.F.; Allard, C.; Godoy, J.A.; Varela-Nallar, L.; Inestrosa, N.C. SIRT1 regulates dendritic development in hippocampal neurons. PLoS ONE 2012, 7, e47073. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.L. Sirt1’s complex roles in neuroprotection. Cell. Mol. Neurobiol. 2009, 29, 1093–1103. [Google Scholar] [CrossRef]
- Srivastava, S.; Haigis, M.C. Role of sirtuins and calorie restriction in neuroprotection: Implications in Alzheimer’s and Parkinson’s diseases. Curr. Pharm. Des. 2011, 17, 3418–3433. [Google Scholar] [CrossRef] [PubMed]
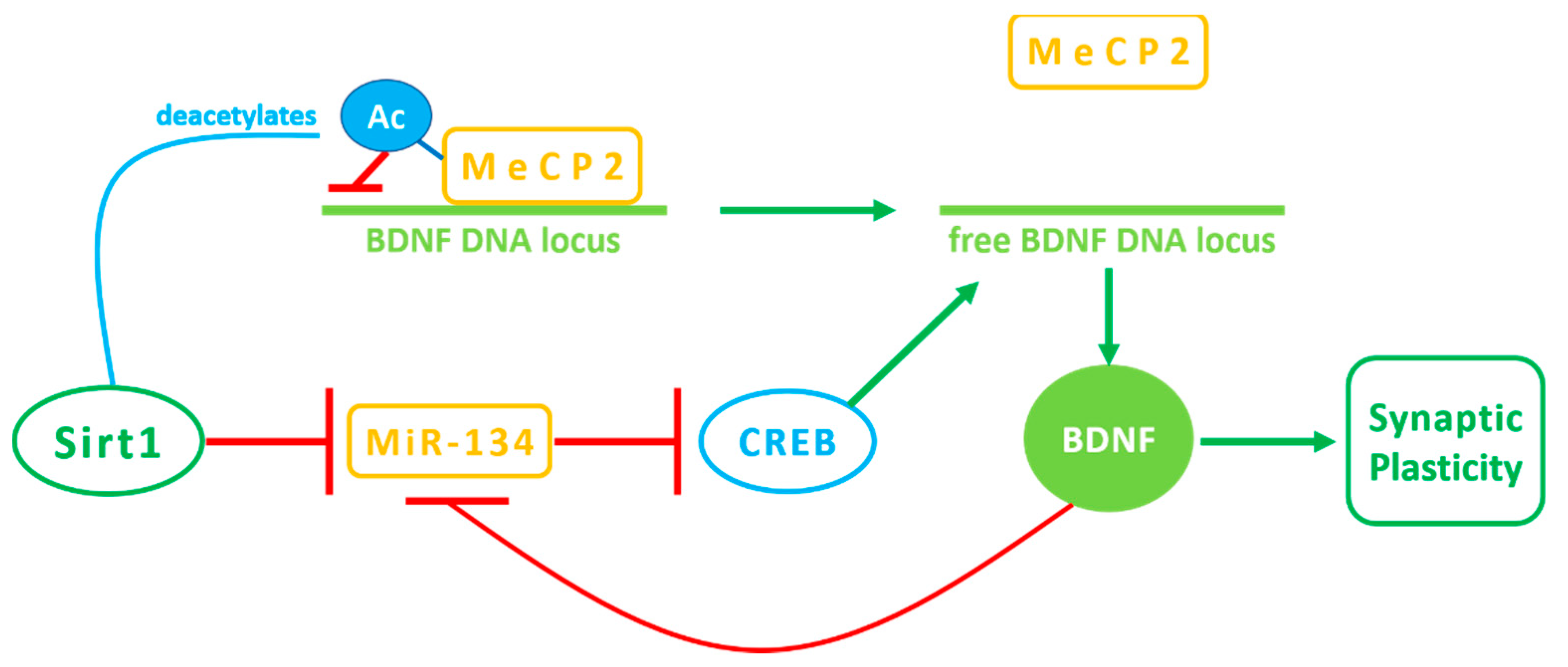
- Zocchi, L.; Sassone-Corsi, P. SIRT1-mediated deacetylation of MeCP2 contributes to BDNF expression. Epigenetics 2012, 7, 695–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Choe, H.K. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech. Ageing Dev. 2019, 177, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. Inflammation, Stem Cells, and the Aging Hypothalamus. Rejuvenation Res. 2017, 20, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.; Song, D.K.; Kim, M.S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 2016, 48, e216. [Google Scholar] [CrossRef] [Green Version]
- Pierantoni, R.; Cobellis, G.; Meccariello, R.; Fasano, S. Evolutionary aspects of cellular communication in the vertebrate hypothalamo-hypophysio-gonadal axis. Int. Rev. Cytol. 2002, 218, 69–141. [Google Scholar]
- Nillni, E.A. The metabolic sensor Sirt1 and the hypothalamus: Interplay between peptide hormones and pro-hormone convertases. Mol. Cell. Endocrinol. 2016, 438, 77–88. [Google Scholar] [CrossRef]
- Vazquez, M.J.; Velasco, I.; Tena-Sempere, M. Novel mechanisms for the metabolic control of puberty: Implications for pubertal alterations in early-onset obesity and malnutrition. J. Endocrinol. 2019, 242, 51–65. [Google Scholar] [CrossRef]
- Pinilla, L.; Aguilar, E.; Dieguez, C.; Millar, R.P.; Tena-Sempere, M. Kisspeptins and reproduction: Physiological roles and regulatory mechanisms. Physiol. Rev. 2012, 92, 1235–1316. [Google Scholar] [CrossRef]
- Cacciola, G.; Chianese, R.; Chioccarelli, T.; Ciaramella, V.; Fasano, S.; Pierantoni, R.; Meccariello, R.; Cobellis, G. Cannabinoids and Reproduction: A Lasting and Intriguing History. Pharmaceuticals 2010, 3, 3275–3323. [Google Scholar] [CrossRef] [Green Version]
- Forte, N.; Fernández-Rilo, A.C.; Palomba, L.; Di Marzo, V.; Cristino, L. Obesity Affects the Microbiota-Gut-Brain Axis and the Regulation Thereof by Endocannabinoids and Related Mediators. Int. J. Mol. Sci. 2020, 21, 1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meccariello, R.; Battista, N.; Bradshaw, H.B.; Wang, H. Updates in reproduction coming from the endocannabinoid system. Int. J. Endocrinol. 2014, 2014, 412354. [Google Scholar] [CrossRef] [PubMed]
- Bovolin, P.; Cottone, E.; Pomatto, V.; Fasano, S.; Pierantoni, R.; Cobellis, G.; Meccariello, R. Endocannabinoids are Involved in Male Vertebrate Reproduction: Regulatory Mechanisms at Central and Gonadal Level. Front. Endocrinol. 2014, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Toorie, A.M.; Nillni, E.A. Minireview: Central Sirt1 Regulates Energy Balance via the Melanocortin System and Alternate Pathways. Mol. Endocrinol. 2014, 28, 1423–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadori, G.; Fujikawa, T.; Anderson, J.; Berglund, E.D.; Frazao, R.; Michán, S.; Vianna, C.R.; Sinclair, D.A.; Elias, C.F.; Coppari, R. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 2011, 14, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Cohen, H.Y.; Miller, C.; Bitterman, K.J.; Wall, N.R.; Hekking, B.; Kessler, B.; Howitz, K.T.; Gorospe, M.; de Cabo, R.; Sinclair, D.A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 2004, 305, 390–392. [Google Scholar] [CrossRef] [Green Version]
- Ramadori, G.; Lee, C.E.; Bookout, A.L.; Lee, S.; Williams, K.W.; Anderson, J.; Elmquist, J.K.; Coppari, R. Brain SIRT1: Anatomical distribution and regulation by energy availability. J. Neurosci. 2008, 28, 9989–9996. [Google Scholar] [CrossRef]
- Cakir, I.; Perello, M.; Lansari, O.; Messier, N.J.; Vaslet, C.A.; Nillni, E.A. Hypothalamic Sirt1 Regulates Food Intake in a Rodent Model System. PLoS ONE 2009, 4, e8322. [Google Scholar] [CrossRef] [Green Version]
- Al-Massadi, O.; Quiñones, M.; Clasadonte, J.; Hernandez-Bautista, R.; Romero-Picó, A.; Folgueira, C.; Morgan, D.A.; Kalló, I.; Heras, V.; Senra, A.; et al. MCH Regulates SIRT1/FoxO1 and Reduces POMC Neuronal Activity to Induce Hyperphagia, Adiposity, and Glucose Intolerance. Diabetes 2019, 68, 2210–2222. [Google Scholar] [CrossRef] [Green Version]
- Ramadori, G.; Fujikawa, T.; Fukuda, M.; Anderson, J.; Morgan, D.A.; Mostoslavsky, R.; Stuart, R.C.; Perello, M.; Vianna, C.R.; Nillni, E.A.; et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell. Metab. 2010, 12, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Kikuchi, O.; Shimpuku, M.; Susanti, V.Y.; Yokota-Hashimoto, H.; Taguchi, R.; Shibusawa, N.; Sato, T.; Tang, L.; Amano, K.; et al. Hypothalamic SIRT1 prevents age-associated weight gain by improving leptin sensitivity in mice. Diabetologia 2014, 57, 819–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rickert, E.; Fernandez, M.O.; Choi, I.; Gorman, M.; Olefsky, J.M.; Webster, N.J.G. Neuronal SIRT1 Regulates Metabolic and Reproductive Function and the Response to Caloric Restriction. J. Endocr. Soc. 2018, 3, 427–445. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Rickert, E.; Fernandez, M.; Webster, N.J.G. SIRT1 in Astrocytes Regulates Glucose Metabolism and Reproductive Function. Endocrinology 2019, 160, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- McBurney, M.W.; Yang, X.; Jardine, K.; Hixon, M.; Boekelheide, K.; Webb, J.R.; Lansdorp, P.M.; Lemieux, M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol. Cell. Biol. 2003, 23, 38–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolthur-Seetharam, U.; Teerds, K.; de Rooij, D.G.; Wendling, O.; McBurney, M.; Sassone-Corsi, P.; Davidson, I. The histone deacetylase SIRT1 controls male fertility in mice through regulation of hypothalamic-pituitary gonadotropin signaling. Biol. Reprod. 2009, 80, 384–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sante, G.; Wang, L.; Wang, C.; Jiao, X.; Casimiro, M.C.; Chen, K.; Pestell, T.G.; Yaman, I.; Di Rocco, A.; Sun, X.; et al. Sirt1-deficient mice have hypogonadotropic hypogonadism due to defective GnRH neuronal migration. Mol. Endocrinol. 2015, 29, 200–212. [Google Scholar] [CrossRef] [Green Version]
- Lannes, J.; L’Hôte, D.; Garrel, G.; Laverrière, J.N.; Cohen-Tannoudji, J.; Quérat, B. Rapid communication: A microRNA-132/212 pathway mediates GnRH activation of FSH expression. Mol. Endocrinol. 2015, 29, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Chianese, R.; Cobellis, G.; Chioccarelli, T.; Ciaramella, V.; Migliaccio, M.; Fasano, S.; Pierantoni, R.; Meccariello, R. Kisspeptins, Estrogens and Male Fertility. Curr. Med. Chem. 2016, 23, 4070–4091. [Google Scholar] [CrossRef]
- Motti, M.L.; Meccariello, R. Minireview: The Epigenetic Modulation of KISS1 in Reproduction and Cancer. Int. J. Environ. Res. Public Health 2019, 16, 2607. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, A.K.; Zavodna, M.; Viljoen, J.L.; Stanton, J.A.; Gemmell, N.J.; Jasoni, C.L. Changes in methylation patterns of kiss1 and kiss1r gene promoters across puberty. Genet. Epigenet. 2013, 5, 51–62. [Google Scholar] [CrossRef]
- Vazquez, M.J.; Toro, C.A.; Castellano, J.M.; Ruiz-Pino, F.; Roa, J.; Beiroa, D.; Heras, V.; Velasco, I.; Dieguez, C.; Pinilla, L.; et al. SIRT1 mediates obesity- and nutrient-dependent perturbation of pubertal timing by epigenetically controlling Kiss1 expression. Nat. Commun. 2018, 9, 4194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barash, I.A.; Cheung, C.C.; Weigle, D.S.; Ren, H.; Kabigting, E.B.; Kuijper, J.L.; Clifton, D.K.; Steiner, R.A. Leptin is a metabolic signal to the reproductive system. Endocrinology 1996, 137, 3144–3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chehab, F.F.; Lim, M.E.; Lu, R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996, 12, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S. Clinical review 94: What’s in a name? In search of leptin’s physiologic role. J. Clin. Endocrinol. Metab. 1998, 83, 1407–1413. [Google Scholar]
- Wahab, F.; Atika, B.; Shahab, M.; Behr, R. Kisspeptin signalling in the physiology and pathophysiology of the urogenital system. Nat. Rev. Urol. 2016, 13, 21–32. [Google Scholar] [CrossRef]
- Skorupskaite, K.; George, J.T.; Anderson, R.A. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update 2014, 20, 485–500. [Google Scholar] [CrossRef] [Green Version]
- Tsatsanis, C.; Dermitzaki, E.; Avgoustinaki, P.; Malliaraki, N.; Mytaras, V.; Margioris, A. The impact of adipose tissue-derived factors on the hypothalamic-pituitary-gonadal (HPG) axis. Hormones 2015, 14, 549–562. [Google Scholar] [CrossRef] [Green Version]
- Dudek, M.; Kołodziejski, P.A.; Pruszyńska-Oszmałek, E.; Sassek, M.; Ziarniak, K.; Nowak, K.W.; Sliwowska, J.H. Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic–pituitary–gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides 2016, 56, 41–49. [Google Scholar] [CrossRef]
- Dai, H.; Sinclair, D.A.; Ellis, J.L.; Steegborn, C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol. Ther. 2018, 188, 140–154. [Google Scholar] [CrossRef]
- Hubbard, B.P.; Sinclair, D.A. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol. Sci. 2014, 35, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Bonkowski, M.S.; Sinclair, D.A. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, L.; Rosset, I.; Roriz-Cruz, M. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed. Res. Int. 2014, 2014, 908915. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S. Current Evidence on the Effect of Dietary Polyphenols Intake on Brain Health. Curr. Nutr. Food Sci. 2020, 16, 1170–1182. [Google Scholar] [CrossRef]
- D’Angelo, S.; Cusano, P. Adherence to the Mediterranean diet in athletes. Sport Sci. 2020, 13, 58–63. [Google Scholar]
- Vuoso, D.C.; Porcelli, M.; Cacciapuoti, G.; D’Angelo, S. Biological Activity of MelAnnurca Flesh Apple Biophenols. Curr. Nutr. Food Sci. 2020, 16, 1149–1162. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Cacciapuoti, G. Effects of Annurca Apple (Malus pumila cv Annurca) Polyphenols on Breast Cancer Cells. Curr. Nutr. Food Sci. 2019, 15, 745–751. [Google Scholar] [CrossRef]
- Martino, E.; Vuoso, D.C.; D’Angelo, S.; Mele, L.; D’Onofrio, N.; Porcelli, M.; Cacciapuoti, G. Annurca apple polyphenol extract selectively kills MDA-MB-231 cells through ROS generation, sustained JNK activation and cellgrowth and survival inhibition. Sci. Rep. 2019, 9, 13045. [Google Scholar] [CrossRef] [PubMed]
- Vuoso, D.C.; D’Angelo, S.; Ferraro, R.; Caserta, S.; Guido, S.; Cammarota, M.; Porcelli, M.; Cacciapuoti, G. Annurca applepolyphenol extract promotes mesenchymal-to-epithelial transition and inhibits migration in triple-negative breast cancercells through ROS/JNK signaling. Sci. Rep. 2020, 10, 15921. [Google Scholar] [CrossRef]
- D’Angelo, S.; Rosa, R. The impact of supplementation with Pomegranate fruit (Punica Granatum L.) on sport performance. Sport Sci. 2020, 13, 29–37. [Google Scholar]
- D’Angelo, S.; Sammartino, D. Protective Effect of Annurca Apple Extract Against Oxidative Damage in Human Erythrocytes. Curr. Nutr. Food Sci. 2015, 11, 248–256. [Google Scholar] [CrossRef]
- D’Angelo, S.; Ascione, A. Guarana and physical performance: A myth or reality? J. Hum. Sport Exerc. 2020, 15, S539–S551. [Google Scholar]
- Sarubbo, F.; Esteban, S.; Miralles, A.; Moranta, D. Effects of Resveratrol and other Polyphenols on Sirt1: Relevance to Brain Function During Aging. Curr. Neuropharmacol. 2018, 16, 126–136. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Tafuri, D. Nutraceutical: Their role in improving sports performance. Sport Sci. 2020, 13, 7–12. [Google Scholar]
- D’Angelo, S. Polyphenols: Potential beneficial effects of these phytochemicals in athletes. Curr. Sports Med. Rep. 2020, 19, 260–265. [Google Scholar] [CrossRef]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Zou, P.; Liu, X.; Li, G.; Wang, Y. Resveratrol pretreatment attenuates traumatic brain injury in rats by suppressing NLRP3 inflammasome activation via SIRT1. Mol. Med. Rep. 2018, 17, 3212–3217. [Google Scholar] [CrossRef]
- Gomes, B.; Silva, J.; Romeiro, C.; Dos Santos, S.M.; Rodrigues, C.A.; Gonçalves, P.R.; Sakai, J.T.; Mendes, P.; Varela, E.; Monteiro, M.C. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid. Med. Cell Longev. 2018, 2018, 8152373. [Google Scholar] [CrossRef]
- Cao, W.; Dou, Y.; Li, A. Resveratrol Boosts Cognitive Function by Targeting SIRT1. Neurochem. Res. 2018, 43, 1705–1713. [Google Scholar] [CrossRef]
- Sarubbo, F.; Ramis, M.R.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D. Chronic Silymarin, Quercetin and Naringenin Treatments Increase Monoamines Synthesis and Hippocampal Sirt1 Levels. J. Neuroimmune Pharmacol. 2018, 13, 24–38. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, S.; Gao, Y.; Wang, R.; Wu, Q.; Wu, H.; Luo, T. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain Res. Bull. 2016, 121, 9–15. [Google Scholar] [CrossRef]
- Ramis, M.R.; Sarubbo, F.; Tejada, S.; Jiménez, M.; Esteban, S.; Miralles, A.; Moranta, D. Chronic Polyphenon-60 or Catechin Treatments Increase Brain Monoamines Syntheses and Hippocampal SIRT1 Levels Improving Cognition in Aged Rats. Nutrients 2020, 12, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; Rahman, I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem. Pharmacol. 2012, 84, 1332–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Shi, J.J.; Fang, J.; Wang, Q.; Chen, Y.; Zhang, S.J. Quercetin ameliorates diabetic encephalopathy through SIRT1/ER stress pathway in db/db mice. Aging 2020, 12, 7015–7029. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.P.; Roggerio, A.; Goes, M.F.S.; Avakian, S.D.; Leal, D.P.; Maranhão, R.C.; Strunz, C.M.C. Serum concentrations and gene expression of sirtuin 1 in healthy and slightly overweight subjects after caloric restriction or resveratrol supplementation: A randomized trial. Int. J. Cardiol. 2017, 15, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.C.; Zhang, Y.F.; Liu, S.S.; Cheng, X.J.; Yang, X.; Cui, X.G.; Zhao, X.R.; Zhao, H.; Hao, M.F.; Li, M.D.; et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J. Cell. Mol. Med. 2020, 24, 12355–12367. [Google Scholar] [CrossRef]
- Wan, J.; Li, J.; Cao, N.; Li, Z.; Han, J.; Li, L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. OncoTargets Ther. 2018, 2, 7777–7786. [Google Scholar]
- Lee, S.H.; Lee, J.H.; Lee, H.Y.; Min, K.J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef] [Green Version]
- McCurey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Yang, L.V.; Murata, R.M.; Rosalen, P.L.; Scalisi, A.; Neri, L.M.; Cocco, L.; et al. Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging 2017, 9, 1477–1536. [Google Scholar] [CrossRef] [Green Version]
- Rizzi, L.; Roriz-Cruz, M. Sirtuin 1 and Alzheimer’s disease: An up-to-date review. Neuropeptides. 2018, 71, 54–60. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [Green Version]
| Cytoplasm | Nucleus | Mitochondrion | Additional Activities with Respect to NAD+—Dependent Removal of Acetyl Groups from Target Proteins [28] | |
|---|---|---|---|---|
| Sirt1 | X | X | - | |
| Sirt2 | X | transient | - | Demyristoylase |
| Sirt3 | - | - | X | |
| Sirt4 | - | - | X | ADP-ribosyltransferase |
| Sirt5 | - | - | X | DemanlonylaseDesuccinylase |
| Sirt6 | - | X | - | ADP-ribosyltransferase |
| Sirt7 | - | X | - |
| Activity | Effect | Reference |
|---|---|---|
| Aging, Neuroprotection, and Neurodegeneration | Sirt1 is implicated in life span extension in mice that are either calorie restricted or on standard diet. | [48] |
| Sirt1 proteinis sensitive to caloric deficit and mediate the beneficial effects of caloric restriction | [49] | |
| A caloric restriction can activate sirtuins via an increase in NAD+ levels. The Sirt1/mTOR signaling pathways in the brain are involved in the mechanisms of neuroprotection of caloric restriction. | ||
| Up-regulation of Sirt1 and PGC-1α expression improved learning and memory abilities | [50] | |
| In Alzheimer’s disease mice models, treadmill exercise inhibited the production of β- amyloid via Sirt1, favoring the non-amyloidogenic pathway of Alzheimer’s disease | [51] | |
| Decline in serum concentration of Sirt1 in healthy individuals as they age | [52] | |
| Sirt1 serum concentration declines in patients diagnosed with Alzheimer’s disease and mild cognitive impairment when compared to elderly and young controls | ||
| In the inducible p25 transgenic mouse, a model of Alzheimer’s disease and tauopathies, enhancement of Sirt1 activity by resveratrol or injection of Sirt1 recombinant lentivirus in the hippocampus resulted in significant protection against neurodegeneration by deacetylation of PGC-1α and P53 | [53] | |
| Protective role of Sirt1 in age-related cognitive decline such as Alzheimer’s disease Parkinson’s disease and Lewybody dementia | [54] | |
| Microglial Sirt1 deficiency is a causative role in cognitive decline and neurodegeneration | [55] | |
| Sirt1 (both mRNA and protein) declines with age in the brain, liver, skeletal muscle and white adipose tissues. SIRT1 expression is age-dependently reduced in microglia | ||
| As pro-survival protein. Sirt1 ameliorates oxidative stress induced neural cell death and is down-regulated in Parkinson’s disease | [56] | |
| Sirt1 was down-regulated in post-mortem brain tissue obtained from patients with Parkinson’s disease, Parkinson’s disease with dementia, dementia with Lewy bodies and Alzheimer’s disease,. | ||
| Circadian clock | Sirt1 plays an important role in translating nutritional cues in the brain | [57] |
| In the ventromedial hypothalamus, Sirt1 was found to control circadian rodent behavior under specific conditions of light and food restriction, which also extends to effect circadian gene expression of the central clock in the suprachiasmatic nucleus. | ||
| Immunity | Deletion of Sirt1 in hypothalamic Agouti-related peptide-expressing neurons creates a pro-inflammatory environment, with enhanced effector T-cell activity and decreased regulatory T-cell function | [58] |
| Hypothalamic inflammation, glial cells activation and learning and memory impairment were alleviated by swimming exercise plus diet control, which was related to the increasing expression of Sirt1 | [59] | |
| Psychiatric disorders | Several Sirt1single nucleotide polymorphisms were over-represented in patients with depression and anxiety disorders | [60] |
| Sirt1 levels correlates with anxiety and exploratory drive and is mechanistically linked with serotonin levels in the brain | [61] | |
| Cocaine or morphine administration increases Sirt1 expression in the nucleus accumbens, a brain region that regulates motivation and reward | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angelo, S.; Mele, E.; Di Filippo, F.; Viggiano, A.; Meccariello, R. Sirt1 Activity in the Brain: Simultaneous Effects on Energy Homeostasis and Reproduction. Int. J. Environ. Res. Public Health 2021, 18, 1243. https://doi.org/10.3390/ijerph18031243
D’Angelo S, Mele E, Di Filippo F, Viggiano A, Meccariello R. Sirt1 Activity in the Brain: Simultaneous Effects on Energy Homeostasis and Reproduction. International Journal of Environmental Research and Public Health. 2021; 18(3):1243. https://doi.org/10.3390/ijerph18031243
Chicago/Turabian StyleD’Angelo, Stefania, Elena Mele, Federico Di Filippo, Andrea Viggiano, and Rosaria Meccariello. 2021. "Sirt1 Activity in the Brain: Simultaneous Effects on Energy Homeostasis and Reproduction" International Journal of Environmental Research and Public Health 18, no. 3: 1243. https://doi.org/10.3390/ijerph18031243
APA StyleD’Angelo, S., Mele, E., Di Filippo, F., Viggiano, A., & Meccariello, R. (2021). Sirt1 Activity in the Brain: Simultaneous Effects on Energy Homeostasis and Reproduction. International Journal of Environmental Research and Public Health, 18(3), 1243. https://doi.org/10.3390/ijerph18031243







