Are Bioactive Molecules from Seaweeds a Novel and Challenging Option for the Prevention of HPV Infection and Cervical Cancer Therapy?—A Review
Abstract
1. Introduction
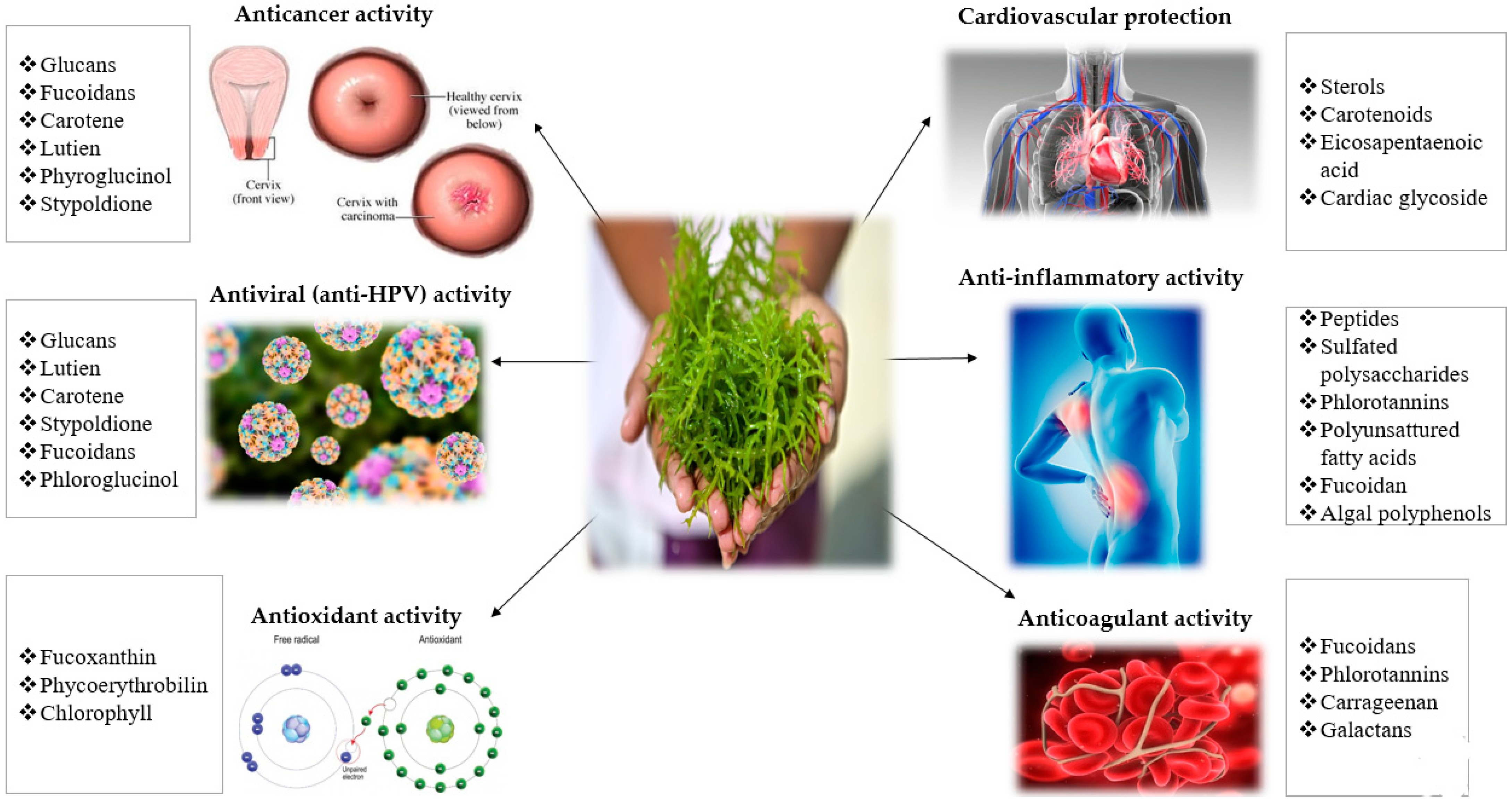
2. Bioactive Compounds of Seaweeds
3. Anti-HPV and Anticancer Mechanisms of Seaweeds Compounds
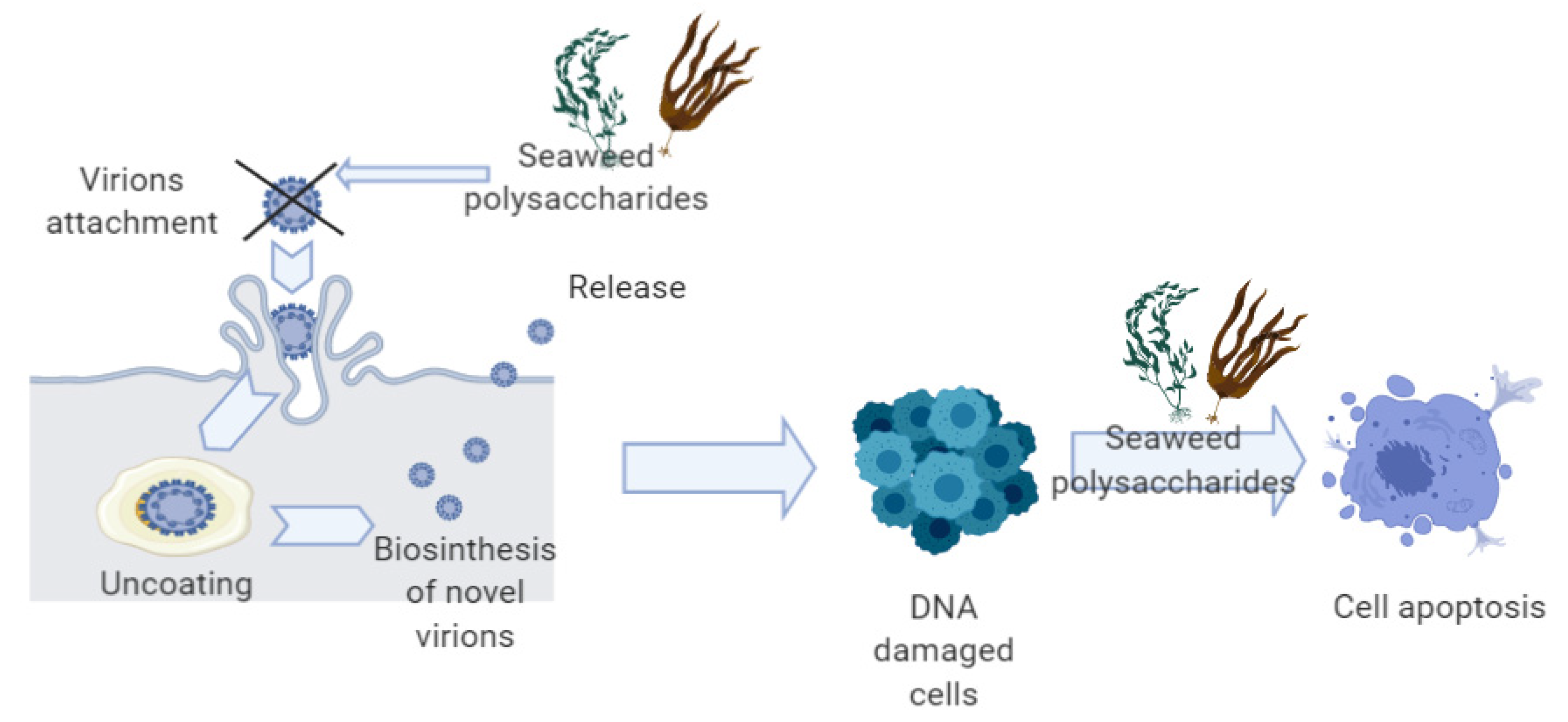
3.1. Anti-HPV Mechanisms of Seaweeds Polysaccharides
3.2. Anti-Cancer Mechanisms of Seaweeds Compounds
4. Material and Method
5. Results and Discussion
5.1. Studies Regarding the Effects of Seaweeds Bioactive Compounds on HPV Infection
5.2. Studies Regarding the Effects of Seaweeds Bioactive Compounds on Cervical Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| HPV | Human papillomavirus |
| DNA | Deoxyribonucleic acid |
| IC50 | The half maximal inhibitory concentration |
| IFN γ | Interferon gamma |
| NF-kB | Nuclear factor kappa light chain enhancer of activated B cells |
| PI3K | Phosphatidylinositol 3-kinase |
| PAI-1 | Plasminogen activator inhibitor-1 |
| PMGS | Polymannuroguluronate sulfate |
| ROS | Reactive oxygen species |
| TH1 | T helper 1 |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand |
| VEGF | Vascular endothelial growth factor |
References
- Moga, M.A.; Bălan, A.; Anastasiu, C.V.; Dimienescu, O.G.; Neculoiu, C.D.; Gavriș, C. An overview on the anticancer activity of azadirachta indica (neem) in gynecological cancers. Int. J. Mol. Sci. 2018, 19, 3898. [Google Scholar] [CrossRef] [PubMed]
- Mammas, I.N.; Sourvinos, G.; Giannoudis, A.; Spandidos, D.A. Human papilloma virus (HPV) and host cellular interactions. Pathol. Oncol. Res. 2008, 14, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Muñoz, N. The viral etiology of cervical cancer. Virus Res. 2002, 89, 183–190. [Google Scholar] [CrossRef]
- Rahman, N.; Riaz, M.; Khan, A.; Lorena, D. Mechanism of anti-inflammatory and anti-nociceptive actions of acacia modesta in animal models. Pakistan J. Zool. 2016, 47, 1723–1730. [Google Scholar]
- Steben, M.; Duarte-Franco, E. Human papillomavirus infection: Epidemiology and pathophysiology. Gynecol. Oncol. 2007, 107, S2–S5. [Google Scholar] [CrossRef]
- Moscicki, A.-B.; Schiffman, M.; Franceschi, S. The natural history of human papillomavirus infection in relation to cervical cancer. In Human Papillomavirus; Jenkins, D., Bosch, F.X., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 149–160. [Google Scholar]
- Lowy, D.R.; Schiller, J.T. Reducing HPV-associated cancer globally. Cancer Prev. Res. 2012, 5, 18–23. [Google Scholar] [CrossRef]
- Syrjanen, S.; Puranen, M. Human papillomavirus infections in children: The potential role of maternal transmission. Crit. Rev. Oral. Biol. Med. 2000, 11, 259–274. [Google Scholar] [CrossRef]
- Yang, Y.J.; Nam, S.J.; Kong, G.; Kim, M.K. A case-control study on seaweed consumption and the risk of breast cancer. Br. J. Nutr. 2010, 103, 1345–1353. [Google Scholar] [CrossRef]
- Kono, S.; Toyomura, K.; Yin, G.; Nagano, J.; Mizoue, T. A case-control study of colorectal cancer in relation to lifestyle factors and genetic polymorphisms: Design and conduct of the Fukuoka colorectal cancer study. Asian Pac. J. Cancer Prev. 2004, 5, 393–400. [Google Scholar]
- Khalid, S.; Abbas, M.; Saeed, F.; Bader Ul Ain, H.; Suleria, H.A.R. Therapeutic potential of seaweed bioactive compounds. Seaweed Biomater. 2018, 1–19. [Google Scholar] [CrossRef]
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A. Diversity of eukaryotic algae. Biodivers. Conserv. 1992, 1, 267–292. [Google Scholar] [CrossRef]
- Abdul, B. Cardioprotective activity of polysaccharides derived from marine algae: An overview. Trends Food Sci. Technol. 2013, 30, 98–104. [Google Scholar] [CrossRef]
- Renn, D. Biotechnology and the red seaweed polysaccharide industry: Status, needs and prospects. Trends Biotechnol. 1997, 15, 9–14. [Google Scholar] [CrossRef]
- Rasmussen, R.S.; Morrissey, M.T. Marine biotechnology for production of food ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292. [Google Scholar] [CrossRef]
- Frestedt, J.L.; Kuskowski, M.A.; Zenk, J.L. A natural seaweed derived mineral supplement (Aquamin F) for knee osteoarthritis: A randomised, placebo controlled pilot study. Nutr. J. 2009, 8, 7. [Google Scholar] [CrossRef]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Kraan, S. Pigments and minor compounds in algae. In Functional Ingredients from Algae For Foods and Nutraceuticals; Dominquez, H., Ed.; Woodhead publishing: Sawston, UK, 2013; pp. 205–251. [Google Scholar]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef]
- Zubia, M.; Payri, C.; Deslandes, E. Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). J. Appl. Phycol. 2008, 20, 1033–1043. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Rioux, L.-E.; Turgeon, S.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Mabeau, S.; Kloareg, B. Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. ceranoides, F. vesiculosus, F. serratus, Bifurcaria bifurcata and Laminaria digitata. J. Exp. Bot. 1987, 38, 1573–1580. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Knutsen, S.; Myslabodski, D.; Larsen, B.; Usov, A.I. A modified system of nomenclature for red algal galactans. Bot. Mar. 1994, 37, 163–170. [Google Scholar] [CrossRef]
- Mayer, A.M.; Rodríguez, A.D.; Berlinck, R.G.; Fusetani, N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.J. Phlorotannins as bioactive agents from brown algae. Process. Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Manivasagan, P.; Bharathiraja, S.; Moorthy, M.S.; Mondal, S.; Seo, H.; Dae Lee, K.; Oh, J. Marine natural pigments as potential sources for therapeutic applications. Crit. Rev. Biotechnol. 2018, 38, 745–761. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Nobel, P.S. Physicochemical & Environmental Plant Physiology; Academic Press: Cambridge, MA, USA, 1999. [Google Scholar]
- Le Bourvellec, C.; Gouble, B.; Bureau, S.; Reling, P.; Bott, R.; Ribas-Agusti, A.; Audergon, J.-M.; Renard, C.M.G.C. Impact of canning and storage on apricot carotenoids and polyphenols. Food Chem. 2018, 240, 615–625. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Matsubara, K. Recent Advances in marine algal anticoagulants. Curr. Med. Chem. 2004, 2, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Jurd, K.M.; Rogers, D.J.; Blunden, G.; McLellan, D.S. Anticoagulant properties of sulfated polysaccharides and a proteoglycan from Codium fragile ssp. atlanticum. J. Appl. Phycol. 1995, 7, 339. [Google Scholar] [CrossRef]
- Vasanthi, H.; Rajamanickam, G.; Saraswathy, A.; Jaswanth, A. Tumoricidal effect of the red algae Acanthophora spicifera on Ehrlich’s ascites carcinoma in mice. Seaweed Res. 2004, 2004, 217–224. [Google Scholar]
- Yang, L.; Zhang, L. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef]
- Chevolot, L.; Foucault, A.; Chaubet, F.; Kervarec, N.; Sinquin, C.; Fisher, A.M.; Boisson-Vidal, C. Further data on the structure of brown seaweed fucans: Relationships with anticoagulant activity. Carbohydr. Res. 1999, 319, 154–165. [Google Scholar] [CrossRef]
- Yabuta, Y.; Fujimura, H.; Kwak, C.S.; Enomoto, T.; Watanabe, F. Antioxidant Activity of the phycoerythrobilin compound formed from a dried korean purple laver during in vitro digestion. Food Sci. Technol. Res. 2010, 16, 347–352. [Google Scholar] [CrossRef]
- Kazłowska, K.; Hsu, T.; Hou, C.C.; Yang, W.C.; Tsai, G.J. Anti-Inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J. Ethnopharmacol. 2010, 128, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Subba Rao, P.V.; Periyasamy, C. Biodiversity, Conservation and Medicinal Uses of Seaweeds: The glimpses; Springer: Singapore, 2020; pp. 21–32. [Google Scholar]
- Bae, J.S. Antithrombotic and profibrinolytic activities of phloroglucinol. Food Chem. Toxicol. 2011, 49, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Hamann, M.T. Marine pharmacology in 1999: Compounds with antibacterial, anticoagulant, antifungal, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities affecting the cardiovascular, endocrine, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 132, 315–339. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453–454, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Talarico, L.B.; Damonte, E.B. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 2007, 363, 473–485. [Google Scholar] [CrossRef]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef]
- Esko, J.D.; Selleck, S.B. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 2002, 71, 435–471. [Google Scholar] [CrossRef]
- Roberts, J.N.; Buck, C.B.; Thompson, C.D.; Kines, R.; Bernardo, M.; Choyke, P.L.; Lowy, D.R.; Schiller, J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nature Med. 2007, 13, 857–861. [Google Scholar] [CrossRef]
- Joyce, J.G.; Tung, J.-S.; Przysiecki, C.T.; Cook, J.C.; Lehman, E.D.; Sands, J.A.; Jansen, K.U.; Keller, P.M. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J. Biol. Chem. 1999, 274, 5810–5822. [Google Scholar] [CrossRef]
- Bienkowska-Haba, M.; Patel, H.D.; Sapp, M. Target cell cyclophilins facilitate human papillomavirus type 16 infection. PLoS Pathog. 2009, 5, e1000524. [Google Scholar] [CrossRef]
- Giroglou, T.; Florin, L.; Schäfer, F.; Streeck, R.E.; Sapp, M. Human papillomavirus infection requires cell surface heparan sulfate. J. Virol. 2001, 75, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.V.; Walsh, N.A. Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol. 2006, 44, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-Cancer activity of porphyran and carrageenan from red seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown seaweed fucoidan: Biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-Y.; Park, P.-J.; Jung, W.-K.; Kim, S.J. Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limanda aspera). Eur. Food Res. Technol. 2004, 219, 20–26. [Google Scholar] [CrossRef]
- Lins, K.O.; Bezerra, D.P.; Alves, A.P.; Alencar, N.M.; Lima, M.W.; Torres, V.M.; Farias, W.R.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer). J. Appl. Toxicol. 2009, 29, 20–26. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Antitumor activity and immune response of Mekabu fucoidan extracted from Sporophyll of Undaria pinnatifida. In Vivo 2003, 17, 245–249. [Google Scholar]
- Boopathy, N.S.; Kathiresan, K. Anticancer drugs from marine flora: An overview. J. Oncol. 2010, 2010, 214186. [Google Scholar] [CrossRef]
- Launay, S.; Hermine, O.; Fontenay, M.; Kroemer, G.; Solary, E.; Garrido, C. Vital functions for lethal caspases. Oncogene 2005, 24, 5137–5148. [Google Scholar] [CrossRef]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Bioph. 2005, 37, 719–727. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamed, S.; Fard, S.G.; Behravan, J.; Mustapha, N.M.; Alitheen, N.B.M.; Othman, F. Polyphenol-Rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem. 2012, 130, 376–382. [Google Scholar] [CrossRef]
- Hyun, J.-H.; Kim, S.-C.; Kang, J.-I.; Kim, M.-K.; Boo, H.-J.; Kwon, J.-M.; Koh, Y.-S.; Hyun, J.-W.; Park, D.-B.; Yoo, E.-S.; et al. Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol. Pharm. Bull. 2009, 32, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.-J.; Nam, T.-J. Chromatographically purified porphyran from Porphyra yezoensis effectively inhibits proliferation of human cancer cells. Food Sci. Biotechnol. 2007, 16, 873–878. [Google Scholar]
- He, D.; Wu, S.; Yan, L.; Zuo, J.; Cheng, Y.; Wang, H.; Liu, J.; Zhang, X.; Wu, M.; Choi, J.-I.; et al. Antitumor bioactivity of porphyran extracted from Pyropia yezoensis Chonsoo2 on human cancer cell lines. J. Sci. Food Agric. 2019, 99, 6722–6730. [Google Scholar] [CrossRef] [PubMed]
- Prasedya, E.S.; Miyake, M.; Kobayashi, D.; Hazama, A. Carrageenan delays cell cycle progression in human cancer cells in vitro demonstrated by FUCCI imaging. BMC Complement. Altern. Med. 2016, 16, 270. [Google Scholar] [CrossRef]
- Ling, N. Growth inhibition and cell cycle arrest of Kappa-Selenocarrageenan and paclitaxel on HepG2 cells. Adv. Mater. Res. 2012, 343–344, 530–534. [Google Scholar] [CrossRef]
- Jin, Z.; Han, Y.-X.; Han, X.-R. Degraded Iota-Carrageenan can induce apoptosis in human osteosarcoma cells via the Wnt/β-Catenin signaling pathway. Nutr. Cancer 2013, 65, 126–131. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Dias, P.F.; Siqueira, J.M., Jr.; Vendruscolo, L.F.; de Jesus Neiva, T.; Gagliardi, A.R.; Maraschin, M.; Ribeiro-do-Valle, R.M. Antiangiogenic and antitumoral properties of a polysaccharide isolated from the seaweed Sargassum stenophyllum. Cancer Chemother. Pharmacol. 2005, 56, 436–446. [Google Scholar] [CrossRef]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Xue, M.; Ge, Y.; Zhang, J.; Wang, Q.; Hou, L.; Liu, Y.; Sun, L.; Li, Q. Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo. PLoS ONE 2012, 7, e43483. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, W.; Wei, J.; Qiu, L.; Lin, X. Marine bromophenol bis (2,3-dibromo-4,5-dihydroxybenzyl) ether, induces mitochondrial apoptosis in K562 cells and inhibits topoisomerase I in vitro. Toxicol. Lett. 2012, 211, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, P.A. PAI-1-a potential therapeutic target in cancer. Curr. Drug Targets 2007, 8, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Croci, D.O.; Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Piccoli, A.; Totani, L.; Ustyuzhanina, N.E.; Bilan, M.I.; Usov, A.I.; Grachev, A.A.; et al. Fucans, but not fucomannoglucuronans, determine the biological activities of sulfated polysaccharides from Laminaria saccharina brown seaweed. PLoS ONE 2011, 6, e17283. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Paper, D.H.; Donaldson, J.; Vogl, H. Inhibition of angiogenesis and murine tumour growth by laminarin sulfate. Br. J. Cancer 1996, 73, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Tsai, Y.C.; Monie, A.; Hung, C.F.; Wu, T.C. Carrageenan as an adjuvant to enhance peptide-based vaccine potency. Vaccine 2010, 28, 5212–5219. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Kleinbeck, K.; Mizenina, O.; Kizima, L.; Levendosky, K.; Jean-Pierre, N.; Villegas, G.; Ford, B.E.; Cooney, M.L.; Teleshova, N.; et al. In vitro and in vivo evaluation of two carrageenan-based formulations to prevent HPV acquisition. Antivir. Res. 2014, 108, 88–93. [Google Scholar] [CrossRef]
- Wang, S.; Lu, Z.; Wang, S.; Liu, W.; Gao, J.; Tian, L.; Wang, L.; Zhang, X.; Zhao, X.; Wang, W.; et al. The inhibitory effects and mechanisms of polymannuroguluronate sulfate against human papillomavirus infection in vitro and in vivo. Carbohydr. Polym. 2020, 241, 116365. [Google Scholar] [CrossRef]
- Santos, S.; Ferreira, T.; Almeida, J.; Pires, M.J.; Colaço, A.; Lemos, S.; Gil da Costa, R.M.; Medeiros, R.; Bastos, M.; Neuparth, M.J.; et al. Dietary supplementation with the red seaweed porphyra umbilicalis protects against DNA damage and pre-malignant dysplastic skin lesions in HPV-Transgenic mice. Mar. Drugs 2019, 17, 615. [Google Scholar] [CrossRef]
- Fujii, T.; Takatsuka, N.; Nagata, C.; Matsumoto, K.; Oki, A.; Furuta, R.; Maeda, H.; Yasugi, T.; Kawana, K.; Mitsuhashi, A.; et al. Association between carotenoids and outcome of cervical intraepithelial neoplasia: A prospective cohort study. Int. J. Clin. Oncol. 2013, 18, 1091–1101. [Google Scholar] [CrossRef]
- Dewi, M.K.; Arsianti, A.; Zahira, C.R.; Zagloel, C.R.Z.; Aziza, Y.A.N.; Kurniasari, K.D.; Mandasari, B.K.D.; Masita, R.; Zulfa, F.R.; Azizah, N.N.; et al. In vitro evaluation of seaweed Gracilaria verrucosa for cytotoxic activity against cervical HeLa Cells. Pharmacogn. J. 2018, 10. [Google Scholar] [CrossRef]
- Ashwini, S.; Babut, S.; Saritha, M.S. Seaweed extracts exhibit anticancer activity against HeLa cell lines. Int. J. Curr. Pharm. Res. 2016, 9, 114–117. [Google Scholar] [CrossRef]
- Gomes, D.L.; Telles, C.B.S.; Costa, M.S.S.P.; Almeida-Lima, J.; Costa, L.S.; Keesen, T.S.L.; Rocha, H.A.O. Methanolic extracts from brown seaweeds Dictyota cilliolata and Dictyota menstrualis induce apoptosis in human cervical adenocarcinoma HeLa cells. Molecules 2015, 20, 6573–6591. [Google Scholar] [CrossRef] [PubMed]
- Saengkhae, C.; Noiraksar, T.; Jongkolnee, J.; Palee, P. Anti-Proliferation and induction of apoptosis by extract of Turbinaria conoides (J. Agardh) Kutzing on human cervical cancer cell line. Chulalongkom Med. J. 2010, 53, 12–24. [Google Scholar]
- Vaseghi, G.; Sharifi, M.; Dana, N.; Ghasemi, A.; Yegdaneh, A. Cytotoxicity of Sargassum angustifolium partitions against breast and cervical cancer cell lines. Adv. Biomed. Res. 2018, 7, 43. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, W.J.; Koo, B.-W.; Kim, D.-W.; Lee, J.H.; Nugroho, W.S.K.; Research, P. Anticancer activity of sulfated polysaccharides isolated from the Antarctic Red seaweed Iridaea cordata. Ocean Polar Res. 2016, 38. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B.S.; Dantas-Santos, N.; Camara, R.B.G.; Cordeiro, S.L.; Costa, M.S.S.P.; Almeida-Lima, J.; Melo-Silveira, R.F.; Oliveira, R.M. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef]
- Arsianti, A.; Aziza, Y.A.N.; Kurniasari, K.D.; Mandasari, B.K.D.; Masita, R.; Zulfa, F.R.; Dewi, M.K.; Zagloel, C.R.Z.; Azizah, N.N.; Putrianingsih, R. Phytochemical test and cytotoxic activity of macroalgae Eucheuma cottonii against cervical HeLa cells. Pharmacogn. J. 2018, 10. [Google Scholar] [CrossRef]
- Ye, G.; Lu, Q.; Zhao, W.; Du, D.; Jin, L.; Liu, Y. Fucoxanthin induces apoptosis in human cervical cancer cell line HeLa via PI3K/Akt pathway. Tumor Biol. 2014, 35, 11261–11267. [Google Scholar] [CrossRef]
- Hou, L.-L.; Gao, C.; Chen, L.; Hu, G.-Q.; Xie, S.-Q. Essential role of autophagy in fucoxanthin-induced cytotoxicity to human epithelial cervical cancer HeLa cells. Acta Pharmacol. Sin. 2013, 34, 1403–1410. [Google Scholar] [CrossRef]
- Jin, Y.; Qiu, S.; Shao, N.; Zheng, J. Fucoxanthin and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically promotes apoptosis of human cervical cancer cells by targeting PI3K/Akt/NF-κB signaling pathway. Int. Med. J. Exp. Clin. Res. 2018, 24, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Shaik, I.; Shameem, A.; Peddamalla, S.B.R. Anti-Cancer activity of selected seaweeds against HeLa, K-562 and MDA-MB cell lines. In Biotechnology and Bioforensics. SpringerBriefs in Applied Sciences and Technology; Springer: Singapore, 2015; pp. 35–42. [Google Scholar]
- Suganya, S.; Dhanalakshmi, B.; Kumar, S.D.; Santhanam, P. Cytotoxic Effect of Silver Nanoparticles Synthesized from Sargassum wightii on Cervical Cancer Cell Line. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 1–8. [Google Scholar] [CrossRef]
- Asik, R.M.; Gowdhami, B.; Mohamed, J.M.S.; Archunan, G.; Suganthy, N. Anticancer potential of zinc oxide nanoparticles against cervical carcinoma cells synthesized via biogenic route using aqueous extract of Gracilaria edulis. Mater. Sci. Eng. 2019, 103, 109840. [Google Scholar] [CrossRef]
- Raubbin, R.; Pushparaj, A.; Ronald, J. Antiproliferative potential of extract from hypnea flagelliformis on hela cancer cell lines. Int. J. Pharm. Biol. Sci. 2018, 8, 391–396. [Google Scholar]
- Ribeiro-Müller, L.; Seitz, H.; Müller, M. Human papillomavirus prophylactic vaccines and alternative strategies for prevention. In Human Papillomavirus Prophylactic Vaccines and Alternative Strategies for Prevention; IntechOpen: London, UK, 2013; Volume 149, p. 149. [Google Scholar]
| Author, Reference | Seaweed | Bioactive Compound |
|---|---|---|
| Matsubara, 2004 [35] | Codium cylindricum | Sulfated galactan |
| Jurd, 1995 [36] | Codium fragile | Arabinogalactans |
| Vasanthi, 2004 [37] | Corallina pilulifera | Ethanolic extract |
| Yang, 2009 [38] | Ecklonia cava | Phlorotannins |
| Shibata, 2002 [39] | Eisenia bicyclis | Phloroglucinol |
| Yan, 1999 [40] | Hijikia Fusiformis | Fucoxanthin |
| Park, 2011 [41] | Lobophora variegate | Fucans |
| Chevolot, 1999 [42] | Phaeophyceae | Fucoidans |
| Yabuta, 2010 [43] | Porphyra spp. | Phycoerythrobilin |
| Kaza-owska, 2010 [44] | Porphyria dentate | Cathecol |
| Subba Rao, 1997 [45] | Rhodophyceae | Galactans |
| Shibata, 2002 [39] | Saccharina japonica | Fucoidans |
| Bae, 2011 [46] | Sargassum thunbergii | Phlorotannins |
| Vasanthi, 2004 [37] | Schizymenia dubyi | Sulfated glucuronogalactan |
| Mayer, 2000 [47] | Taonamaria atomaria | Stypoldione |
| Yan, 1999 [40] | Undaria pinnatifida | Fucoxanthin |
| STUDIES ON ANTI-HPV EFFECT | |||||
|---|---|---|---|---|---|
| Author, Reference | Health-Promoting Effect | Seaweeds | Bioactive Compounds | Methods | Results |
| Rodriguez et al., 2014 [81] | Anti-HPV | Carrageenan | Carrageenan 3% was applied as intravaginal gel in a mouse model, infected with HPV16, HPV18 and HPV45 pseudoviruses. In vitro, PC-515 gel containing carrageenan was tested on a murine model. | Carrageenan formulations were effective as anti-HPV agents both in vitro and in vivo. Physicochemical properties of carrageenan lubricants influence the protection against HPV infection in vivo. | |
| Santos et al., 2019 [83] | Anti-HPV | Porphyra umbilicalis | The diet of 44 mice infected with HPV16 was supplemented with 10% seaweed. After the study period, the skin of the animal models was examined in order to classify HPV16-induced lesions. | Porphyra umbilicalis dietary intake significantly reduced the incidence of dysplastic lesions in HPV16 infected mice. | |
| Wang et al., 2020 [82] | Anti-HPV | Brown seaweeds | Polymannuroguluronate sulfate (PMGS) | The effects of PMGS were tested in mice and in vitro, using HeLa cells. | PMGS inhibited skin infection with HPV in mice. PMGS allowed the downregulation of E6 and E7 proteins of HPV. PMGS targeted L1 protein from the viral capsid and blocked the HPV infection. |
| STUDIES ON ANTI-CERVICAL CANCER EFFECTS | |||||
| Saengkhae et al., 2010 [88] | Antiproliferative effects Cytotoxic effects | Turbinaria conoides | HeLa cells were treated with fresh samples of T. conoides. | T. conoides exhibited cytotoxic effects against HeLa cells (IC50 20.92 ± 3.15 μg/mL) in a dose-dependent manner. | |
| Costa et al., 2011 [91] | Antiproliferative effects Antioxidant effects | Sargassum filipendula | Fucans | Antiproliferative effects of S. filipendula were tested in vitro, on HeLa, HepG2 and PC-3 cells. | The strongest antiproliferative effect was exhibited against HeLa cells. S. filipendula also exerted antioxidant effects in vitro. |
| Hou et al., 2013 [94] | Pro-apoptotic effects | Fucoxanthin | HeLa cells were used to evaluate the cytotoxicity of fucoxanthin (doses ranged between 10 and 80 μmol/L), for 48 h. | Fucoxanthin induced G0/G1 arrest in a dose-dependent manner, and also increased the expression of LC3 II (autophagosome marker). Fucoxanthn inhibited the phosphorylation of Akt and increased PTEN expression in tumoral cervical cells. | |
| Ye et al., 2014 [93] | Pro-apoptotic effects | Fucoxanthin | HeLa cells were treated with fucoxanthin for 24 h. | Fucoxanthin induced apoptosis in tumoral cells. The phosphorylation of Akt significantly decreased depending on the dose of fucoxanthin. Fucoxanthin interfered with the mitochondrial signal transduction pathway. | |
| Shaik et al., 2014 [96] | Cytotoxic effects | Sargassum wightii Ulva fasciata Gracillaria corticata | Methanolic, butanolic and hexanoic extracts of selected seaweeds were used on HeLa cells and their cytotoxic effects were registered. | Butanolic extracts of G. corticata showed the most potent cytotoxic activity. | |
| Kim et al., 2016 [90] | Cytotoxic effects | Iridaea cordata | Sulfated polysaccharides | HeLa, HT-29 and PC-3 cells were treated with polysaccharides extracted from Iridaea cordata seaweed. | Iriaea Cordata polysaccharides exerted a weak antitumor activity against HeLa, Pc-3 and HT-29 tumoral cells. |
| Ashwini et al., 2016 [86] | Cytotoxic effects | Gracilaria corticata | Chloroform and ethanol extracts | The anticancer activity of chloroform and ethanol extracts from seaweeds on HeLa cells was observed after 24, 48 and 72 h. | IC50 for chloroform extracts 341.82 µg/mL. IC50 for ethanol extracts 244.7 µg/mL. |
| Arsianti et al., 2018 [92] | Cytotoxic effects | Eucheuma cottonii | Ethanol, chloroform, and ethyl acetate extracts | Cytotoxic activity of ethanol, chloroform, and ethylacetate extracts from Eucheuma cottonii were tested on HeLa cells. | IC50 for ethanol extract—7.54 μg/mL. IC50 for ethylacetate extract—4.34 μg/mL. IC50 for chloroform extract—4.82 μg /mL. |
| Micheylla et al., 2018 [85] | Cytotoxic effects | Gracilaria verrucosa | Hexane, chloroform, ethyl acetate, ethanol extracts | Seaweed extracts were diluted into 8 concentrations and their anticancer activity was investigated in vitro, using HeLa cells. | The most potent cytotoxic activity against HeLa cells was exhibited by hexane extract. IC50 for hexane extract 14.94 μg/mL. IC50 for chloroform extract 15.74 μg/mL. IC50 for ethyl acetate 16.18 μg/mL. IC50 for ethanol extract 19.43 μg/mL. |
| Vaseghi et al., 2018 [89] | Cytotoxic effects | Sargassum angustifolium | Methanol-ethyl acetate extracts | HeLa and MCF-7 cells were treated with methanol-ethyl acetate extracts from Sargassum angustifolium (150, 450, and 900 μg/mL). | Seaweed extracts exhibited cytotoxic effects against HeLa and MCF-7 cells in a dose-dependent manner. |
| Jin et al., 2018 [95] | Pro-apoptotic effects | Fucoxanthin | HeLa cells were treated with fucoxanthin or TRAIL (Tumor necrosis factor-related apoptosis-inducing ligand) | The combination of TRAIL and fucoxanthin induced apoptosis in HeLa cells Fucoxanthin inhibited PI3K/Akt and NF-κB pathways for apoptosis. TRAIL upregulated PI3K/Akt and NF-κB pathways in HeLa cells. | |
| Raubbin et al., 2018 [99] | Cytotoxic effects | Hypnea flagelliformis | The effects of different concentrations of ethyl acetate extract from H. flageliformis on HeLa cells were observed after 48 h of incubation. | IC50 for ethyl acetate extract was 138.321 μg/mL. | |
| Suganya et al., 2019 [97] | Pro-apoptotic effects | Sargassum wightii | Silver nanoparticles containing seaweed S. wightii extracts were incubated for 24 or 48 h with HeLa cells. Cytotoxic activity of silver nanoparticles with different concentrations was investigated. | Silver nanoparticles showed a significant decrease in cell viability after 24 h. The complete loss of cell viability was observed after 48 h. Silver nanoparticles showed a 50% inhibition of HeLA viability at 47.48 μg/mL in 24 h. | |
| Asik et al., 2019 [98] | Cytotoxic and pro-apoptotic effects | Gracilaria edulis | Zinc nanoparticles were synthetized using an aqueous extract from Gracilaria edulis seaweed. Their anticancer effects were investigated on SiHa cells. | IC50 for zinc nanoparticles—35 ± 0.03 μg/mL. Zinc nanoparticles induced apoptosis and necrosis in SiHa cells in a dose-dependent manner. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moga, M.A.; Dima, L.; Balan, A.; Blidaru, A.; Dimienescu, O.G.; Podasca, C.; Toma, S. Are Bioactive Molecules from Seaweeds a Novel and Challenging Option for the Prevention of HPV Infection and Cervical Cancer Therapy?—A Review. Int. J. Mol. Sci. 2021, 22, 629. https://doi.org/10.3390/ijms22020629
Moga MA, Dima L, Balan A, Blidaru A, Dimienescu OG, Podasca C, Toma S. Are Bioactive Molecules from Seaweeds a Novel and Challenging Option for the Prevention of HPV Infection and Cervical Cancer Therapy?—A Review. International Journal of Molecular Sciences. 2021; 22(2):629. https://doi.org/10.3390/ijms22020629
Chicago/Turabian StyleMoga, Marius Alexandru, Lorena Dima, Andreea Balan, Alexandru Blidaru, Oana Gabriela Dimienescu, Cezar Podasca, and Sebastian Toma. 2021. "Are Bioactive Molecules from Seaweeds a Novel and Challenging Option for the Prevention of HPV Infection and Cervical Cancer Therapy?—A Review" International Journal of Molecular Sciences 22, no. 2: 629. https://doi.org/10.3390/ijms22020629
APA StyleMoga, M. A., Dima, L., Balan, A., Blidaru, A., Dimienescu, O. G., Podasca, C., & Toma, S. (2021). Are Bioactive Molecules from Seaweeds a Novel and Challenging Option for the Prevention of HPV Infection and Cervical Cancer Therapy?—A Review. International Journal of Molecular Sciences, 22(2), 629. https://doi.org/10.3390/ijms22020629






