Biotransformation of Citrus Waste-I: Production of Biofuel and Valuable Compounds by Fermentation
Abstract
1. Introduction
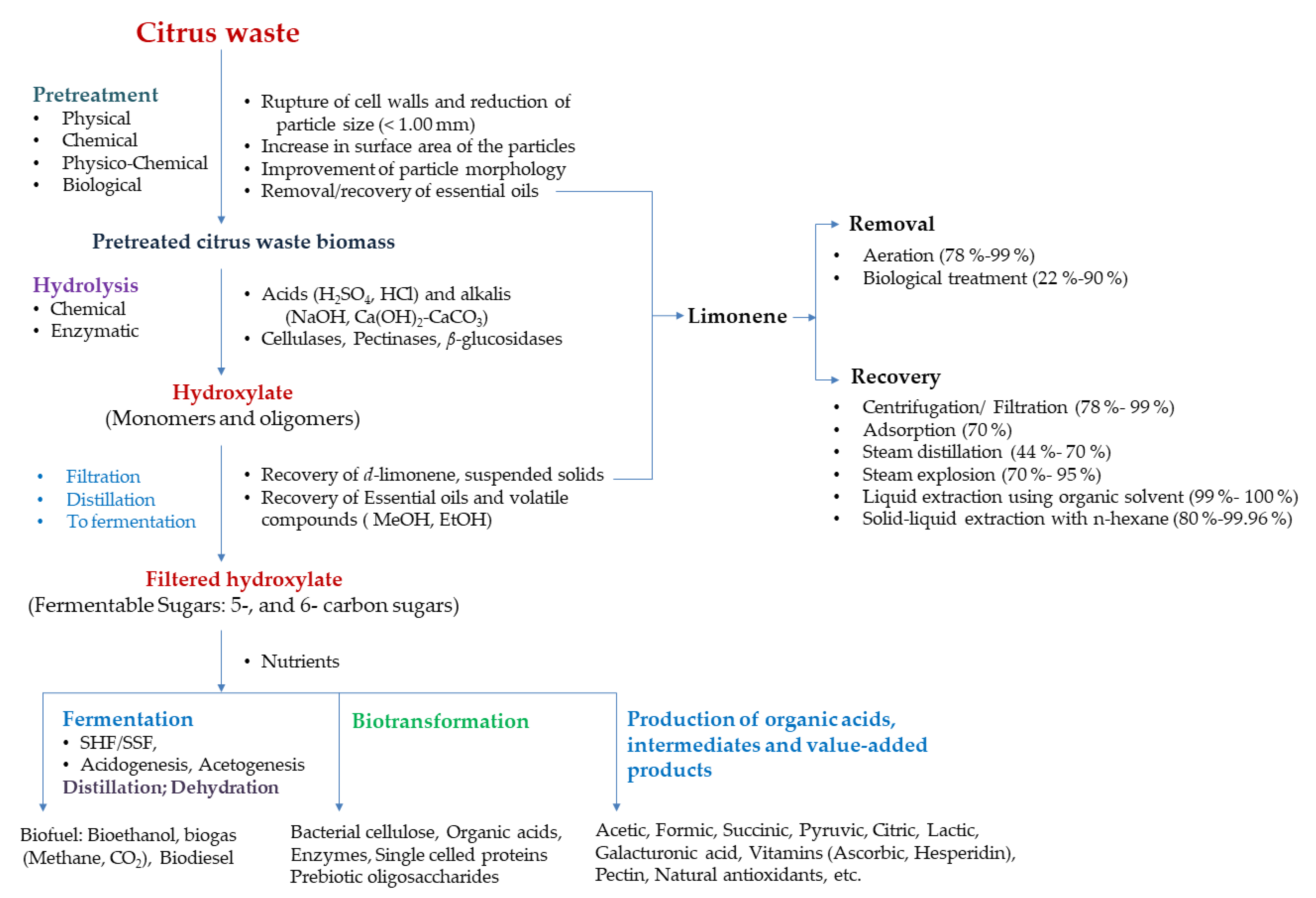
2. Biofuel: Bio-Ethanol, Biodiesel and Biogas
2.1. Pre-Treatment
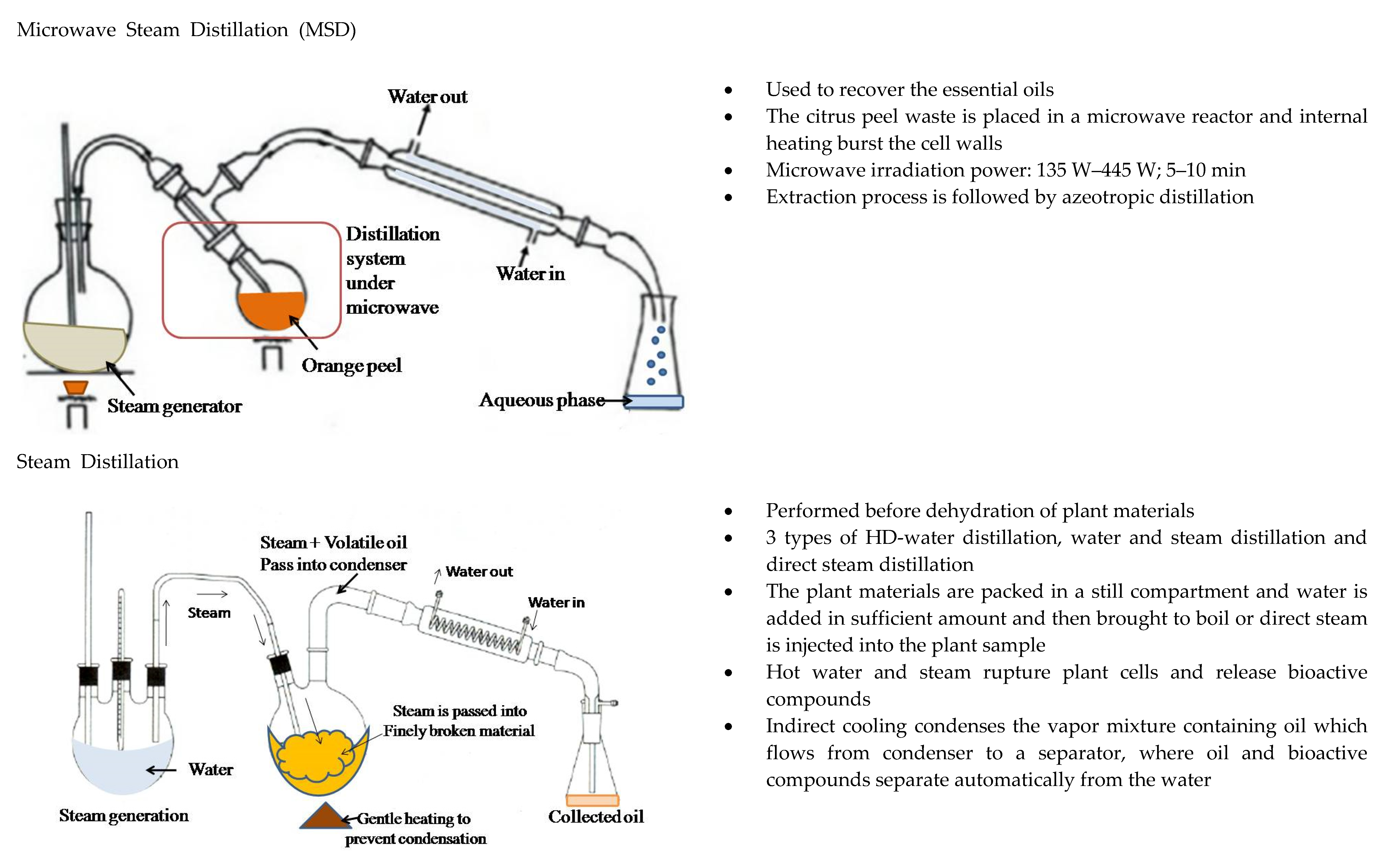
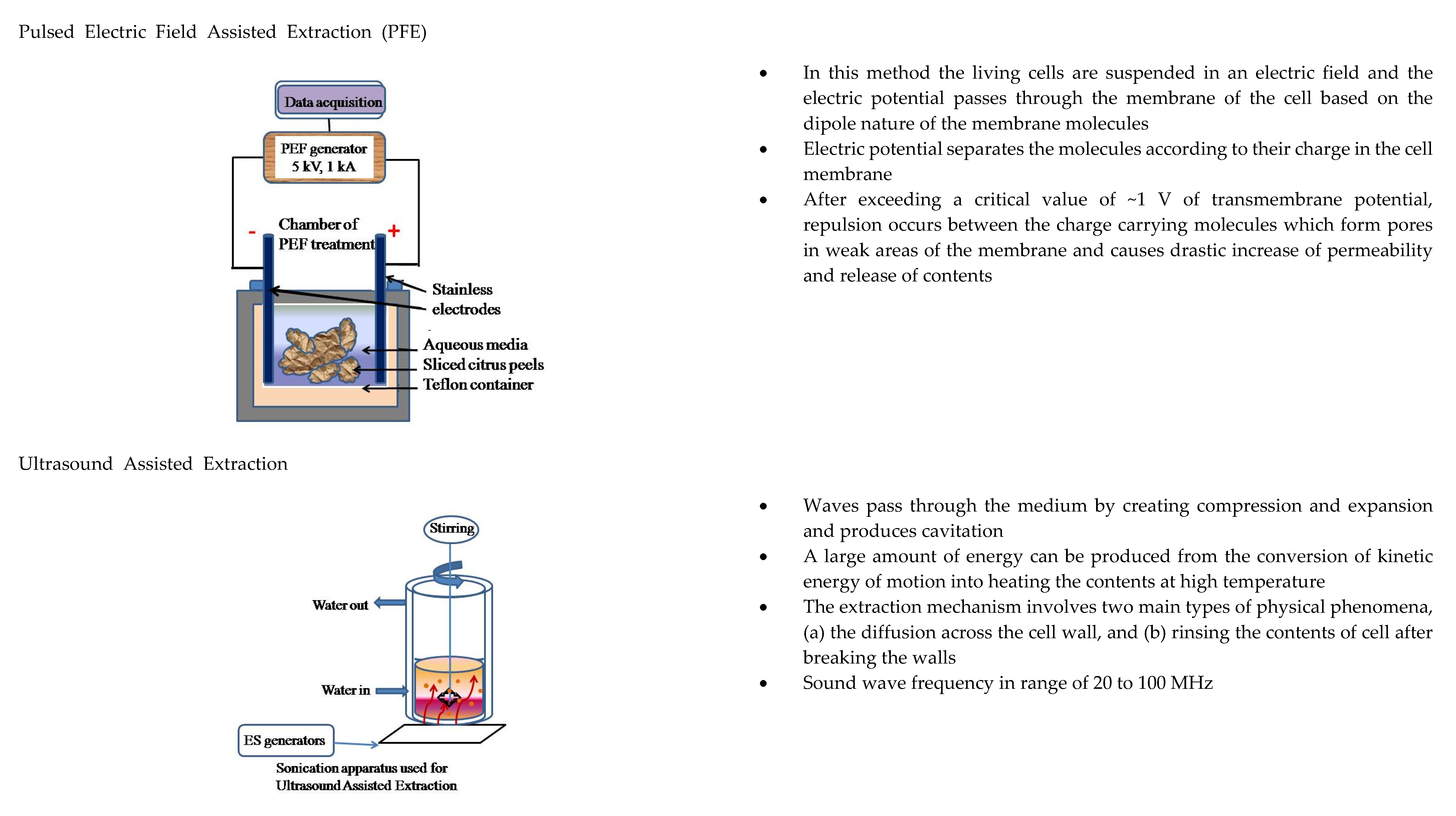
2.2. Esential Oils: Removal and Recovery of Limonene
2.3. Hydrolysis
- (a)
- Chemical hydrolysis: Chemical hydrolysis can be an integral part of both the processes, namely pretreatment and hydrolysis assisted fermentation. It can be carried out by using either dilute or concentrated acids, namely, hydrochloric acid or sulfuric acid. Sulfuric acid at a pH of 2.0 is generally considered to be highly effective for hydrolysis of citrus waste biomass in terms of less acid consumption and lower destruction of fructose [65]. Increasing the acid concentration from 0.05% to 1.0%, (w/v) has been observed to increase the release of sugars into the slurry. However, there is also observed an increase in the amounts of HMF, acetic acid and phenolics which are inhibitory to the fermentation process. Hence, the chemical hydrolysis process is proposed to be carried out in two stages with dilute acid (H2SO4, 0.5% w/v in the second stage) to improve the yields. In addition, longer duration of hydrolysis than the optimized duration has also been witnessed with formation of inhibitory HMF in the medium [31].
- (b)
- Enzymatic hydrolysis: This is the most favored mode of hydrolysis for the citrus processing waste in order to produce ethanol. Enzyme hydrolysis is also known as enzymatic saccharification in which the cellulosic and hemicellulose fraction in the biomass is digested by enzymes to generate pentose and hexose sugars and utilized by microbes in the fermentation process. Researchers have employed cocktails of enzymes in different proportions and recorded the effects of different amounts of loading. The commonly employed enzymes for hydrolysis process are cellulase (1.9 mg of protein/g of total sugar), pectinase (0.8 mg of protein/g of total sugar), and β-glucosidase (1.6 mg of protein/g of total sugar). Cellulase breakdown cellulose and the commonly employed cellulase enzymes are, endo-1,4-β-glucanase (EC 3.2.1.4), exo-1,4-β-glucanase (EC 3.2.1.91), and β-glucosidase (EC 3.2.1.21). Pectinase breakdown pectic substances present in the cell wall; decrease the viscosity of the medium and soften the tissues. The commonly employed pectinase enzymes are polygalacturonase (EC 3.2.1.15), pectin lyase (EC 4.2.2.10), and pectin esterase (EC 3.1.1.11). Enzymatic hydrolysis is an environment-friendly process and does not generate any toxic products as a result of degradation. The moderate conditions for carrying out enzymatic hydrolysis are; temperature 45–50 °C, pH of 4.8–5.0 for 24 h. When xylanase was employed along with cellulase, pectinase and β-glucosidase for the hydrolysis of mandarin peel waste, the hydrolysis generated galacturonic acid, rhamnose, arabinose, glucose, galactose, xylose, fructose and sucrose. The pectinolytic and xylanolytic activities are facilitated by Aspergillus sp., and cellulolytic activities are facilitated by Trichoderma sp. The enzymatic hydrolysis is pH sensitive. To obtain best results, the mixture is supplemented with acetate (sodium acetate) buffer of pH 4.8. As the enzymes and nutritional supplements are added, the pH of the mixture becomes stable between 4.8 and 5.2 and the constituents are incubated at 35 °C with constant rotation. Since, commercial enzymes are expensive; the overall production of ethanol becomes expensive too. One of the successful resolutions in this case has been in-house production of these enzymes using microorganisms [31,34,41,66,67,68,69].
2.4. Fermentation
2.4.1. Ethanol
- (a)
- Batch Fermentation: In the batch process, the reactor vessel is supplied with a limited-set amount of nutrients and microbial inoculation at the initial stage or at zero time and the fermentation process is allowed to run under a controlled environment of temperature, pH and pressure until maximum yield of end products (concentration) is achieved. During the entire process, no additional nutrients are added, and hence it is a closed system. At the end, all the nutrients are consumed and it is possible to characterize the microbial strains and optimize the nutrient medium to develop a rapid mechanism. However, the product yield from the biomass remains limited. In this process, the microbes have limited time to remain in the exponential growth phase because the carbon sources (biomass and the nutrients) and oxygen supply act as limiting factors. The process is usually facilitated with increasing the stirring speed, the oxygen gas flow into the medium. At the end, the medium is collected and proceeds to further processing to obtain the desired form of the product. The main advantages of this method are; (i) short duration of operation (ii) reduced or limited chances of contamination as no biomass substrate or nutrients are added during the process, (iii) ease of separation of batch material for traceability, and (iv) convenient to handle and operate. On the other hand, the disadvantages are (i) the end product is mixed with substrate biomass, nutrients cell debris and metabolic toxins, and (ii) productive time is short, resulting in low yields [14,31].
- (b)
- Fed-batch fermentation: In this method, a controlled addition of substrate and nutrients is carried out at desired intervals during the entire fermentation process. The system is partly open unlike the batch system which is completely closed. The substrate is pumped from the supply vessel to the culture reactor through a silicone tube attached to it. The fed-batch process allows specific growth conditions to the microorganisms to a prolonged duration and an exponential growth curve is maintained for a relatively longer duration. Eventually, an increased feed rate is required. Although, the fed-batch method allows a range of control strategies and extended operational durations, the fermentation process faces inhibition because of the formation of toxic by-products. The fed-batch method is also known as semi-continuous process. The main advantage of the fed-batch method is that it allows an increase in the concentration of viable microbial colonies, product accumulation and sufficient culture life time [31,73].
- (c)
- Continuous culture fermentation: In this method, the substrate and nutrients are continuously fed to the reactor after a batch growth phase attains an equilibrium stage or a steady-state and products are removed. The removed medium containing desired products as well as by-products is utilized to harvest microbial cells and residues besides ethanol. Hence, it is usually run with a programed inflow and out flow mechanism adjusted with intervals less than doubling time of the microorganisms employed. Although, this method is an advantage to the fed-batch mechanism, however, the prolonged cultivation period with repeated feeding of fresh substrate to the medium in the reactor increases the risk of contamination. Furthermore, it is difficult to (i) maintain a constant microbial population density inside the reactor medium over sustained duration, (ii) carry out separation of products from the batches and control for changes in the genetic material/mutation of the microorganism [31,74,75].
- (d)
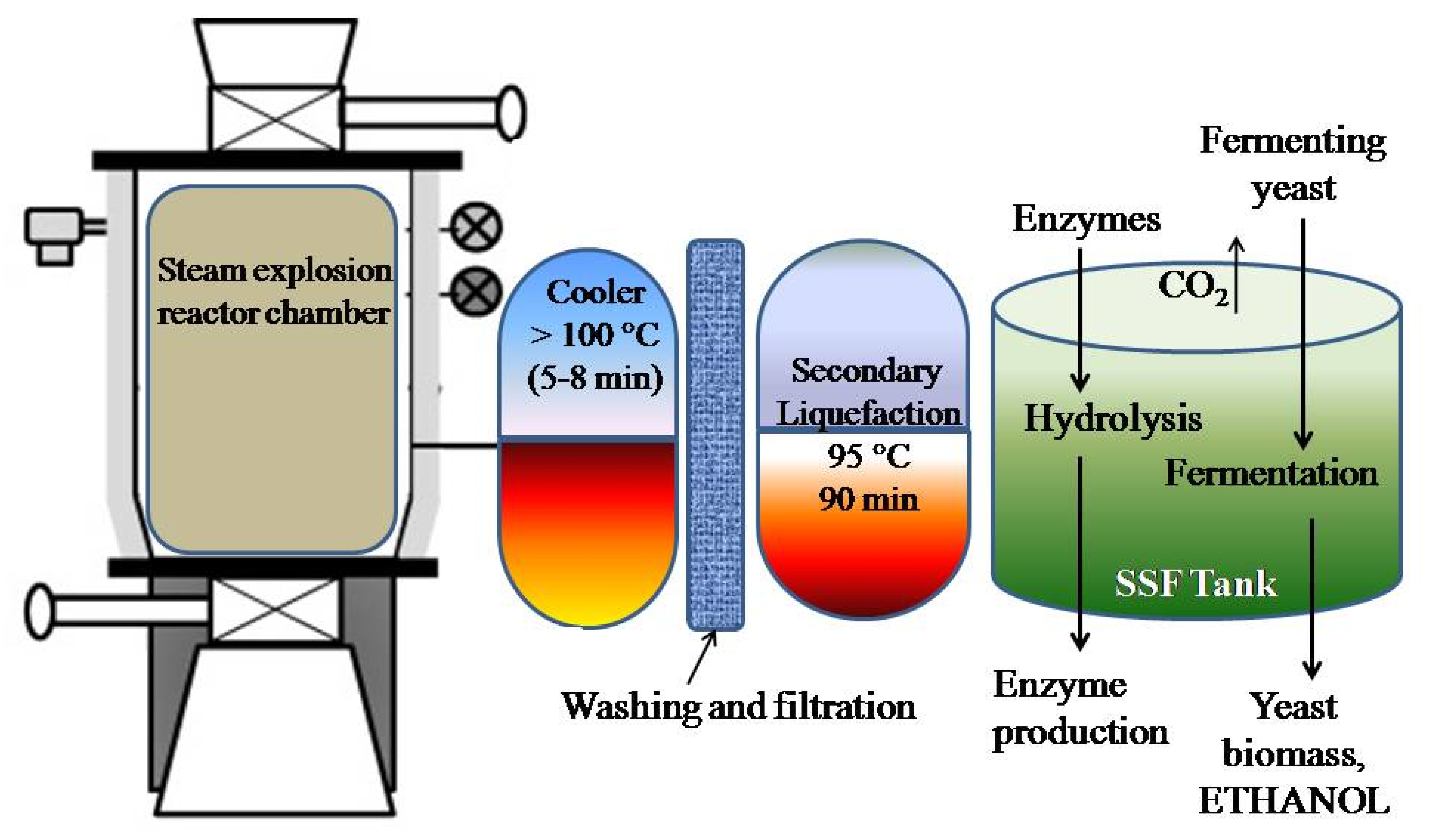
- Separate hydrolysis and fermentation (SSF): In this method, the enzymatic hydrolysis is carried out separately from the microbial fermentation process. Here, the medium from hydrolysis reactor is transferred to the fermentation tank and the ethanol is distilled off leaving behind unconverted sugars and microbial cell mass. The separation of the two fundamental processes, viz., enzymatic hydrolysis (at 45 °C–50 °C) and fermentation (at 30 °C), facilitates optimum favorable conditions for both adequately. On the other hand, the inhibitory effect caused by the sugars (e.g., glucose), produced from hydrolysis, to the enzymatic actions of cellulase and β-glucosidase enzymes retards completion of the hydrolysis process and might require higher or additional loadings of enzymes to the reactor to achieve good yields of fermentable sugars. Choi et al. have observed an ethanol yield of 90.6% from pretreated mandarin peels by SHF process. They reported an ethanol concentration of 46.2 g/L with a productivity of 3.85 g/L h [34].
- (e)
- Simultaneous saccharification and fermentation (SSF): In this process, both the enzymatic hydrolysis and fermentation processes are carried out in the same tank. Since, the products released from hydrolysis, viz., glucose, xylose, and cellobiose by the action of cellulase, xylanase, etc., cause inhibition to further hydrolysis and saccharification process, therefore, these are required to be consumed by the microbial fermentation process. Due to the combined processes running simultaneously, the system requires a relatively shorter duration of operation, lower enzyme requirement, facilitates higher product yields, effective conversion of fermentable sugars to ethanol and eventually regulating the inhibitory effects of the sugars on the enzymatic action. In addition, there is a lower requirement of sterile conditions in this process as the sugars produced during the hydrolysis by enzymes are quickly consumed by the microbes for producing ethanol. Boluda-Aguilar et al. investigated the yield capacities of the two processes, and reported that SSF resulted in higher yields (50 L/1000 kg of citrus peels) compared to the SHF (50 L/1000 kg of citrus peels). The residue remaining after the distillation of ethanol have been found to be lower in SSF than SHF which is converted to citrus pulp pellet or animal feed and manure. Most importantly, the production of ethanol by either of the fermentation processes also greatly depends upon pre-treatment. The hydrolysis of citrus cellulosic biomass under SSF to produce ethanol has been reported to be the least expensive method. Moreover, the by-products of ethanol, viz., syngas (CO+H2) can also be converted back to ethanol [5,31,66,67].
- (f)
- Continuous immobilized fermentation: In this method, a continuous fermentation process is carried out using immobilized microorganisms. The immobilized microbes can be recovered post-fermentation process and reused again. The microbes are immobilized by housing them inside a column. Choi et al. developed an Immobilized Cell Reactor (ICR) by housing immobilized Saccharomyces cerevisiae cells, packed up to 70% of the column volume (column dimension 25 cm long with an internal diameter of 2.1 cm). The fermentation medium was introduced to the ICR from limonene removal column (LRC) and fermentation was run for 10 days at 30 °C in an incubator. The process was reported to yield a 12 folds higher yields of ethanol [31,41].
2.4.2. Biogas
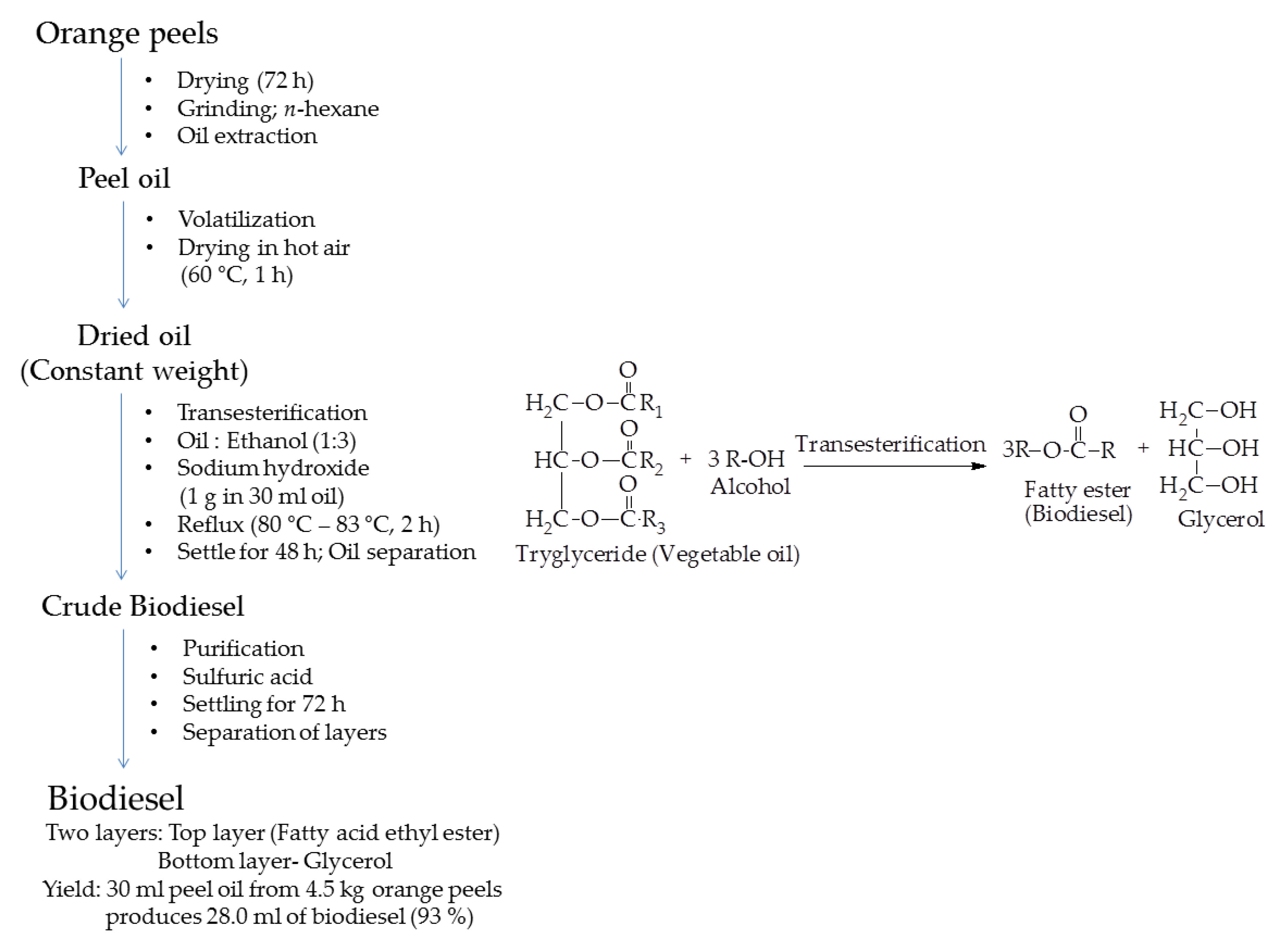
2.4.3. Biodiesel
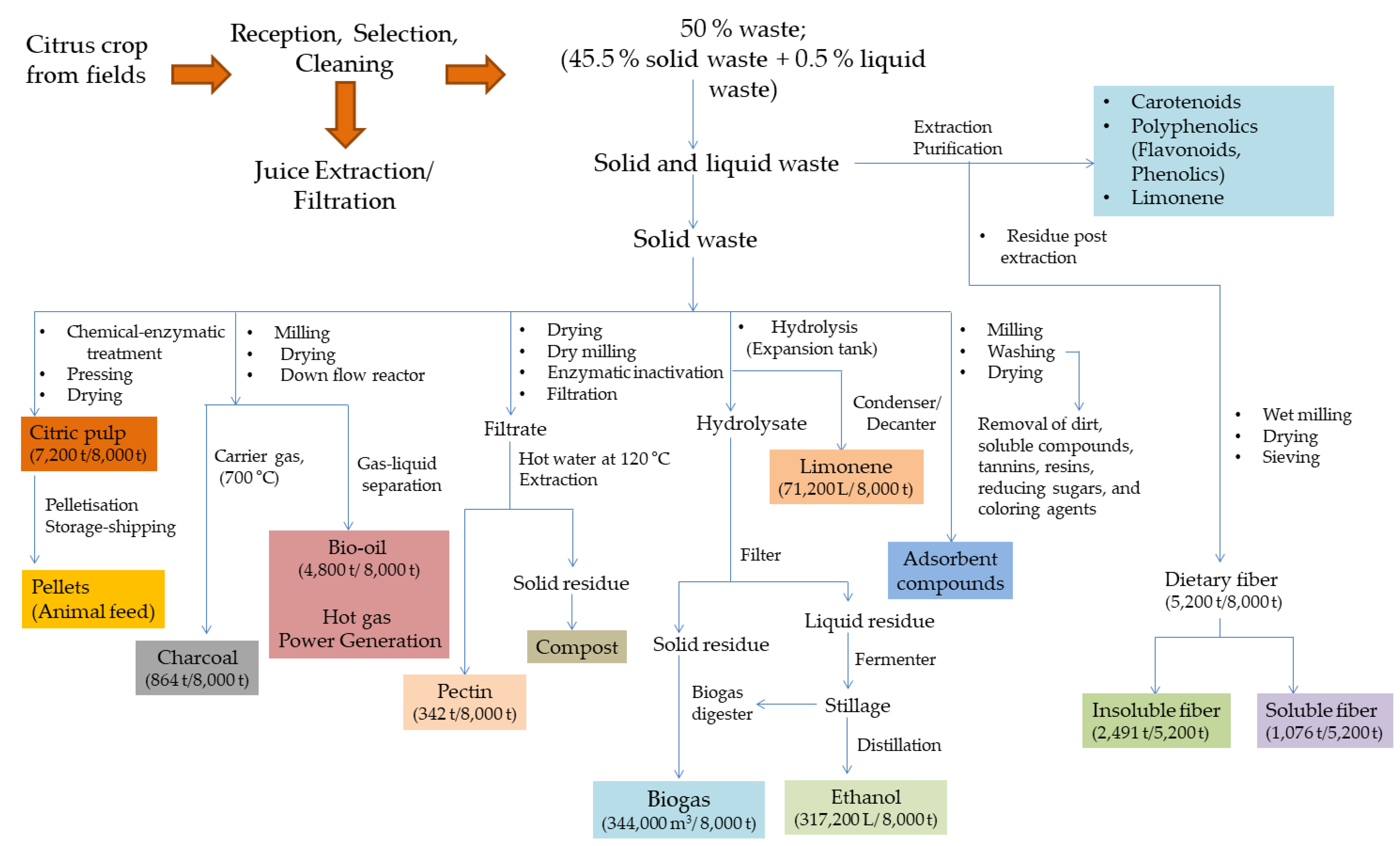
2.5. Biorefinery
3. Organic Acids
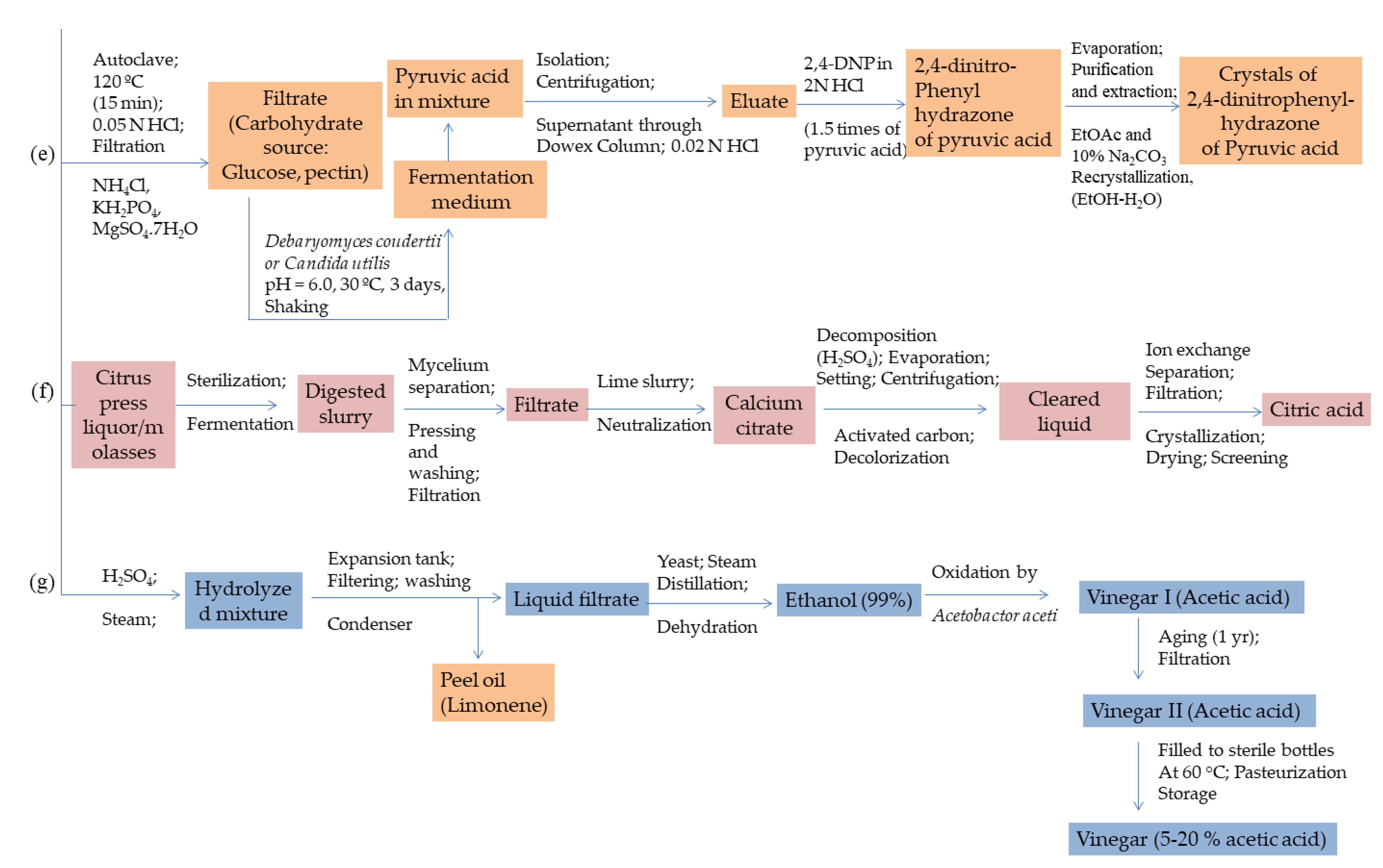
3.1. Citric Acid
3.2. Lactic, Succinic, Pyruvic and Acetic Acids
4. Valuable Products
- (a)
- Vitamins: Similar to many different carboxylic acids, viz., citric, pyruvic, succinic and acetic (in the form of vinegar), the vitamins, namely, vitamin-C (ascorbic acid + dehydroascorbic acid) in ample amounts and pro-vitamin A, B-complex, riboflavin and co-factors in trace amounts are also found in citrus fruit juices. Riboflavin is commercially produced from molasses employing yeast strains, viz., Ashbya gossypi and Eremothecium ashbyii. The microbes in the fermentation media require additional nutrient supplements, such as proteins and carbohydrate sources to produce good yields. A near neutral pH of 6.6 to 8.0 is generally required for optimal microbial action. Gaden et al. reported a maximum yield of 0.7 g of riboflavin per liter of diluted molasses obtained from citrus waste residue. The fermentation medium contained, A. gossypii was found to be incapable of transforming riboflavin from the fermentable ingredients present in citrus molasses [119]. Hesperidin, also known as Vitamin-P, is another high-valued by-product is recovered from the solid residue collected after an acidic pretreatment of peel waste in quantities ranging between 3.7% and 4.5% of dry mass [4,14,115].
- (b)
- Pectin: Pectin has been reported to be composed of 17 different monosaccharides. Annual consumption of pectin worldwide exceeds 45 million kilograms with a global market value of 1.0 billion USD in 2019 and expected to achieve 1.5 billion USD by the year 2025. Pectin is conventionally extracted by using chemicals, such as, strong acids, e.g., oxalic acid, HCl. HNO3 and H2SO4. In current times, there has been a shift from chemical to green methods regards extraction of pectin. Commercial enzymes, e.g., multienzyme complexes containing pectinolytic, cellulolytic, hemicellulolytic and proteolytic enzymes have been used to extract pectin from citrus waste biomass [120,121,122,123]. Besides enzymatic extraction, several thermal and mechanical techniques, viz., ultrasound assisted extraction, autoclave extraction, extrusion cooking or microwave assisted extraction and subcritical water extraction [4,14,92,124,125,126,127,128,129].
- (c)
- Single cell protein: Single cell protein is a crude, refined and edible microbial protein obtained from biological substrates from agricultural and industrial processing wastes. Single cell proteins are extracted commercially from algae, fungi, yeast and bacteria which possesses very high quantities of proteins in their cell bodies. Bacterial single cell proteins contain 50–80% protein by dry weight. Spirulina and Chlorella are major sources of revenue generation in this market. Citrus waste biomass can be a relatively inexpensive feedstock to manufacture single cell proteins. Citrus processing waste is rich in cellulose, hemicellulose but have low quantities of lignin. The composition is ideal for producing feed for ruminants and microbial proteins or single cell proteins. The methods for obtaining single cell proteins are simultaneous saccharification and fermentation, solid state fermentation and separate hydrolysis and fermentation employing unicellular microorganisms. Fermentation of bergamot peels using strains of Penicillium sp. have been observed to improve nutritional value of the feed by increase in the amounts of crude proteins, crude fats and structural carbohydrates [130]. Solid-state fermentation of orange and lemon pulps by P. roqueforti Pr2 have been also observed to be an effective bioconversion in terms of enhancing quantities of proteins and lipid content in the yield [131]. Significant amounts of single cell proteins and high-activity crude pectinases have been produced from hydrolysis of lemon pulps by A. niger and T. viride. The highest protein level in the yield was observed after 14 days by using A. niger, whereas, T. viride yielded higher nitrogen content (31.9%) compared to A. niger which yielded (25.6%) [132].
- (d)
- Prebiotic oligosaccharides: Prebiotics are selectively fermented ingredients which allow specific changes in the composition and/or activities of microbial flora in the gastrointestinal tract and helps in sustaining well-being and health. The best-known prebiotic oligosaccharides are fructooligosaccharides, galactooligosaccharides, lactulose, pectic oligosaccharides (POS), etc. POS helps in regulating lipid and glucose metabolism with reduced glycemic response and blood cholesterol levels. Besides this, POS have also been observed to exhibit anti-cancer, anti-obesity, antibacterial, antioxidant and immunological properties. These also help in growth of friendly bacteria, such as bifidobacteria and lactobacilli in the gastrointestinal tract and limit the growth of pathogenic bacteria. Citrus peel albedo, and pectin obtained from citrus are considered as good source materials for obtaining prebiotics. The common methods are hydrolysis by enzymes, microwave and autoclave extraction, non-isothermal processing with hot compressed water (autohydrolysis or hydrothermal treatments of citrus peel substrates [133,134,135,136,137]. Olano-Martin et al. obtained POS from hydrolysis by pectin enzymes from citrus and apples in an enzyme membrane reactor [134]. Simultaneously, pectinolytic enzymes as well as pectinase and cellulase were produced from bergamot peels and orange peels, respectively, along with POS production. Furthermore, the solid residue obtained after the extraction of pectin from citrus waste is a good resource for the extraction of pectic oligosaccharides which is present in the soluble fraction [122].
- (e)
- Bacterial cellulose: It is a linear homopolymer composed of β-1,4-linked D-glucopyranose. Citrus fruits are rich in soluble sugars, cellulose, hemicellulose and pectin and can be used as a sustainable and renewable feedstock for the production of bacterial cellulose (BC). One of the recently reported methods for obtaining bacterial cellulose utilized citrus peels citrus peels (lemon, mandarin, orange and grapefruit). The citrus peel waste biomass was hydrolyzed using dilute acid subjected for microbial biotransformation by Komagataeibacter hansenii GA2016 for 21 days at 28–32 °C under static conditions. The yield was found to be between 2.06 and 3.92% and the BCs produced from citrus peel hydrolysates were examined to be similar to the BC produced from commercial methods, possessed high water holding capacity, thin fiber diameter, high the thermal stability and high crystallinity [138]. Kuo et al. employed Gluconacetobacter xylinus for the production of bacterial cellulose. The nutrient medium consisted acetic acid buffer or nitrogen source and added to the orange peel media. G. Xylinus directly utilized soluble sugars and the production was found to be 4.2–6.32 times higher than that achieved with traditional Hestrin and Schramm (HS) medium [139]. The bacterial cellulose has been found to be free from other polysaccharide contamination, e.g., lignin or hemicellulose. It is an ultrafine 3D structural network of cellulose nanofibers (3–8 nm in diameter). It has specific high water holding capacity, great elasticity, significantly high wet strength and remarkable conformability. It can be recovered as a pure compound and find useful applications in biomaterials, viz., artificial skin, artificial cardiovascular tissues, scaffolds for tissue regeneration and wound coverage [14].
- (f)
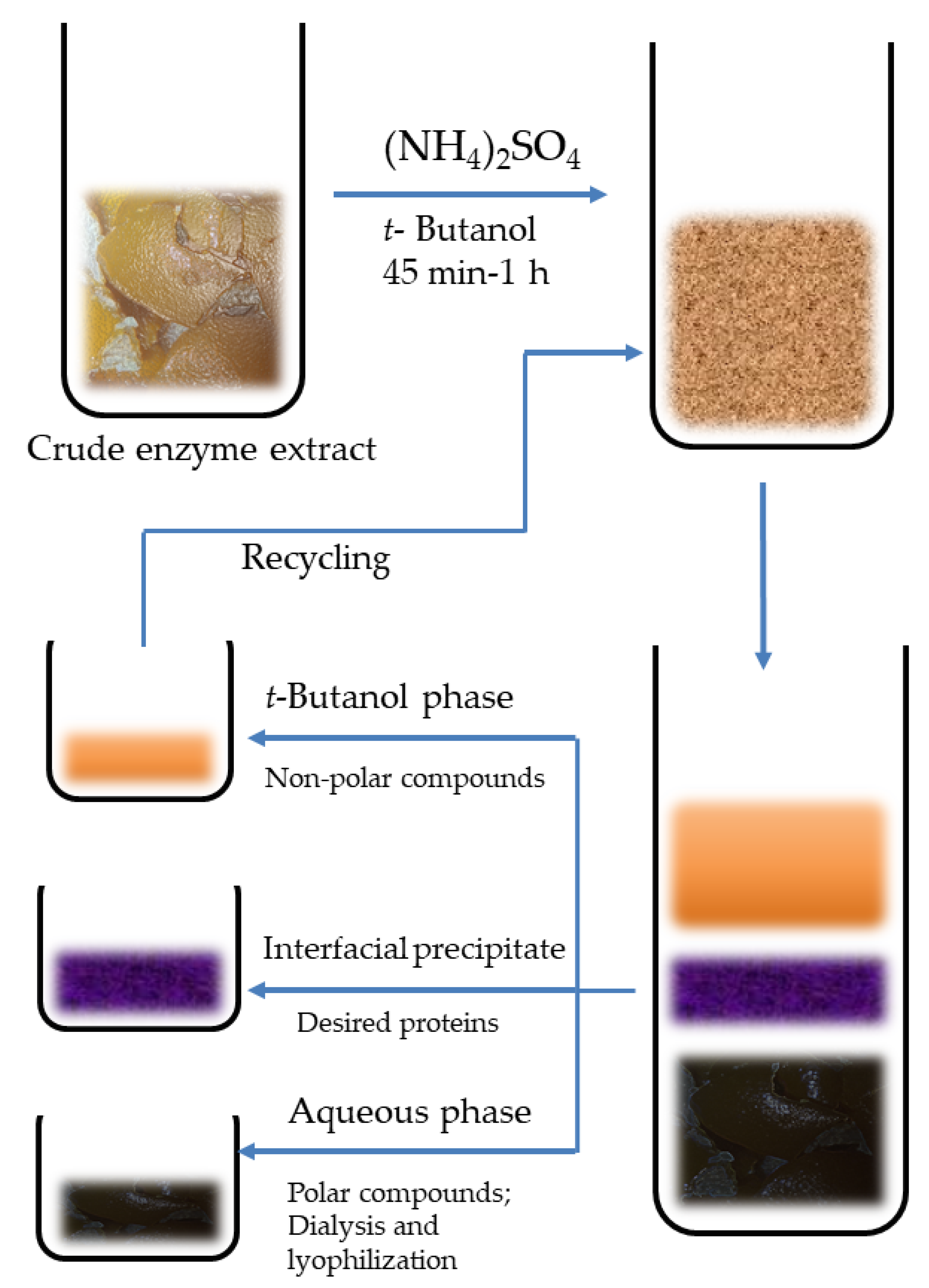
- Enzymes: Enzymes are biocatalysts and find immense applications in biotransformation of complex organic molecules into simple ones. Citrus peels have unique composition and a natural resource for extraction of a number of enzymes. Peroxidases are one of the most important biocatalysts, but its synthesis is low yielding as well as expensive. This limits its application. In recent years, many modern techniques have been introduced for enzyme bio-separation from natural resources. Three-phase partitioning (TPP) is one of the modest bio-separation techniques which bring satisfactory results. This method involves addition of a suitable salt, mostly ammonium sulfate to the aqueous suspension of crude extract obtained from citrus waste biomass followed by addition of tertiary butanol (Figure 12). Normally, tertiary butanol is completely miscible with aqueous phase, but it separates out at the top of the aqueous phase upon addition of ammonium sulfate in adequate concentration to the reaction medium. Besides peroxide extraction from orange peels (Citrus sinensis), the triple phase partitioning technique has also been used in extraction, separation, isolation and purification of a number of enzymes from natural resources, viz., invertase from tomato and yeasts; protease from papaya; cellulase, pectinase, xylanase from a number of plant resources, and so on [140,141,142]. The factors affecting the extraction process are duration of extraction, concentration of ammonium sulfate in the reaction mixture, pH of the medium, ratio of substrate to t-butanol, temperature and speed of rotation organization of the reaction mixture [143]. This technique has been reported to obtain peroxidases with purity up to 93.96% from the orange peel waste biomass in the reaction mixture containing 50% of ammonium sulfate at a pH of 6 and temperature 30 °C. The mixture was supplied with tertiary butanol (feed to t-butyl ratio of 1:1.5 v/v) and centrifuged for 80 min. Besides pectinases and peroxidases, cellulases and hemicellulases are also extracted from citrus peels. These are included in multienzyme complexes. Cellulase enzymes comprise endo-1,4-β-D-glucanase, exo-1,4-β-glucanase and β-D-glucanase. These enzymes find applications in feed, fuel and chemical industries and employed in processing of lignocellulose materials. Hemicellulytic enzymes comprise β-1,4-endoxylanase, acetyl xylan esterase and phenolic acid (ferulic and p-coumaric acid) esterase. For extraction of these enzymes, both fermentation methods, viz., submerged and simultaneous saccharification and fermentation [14].
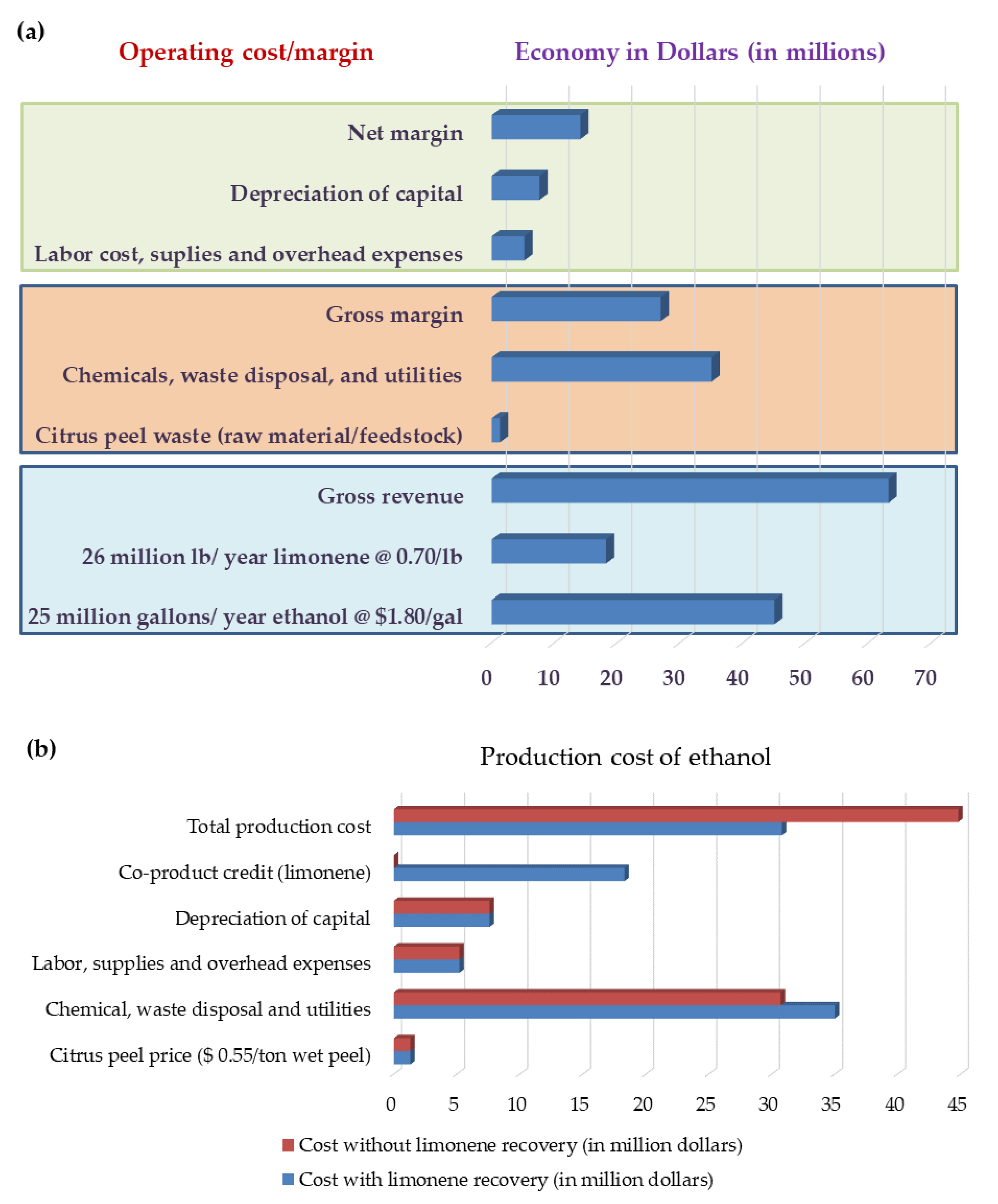
5. Economic Aspects
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value added products: Economical and environment friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M.H. Citrus essential oils: Extraction, authentication and application in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 611–625. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern extraction and purification techniques for obtaining high purity food-grade bioactive compounds and value-added co-products from citrus wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef]
- Zhou, W.; Widmer, W.; Grohmann, K. Economic analysis of ethanol production from citrus peel waste. Proc. Flo. State Hort. Soc. 2007, 120, 310–315. [Google Scholar]
- Grohmann, K. Project Title: Citrus Waste Biomass Program; Final Technical Report; CRADA 58-3K95-4-1053; USDA Agricultural Research Service, 2007. Available online: www.osti.gov/bridge/servlets/purl/898345–9P4Slw/898345.pdf (accessed on 3 February 2016).
- Braddock, R.J.; Miller, W. Volatile organic compounds from citrus feed mill emissions. J. Food Process Eng. 2001, 24, 1–15. [Google Scholar]
- Buff, D. VOC emissions from citrus processing plants. Trans. Citrus Eng. Conf. 1997, 43, 65–79. [Google Scholar]
- Odio, C. D-limonene recovery. Citrus Eng. Conf. 1996, 42, 45–54. [Google Scholar]
- Rivas-Cantu, R.C.; Jones, K.D.; Mills, P.L. A citrus waste-based biorefinery as a source of renewable energy: Technical advances and analysis of engineering challenges. Waste Manag. Res. 2013, 31, 413–420. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Lee, Y.R. Extraction, characterization and biological activity of citrus flavonoids. Rev. Chem. Eng. 2018, 35, 265–284. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Baral, E.R.; Koteswararao, R.; Dhyani, A.; Cho, M.H.; Cho, S. Bio-sorbents, industrially important chemicals and novel materials from citrus processing waste as a sustainable and renewable Bioresource: A review. J. Adv. Res. 2020, 23, 61–82. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Nabybaccus, F.; Cho, M.H. Citrus waste reuse for health benefits and pharma-/neutraceutical applications. Era’s J. Med. Res. 2016, 3, 20–32. [Google Scholar]
- Mamma, D.; Christakopoulos, P. Biotechnological potential of citrus peels. In Industrial Microbiology: Microbes in Process; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2014; pp. 59–92. [Google Scholar]
- Fagbohungbe, M.O.; Herbert, B.M.J.; Hurst, L.; Li, H.; Usmani, S.Q.; Semple, K.T. Impact of biochar on the anaerobic digestion of citrus peel waste. Biores. Technol. 2016, 216, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Biores. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef]
- Su, H.; Tan, F.; Xu, Y. Enhancement of biogas and methanization of citrus waste via biodegradation pretreatment and subsequent optimized fermentation. Fuel 2016, 181, 843–851. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef]
- Anonymous. What a Life Cycle Assessment? National Renewable Energy Lab. Tech. Rpt., NREL/PD-510-31792; 2002. Available online: http://www.nrel.gov/docs/gen/fy02/31792.pdf (accessed on 14 January 2013).
- Gunaseelan, V.N. Biochemical methane potential of fruits and vegetable solid waste feedstocks. Biomass Bioenergy 2004, 26, 389–399. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Widmer, W.W.; Grohmann, K. Simultaneous saccharification and fermentation of citrus peel waste by Saccharomyces cerevisiae to produce ethanol. Process Biochem. 2007, 42, 1614–1619. [Google Scholar] [CrossRef]
- Pourbafrani, M.; Forgács, G.; Horváth, I.S.; Niklasson, C.; Taherzadeh, M.J. Production of biofuels, limonene and pectin from citrus wastes. Biores. Technol. 2010, 101, 4246–4250. [Google Scholar] [CrossRef]
- Lyons, T.R. Ethanol around the World: Rapid Growth in Policies, Technology and Production; Nottingham University Press: Nottingham, UK, 2004; pp. 1–8. [Google Scholar]
- Mazumdar, A.; Srivastava, R. Citrus Peel Gasification Using Molten Sodium Heat Pipes. In Proceedings of the Extended Abstract Prepared for Presentation at the AIChE 2004 Annual Meeting, Austin, TX, USA, 7–12 November 2004. [Google Scholar]
- Volpe, M.; Panno, D.; Volpe, R.; Messineo, A. Upgrade of citrus waste as a biofuel via slow pyrolysis. J. Anal. Appl. Pyrolysis 2015, 115, 66–76. [Google Scholar] [CrossRef]
- Volpe, R.; Messineo, S.; Volpe, M.; Messineo, A. Catalytic effect of char for tar cracking in pyrolysis of citrus wastes, design of a novel experimental set up and first results. Chem. Eng. Trans. 2016, 50, 181–186. [Google Scholar]
- Saha, N.; Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A.; Reza, M.T. Cationic dye adsorption on hydrochars of winery and citrus juice industries residues: Performance, mechanism, and thermodynamics. Energies 2020, 13, 4686. [Google Scholar] [CrossRef]
- Grohmann, K.; Baldwin, E.A.; Buslig, B.S. Production of ethanol from enzymatically hydrolyzed orange peel by the yeast Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 1994, 45/46, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, K.; Cameron, R.G.; Buslig, B.S. Fermentation of sugars in orange peel hydrolysates to ethanol by recombinant Escherichia coli K011. Appl. Biochem. Biotechnol. 1995, 51/52, 423–435. [Google Scholar] [CrossRef]
- Stewart, D.S.; Widmer, W.W.; Grohmann, K.; Wilkins, M.R. Ethanol Production from Citrus Processing Waste. U.S. Patent US20060177916A1, 10 August 2006. [Google Scholar]
- John, I.; Muthukumar, K.; Arunagiri, A. A review on the potential of citrus waste for d-limonene, pectin, and bioethanol production. Int. J. Green Energy. 2017, 14, 599–612. [Google Scholar] [CrossRef]
- Forgács, G. Biogas Production from Citrus Wastes and Chicken Feather: Pretreatment and Co-Digestion; Chalmers University of Technology: Göteborg, Sweden, 2012. [Google Scholar]
- Forgács, G.; Pourbafrani, M.; Niklasson, C.; Taherzadeh, M.J.; Hováth, I.S. Methane production from citrus wastes: Process development and cost estimation. J. Chem. Technol. Biotechnol. 2011, 87, 250–255. [Google Scholar] [CrossRef]
- Choi, I.S.; Kim, J.-H.; Wi, S.G.; Kim, K.H.; Bae, H.-J. Bioethanol production from mandarin (Citrus unshiu) peel waste using popping pretreatment. Appl. Energy 2013, 102, 204–210. [Google Scholar] [CrossRef]
- Ruiz, B.; Flotats, X. Citrus essential oils and their influence on the anaerobic digestion process: An overview. Waste Manag. 2014, 34, 2063–2079. [Google Scholar] [CrossRef]
- Ruiz, B.; de Benito, A.; Rivera, J.D.; Flotats, X. Assessment of different pre-treatment methods for the removal of limonene in citrus waste and their effect on methane potential and methane production rate. Waste Manag. Res. 2016, 34, 1249–1257. [Google Scholar] [CrossRef]
- Ruiz, B.; Flotats, X. Effect of limonene on batch anaerobic digestion of citrus peel waste. Biochem. Eng. J. 2016, 109, 9–18. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Rezzoug, S.-A.; Louka, N. Thermomechanical process intensification for oil extraction from orange peels. Innov. Food Sci. Emerg. Technol. 2009, 10, 530–536. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; López-López, L.I.; Rodríguez-Herrera, R.; Contreras-Esquivel, J.C.; Aguilar, C.N. Enzyme-assisted extraction of citrus essential oil. Chem. Pap. 2016, 70, 412–417. [Google Scholar] [CrossRef]
- Choi, I.S.; Lee, Y.G.; Khanal, S.K.; Park, B.J.; Bae, H.-J. A low-energy, cost-effective approach to fruit and citrus peel waste processing for bioethanol production. Appl. Energy 2015, 140, 65–74. [Google Scholar] [CrossRef]
- Akao, T.; Mizuki, E.; Saito, H.; Okumura, S. The methane fermentation of Citrus unshiu peel pretreated with fungus enzymes. Biores. Technol. 1992, 41, 35–39. [Google Scholar] [CrossRef]
- Srilatha, H.R.; Nand, K.; Babu, K.S.; Madhukara, K. Fungal pretreatment of orange processing waste by solid-state fermentation for improved production of methane. Process Biochem. 1995, 30, 327–331. [Google Scholar] [CrossRef]
- Bausbia, N.; Vian, M.A.; Ferhat, M.A.; Meklati, B.Y.; Chemat, F. A new process for extraction of essential oil from Citrus peels: Microwave hydrodiffusion and gravity. J. Food Eng. 2009, 90, 409–413. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Mizuki, E.; Akao, T.; Saruwatari, T. Inhibitory effect of Citrus unshiu peel on anaerobic-digestion. Biol. Wastes 1990, 33, 161–168. [Google Scholar] [CrossRef]
- Bowen, E. Potential By-Products from Microbial Transformation of D-Limonene; Florida State Horticultural Society: Alexandria, VA, USA, 1975; p. 304. [Google Scholar]
- Badee, A.; Helmy, S.; Morsy, N. Utilisation of orange peel in the production of α-terpineol by Penicillium digitatum (NRRL 1202). Food Chem. 2011, 126, 849–854. [Google Scholar] [CrossRef]
- Lane, A. Production of aromatic acids during anaerobic digestion of citrus peel. J. Chem. Technol. Biotechnol. 1980, 30, 345–350. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.; Pisano, B. In vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Sonboli, A.; Eftekhar, F.; Yousefzadi, M.; Kanani, M. Antibacterial activity and chemical composition of the essential oil of Grammosciadium platycarpum Boiss. from Iran. Zeitschrift für Naturforschung C 2005, 60, 30–34. [Google Scholar] [CrossRef]
- Pasqua, R.D.; Hoskins, N.; Betts, G. Changes in membrane fatty acids composition of microbial cells induced by addition of thymol, carvacrol, limonene, cinnamaldehyde, and eugenol in the growing media. J. Agric. Food Chem. 2006, 54, 2745–2749. [Google Scholar] [CrossRef]
- Kaparaju, P.; Rintala, J. Thermophilic anaerobic digestion of industrial orange waste. Environ. Technol. 2006, 27, 623–633. [Google Scholar] [CrossRef]
- Braddock, R.J.; Temelli, F.; Cadwallader, K.R. Citrus essential oils—A dossier for material safety data sheets. Food Technol. 1986, 40, 114–116. [Google Scholar]
- Anagnostopoulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- Chemat, F. Techniques for oil extraction. In Citrus Essential Oils: Flavor and Fragrance; Sawamura, M., Ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 9–36. [Google Scholar]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- de Castro, M.; Carmona, M.J.; Fernandez-Prez, V. Towards more rational techniques for the isolation of valuable essential oils from plants. Trends Anal. Chem. 1999, 18, 708–716. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Meklati, B.Y.; Chemat, F. Comparison of different isolation methods of essential oil from citrus fruits: Cold pressing, hydrodistillation and microwave ‘dry’ distillation. Flavour Frag. J. 2007, 22, 494–504. [Google Scholar] [CrossRef]
- Galanakis, G.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Gámez, S.; Ramírez, J.A.; Garrote, G.; Vázquez, M. Manufacture of fermentable sugar solutions from sugar cane bagasse hydrolyzed with Phosphoric acid at atmospheric pressure. J. Agric. Food Chem. 2004, 52, 4172–4177. [Google Scholar] [CrossRef]
- Demirbas, A. Products from Lignocellulosic materials via degradation processes. In Energy Sources, Part A: Recovery, Utilization, and Environmental Effects; Taylor & Francis: Abingdon, UK, 2007; Volume 30, pp. 27–37. [Google Scholar]
- Gullu, D. Effect of catalyst on yield of liquid products from biomass via pyrolysis. In Energy Sources; Taylor & Francis: Abingdon, UK, 2003; Volume 25, pp. 753–765. [Google Scholar]
- Dunlop, A.P. Furfural formation and behavior. Ind. Eng. Chem. 1948, 40, 204–209. [Google Scholar] [CrossRef]
- Grohmann, K.; Cameron, R.; Buslig, B. Fractionation and pretreatment of orange peel by dilute acid hydrolysis. Biores. Technol. 1995, 54, 129–141. [Google Scholar] [CrossRef]
- Boluda-Aguilar, M.; García-Vidal, L.; González-Castañeda, F.d.P.; López-Gómez, A. Mandarin peel wastes pretreatment with steam explosion for bioethanol production. Biores. Technol. 2010, 101, 3506–3513. [Google Scholar] [CrossRef]
- Boluda-Aguilar, M.; López-Gómez, A. Production of bioethanol by fermentation of lemon (Citrus limon L.) peel wastes pretreated with steam explosion. Ind. Crops Prod. 2013, 41, 188–197. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Madl, R.L.; Saida, L.; Abeykoon, J.P. Ethanol production from orange peels: Two-stage hydrolysis and fermentation studies using optimized parameters through experimental design. J. Agric. Food Chem. 2010, 58, 3422–3429. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Nanjundaswamy, A.; Bansal, S.; Singh, S.; Kaur, S.; Babbar, N. Enhanced ethanol production from Kinnow Mandarin (Citrus Reticulata) waste via a statistically optimized simultaneous saccharification and fermentation process. Biores. Technol. 2011, 102, 1593–1601. [Google Scholar] [CrossRef]
- Grohmann, K.; Baldwin, E.A. Fermentation of galacturonic acid and other sugars in orange peel hydrosylates by the ethanologenic strain of Escherichia coli. Biotechnol. Lett. 1994, 16, 281–286. [Google Scholar] [CrossRef]
- Widmer, W.; Zhou, W.; Grohmann, K. Pretreatment effects on orange processing waste for making ethanol by simultaneous saccharification and fermentation. Biores. Technol. 2010, 101, 5242–5249. [Google Scholar] [CrossRef]
- Widmer, W.W.; Narciso, J.A.; Grohmann, K.; Wilkins, M.R. Simultaneous saccharification and fermentation of orange processing waste to ethanol using Kluyveromyces marxianus. Biol. Eng. 2009, 2, 17–29. [Google Scholar] [CrossRef]
- Frison, A.; Memmert, K.; Pharma, A.N. Fed-batch process development for monoclonal antibody production with Cellferm-Pro. Genet. Eng. Biotechnol. News 2002, 22, 66–67. [Google Scholar]
- Çaylak, B.; Sukan, F.V. Comparison of different production processes for bioethanol. Turk. J. Chem. 1998, 22, 351–360. [Google Scholar]
- Lawford, H.G. A new approach to improving the performance of Zymomonas in continuous ethanol fermentations. Appl. Biochem. Biotechnol. 1988, 17, 203–219. [Google Scholar] [CrossRef]
- Parawira, W.; Murto, M.; Read, J.; Mattiasson, B. Profile of hydrolases and biogas production during two-stage mesophilic anaerobic digestion of solid potato waste. Process Biochem. 2005, 40, 2945–2952. [Google Scholar] [CrossRef]
- Weiland, P. Applied Microbiology and Biotechnology. Biogas Prod. Curr. State Perspect. 2010, 85, 849–860. [Google Scholar]
- Schnürer, A.; Nordberg, A. Ammonia, a selective agent for methane production by syntrophic acetate oxidation at mesophilic temperature. Water Sci. Technol. 2008, 57, 735–740. [Google Scholar] [CrossRef]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: Formation of Biogas; Wiley-VCH: Weinheim, Germany, 2008; pp. 87–147. [Google Scholar]
- Martín, M.A.; Siles, J.A.; Chica, A.F.; Martín, A. Biomethanization of orange peel waste. Biores. Technol. 2010, 101, 8993–8999. [Google Scholar] [CrossRef]
- Talebnia, F.; Pourbafrani, M.; Lundin, M.; Taherzadeh, M. Optimization study of citrus wastes saccharification by dilute-acid hydrolysis. Bioresources 2008, 3, 108–122. [Google Scholar]
- Rezzadori, K.; Benedetti, S.; Amante, E.R. Proposals for the residues recovery: Orange waste as raw material for new products. Food Bioprod. Process. 2012, 90, 606–614. [Google Scholar] [CrossRef]
- Marín, F.R.; Soler-Rivas, C.; Benavente-García, O.; Castillo, J.; Pérez-Alvarez, J.A. By-products from different citrus processes as a source of customized functional fibres. Food Chem. 2007, 100, 736–741. [Google Scholar] [CrossRef]
- Chun, J.A.; Lee, J.W.; Yi, Y.B.; Hong, S.S.; Chung, C.H. Catalytic production of hydroxymethylfurfural from sucrose using 1-methyl-3-octylimidazolium chloride ionic liquid. Korean J. Chem. Eng. 2010, 27, 930–935. [Google Scholar] [CrossRef]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An overview of biorefinery-derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Reports, M. Global 5-hydroxymethylfurfural (5-HMF) Market Report 2018. SKU ID: BIS-11466113. 2018, p. 123. Available online: https://www.marketreportsworld.com/-global-5-hydroxymethylfurfural-5-hmf-market-11466113 (accessed on 12 December 2020).
- Kamm, B. Biorefineries-Industrial Processes and Products: Status Quo and Future Directions; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; Volume 1, pp. 3–40. [Google Scholar]
- Lee, J.W.; Shin, J.Y.; Chun, Y.S.; Jang, H.B.; Song, C.E.; Lee, S.G. Toward understanding the origin of positive effects of ionic liquids on catalysis: Formation of more reactive catalysts and stabilization of reactive intermediates and transition states in ionic liquids. Acc. Chem. Res. 2010, 43, 985–994. [Google Scholar] [CrossRef]
- Yi, Y.B.; Ha, M.G.; Lee, J.W.; Park, S.M.; Choi, Y.H.; Chung, C.H. Direct conversion of citrus peel waste into hydroxymethylfurfural in ionic liquid by mediation of fluorinated metal catalysts. J. Ind. Eng. Chem. 2013, 19, 523–528. [Google Scholar] [CrossRef]
- Pourbafrani, M.; McKechnie, J.; MacLean, H.L.; Saville, B.A. Life cycle greenhouse gas impacts of ethanol, biomethane and limonene production from citrus waste. Environ. Res. Lett. 2013, 8, 015007. [Google Scholar] [CrossRef]
- Bull, O.; Obunwo, C. Bio-diesel production from oil of orange (Citrus sinensis) peels as feedstock. J. Appl. Sci. Environ. Manag. 2014, 18, 371–374. [Google Scholar]
- Ueno, H.; Tanaka, M.; Hosino, M.; Sasaki, M.; Gota, M. Extraction of valuable compounds from the flavedo of Citrus junos using subcritical water. Sep. Purif. Technol. 2008, 62, 513–516. [Google Scholar] [CrossRef]
- Bozell, J.J.; Moens, L.; Elliott, D.; Wang, Y. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; West, R.M.; Dumesic, A. Catalytic conversion of renewable biomass resources to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Stankovikj, F.; Mcdonald, A.G.; Helms, G.L.; Olarte, M.V.; Garciaperez, M. Characterization of woody biomass pyrolysis oils’ water soluble fraction. Energy Fuels 2017, 31, 1650–1664. [Google Scholar] [CrossRef]
- Bayerbach, R.; Meier, D. Characterization of the water-insoluble fraction from fast pyrolysis liquids (pyrolytic lignin). Part IV: Structure elucidation of oligomeric molecules. J. Anal. Appl. Pyrolysis 2009, 85, 98–107. [Google Scholar] [CrossRef]
- Patil, S.K.; Heltzel, J.; Lund, C.R. Comparison of structural features of humins formed catalytically from glucose, fructose, and 5-hydroxymethylfurfuraldehyde. Energy Fuels 2012, 26, 5281–5293. [Google Scholar] [CrossRef]
- Srivastava, R.; Mazumdar, A.; Conklin, W.; Chen, Z.; Philippidis, G. Development and Demonstration of a Pilot-Scale Biomass Gasification Unit for Hydrogen Production; Florida Solar Energy Center, NASA Hydrogen Research at Florida Universities: Cocoa, FL, USA, 2006. [Google Scholar]
- Muradov, N.; Smith, F. Local Hydrogen Production via Catalytic Reformation of Fossil and Renewable Feedstocks; Florida Solar Energy Center, Florida Universities Hydrogen Review: Cocoa, FL, USA, 2007. [Google Scholar]
- Wikandari, R.; Millati, R.; Cahyanto, M.N.; Taherzadeh, M.J. Biogas production from citrus waste by membrane bioreactor. Membranes 2014, 4, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Ylitervo, P. Production of Ethanol and Biomass from Orange Peel Waste by Mucor indicus. Master’s Thesis, University College of Borås, Borås, Sweden, 2008. [Google Scholar]
- Santos, C.M.; Dweck, J.; Viotto, R.S.; Rosa, A.H.; de Morais, L.C. Application of orange peel waste in the production of solid biofuels and biosorbents. Biores. Technol. 2015, 196, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Torrado, A.M.; Cortés, S.; Salgado, J.M.; Max, B.; Rodríguez, N.; Bibbins, B.P.; Converti, A.; Domínguez, J.M. Citric acid production from orange peel wastes by solid-state fermentation. Braz. J. Microbiol. 2011, 42, 394–409. [Google Scholar] [CrossRef] [PubMed]
- Aravantinos-Zafiris, G.; Tzia, C.; Oreopoulou, V.; Thomopoulos, C.D. Fermentation of orange processing wastes for citric acid production. J. Sci. Food Agric. 1994, 65, 117–120. [Google Scholar] [CrossRef]
- Kristiansen, B.; Sinclair, C.G. Production of citric acid in continuous culture. Biotechnol. Bioeng. 1979, 21, 297–315. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, X.; Feng, X.; Wang, J.; Sun, C.; Zhang, H.; Xian, M.; Liu, H. Recovery processes of organic acids from fermentation broths in the biomass-based industry. J. Microbiol. Biotechnol. 2016, 26, 1–8. [Google Scholar] [CrossRef]
- Soccol, C.R.; Vandenberghe, L.P.S.; Rodrigues, C.; Pandey, A. A new perspective for citric acid production and application. Food Technol. Biotechnol. 2006, 44, 141–149. [Google Scholar]
- Yi, M.; Pen, Q.; Chen, D.; Pen, L.; Zhang, M.; Wen, R.; Mou, X.; Wang, W. Extraction of citric acid by N,N-disubstituted alkyl amides from fermentation aqueous solution. Beiji Dax Xue 1987, 4, 30–37. [Google Scholar]
- Shishikura, A.; Takuhashi, H.; Hirohama, S.; Arai, K. Citric acid purification process using compressed carbon dioxide. J. Supercrit. Fluids 1992, 5, 303–312. [Google Scholar] [CrossRef]
- Pazouki, M.; Panda, T. Recovery of citric acid—A review. Bioprocess Eng. 1998, 19, 435–439. [Google Scholar] [CrossRef]
- Doores, S. Organic Acids, 2nd ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 95–136. [Google Scholar]
- Kagan, J.J.; Pilnik, W.; Smith, M.D. Citrus by-products, lactic acid production by fermentation of citrus peel juice. J. Agric. Food Chem. 1960, 8, 236–238. [Google Scholar] [CrossRef]
- Patsalou, M.; Menikea, K.K.; Makri, E.; Vasquez, M.I.; Drouza, C.; Koutinas, M. Development of a citrus peel-based biorefinery strategy for the production of succinic acid. J. Clean. Prod. 2017, 166, 706–716. [Google Scholar] [CrossRef]
- Li, Q.; Siles, J.A.; Thompson, I. Succinic acid production from orange peel and wheat straw by batch fermentations of Fibrobacter succinogenes S85. Appl. Microbiol. Biotechnol. 2010, 88, 671–678. [Google Scholar] [CrossRef]
- Locurto, R.; Tripodo, M.; Leuzzi, U.; Giuffre, D.; Vaccarino, C. Flavonoids recovery and SCP production from orange peel. Biores. Technol. 1992, 42, 83–87. [Google Scholar] [CrossRef]
- Vaccarino, C.; Locurto, R.; Tripodo, M.; Patane, R.; Lagana, G.; Ragno, A. SCP from orange peel by fermentation with fungi-acid-treated peel. Biol. Wastes 1989, 30, 1–10. [Google Scholar] [CrossRef]
- Moriguchi, M. Fermentative production of pyruvic acid from citrus peel extract by Debaryomyces coudertii. Agric. Biol. Chem. 1982, 46, 955–961. [Google Scholar] [CrossRef]
- Moriguchi, M.; Shuto, K.; Hashimoto, T. Production of pyruvic acid from saccharified citrus peel extract by dried cells of Debaryomyces coudertii. J. Ferment. Technol. 1984, 62, 243–248. [Google Scholar]
- Gaden, E.L.; Petsiavas, D.N.; Winoker, J. Citrus waste utilization: Microbiological production of riboflavin and citric acid from citrus molasses. Agric. Food Chem. 1954, 2, 632–638. [Google Scholar] [CrossRef]
- Contreras-Esquivel, J.C.; Voget, C.; Vita, C.; Espinoza-Perez, J.; Renard, C. Enzymatic extraction of lemon pectin by endo-polygalacturonase from Aspergillus niger. Food Sci. Biotechnol. 2006, 15, 163–167. [Google Scholar]
- Mandalari, G.; Bennett, R.; Kirby, A.; Curto, R.L.; Bisignano, G.; Waldron, K.; Faulds, G. Enzymatic hydrolysis of flavonoids and pectic oligosaccharides from Bergamot (Citrus bergamia Risso) peel. J. Agric. Food Chem. 2006, 54, 8307–8313. [Google Scholar] [CrossRef]
- Zykwinska, A.; Boiffard, M.H.; Kontkanen, H.; Buchert, J.; Thibault, J.F.; Bonnin, E. Extraction of green labeled pectins and pectic oligosaccharides from plant byproducts. J. Agric. Food Chem. 2008, 56, 8926–8935. [Google Scholar] [CrossRef]
- Lim, J.; Yoo, J.; Ko, S.; Lee, S. Extraction and characterization of pectin from Yuza (Citrus junos) pomace: A comparison of conventional-chemical and combined physical-enzymatic extractions. Food Hydrocoll. 2012, 29, 160–165. [Google Scholar] [CrossRef]
- Panchev, I.; Kirchev, N.; Kratchanov, C. Improving pectin technology. Int. J. Food Sci. Technol. 1988, 23, 337–341. [Google Scholar] [CrossRef]
- Oosterveld, A.; Beldman, G.; Schols, H.; Voragen, A. Characterization of arabinose and ferulic acid rich pectic polysaccharides and hemicelluloses from sugar beet pulp. Carbohydr. Res. 2000, 328, 185–197. [Google Scholar] [CrossRef]
- Fishman, M.L.; Chau, H.; Hoagland, P.; Ayyad, K. Characterization of pectin, flash-extracted from orange albedo by microwave heating, under pressure. Carbohydr. Res. 2000, 323, 126–138. [Google Scholar] [CrossRef]
- Fishman, M.L.; Walker, P.; Chau, H.; Hotchkis, A. Flash extraction of pectin from orange albedo by steam injection. Biomacromolecules 2003, 4, 880–889. [Google Scholar] [CrossRef]
- Kratchanova, M.; Pavlovaa, E.; Panchev, I. The effect of microwave heating of fresh orange peels on the fruit tissue and quality of extracted pectin. Carbohydr. Polym. 2004, 56, 181–185. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.; Langrish, T. Water-based extraction of pectin from flavedo and albedo of orange peels. Chem. Eng. J. 2006, 120, 203–209. [Google Scholar] [CrossRef]
- Scerra, V.; Caridi, A.; Foti, F.; Sinatra, M. Influence of dairy Penicillium spp. on nutrient content of citrus fruit peel. Anim. Feed Sci. Technol. 1999, 78, 169–176. [Google Scholar] [CrossRef]
- Scerra, V.; Caridi, A.; Foti, F.; Sinatra, M.; Caparra, P. Changes in chemical composition during the colonisation of citrus pulps by a dairy Penicillium roqueforti strain. Biores. Technol. 2000, 72, 197–198. [Google Scholar] [CrossRef]
- Gregorio, A.D.; Mandalari, G.; Arena, N.; Nucita, F.; Tripodo, M.; Curto, R.L. SCP and crude pectinase production by slurry-state fermentation of lemon pulps. Biores. Technol. 2002, 83, 89–94. [Google Scholar] [CrossRef]
- Mandalari, G.; Palop, C.N.; Tuohy, K.; Gibson, G.; Bennett, R.; Waldron, K.; Bisignano, G.; Narbad, A.; Faulds, C. In vitro evaluation of the prebiotic activity of a pectic oligosaccharide-rich extract enzymatically derived from bergamot peel. Appl. Microbiol. Biotechnol. 2007, 73, 1173–1179. [Google Scholar] [CrossRef]
- Olano-Martín, E.; Mountzouris, K.; Gibson, G.; Rastall, R. Continuous production of oligosaccharides from pectin in an enzyme membrane reactor. J. Food Sci. 2001, 66, 966–971. [Google Scholar] [CrossRef]
- Sabajanes, M.M.N.; Yáñez, R.; Alonso, J.; Parajo, J. Pectic oligosaccharides production from orange peel waste by enzymatic hydrolysis. Int. J. Food Sci. Technol. 2012, 47, 747–754. [Google Scholar] [CrossRef]
- Hotchkiss, A.; Olano-Martín, E.; Grace, W.; Williams, M.; Gibson, G.; Rastall, R. Pectic oligosaccharides as prebiotics. In Oligosaccharides in Food and Agriculture; ACS Symposium Series 849; ACS: Washington, DC, USA, 2003; pp. 54–62. [Google Scholar]
- Martínez, M.M.; Yáñez, R.; Alonso, J.; Parajo, J. Chemical production of pectic oligosaccharides from orange peel wastes. Ind. Eng. Chem. Res. 2010, 49, 8470–8476. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Production and characterization of bacterial cellulose from citrus peels. Waste Biomass Valorization 2019, 10, 2165–2175. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, C.-Y.; Shieh, C.-J.; Wang, H.-M.D.; Tseng, C.-Y. Hydrolysis of orange peel with cellulase and pectinase to produce bacterial cellulose using Gluconacetobacter xylinus. Waste Biomass Valorization 2019, 10, 85–93. [Google Scholar] [CrossRef]
- Akardere, E.; Ozer, B.; Celem, E.B.; Onal, S. Three-phase partitioning of invertase from baker’s yeast. Sep. Purif. Technol. 2010, 72, 335–339. [Google Scholar] [CrossRef]
- Ozer, B.; Akardere, E.; Celem, E.B.; Onal, S. Three-phasepartitioning as a rapid and efficient method for purification ofinvertase from tomato. Biochem. Eng. J. 2010, 50, 110–115. [Google Scholar] [CrossRef]
- Chaiwuta, P.; Pintathonga, P.; Rawdkuen, S. Extraction and three-phase partitioning behavior of proteases from papaya peels. Process Biochem. 2010, 45, 1172–1175. [Google Scholar] [CrossRef]
- Vetal, M.D.; Rathod, V.K. Three phase partitioning a novel technique for purification of peroxidase from orange peels (Citrus sinenses). Food Bioprod. Process. 2015, 94, 284–289. [Google Scholar] [CrossRef]
- Jayani, R.; Saxena, S.; Gupta, R. Microbialpectinolytic enzymes: A review. Process Biochem. 2005, 40, 2931–2944. [Google Scholar] [CrossRef]
- Castilho, L.R.; Alves, T.L.M.; Medronho, R.A. Recovery of pectinolytic enzymes produced by solid state culture of Aspergillus niger. Process Biochem. 1999, 34, 181–186. [Google Scholar] [CrossRef]
- Hang, Y.D.; Woodanms, E.E. Production of fungal polygalacturonase from apple pomace. Lebensmittel-Wissenschaft und-Technologie 1994, 27, 194–196. [Google Scholar] [CrossRef]
- Zheng, Z.; Shetty, K. Solid state production of polygalacturonase by Lentinus edodes using fruit processing wastes. Process Biochem. 2000, 35, 825–830. [Google Scholar] [CrossRef]
- Schwan, R.F.; Cooper, R.M.; Wheals, A.E. Endopolygalacturonase secretion by Kluyveromyces marxianus and other cocoa pulp-degrading yeasts. Enzym. Microb. Technol. 1997, 21, 234–244. [Google Scholar] [CrossRef]
- Fonseca, M.J.V.; Said, S. The pectinase produced by Tubercularia vulgarisin submerged culture using pectin or orange-pulp pellets as inducer. Appl. Microbiol. Biotechnol. 1994, 42, 32–35. [Google Scholar] [CrossRef]
- Acuna-Arguelles, M.E.; Gutiérrez-Rojas, M.; Viniegra-Gonzáles, G.; Favela-Torres, E. Effect of water activity on exo-pectinase production by Aspergillus niger CH4 on solid state fermentation. Biotechnol. Lett. 1994, 16, 23–28. [Google Scholar] [CrossRef]
- Perez-Guerra, N.; Torrado-Agrassar, A.; Lopez-Macias, C.; Pastrana, L. Main characteristics and applications of solid state fermentation. Electron. J. Environ. Agric. Food Chem. 2003, 2, 343–350. [Google Scholar]
- Ahmed, I.; Zia, M.A.; Hussain, M.A.; Akram, Z.; Naveed, M.T.; Nowrouzi, A. Bioprocessing of citrus waste peel for inducedpectinase production by Aspergillus niger; itspurification and characterization. Electron. J. Environ. Agric. Food Chem. 2016, 9, 148–154. [Google Scholar]
- Mamma, D.; Kourtoglou, E.; Christakopoulos, P. Fungal multienzyme production on industrial by-products of the citrus-processing industry. Biores. Technol. 2008, 99, 2373–2383. [Google Scholar] [CrossRef]
- Ismail, A. Utilization of orange peels for the production of multienzyme complexes by some fungal Strains. Process Biochem. 1996, 31, 645–650. [Google Scholar] [CrossRef]
- Yalemtesfa, B.; Alemu, T.; Santhanam, A. Solid substrate fermentation and conversion of orange waste in to fungal biomass using Aspergillus niger KA-06 and Chaetomium spp KC-06. Afr. J. Microbiol. Res. 2010, 4, 1275–1281. [Google Scholar]
- Inácio, F.; Ferreira, R.; Araujo, C.; Peralta, R.; de Souza, C. Production of Enzymes and Biotransformation of Orange Waste by Oyster Mushroom, Pleurotus pulmonarius (Fr.) Quél. Adv. Microbiol. 2015, 5, 1–8. [Google Scholar]
- Hart, H.; Parish, M.; Burns, J.; Wicker, L. Orange finisher pulp as substrate for polygalacturonase production by Rhizopus oryzae. J. Food Sci. Technol. 1991, 56, 480–483. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K.; Taherzadeh, M.; Zamani, A. Co-production of fungal biomass derived constituents and ethanol from citrus wastes free sugars without auxiliary nutrients in airlift bioreactor. Int. J. Mol. Sci. 2016, 17, 302. [Google Scholar] [CrossRef]
- Pedrolli, D.; Gomes, E.; Monti, R.; Carmona, E. Studies on productivity and characterization of polygalacturonase from Aspergillus giganteus submerged culture using citrus pectin and orange waste. Appl. Biochem. Biotechnol. 2008, 144, 191–200. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tian, M.; Wang, K.; Pan, S. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataei bacterxylinus. Carbohydr. Polym. 2016, 151, 1068–1072. [Google Scholar] [CrossRef]
- Patsalou, M.; Samanides, C.G.; Protopapa, E.; Stavrinou, S.; Vyrides, I.; Koutinas, M. A citrus peel waste biorefinery for ethanol and methane production. Molecules 2019, 24, 2451. [Google Scholar] [CrossRef]
- Limonene Market Size and Forecast, By Application (Food & Beverage, Pharmaceutical, Personal Care, Industrial) and Trend Analysis, 2015–2025; Bulk Chemicals: Pune, India, 2019; p. 65, ID: 4760086 February 2019, pp. 65 (Electronic Report); Available online: https://www.researchandmarkets.com/reports/4760086/limonene-market-size-and-forecast-by-application (accessed on 14 December 2020).
- Global Lactic Acid Market Size Worth around USD 1156.5 Million by 2026, from USD 997.2 Million in 2020, at a CAGR of 2.5% during 2020–2026 with Top Countries Data. November 2020. (Electronic Report). Available online: https://www.prnewswire.com/in/news-releases/lactic-acid-market-size-usd-1156-5-million-by-2026-at-a-cagr-2-5-valuates-reports-818131313.html (accessed on 14 December 2020).
- Pectin Market by Type (HM Pectin, LM Pectin), Raw Material (Citrus Fruits, Apples, Sugar Beet), Function, Application (Food & Beverages, Pharmaceutical & Personal Care Products, Industrial Applications), and Region—Global Forecast to 2025. Report Code FB 7357. 2019, p. 181, (Electronic Report). Available online: https://www.marketsandmarkets.com/Market-Reports/pectin-market-139129149.html (accessed on 14 December 2020).
- Succinic Acid Market Analysis by Application (1,4 BDO, Resins, Coatings, Dyes & Inks, Pharmaceuticals, Polyurethanes, Food, Plasticizers, Cosmetics, Solvents & Lubricants, De-Icing Solutions) and Segment Forecasts to 2022. 2016, p. 93, (Electronic Report). Available online: https://www.prnewswire.com/news-releases/succinic-acid-market-for-14-bdo-resin-coatings-dyes--inks-pharmaceutical-polyurethane-food-plasticizers-cosmetics-solvents--lubricants-and-de-icing-solutions-applications---global-industry-analysis-size-share-growt-224449951.html (accessed on 14 December 2020).
- Prebiotic Ingredients Market—Forecast (2021–2026). 2019. (Electronic Report). Available online: https://www.industryarc.com/Report/7481/prebiotics-ingredients-market.html (accessed on 14 December 2020).
- Enzymes Market Size Worth $17.2 Billion By 2027 | CAGR: 7.1%. 2020. (Electronic Report). Available online: https://www.prnewswire.com/news-releases/enzymes-market-size-worth-17-2-billion-by-2027--cagr-7-1-grand-view-research-inc-301024575.html (accessed on 14 December 2020).
- Enzymes Market Type (Protease, Carbohydrase, Lipase, Polymerase and Nuclease, and Other Types), Source (Microorganisms, Plants, and Animals), Reaction Type (Hydrolase, Oxidoreductase, Transferase, Lyase, and Other Reaction Types), and Application (Food and Beverages, Household Care, Bioenergy, Pharmaceutical and Biotechnology, Feed, and Other Applications)—Global Opportunity Analysis and Industry Forecast, 2017–2024. 2018, p. 344, (Electronic Report). Available online: https://www.alliedmarketresearch.com/enzymes-market (accessed on 14 December 2020).
- Citric Acid Market by Form (Anhydrous and Liquid), Application (Food, Pharmaceuticals, and Cosmetics), Function (Acidulant, Antioxidant, Preservative, and Sequestrant), and by Region (North America, Europe, Asia-Pacific, and RoW)—Global Forecast to 2020. 2015. (Electronic Report). Available online: https://www.marketsandmarkets.com/Market-Reports/citric-acid-market-185568353.html (accessed on 14 December 2020).
- Protein Extracts from Single Cell Protein Sources Market by Source (Algae, Yeast, Bacteria, Fungi), by Application (Animal Feed, Biotechnology, Agriculture & Fertilizers), by Geography (U.S., Canada, China, India, Japan, Germany, U.K., France, Italy, Spain, Brazil, Mexico, South Africa)—Global Market Size, Share, Development, Growth and Demand Forecast, 2013–2023. 2018, p. 127, (Electronic Report). Available online: https://www.psmarketresearch.com/market-analysis/protein-extracts-from-single-cell-protein-sources-market (accessed on 14 December 2020).
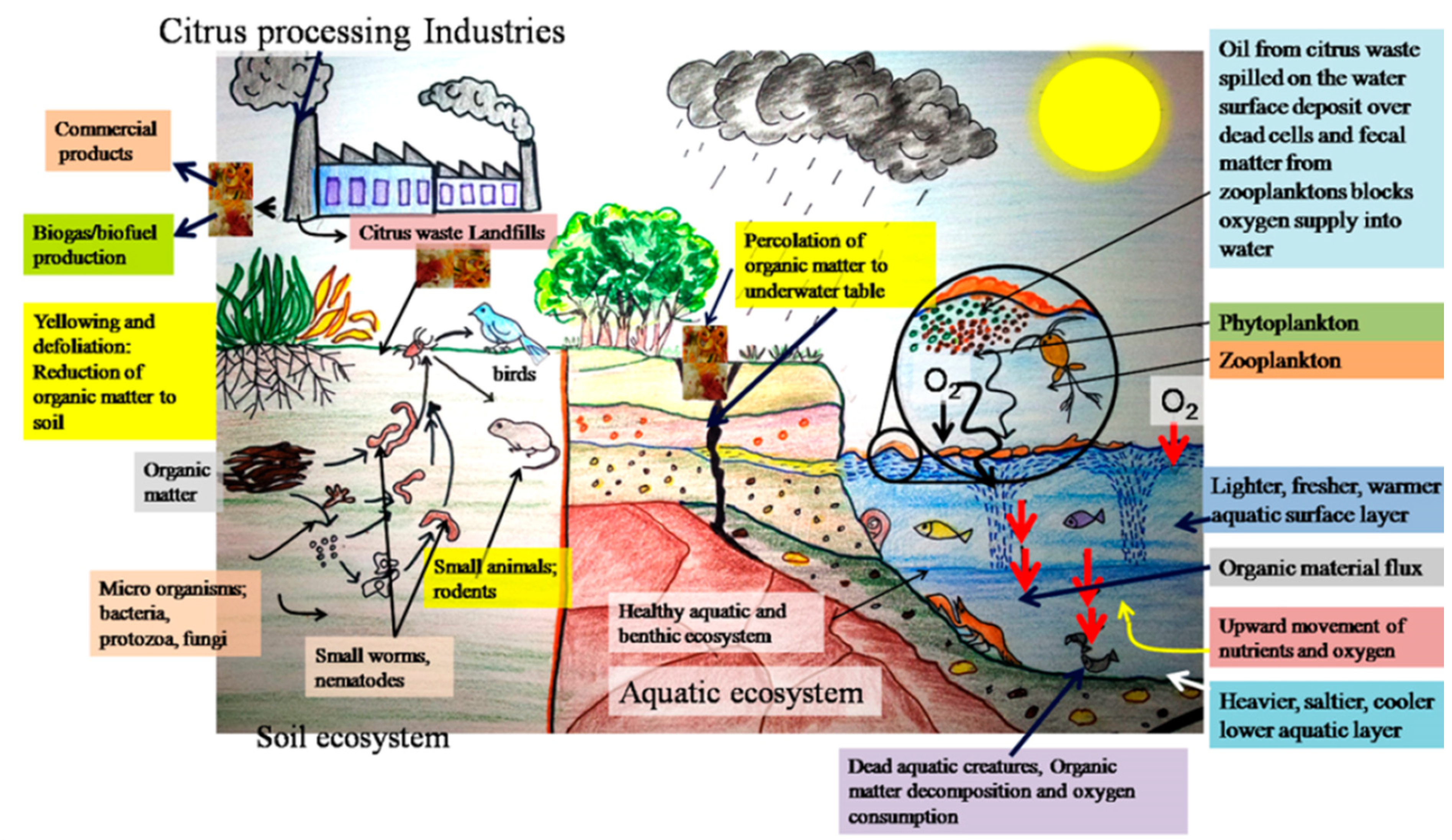








| Citrus Part | Procedure | Product and Yield | Application | Ref. |
|---|---|---|---|---|
| Citrus Peel and pulp | Peel and pulp is pulverized and pelletized; pyrolysis and gasification at 550–600 °C in molten sodium heat pipe furnace | Synthesis gas (H2+CO), CO2; biochar; H2 yield ~51% | Hydrogen for clean energy | [98] |
| Citrus waste | Pyrolysis, gassification and catalytic reforming | H2; biochar; H2 yield 0.55 l/g of citrus pulp pellets | H2 and clean carbon | [99] |
| Orange peels | Oil extraction in n-hexane using soxhelet; Drying at 60 °C, 1 h; Transesterification, 80–83 °C with ethanol, NaOH, 2 h; Settle for 48 h; Separation; | Biodiesel; fatty esters; 93% yield; in accordance with ASTM standards | Fuel in diesel engines | [91] |
| Mandarin peels (Citrus unshiu) | Grinding and lyophilisation at −50 °C; popping treatment, 150 °C, 10 min, 15 Kgf/cm pressure; enzymatic hydrolysis (cellulase, pectinase, xylanase, glucosidase); 50 mM sodium acetate, pH 4.8, 45 °C, 6 h; vacuum evaporation (condensation 1–10%); yeast fermentation, 30 °C, pH = 5 (Saccharomyces cerevisae) KCTC 7906; ssimultaneous saccharification and fermentation | Bioethanol; production: 46.2% (w/v) 90% yield; greater yield in shorter time (conventional methods take 12 h in enzymatic hydrolysis and 24–48 h during fermentation); low d -limonene; low cost | Fuel and industrial solvent | [34] |
| Mandarin peels | Peel waste is ground; sSteam explosion treatment—5 min, 160 °C, 6 Bar (removal of d-limonene); enzymatic hydrolysis, 24 h, 45 °C; yeast fermentation (Saccharomyces cerevisae CECT 1329), 37 °C, 4 days; ssimultaneous saccharification and fermentation | Bioethanol; production: 59.3% (w/v) d-limonene (90% recovery), galacturonic acid, citrus peel pulp; ethanol content of 50–60 L/1000 kg raw peel waste; steam explosion method reduces enzymatic dose requirement | Biofuel and industrial solvent/ chemical; | [66] |
| Orange peel (Citrus sinensis) | Dry at 40 °C; Steam treatment, 160 °C, 7 bar, 3–7 min; decompression at 50 mBar; oil extraction by thermochemical technique | d-limonene; 99% peel oil extraction | Industrial solvent for making soaps, perfumes, toiletries, etc. | [39] |
| Citrus waste | Ground and made slurry with H2O; steam explosion, 150 °C, 20 min, 60 Bar; batch anaerobic digestion, 55 °C, microbe inoculum, 80% N2 + 20% CO2; shaking, 1–21 days | Methane; 107.4 m3 methane and 8.4 L d-limonene per ton of citrus waste; steam explosion extracts d-limonene by 94.3% | Biogas; | [32] |
| Citrus Waste | Thawing; grinding and slurry making in H2O; digestion by methane producing bacteria in a membrane bioreactor, 55 °C, 30 days; organic biomass loading rate = 0.3–3.0 kg volatile solid/m3/day | Methane; 73% methane production; methane production of 0.33 Nm3/kg vs. compared with 0.05 Nm3/kg vs. in traditional method | Biogas; | [100] |
| Orange peel | Dilute acid hydrolysis−0.5% w/v H2SO4, 150 °C; enzymatic hydrolysis; (cellulase, pectinase, β-glucosidase); fermentation (Mucor indicus) | Ethanol, glycerol; EtOH yield = 0.36 g per gram peel waste after 24 h; glycerol yield = 0.048 g/g in 24 h; chitosan recovery | Fuel and industrial solvent; | [101] |
| Citrus waste | Dilute acid hydrolysis; Explosive drainage, 150 °C, 6 min; Flashing; Anaerobic Digestion | Ethanol, pectin, d-limonene, biogas | Biofuel, biogas, | [22] |
| Orange peels (Citrus sinensis) | Biological treatment (Penicillium digitatum, Penicillium italicum); steam distillation, 100 °C, 1 atm., 60–180 min; ethanol extraction (70% ethanol), 60 min biochemical methane production post-treatments (1–3); 20–40 days, organic biomass loading rate 3 Kg volatile solid m−3d−1 | Limonene, methane; yield per ton of citrus waste with 20% dry weight ethanol-39.64 L; methane-45 m3; limonene- 8.9 L; pectin-38.8 Kg, 1. 22% 2. 44%; (BMP-36%, MPR-74%) 3. 100%; (BMP-34%, MPR-74%) | Biofuel and industrial chemical; limonene extraction; | [36] |
| Citrus Peel Waste | In-house enzyme production—Aspergillus citrisporus (KCCM11449); Trichoderma longibrachiatum (KCTC6507); limonene removal column (LRC) continuous immobilization yeast fermentation-immobilized reactor cell (ICR)—Saccharomyces cerevisiae KCTC7906 | Ethanol, limonene; yield-93% LRC-ICR give 12 fold greater ethanol than ICR fermentation alone | Biofuel, industrial solvent/ chemical; | [41] |
| Orange Peel | Drying, 110 °C, 3d; pyrolysis, 1 Bar, inert atmosphere, 450 °C | Biochar cum biosorbent; Biosorption from aqueous solution in the following order→ Pb > Cu > Ni > Cd > Zn > Al; Biochar Calorific value- 10.9–19.3 MJ/Kg | Solid fuel; biosorbent; | [102] |
| Orange peel (Citrus sinensis cv Valencia) | Milling; enzymes: pectinase (Pectinex Ultra SP), cellulase (Celluclast 1.5 L), glucosidase (Novozym 188); sodium acetate buffer with pH 4.8 hydrolysis and fermentation; microorganism (Saccharomyces cerevisiae | Enzymatic hydrolysis doubles the yield of total reducing sugars. Sugars fermentable by S. cerevisiae increases by 25–35%. Ethanol yield −4.7% w/v hydrolysis and fermentation produces 55–60 galons of ethanol per ton of dry peel waste compared with 40–50 galons of ethanol per ton of peels from fermentation alone | Ethanol | [28,41] |
| Orange peel | Milling; enzymes (pectinase, cellulase, glucosidase); hydrolysis and fermentation; microorganism (Escherichia coli KO11) | Fermentation by recombinant E coli KO11 increases the ethanol yield by 25–35% compared with fermentation by yeast; ethanol yield-2.76% w/v | Ethanol; small amounts of acetic acid and traces of lactic and formic acid | [28,41,70] |
| Orange peel | Steam explosion; enzymes: pectinase (Pectinex Ultra SP), cellulase (Celluclast 1.5 L), glucosidase (Novozym188); simultaneous saccharification and fermentation; microorganism (S. cerevisiae); yeast concentration of 0.7 g cells/100 g CPW; Temperature 37 °C; pH 6.0 | Ethanol yield-3.96% w/v; ethanol production were reduced at pectinase loadings less than 25 IU/g peel dry matter, cellulase loadings less than 0.02 FPU/g peel dry matter, and beta-glucosidase loadings less than 13 IU/g peel dry matter; steam explosion removes 90% of d-limonene | Ethanol, d-limonene | [21,41] |
| Orange peel | Steam explosion (155 °C under 410 to 550 kPa, 2 min); enzymes (pectinase, cellulase, glucosidase); enzyme loading of 1 mL solution in water containing 60 IU pectinase, 0.035 FPU cellulase, and 0.81 IU beta-glucosidase g−1 dry CPW; simultaneous saccharification and fermentation; microorganism: (Kluyveromyces marxianus)-thermotolerant yeast (loading—1.26 ± 0.27 mg cells g-1 peel waste); temperature—40 °C; pH—4.8; 48 h | Ethanol yield-3.45% w/v (86%) high inoculum density increases the ethanol yield from 45% to 71% at 37 °C in 24 h and 86% in 48 h; Kluyveromyces marxianus is thermotolerant and can ferment at an elevated temperatures of 42 °C–45 °C | Ethanol, d-limonene | [41,72] |
| Orange peel | Acidic steam explosion (160 °C for more than 4 min); acid treatment (pH 2.8); enzymes (1 mL per 100 g CPW): pectinase (Rapidase PNS), cellulase (Celluclast 1.5 L), glucosidase (Novozym 188); simultaneous saccharification and fermentation; microorganism (S. cerevisiae); addition of calcium carbonate to the SSF mixture (0.75 g for acid and untreated CPW, 0.3 g for CPW at initial 6.8 pH, no carbonate for CPW at initial 8.2 pH), 1 mL of antibiotics per 100 g CPW (5 mg/mL each of Neomycin and Streptomycin in water) | Ethanol-2.7% w/v (76% to 94%); ethanol yields were observed to be not affected after SSF using either acid or base adjustment to CPW prior to pretreatment at 160 °C for limonene removal | Ethanol, d-limonene | [41,71] |
| Lemon peel | Steam-explosion; enzymes (pectinase, cellulase, glucosidase); simultaneous saccharification and fermentation; microorganism (S. cerevisiae) | Ethanol- 67.8% w/v; ethanol production of 60 L/1000 kg fresh lemon peel biomass; the pre-treatment reduces the residual content of essential oils below 0.025% and significantly decreases the hydrolytic enzyme requirements | Ethanol, lemon EOS, d-limonene, galacturonic acid; citrus pulp pellets | [41,67] |
| Enzyme (Name and Type) | Function/Applications | Citrus Waste Part Utilized | Microbe Strain Applied; Reaction/Process Parameters | Ref. |
|---|---|---|---|---|
| Pectinase, Cellulase, Xylanase, Amylase and Lipase | In the extraction of the major components (starch and lipids) of plant materials | Orange (Citrus sinensis var. Balady) peels | Aspergillus niger A-20; pH 4.0–5.0; Temperature 45–50 °C | [152,154,155] |
| Polygalacturonase, Pectate, Lyase, Xylanase, Invertase | Degrade orange waste to various useful products | Dry citrus peels | Aspergillus niger BTL, pH 5.0; Temperature 49 ± 1 °C | [153] |
| Pectinase, Cellulase | Production of sugars, cellulose, hemicellulose and pectin from citrate waste | Orange peel and pulp | Chaetomium spp. (KC-06) pH 5.0–7.0; Temperature 25 °C | [155] |
| Hydrolytic (Pectinase), Oxidative (Laccase) enzymes | Bioconversion of lignocellulose | Orange (Citrus sinensis (L.) Osb.) pulp, including membrane tissueand peel | Pleurotus pulmonarius Temperature-28 °C in the dark | [156] |
| Endoglucanase, xylanase, invertase | Production of sugar | Dry orange peels | Fusarium oxysporum F3 pH 6.0; Temperature 49 ± 1 °C | [153] |
| Endoglucanase | Degradation of cellulose | Dry orange peels | Neurospora crassa DSM 1129 pH 5.0; Temperature 49 ± 1 °C | [153] |
| Endoglucanase, invertase | Production of sugar | Dry orange peels | Penicillium decumbens pH 5.0; Temperature 49 ± 1 °C | [153] |
| Polygalacturonase | Liquefaction and solubilization of uranic acid and decrease in pectin content | Orange finisher pulp | Rhizopus oryzae pH 5.0 | [157] |
| Cellulase, Pectinase | Extraction of free sugars from citrus waste | Citrus waste | Mucor indicus pH 5.5; Temperature 30 °C | [158] |
| Cellulase, Pectinase | Extraction of free sugars from citrus waste | Citrus waste | Rhizopus oryzae pH 5.5; Temperature 35 °C | [158] |
| Polygalacturonase, Pectin lyase | Pectin degradation | Orange bagasse | Aspergillus giganteus pH 3.5; Temperature 30 °C | [159] |
| Cellulase | Bacterial cellulose production | Citrus peel and pomace | Komagataeibacter xylinus pH 3.5; Temperature 30 °C | [160] |
| Pectin lyase | Pectin degradation | Orange bagasse | Aspergillus giganteus pH 3.5; Temperature 30 °C | [159] |
| By-Products | Application | Global Market Size * | Ref. |
|---|---|---|---|
| Limonene | Solvent, domestic household products, feedstock for new chemicals, cosmetics, pharmaceutical, food and beverage, personal care | 323.2 million USD in 2020 379.2 million USD in 2026 | [162] |
| Enzyme | Extraction of fruit juices, degumming of plant fibers, waste water treatment, vegetable oil extraction, fermentation of tea and coffee, bleaching of paper, | 7.1 billion USD in 2017 17.2 billion USD by 2027 | [167,168] |
| Citric acid | Medicinal citrates, confectionary, soft drinks, effervescent salts, silvering and engravings, dying and calico printing | 2.5 billion USD in 2016 3.6 billion USD by 2020 | [169] |
| Succinic acid | polymers, polyesters, polyols, polybutylene, surfactants, solvents, detergents, flavors, fragrances, succinate-terephthalate resins, pharmaceutics | 181.6 million USD in 2019 237.8 million USD in 2022 | [165] |
| Lactic acid | Flavoring agent, pH regulator, and preservative, cosmetic and food processing | 997.2 million USD in 2020 1156.5 million USD by 2026 | [163] |
| Single cell protein | Nutritional supplements, animal feed, food and beverage, pharmaceutical and biotechnology, cosmetic, and agriculture | 5.3 billion USD in 2017 8.7 billion USD by 2023. | [170] |
| Prebiotic oligosaccharides | Healthy drinks, snack bars, bakery and confectionery, dietary supplements | 4.0 billion USD in 2017 7.2 billion USD by 2023 | [166] |
| Pectin | Food and beverages (gelling agents, thickener, stabilizer, fat replacer, jams, jellies, dairy products, beverages, bakery and confectionery) | 1.0 billion USD in 2019 1.5 billion USD by 2025 | [164] |
| 5-hydroxy- methylfurfural (5-HMF) | Building block for new molecules for packaging, construction, textile, cosmetics, formaldehyde replacement in resins | 145 million USD in 2022 120 million USD in 2017 | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahato, N.; Sharma, K.; Sinha, M.; Dhyani, A.; Pathak, B.; Jang, H.; Park, S.; Pashikanti, S.; Cho, S. Biotransformation of Citrus Waste-I: Production of Biofuel and Valuable Compounds by Fermentation. Processes 2021, 9, 220. https://doi.org/10.3390/pr9020220
Mahato N, Sharma K, Sinha M, Dhyani A, Pathak B, Jang H, Park S, Pashikanti S, Cho S. Biotransformation of Citrus Waste-I: Production of Biofuel and Valuable Compounds by Fermentation. Processes. 2021; 9(2):220. https://doi.org/10.3390/pr9020220
Chicago/Turabian StyleMahato, Neelima, Kavita Sharma, Mukty Sinha, Archana Dhyani, Brajesh Pathak, Hyeji Jang, Seorin Park, Srinath Pashikanti, and Sunghun Cho. 2021. "Biotransformation of Citrus Waste-I: Production of Biofuel and Valuable Compounds by Fermentation" Processes 9, no. 2: 220. https://doi.org/10.3390/pr9020220
APA StyleMahato, N., Sharma, K., Sinha, M., Dhyani, A., Pathak, B., Jang, H., Park, S., Pashikanti, S., & Cho, S. (2021). Biotransformation of Citrus Waste-I: Production of Biofuel and Valuable Compounds by Fermentation. Processes, 9(2), 220. https://doi.org/10.3390/pr9020220










