Potential Uses of Olive Oil Secoiridoids for the Prevention and Treatment of Cancer: A Narrative Review of Preclinical Studies
Abstract
1. Introduction
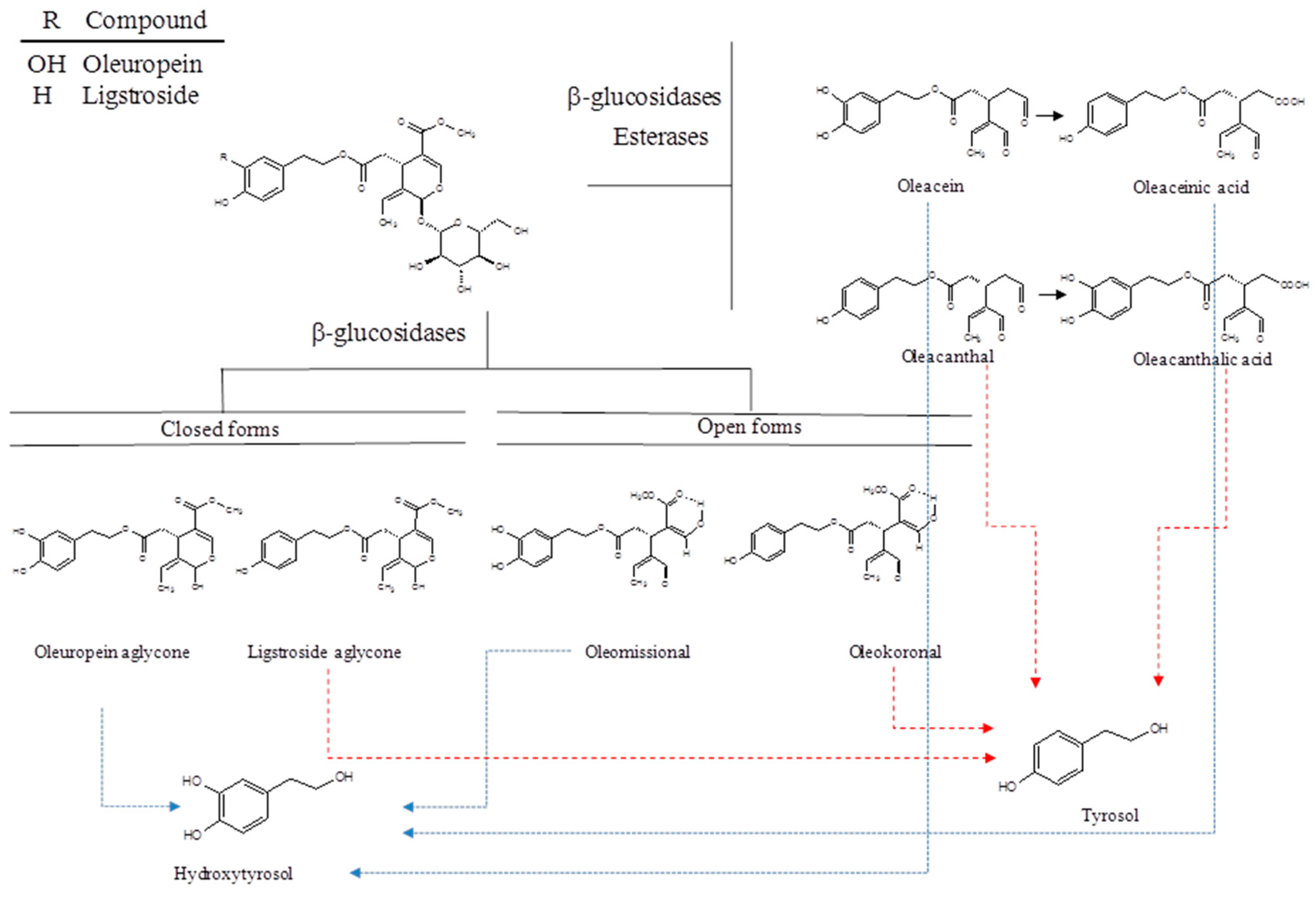
2. Chemistry of Secoiridoids
3. Extraction of Phenolic Alcohols and Secoiridoids
4. Oleocanthal
5. Oleacein
6. Tyrosol and Hydroxytyrosol
7. Oleuropein
8. Ligstroside Aglycone
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Danaei, G.; Ding, E.L.; Mozaffarian, D.; Taylor, B.; Rehm, J.; Murray, C.J.L.; Ezzati, M. The preventable causes of death in the United States: Comparative risk assessment of dietary; lifestyle; and metabolic risk factors. PLoS Med. 2009, 6, e1000058. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, M. Nutrition and cancer: A review of the evidence for an anti-cancer diet. Nutr. J. 2004, 3, 19. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Critselis, E. Mediterranean diet and longevity. Eur. J. Cancer Prev. 2004, 13, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean Diet Foundation Expert Group; Mediterranean diet pyramid today. Sci. Cult. Updates Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Mentella, M.C.; Scaldaferri, F.; Ricci, C.; Gasbarrini, A.; Miggiano, G.A.D. Cancer and Mediterranean Diet: A review. Nutrients 2019, 11, 2059. [Google Scholar] [CrossRef]
- Grosso, G.; Buscemi, S.; Galvano, F.; Mistretta, A.; Marventano, S.; La Vela, V.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Med-iterranean diet and cancer: Epidemiological evidence and mechanism of selected aspects. BMC Surg. 2013, 13, S14. [Google Scholar] [CrossRef]
- Schwingshack, L.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis of observational studies. Cancer Med. 2015, 4, 1933–1947. [Google Scholar] [CrossRef]
- Owen, R.; Giacosa, A.; Hull, W.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on pro-tection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Alarcón-De-La-Lastra, C. An up-date of olive oil phenols in inflammation and cancer: Molecular mechanisms and clinical implications. Curr. Med. Chem. 2013, 20, 4758–4776. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Iacono, A.; Gómez, R.; Sperry, J.; Conde, J.; Bianco, G.; Meli, R.; Gómez-Reino, J.J.; Smith, A.B., III; Gualillo, O. Effect of oleocanthal and its derivatives on inflammatory response induced by lipopolysaccharide in a murine chondrocyte cell line. Arthritis Rheum. 2010, 62, 1675–1682. [Google Scholar] [CrossRef]
- Li, W.; Sperry, J.B.; Crowe, A.; Trojanowski, J.Q.; Iii, A.B.S.; Lee, V.M.-Y. Inhibition of tau fibrillization by oleocanthal via reaction with the amino groups of tau. J. Neurochem. 2009, 110, 1339–1351. [Google Scholar] [CrossRef]
- Pitt, J.; Roth, W.; Lacor, P.; Smith, A.B., III; Blankenship, M.; Velasco, P.; De Felice, F.; Breslin, P.; Klein, W.L. Alzhei-mer’s-associated Aβ oligomers show altered structure; immunoreactivity and synaptotoxicity with low doses of oleocanthal. Toxicol. Appl. Pharmacol. 2009, 240, 189–197. [Google Scholar] [CrossRef]
- Qosa, H.; Batarseh, Y.S.; Mohyeldin, M.M.; El Sayed, K.A.; Keller, J.N.; Kaddoumi, A. Oleocanthal Enhances Amyloid-β Clearance from the Brains of TgSwDI Mice and in Vitro across a Human Blood-Brain Barrier Model. ACS Chem. Neurosci. 2015, 6, 1849–1859. [Google Scholar] [CrossRef]
- Grilo, F.; Sedaghat, S.; Di Stefano, V.; Schicchi, R.; Caruso, T.; Lo Bianco, R. Tree Planting Density and Canopy Position Affect ‘Cerasuola’ and ‘Koroneiki’ Olive Oil Quality. Horticulturae 2021, 7, 11. [Google Scholar] [CrossRef]
- Huang, Y.L.; Oppong, M.B.; Guo, Y.; Wang, L.Z.; Fang, S.M.; Deng, Y.R.; Gao, X.M. The Oleaceae family: A source of secoiridoids with multiple biological activities. Fitoterapia 2019, 136, 104155. [Google Scholar] [CrossRef]
- Boussahel, S.; Di Stefano, V.; Muscarà, C.; Cristani, M.; Melilli, M.G. Phenolic Compounds Characterization and Antioxidant Properties of Monocultivar Olive Oils from Northeast Algeria. Agriculture 2020, 10, 494. [Google Scholar] [CrossRef]
- Grilo, F.; Novara, M.E.; D’Oca, M.C.; Rubino, S.; Bianco, R.L.; Di Stefano, V. Quality evaluation of extra-virgin olive oils from Sicilian genotypes grown in a high-density system. Int. J. Food Sci. Nutr. 2019, 71, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.A.; Hernández, J.M.; Trujillo, J.M.; Lopez, H. Iridoids and secoiridoids from Oleaceae. Bioact. Nat. Prod. 2005, 32, 303–363. [Google Scholar] [CrossRef]
- Di Stefano, V.; Melilli, M.G. Effect of storage on quality parameters and phenolic content of Italian extra-virgin olive oils. Nat. Prod. Res. 2020, 34, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Diamantakos, P.; Velkou, A.; Killday, K.B.; Gimisis, T.; Melliou, E.; Magiatis, P. Oleokoronal and oleomissional: New major phenolic ingredients of extra virgin olive oil. Olivae 2015, 122, 22–33. [Google Scholar]
- Angelis, A.; Antoniadi, L.; Stathopoulos, P.; Halabalaki, M.; Skaltsounis, L.A. Oleocanthalic and Oleaceinic acids: New compounds from Extra Virgin Olive Oil (EVOO). Phytochem. Lett. 2018, 26, 190–194. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A. Simple and hydrolyzable compounds in virgin olive oil. 3. Spectroscopic characterizations of the secoiridoid derivatives. J. Agric. Food Chem. 1993, 41, 2228–2234. [Google Scholar] [CrossRef]
- Andrewes, P.; Busch, J.; de Joode, T.; Groenewegen, A.; Alexandre, H.J. Sensory properties of virgin olive oil polyphenols: Identification of deacetoxy-ligstroside aglycon as a key contributor to pungency Agric. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef]
- Impellizzeri, J.; Lin, J. A Simple High-Performance Liquid Chromatography Method for the Determination of Throat-Burning Oleocanthal with Probated Antiinflammatory Activity in Extra Virgin Olive Oils. J. Agric. Food Chem. 2006, 54, 3204–3208. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Mulinacci, N.; Galardi, C.; Vincieri, F.F.; Liberatore, L.; Cichelli, A. HPLC and HRGC Analyses of Pol-yphenols and Secoiridoid in Olive Oil. Chromatographia 2001, 53, 279–284. [Google Scholar] [CrossRef]
- Adhami, H.-R.; Zehl, M.; Dangl, C.; Dorfmeister, D.; Stadler, M.; Urban, E.; Hewitson, P.; Ignatova, S.; Krenn, L. Preparative isolation of oleocanthal, tyrosol, and hydroxytyrosol from olive oil by HPCCC. Food Chem. 2015, 170, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Angelis, A.; Michailidis, D.; Antoniadi, L.; Stathopoulos, P.; Tsantila, V.; Nuzillard, J.M.; Renault, J.H.; Skaltsounis, L.A. Pilot continuous centrifugal liquid-liquid extraction of extra virgin olive oil biophenols and gram-scale recovery of pure oleocanthal, oleacein, MFOA, MFLA and hydroxytyrosol. Sep. Purif. Technol. 2021, 255, 117692. [Google Scholar] [CrossRef]
- Smith, A.B., III; Han, Q.; Breslin, P.A.S.; Beauchamp, G.K. Synthesis and Assignment of Absolute Configuration of (−)-Oleocathal: A Potent, Naturally Occurring Non-steroidal Anti-inflammatory and Anti-oxidant Agent Derived from Extra Virgin Olive Oils. Org. Lett. 2005, 7, 5075–5078. [Google Scholar] [CrossRef]
- English, B.J.; Williams, R.M. Synthesis of (±)-oleocanthal via a tandem intramolecular Michael cyclization–HWE olefination. Tetrahedron Lett. 2009, 50, 2713–2715. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuch, J.T.B.; O’Connor, P.D.; Hügel, H.; Brimble, M.A. Synthetic studies towards the anti-inflammatory agent, oleocanthal using a Johnson–Claisen (orthoester) rearrangement strategy. ARKIVOC 2009, 7, 58–71. [Google Scholar]
- Takahashi, K.; Morita, H.; Honda, T. Formal synthesis of (−)-oleocanthal by means of a SmI2-promoted intramolecular cou-pling of bromoalkyne with α,β-unsaturated ester. Tetrahedron Lett. 2012, 53, 3342–3345. [Google Scholar] [CrossRef]
- Valli, M.; Peviani, E.G.; Porta, A.; D’Alfonso, A.; Zanoni, G.; Vidari, G. A Concise and Efficient Total Synthesis of Oleocan-thal. Eur. J. Org. Chem. 2013, 2013, 4332–4336. [Google Scholar] [CrossRef]
- Sarikaki, G.; Christoforidou, N.; Gaboriaud-Kolar, N.; Smith, A.B., III; Kostakis, I.K.; Skaltsounis, A.L. Biomimetic Synthesis of Oleocanthal, Oleacein, and Their Analogues Starting from Oleuropein, A Major Compound of Olive Leaves. J. Nat. Prod. 2020, 83, 1735–1739. [Google Scholar] [CrossRef]
- Calero, J.; Martínez, L.; García-Granados, A. Procedimiento Deaprovechamiento del Alpechin Para la Obtención Deácidos; Fenoles; Alcohols y Derivados Mediante Extracciónen Contracorriente. ES2051238, 16 May 1994. [Google Scholar]
- Cuomo, J.; Rabovskiy, A.B. Antioxidant Compositions Extracted from Olives and Olive by-Products. U.S. Patent WO2001045514A1, 28 June 2001. [Google Scholar]
- Crea, R. Method of Obtaining a Hydroxytyrosol-Rich Composition from Vegetation Water. U.S. Patent WO0218310, 7 March 2002. [Google Scholar]
- Brenes, M.; Castro, A. Procedure is for Obtaining Phenolics Extract with High Concentration of Anti-Oxidants and Involves Ul-tra-Filtration of Solutions Derived from Preparation Process of Preserved Table Olives. ES2186467, 1 May 2003. [Google Scholar]
- Fernández-Bolaños, J.; Rodríguez, G.; Rodríguez, R.; Guillén, R.; Jimenez-Araujo, A. Extraction of interesting organic compounds from olive oil waste. Grasas Aceites 2006, 57, 95–106. [Google Scholar] [CrossRef]
- Yasemi, M.; Heydarinasab, A.; Rahimi, M.; Ardjmand, M. Microchannels Effective Method for the Extraction of Oleuropein Compared with Conventional Methods. J. Chem. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Sengling Cebin Coppa, C.F.; Gonçalves, B.L.; Hwa, S.; Lee, I.; Martinelli, V.; Nunes, R.; Gonçalves, C.B.; Costa Rodrigues, C.E.; Oliveira, C.A.F. Extraction of oleuropein from olive leaves and applicability in foods. Qual. Assur. Saf. Crop. Foods 2020, 12, 50–62. [Google Scholar] [CrossRef]
- Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave; ultrasonic and conventional techniques for ex-traction of bioactive compounds from olive leaves Olea europaea L. Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Khemakhem, I.; Gargouri, O.D.; Dhouib, A.; Ayadi, M.A.; Bouaziz, M. Oleuropein rich extract from olive leaves by combining microfiltration, ultrafiltration and nanofiltration. Sep. Purif. Technol. 2017, 172, 310–317. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Baldino, L.; Della Porta, G.; Osseo, L.S.; Reverchon, E.; Adami, R. Concentrated oleuropein powder from olive leaves using alcoholic extraction and supercritical CO2 assisted extraction. J. Supercrit. Fluids 2018, 133, 65–69. [Google Scholar] [CrossRef]
- Lamprou, G.K.; Vlysidis, A.; Tzathas, K.; Vlyssides, A.G. Statistical optimization and kinetic analysis of the extraction of phenolic compounds from olive leaves. J. Chem. Technol. Biotechnol. 2020, 95, 457–465. [Google Scholar] [CrossRef]
- Murowaniecki, D.O.; Lorini, A.; Antunes, B.d.F.; Oliveira, R.M.; Zambiazi, R. Oleuropein: Methods for extraction; purifying and applying. Rev. Ceres. 2020, 67, 4. [Google Scholar]
- Cicerale, S.; Breslin, P.A.; Beauchamp, G.K.; Keast, R. Sensory Characterization of the Irritant Properties of Oleocanthal, a Natural Anti-Inflammatory Agent in Extra Virgin Olive Oils. Chem. Senses 2009, 34, 333–339. [Google Scholar] [CrossRef]
- Abuznait, A.H.; Qosa, H.; Busnena, B.A.; El Sayed, K.A.; Kaddoumi, A. Olive-Oil-Derived Oleocanthal Enhances β-Amyloid Clearance as a Potential Neuroprotective Mechanism against Alzheimer’s Disease: In Vitro and in Vivo Studies. ACS Chem. Neurosci. 2013, 4, 973–982. [Google Scholar] [CrossRef]
- Gachons, C.; Uchida, K.; Bryant, B.; Shima, A.; Sperry, J.B.; Dankulich-Nagrudny, L.; Tominaga, M.; Smith, A.B., III. Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. Neuroscience 2011, 31, 999–1009. [Google Scholar] [CrossRef]
- García-Villalba, R.; Carrasco-Pancorbo, A.; Nevedomskaya, E.; Mayboroda, O.A.; Deelder, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Exploratory analysis of human urine by LC–ESI-TOF MS after high intake of olive oil: Understanding the metabolism of polyphenols. Anal. Bioanal. Chem. 2010, 398, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Romero, C.; Medina, E.; Vargas, J.; Brenes, M.; De Castro, A. In Vitro Activity of Olive Oil Polyphenols against Helicobacter pylori. J. Agric. Food Chem. 2007, 55, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.chemspider.com/Chemical-Structure.9827154.html (accessed on 25 January 2021).
- Cusimano, A.; Balasus, D.; Azzolina, A.; Augello, G.; Emma, M.R.; Di Sano, C.; Gramignoli, R.; Strom, S.C.; McCubrey, J.A.; Montalto, G.; et al. Oleocanthal exerts antitumor effects on human liver and colon cancer cells through ROS genera-tion. Int. J. Oncol. 2017, 51, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Oh, W.-K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.-G.; Kwon, S.-M.; Choi, H.S. p-HPEA-EDA, a phenolic compound of virgin olive oil, activates AMP-activated protein kinase to inhibit carcinogenesis. Carcinogenesis 2011, 32, 545–553. [Google Scholar] [CrossRef]
- Hardie, D.G.; Iwadate, Y.; Yumura, S. The AMP-activated protein kinase pathway-new players upstream and downstream. J. Cell Sci. 2004, 117, 5479–5487. [Google Scholar] [CrossRef]
- Elnagar, A.Y.; Sylvester, P.W.; El Sayed, K.A. (−)-Oleocanthal as a c-Met Inhibitor for the Control of Metastatic Breast and Prostate Cancers. Planta Med. 2011, 77, 1013–1019. [Google Scholar] [CrossRef]
- Ho-Yen, C.M.; Jones, J.L.; Kermorgant, S. The clinical and functional significance of c-Met in breast cancer: A review. Breast Cancer Res. 2015, 17, 1–11. [Google Scholar] [CrossRef]
- Akl, M.R.; Ayoub, N.M.; Mohyeldin, M.M.; Busnena, B.A.; Foudah, A.I.; Liu, Y.Y.; Ei Sayed, K.A. Olive phenolics as c-Met inhibi-tors: (−)-Oleocanthal attenuates cell proliferation; invasiveness; and tumor growth in breast cancer models. PLoS ONE 2014, 9, e97622. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Mohyeldin, M.M.; Akl, M.R.; Mady, M.S.; Dragoi, A.M.; Dykes, S.; Cardelli, J.A.; El Sayed, K.A. The oleocanthal-based homovanillyl sinapate as a novel c-Met inhibitor. Oncotarget 2016, 7, 32247–32273. [Google Scholar] [CrossRef]
- Ayoub, N.M.; Siddique, A.B.; Ebrahim, H.Y.; Mohyeldin, M.M.; El Sayed, K.A. The olive oil phenolic (−)-oleocanthal modu-lates estrogen receptor expression in luminal breast cancer in vitro and in vivo and synergizes with tamoxifen treatment. Eur. J. Pharmacol. 2017, 810, 100–111. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ebrahim, H.Y.; Akl, M.R.; Ayoub, N.M.; Goda, A.A.; Mohyeldin, M.M.; Nagumalli, S.K.; Hananeh, W.M.; Liu, Y.Y.; Meyer, S.A.; et al. (−)-Oleocanthal as a Dual c-MET-COX2 Inhibitor for the Control of Lung Cancer. Nutrients 2019, 11, 412. [Google Scholar] [CrossRef]
- Samiee, S.; Berardi, P.; Bouganim, N.; Vandermeer, L.; Arnaout, A.; Dent, S.; Mirsky, D.; Chasen, M.; Caudrelier, J.M.; Clemons, M. Excision of the primary tumor in patients with metastatic breast cancer: A clinical dilemma. Curr. Oncol. 2012, 19, 270–279. [Google Scholar] [CrossRef]
- Isakoff, S.J. Triple negative breast cancer: Role of specific chemotherapy agents. Cancer J. 2010, 16, 53–61. [Google Scholar] [CrossRef]
- Siddique, A.B.; Ayoub, N.M.; Tajmim, A.; Meyer, S.A.; Hill, R.A.; El Sayed, K.A. (−)-Oleocanthal Prevents Breast Cancer Lo-coregional Recurrence After Primary Tumor Surgical Excision and Neoadjuvant Targeted Therapy in Orthotopic Nude Mouse Models. Cancers 2019, 11, 637. [Google Scholar] [CrossRef]
- Siddique, A.B.; Kilgore, P.; Tajmim, A.; Singh, S.S.; Meyer, S.A.; Jois, S.; Cvek, U.; Trutschl, M.; El Sayed, K.A. (−)-Oleocanthal as a Dual c-MET-COX2 Inhibitor for the Control of Lung Cancer. Nutrients 2020, 12, 1749. [Google Scholar] [CrossRef]
- Diez-Bello, R.; Jardin, I.; Lopez, J.; El Haouari, M.; Ortega-Vidal, J.; Altarejos, J.; Salido, G.; Salido, S.; Rosado, J.A. (−)-Oleocanthal inhibits proliferation and migration by modulating Ca2+ entry through TRPC6 in breast cancer cells. Biochim. Biophys. Acta Bioenergy 2019, 1866, 474–485. [Google Scholar] [CrossRef]
- Miller, T.W.; Rexer, B.N.; Garrett, J.T.; Arteaga, C.L. Mutations in the phosphatidylinositol 3-kinase pathway: Role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011, 13, 1–12. [Google Scholar] [CrossRef]
- Khanfar, M.A.; Bardaweel, S.K.; Akl, M.R.; El Sayed, K.A. Olive Oil-derived Oleocanthal as Potent Inhibitor of Mammalian Target of Rapamycin: Biological Evaluation and Molecular Modeling Studies. Phytother. Res. 2015, 29, 1776–1782. [Google Scholar] [CrossRef]
- Legendre, O.; Breslin, P.A.; Foster, D.A. (−)-Oleocanthal rapidly and selectively induces cancer cell death via lysosomal membrane permeabilization. Mol. Cell. Oncol. 2015, 2, e1006077. [Google Scholar] [CrossRef]
- Wang, F.; Gómez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Goren, L.; Zhang, G.; Kaushik, S.; Breslin, P.A.S.; Du, Y.N.; Foster, D.A. (−)-Oleocanthal and (−)-oleocanthal-rich olive oils in-duce lysosomal membrane permeabilization in cancer cells. PLoS ONE 2019, 14, e0216024. [Google Scholar] [CrossRef] [PubMed]
- Scotece, M.; Gómez, R.; Conde, J.; Lopez, V.; Gómez-Reino, J.J.; Lago, F.; Smith, A.B., III; Gualillo, O. Oleocanthal inhibits proliferation and MIP-1α expression in human multiple myeloma cells. Curr. Med. Chem. 2013, 20, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Arena, C.; Carpi, S.; Polini, B.; Bertini, S.; Digiacomo, M.; Gado, F.; Saba, A.; Saccomanni, G.; Breschi, M.C.; et al. Cytotoxic Activity of Oleocanthal Isolated from Virgin Olive Oil on Human Melanoma Cells. Nutr. Cancer 2016, 68, 873–877. [Google Scholar] [CrossRef]
- Lopez-Bergami, P.; Fitchman, B.; Ronai, Z.A. Understanding Signaling Cascades in Melanoma. Photochem. Photobiol. 2007, 84, 289–306. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, J.; Peng, L. (−)-Oleocanthal exerts anti-melanoma activities and inhibits STAT3 signaling pathway. Oncol. Rep. 2016, 37, 483–491. [Google Scholar] [CrossRef]
- Margarucci, L.; Cassiano, C.; Mozzicafreddo, M.; Angeletti, M.; Riccio, R.; Monti, M.C.; Tosco, A.; Casapullo, A. Chemical proteomics-driven discovery of oleocanthal as an Hsp90 inhibitor. Chem. Commun. 2013, 49, 5844–5846. [Google Scholar] [CrossRef]
- De Stefanis, D.; Scimè, S.; Accomazzo, S.; Catti, A.; Occhipinti, A.; Bertea, C.M.; Costelli, P. Anti-Proliferative Effects of an Extra-Virgin Olive Oil Extract Enriched in Ligstroside Aglycone and Oleocanthal on Human Liver Cancer Cell Lines. Cancers 2019, 11, 1640. [Google Scholar] [CrossRef]
- Pei, T.; Meng, Q.; Han, J.; Sun, H.; Li, L.; Song, R.; Sun, B.; Pan, S.; Liang, D.; Liu, L. (−)-Oleocanthal inhibits growth and me-tastasis by blocking activation of STAT3 in human hepatocellular carcinoma. Oncotarget 2016, 7, 43475–43491. [Google Scholar] [CrossRef]
- Ünsal, Ü.Ü.; Mete, M.; Aydemir, I.; Duransoy, Y.K.; Umur, A.Ş.; Tuglu, M.I. Inhibiting effect of oleocanthal on neuroblastoma cancer cell proliferation in culture. Biotech. Histochem. 2020, 95, 233–241. [Google Scholar] [CrossRef]
- Briante, R.; Patumi, M.; Terenziani, S.; Bismuto, E.; Febbraio, F.; Nucci, R.J. Olea europaea L. Leaf Extract and Derivatives: Antioxidant PropertiesAgric. Food Chem. 2002, 50, 4934–4940. [Google Scholar] [CrossRef]
- Czerwinska, M.; Kiss, A.K.; Naruszewicz, M. A comparison ofantioxidant activities of oleuropein and its dialdehydic deriva-tive from olive oil, oleacein. Food Chem. 2012, 131, 940–947. [Google Scholar] [CrossRef]
- Polini, B.; Digiacomo, M.; Carpi, S.; Bertini, S.; Gado, F.; Saccomanni, G.; Macchia, M.; Nieri, P.; Manera, C.; Fogli, S. Oleocanthal and oleacein contribute to the in vitro therapeutic potential of extra virgin oil-derived extracts in non-melanoma skin cancer. Toxicol. In Vitro 2018, 52, 243–250. [Google Scholar] [CrossRef]
- Cirmi, S.; Celano, M.; Lombardo, G.E.; Maggisano, V.; Procopio, A.; Russo, D.; Navarra, M. Oleacein inhibits STAT3; acti-vates the apoptotic machinery; and exerts anti-metastatic effects in the SH-SY5Y human neuroblastoma cells. Food Funct. 2020, 11, 3271–3279. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Poten-tial Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Fabiani, R.; Sepporta, M.V.; Rosignoli, P.; De Bartolomeo, A.; Crescimanno, M.; Morozzi, G. Anti-proliferative and pro-apoptotic activities of hydroxytyrosol on different tumour cells: The role of extracellular production of hydrogen peroxide. Eur. J. Nutr. 2012, 51, 455–464. [Google Scholar] [CrossRef]
- Corona, G.; Deiana, M.; Incani, A.; Vauzour, D.; Dessi, M.A.; Spencer, J.P.E. Hydroxytyrosol inhibits the proliferation of hu-man colon adenocarcinoma cells through inhibition of ERK1/2 and cyclin D1. Mol. Nutr. Food Res. 2009, 53, 897–903. [Google Scholar] [CrossRef]
- Sun, L.; Luo, C.; Liu, J. Hydroxytyrosol induces apoptosis in human colon cancer cells through ROS generation. Food Funct. 2014, 5, 1909–1914. [Google Scholar] [CrossRef]
- Terzuoli, E.; Giachetti, A.; Ziche, M.; Donnini, S. Hydroxytyrosol, a product from olive oil, reduces colon cancer growth by enhancing epidermal growth factor receptor degradation. Mol. Nutr. Food Res. 2016, 60, 519–529. [Google Scholar] [CrossRef]
- Wang, D.; Wang, H.; Ning, W.; Backlund, M.G.; Dey, S.K.; Dubois, R.N. Loss of Cannabinoid Receptor 1 Accelerates Intestinal Tumor Growth. Cancer Res. 2008, 68, 6468–6476. [Google Scholar] [CrossRef]
- Di Francesco, A.; Falconi, A.; Di Germanio, C.; Di Bonaventura, M.V.M.; Costa, A.; Caramuta, S.; Del Carlo, M.; Compagnone, D.; Dainese, E.; Cifani, C.; et al. Extravirgin olive oil up-regulates CB1 tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J. Nutr. Biochem. 2015, 26, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Hormozi, M.; Marzijerani, A.S.; Baharvand, P. Effects of Hydroxytyrosol on Expression of Apoptotic Genes and Activity of Antioxidant Enzymes in LS180 Cells. Cancer Manag. Res. 2020, 12, 7913–7919. [Google Scholar] [CrossRef] [PubMed]
- Calahorra, J.; Martínez-Lara, E.; De Dios, C.; Siles, E. Hypoxia modulates the antioxidant effect of hydroxytyrosol in MCF-7 breast cancer cells. PLoS ONE 2018, 13, e0203892. [Google Scholar] [CrossRef] [PubMed]
- Calahorra, J.; Martínez-Lara, E.; Granadino-Roldán, J.M.; Martí, J.M.; Cañuelo, A.; Blanco, S.; Oliver, F.J.; Siles, E. Crosstalk between hydroxytyrosol, a major olive oil phenol, and HIF-1 in MCF-7 breast cancer cells. Sci. Rep. 2020, 10, 6361. [Google Scholar] [CrossRef] [PubMed]
- El-Azem, N.; Pulido-Moran, M.; Ramirez-Tortosa, C.L.; Quiles, J.L.; Cara, F.E.; Sanchez-Rovira, P.; Granados-Principal, S.; Ramirez-Tortosa, M. Modulation by hydroxytyrosol of oxidative stress and antitumor activities of paclitaxel in breast cancer. Eur. J. Nutr. 2019, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.A.; Elkady, M. A Prospective Study to Evaluate the Effect of Paclitaxel on Cardiac Ejection Fraction. Breast Care 2017, 12, 255–259. [Google Scholar] [CrossRef]
- Schlitt, A.; Jordan, K.; Vordermark, D.; Schwamborn, J.; Langer, T.; Thomssen, C. Cardiotoxicity and Oncological Treatments. Dtsch. Aerzteblatt Online 2014, 111, 161–168. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Zhu, J.-S.; Zhang, Z.; Shen, W.-J.; Jiang, S.; Long, Y.-F.; Wu, B.; Ding, T.; Huan, F.; Wang, S.-L. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion of MDA-MB-231 Triple-Negative Breast Cancer Cell via Induction of Autophagy. Anti-Cancer Agents Med. Chem. 2020, 19, 1983–1990. [Google Scholar] [CrossRef]
- Lu, H.Y.; Zhu, J.S.; Xie, J.; Zhang, Z.; Zhu, J.; Jiang, S.; Shen, W.J.; Wu, B.; Ding, T.; Wang, S.L. Hydroxytyrosol and Oleuropein Inhibit Migration and Invasion via Induction of Autophagy in ER-Positive Breast Cancer Cell Lines (MCF-7 and T47D). Nutr. Cancer 2020, 1–11. [Google Scholar] [CrossRef]
- Cruz-Lozano, M.; González-González, A.; Marchal, J.A.; Muñoz-Muela, E.; Molina, M.P.; Cara, F.E.; Brown, A.M.; García-Rivas, G.; Hernández-Brenes, C.; Lorente, J.A.; et al. Hydroxytyrosol inhibits cancer stem cells and the metastatic capacity of triple-negative breast cancer cell lines by the simultaneous targeting of epithelial-to-mesenchymal transition, Wnt/β-catenin and TGFβ signaling pathways. Eur. J. Nutr. 2019, 58, 3207–3219. [Google Scholar] [CrossRef]
- Zubair, H.; Bhardwaj, A.; Ahmad, A.; Srivastava, S.K.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Hydroxytyrosol Induces Apoptosis and Cell Cycle Arrest and Suppresses Multiple Oncogenic Signaling Pathways in Prostate Cancer Cells. Nutr. Cancer 2017, 69, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, Y.; Xu, Z.; Wang, J.; Wang, F.; Wang, D.; Pan, S.; Wu, Y.; Pan, H.; Xu, D.; et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014, 347, 79–87. [Google Scholar] [CrossRef]
- Tutino, V.; Caruso, M.G.; Messa, C.; Perri, E.; Notarnicola, M. Antiproliferative, antioxidant and anti-inflammatory effects of hydroxytyrosol on human hepatoma HepG2 and Hep3B cell lines. Anticancer Res. 2012, 32, 5371–5377. [Google Scholar]
- Lamy, S.; Ben Saad, A.; Zgheib, A.; Annabi, B. Olive oil compounds inhibit the paracrine regulation of TNF-α-induced endo-thelial cell migration through reduced glioblastoma cell cyclooxygenase-2 expression. J. Nutr. Biochem. 2016, 27, 136–145. [Google Scholar] [CrossRef]
- Shamshoum, H.; Vlavcheski, F.; Tsiani, E. Anticancer effects of oleuropein. BioFactors 2017, 43, 517–528. [Google Scholar] [CrossRef]
- Torić, J.; Marković, A.K.; Brala, C.J.; Barbarić, M. Anticancer effects of olive oil polyphenols and their combinations with an-ticancer drugs. Acta Pharm. 2019, 69, 461–482. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Bianchini, F.; Andreucci, E.; Urciuoli, S.; Romani, A.; Tortora, K.; Caderni, G.; Nediani, C.; Calorini, L. Cancer Glycolytic Dependence as a New Target of Olive Leaf Extract. Cancers 2020, 12, 317. [Google Scholar] [CrossRef]
- Notarnicola, M.; Pisanti, S.; Tutino, V.; Bocale, D.; Rotelli, M.T.; Gentile, A.; Memeo, V.; Bifulco, M.; Perri, E.; Caruso, M.G. Effects of olive oil polyphenols on fatty acid synthase gene expression and activity in human colorectal cancer cells. Genes Nutr. 2010, 6, 63–69. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vazquez-Martin, A.; Colomer, R.; Brunet, J.; Carrasco-Pancorbo, A.; Garcia-Villalba, R.; Fernan-dez-Gutierrez, A.; Segura-Carretero, A. Olive oil’s bitter principle reverses acquired autoresistance to trastuzumab (Her-ceptin) in HER2-overexpressing breast cancer cells. BMC Cancer 2007, 7, 80. [Google Scholar] [CrossRef]
- Anter, J.; Fernández-Bedmar, Z.; Villatoro-Pulido, M.; Demyda-Peyras, S.; Moreno-Millán, M.; Alonso-Moraga, A.; Muñoz-Serrano, A.; Luque de Castro, M.D. A pilot study on the DNA-protective, cytotoxic, and apoptosis-inducing properties of olive-leaf extracts. Mutat Res. 2011, 723, 165–170. [Google Scholar] [CrossRef]
- Cárdeno, A.; Sánchez-Hidalgo, M.; Rosillo, M.A.; Alarcón-De-La-Lastra, C. Oleuropein, a Secoiridoid Derived from Olive Tree, Inhibits the Proliferation of Human Colorectal Cancer Cell Through Downregulation of HIF-1α. Nutr. Cancer 2013, 65, 147–156. [Google Scholar] [CrossRef]
- Sepporta, M.V.; Fuccelli, R.; Rosignoli, P.; Ricci, G.; Servili, M. Oleuropein prevents azoxymethane-induced colon crypt dys-plasia and leukocytes DNA damage in A/J mice. J. Med. Food 2016, 19, 983–989. [Google Scholar] [CrossRef]
- Giner, E.; Recio, M.C.; Ríos, J.L.; Cerdá-Nicolás, J.M.; Giner, R.M. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol. Nutr. Food Res. 2015, 60, 242–255. [Google Scholar] [CrossRef]
- Yan, C.-M.; Chai, E.-Q.; Cai, H.-Y.; Miao, G.-Y.; Ma, W. Oleuropein induces apoptosis via activation of caspases and suppression of phosphatidylinositol 3-kinase/protein kinase B pathway in HepG2 human hepatoma cell line. Mol. Med. Rep. 2015, 11, 4617–4624. [Google Scholar] [CrossRef]
- Sherif, I.O.; Al-Gayyar, M.M. Oleuropein potentiates anti-tumor activity of cisplatin against HepG2 through affecting proNGF/NGF balance. Life Sci. 2018, 198, 87–93. [Google Scholar] [CrossRef]
- Secme, M.; Eroglu, C.; Dodurga, Y.; Bagc, G. Investigation of anticancer mechanism of oleuropein via cell cycle and apoptotic pathways in SH-SY5Y neuroblastoma cells. Gene 2016, 585, 93–99. [Google Scholar] [CrossRef]
- Bulotta, S.; Corradino, R.; Celano, M.; Maiuolo, J.; D’Agostino, M.; Oliverio, M.; Procopio, A.; Filetti, S.; Russo, D. Antioxidant and antigrowth action of peracetylated oleuropein in thyroid cancer cells. J. Mol. Endocrinol. 2013, 51, 181–189. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Zhang, Q.; Li, X.; Zhu, X.; Wang, Q.; Cao, S.; Du, L. Mitochondria-mediated apoptosis was induced by oleuropein in H1299 cells involving activation of p38 MAP kinase. J. Cell. Biochem. 2019, 120, 5480–5494. [Google Scholar] [CrossRef]
- Aktas, H.G.; Ayan, H. Oleuropein: A Potential Inhibitor for Prostate Cancer Cell Motility by Blocking Voltage-Gated Sodium Channels. Nutr. Cancer 2020, 26, 1–10. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M. Olive leaf extract and its main component oleuropein prevent chronic ultraviolet B radiation-induced skin damage and carcinogenesis in hairless mice. J. Nutr. 2009, 139, 2079–2086. [Google Scholar] [CrossRef]
- Przychodzen, P.; Wyszkowska, R.; Gorzynik-Debicka, M.; Kostrzewa, T.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. An-ticancer Potential of Oleuropein, the Polyphenol of Olive Oil, with 2-Methoxyestradiol, Separately or in Combination, in Human Osteosarcoma Cells. Anticancer Res. 2019, 39, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.D.; Bond, D.R.; Jankowski, H.; Weidenhofer, J.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. The Olive Bio-phenols Oleuropein and Hydroxytyrosol Selectively Reduce Proliferation, Influence the Cell Cycle and Induce Apoptosis in Pancreatic Cancer Cells. Int. J. Mol. Sci. 2018, 19, 1937. [Google Scholar] [CrossRef]
- Fengli, Z.; Mei, Z. Oleuropein inhibits esophageal cancer through hypoxic suppression of BTG3 mRNA. Food Funct. 2019, 10, 978–985. [Google Scholar]
- Menendez, J.A.; Vazquez-Martin, A.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Oliveras-Ferraros, C.; Fernandez-Gutierrez, A.; Segura-Carretero, A. tabAnti-HER2 (erbB-2) oncogene effects of phenolic compounds directly isolated from commercial Extra- Virgin Olive Oil (EVOO). BMC Cancer 2008, 8, 377. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Analyzing effects of extra-virgin olive oil polyphenols on breast cancer-associated fatty acid synthase protein expression using reverse-phase protein microarrays. Int. J. Mol. Med. 2008, 22, 433–439. [Google Scholar] [CrossRef]
- Elamin, M.H.; Elmahi, A.B.; Daghestani, M.; Al-Olayan, E.M.; Al-Ajmi, R.A.; Alkhuriji, A.F.; Hamed, S.S.; El-Khadragy, M.F. Synergistic Anti-Breast-Cancer Effects of Combined Treatment with Oleuropein and Doxorubicin In Vivo. Altern. Ther. Health Med. 2017, 25, 17–24. [Google Scholar]
- Liu, L.; Ahn, K.S.; Shanmugam, M.K.; Wang, H.; Shen, H.; Arfuso, F.; Chinnathambi, A.; Alharbi, S.A.; Chang, Y.; Sethi, G.; et al. Oleuropein induces apoptosis via abrogating NF-κB activation cascade in estrogen receptor-negative breast cancer cells. J. Cell Biochem. 2019, 120, 4504–4513. [Google Scholar] [CrossRef]
- Przychodzen, P.; Kuban-Jankowska, A.; Wyszkowska, R.; Barone, G.; Bosco, G.L.; Celso, F.L.; Kamm, A.; Daca, A.; Kostrzewa, T.; Gorska-Ponikowska, M. PTP1B phosphatase as a novel target of oleuropein activity in MCF-7 breast cancer model. Toxicol. In Vitro 2019, 61, 104624. [Google Scholar] [CrossRef]
- Abtin, M.; Alivand, M.R.; Khaniani, M.S.; Bastami, M.; Zaeifizadeh, M.; Derakhshan, S.M. Simultaneous downregulation of miR-21 and miR-155 through oleuropein for breast cancer prevention and therapy. J. Cell. Biochem. 2018, 119, 7151–7165. [Google Scholar] [CrossRef]
- Rigacci, S.; Miceli, C.; Nediani, C.; Berti, A.; Cascella, R.; Pantano, D.; Nardiello, P.; Luccarini, I.; Casamenti, F.; Stefania, R. Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: A mechanistic insight. Oncotarget 2015, 6, 35344–35357. [Google Scholar] [CrossRef]
- Kikuchi, M.; Mano, N.; Uehara, Y.; Machida, K.; Kikuchi, M. Cytotoxic and EGFR tyrosine kinase inhibitory activities of aglycone derivatives obtained by enzymatic hydrolysis of oleoside-type secoiridoid glucosides, oleuropein and ligustroside. J. Nat. Med. 2011, 65, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Busnena, B.A.; Foudah, A.I.; Melancon, T.; El Sayed, K.A. Olive secoiridoids and semisynthetic bioisostere analogues for the control of metastatic breast cancer. Bioorg. Med. Chem. 2013, 21, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
| Oleocanthal | ||||
|---|---|---|---|---|
| Tumor | Model Cell Line | Molecular Target | Ref | In Vivo Model |
| Colon Cancer | SW480 | ↑ROS | [58] | |
| HT29 | ↓COX2 | |||
| ↑γH2AX | ||||
| HT29 | ↑AMP | [59] | HT29 cells CAM assay | |
| Caco | ↓p-mTOR | [74] | ||
| Breast Cancer | MCF-7 | ↓p-cMET | [61] | |
| MDA-MB-231 | ||||
| ↓AKT | [63] | MDA-MB-231 cells xenografts in athymic nude mice | ||
| ↓MAPK | ||||
| BT-474 | ↓Brk/paxillin/Rac1 | |||
| MCF-7 | ↑E-cadherin | |||
| MDA-MB-231 | ↑Zo-1 | |||
| ↓vimentin | ||||
| ↓ERα receptor | ||||
| MCF-7 | ↓p-mTOR | [74] | ||
| T-47D | ||||
| MDA-MB-231 | ||||
| ↓c-MET | [66] | BT-474 orthotopic model in athymic nude mice | ||
| ↓HER2 | ||||
| BT-474 | ↓EGFR | |||
| SK-BR-3 | ↓AKT | |||
| ↓PI3H | ||||
| ↓MAPK | ||||
| ↓STAT3 | ||||
| ↓vimentin | ||||
| MDA-MB-231 | ↑LMP | [75] | ||
| MCF-7 | ↓TRPC6 | [72] | ||
| MDA-MB-231 | ||||
| Prostate Cancer | PC3 | ↓p-cMET | [61] | |
| PC3 | ↑LMP | [75] | ||
| HCC | HepG2 | ↑ROS | [58] | |
| Huh7 | ↓COX2 | |||
| Hep3B | ↑γH2AX | |||
| PLC/PRF/5 | ||||
| HepG2 | ↑AKT | [83] | ||
| Huh7 | ↑ERK | |||
| Hep3B | ↑LC3-II/LC3-I | |||
| ↓p62 | ||||
| HepG2 | ↓STA3 | [84] | ||
| Huh7 | ↓JAK1, JAK2 | |||
| HCCLM3 | ↓TWIST | |||
| Melanoma | ↓Mcl1 | [80] | A375 cells xenografts in nude mice | |
| ↓BCL-xl | ||||
| A375 | ↓MMP9 | |||
| A2058 | ↓MMP2 | |||
| ↓STAT3 | ||||
| ↓JAK2 | ||||
| Lung Cancer | A549 | ↓p-cMET | [71] | orthotopic model of A549 cells in athymic nude mice |
| NCI-H322M | ↓COX2 | |||
| Pancreatic Cancer | BxPC3 | ↑LMP | [75] | PNET RIP-Tag mice |
| Multiple Myeloma | ARH-77 | ↓AKT | [78] | |
| ↓ERK | ||||
| ↑p38 | ||||
| ↓MIP-1α | ||||
| Histiocytic Lymphoma | U937 | ↓Hsp90 | [82] | |
| ↓AKT | ||||
| ↓Cdk4 | ||||
| Oleacein | ||||
| Tumor | Model Cell Line | Molecular Target | Ref | In Vivo Model |
| Non-melanoma Skin Cancer | A43 | ↓AKT | [88] | |
| ↓ERK | ||||
| ↓B-Raf | ||||
| Neuroblastoma | SH-SY5Y | ↑Bax | [89] | |
| ↑p53 | ||||
| ↓Bcl2 | ||||
| ↓STAT3 | ||||
| Hydroxytyrosol | ||||
|---|---|---|---|---|
| Tumor | Model Cell Line | Molecular Target | Ref | In Vivo Model |
| Prostate Cancer | ↓AKT | [106] | ||
| ↓STA3 | ||||
| LNCaP | ↓MCT4 | |||
| C4-2 | ↑Bax | |||
| ↑Bcl-2 | ||||
| ↓NF-κB | ||||
| Colon Cancer | Caco-2 | ↓pERK | [92] | |
| ↓cyclin D1 | ||||
| DLD1 | ↑ROS | [93] | ||
| HT-29 | ↓EGFR | [94] | HT-29 cells xenografts in immunodeficient mice | |
| CaCo2 | ↑pCBL | |||
| Caco-2 | ↑CB1 | [96] | ||
| LS180 | ↑CASP3 | [97] | ||
| ↑Bax | ||||
| HCC | HepG2 | ↓AKT | [107] | orthotopic HCC model in 4–6-week old nude mice |
| Huh7 | ↓NF-κB | |||
| Hep3B | ||||
| SK-HEP-1 | ||||
| HepG2 | ↓FAS | [108] | ||
| Hep3B | ↓FPPS | |||
| Breast Cancer | MCF-7 | ↓PGC1a/Nrf2 | [98] | |
| ↓PGC1a/ERRa | ||||
| MCF-7 | ↑ROS | [100] | ||
| MDA-MB-231 | ↓DNA damage | |||
| MCF-7 | ↑LC3-II/LC3-I | [104] | ||
| MDA-MB-231 | ↓p62 | |||
| T47D | ||||
| SUM159PT | ↓β-catenin | [105] | breast tumor-bearing rats | |
| MDA-MB-231 | ↓cyclin D1 | |||
| Hs578T | ↓Snail | |||
| BT549 | ↓vimentin | |||
| ↓SMAD2/3 | ||||
| Tyrosol | ||||
| Tumor | Model Cell Line | Molecular target | Ref | In Vivo Model |
| Glioblastoma | U-87 MG | ↓pJNK | [109] | |
| ↓pERK | ||||
| ↓NF-κB | ||||
| ↓COX2 | ||||
| Oleuropein | ||||
|---|---|---|---|---|
| Tumor | Model Cell Line | Molecular Target | Ref | In Vivo Model |
| Melanoma | A375 | ↓GLUT1 | [112] | |
| ↓PKM2 | ||||
| ↓MCT4 | ||||
| Colon Cancer | SW620 | ↓FAS | [113] | |
| HT-29 | ||||
| HT-29 | ↓HIF-1α | [116] | ||
| ↑p53 | ||||
| ↑PPARγ | ||||
| ↓NF-κB | ||||
| HT-29 | ↓DNA damage | [117] | Azoxymethane (AOM)-treated mice | |
| HT-29 | ↓IL-6 | AOM and d extran sulfate sodium (DSS)-treated mice | ||
| ↓IFN-γ | ||||
| ↓TNF-α | ||||
| ↓IL-17 | [118] | |||
| ↓COX-2 | ||||
| ↓NF-κB | ||||
| ↓Wnt/β-catenin | ||||
| ↑STAT3 | ||||
| Chronic Myeloid Leukemia | HL60 | ↑Apoptosis | [115] | |
| HCC | HepG2 | ↑Bax | [119] | |
| ↑Bcl-2 | ||||
| ↓AKT | ||||
| HepG2 | ↑MMP-7 | [120] | ||
| Neuroblastoma | SH-SY5Y | ↓cylin D1 | [121] | |
| ↓cylin D2 | ||||
| ↓cyclin D3 | ||||
| ↓CDK4 | ||||
| ↓CDK6 | ||||
| ↑p53 | ||||
| ↑CDKN2A | ||||
| ↑CDKN2B | ||||
| ↑CDKN1A | ||||
| Thyroid Cancer | TPC-1 | ↓p-AKT | [122] | |
| BCPAP | ↓p-ERK | |||
| ↑ROS | ||||
| Lung Cancer | H1299 | ↑p38 | [123] | |
| Prostate Cancer | MAT-LyLu | ↓SCN9A | [124] | |
| Skin Cancer | ↓VEGF | [125] | skin damage and carcinogenesis in hairless mice exposed to UVB irradiation | |
| ↓MMP-2 | ||||
| ↓MMP-9 | ||||
| ↓MMP-13 | ||||
| ↓COX-2 | ||||
| Breast Cancer | MDA-MB-231 | ↑LC3II | [103] | |
| ↓p62 | ||||
| MCF-7 | ↓HER2 | [113] | ||
| SKBR3 | ↓cyclin D1 | [131] | MDA-MB-231 cells xenografts in nude mice | |
| ↓NF-κB | ||||
| ↓Bcl-2 | ||||
| ↓survivin | ||||
| MCF-7 | ↓NF-κB | [132] | ||
| MDA-MB-231 | ||||
| MCF-7 | ↓PTP1B | [133] | ||
| MCF-7 | ↓miR-21 | [134] | ||
| ↓miR-155 | ||||
| Osteosarcoma | 143B OS | ↑LC3-II/LC3-I | [126] | |
| Pancreatic Cancer | MIAPaCa-2 | ↑c-Jun | [127] | |
| BxPC-3 | ↑c-Fos | |||
| CFPAC-1 | ||||
| Esophageal Cancer | EC | ↓HIF-1α | [128] | EC cells xenografts in nude mice |
| ↑BTG3 | ||||
| Ligstroside | ||||
| Tumor | Model Cell Line | Molecular Target | Ref | In Vivo Model |
| Breast Cancer | MDA-MB231 | ↓c-MET | [137] | |
| HCC | ↑AKT | [83] | ||
| ↑ERK | ||||
| HepG2 | ↑mTOR | |||
| Huh7 | ↑p70 | |||
| Hep3B | ↑P-4E-BP1 | |||
| ↑LC3-II/LC3-I | ||||
| ↑Beclin-1 | ||||
| ↑p62 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emma, M.R.; Augello, G.; Di Stefano, V.; Azzolina, A.; Giannitrapani, L.; Montalto, G.; Cervello, M.; Cusimano, A. Potential Uses of Olive Oil Secoiridoids for the Prevention and Treatment of Cancer: A Narrative Review of Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 1234. https://doi.org/10.3390/ijms22031234
Emma MR, Augello G, Di Stefano V, Azzolina A, Giannitrapani L, Montalto G, Cervello M, Cusimano A. Potential Uses of Olive Oil Secoiridoids for the Prevention and Treatment of Cancer: A Narrative Review of Preclinical Studies. International Journal of Molecular Sciences. 2021; 22(3):1234. https://doi.org/10.3390/ijms22031234
Chicago/Turabian StyleEmma, Maria Rita, Giuseppa Augello, Vita Di Stefano, Antonina Azzolina, Lydia Giannitrapani, Giuseppe Montalto, Melchiorre Cervello, and Antonella Cusimano. 2021. "Potential Uses of Olive Oil Secoiridoids for the Prevention and Treatment of Cancer: A Narrative Review of Preclinical Studies" International Journal of Molecular Sciences 22, no. 3: 1234. https://doi.org/10.3390/ijms22031234
APA StyleEmma, M. R., Augello, G., Di Stefano, V., Azzolina, A., Giannitrapani, L., Montalto, G., Cervello, M., & Cusimano, A. (2021). Potential Uses of Olive Oil Secoiridoids for the Prevention and Treatment of Cancer: A Narrative Review of Preclinical Studies. International Journal of Molecular Sciences, 22(3), 1234. https://doi.org/10.3390/ijms22031234







