Wear Behavior Characterization of Hydrogels Constructs for Cartilage Tissue Replacement
Abstract
:1. Introduction
2. Materials and Methods
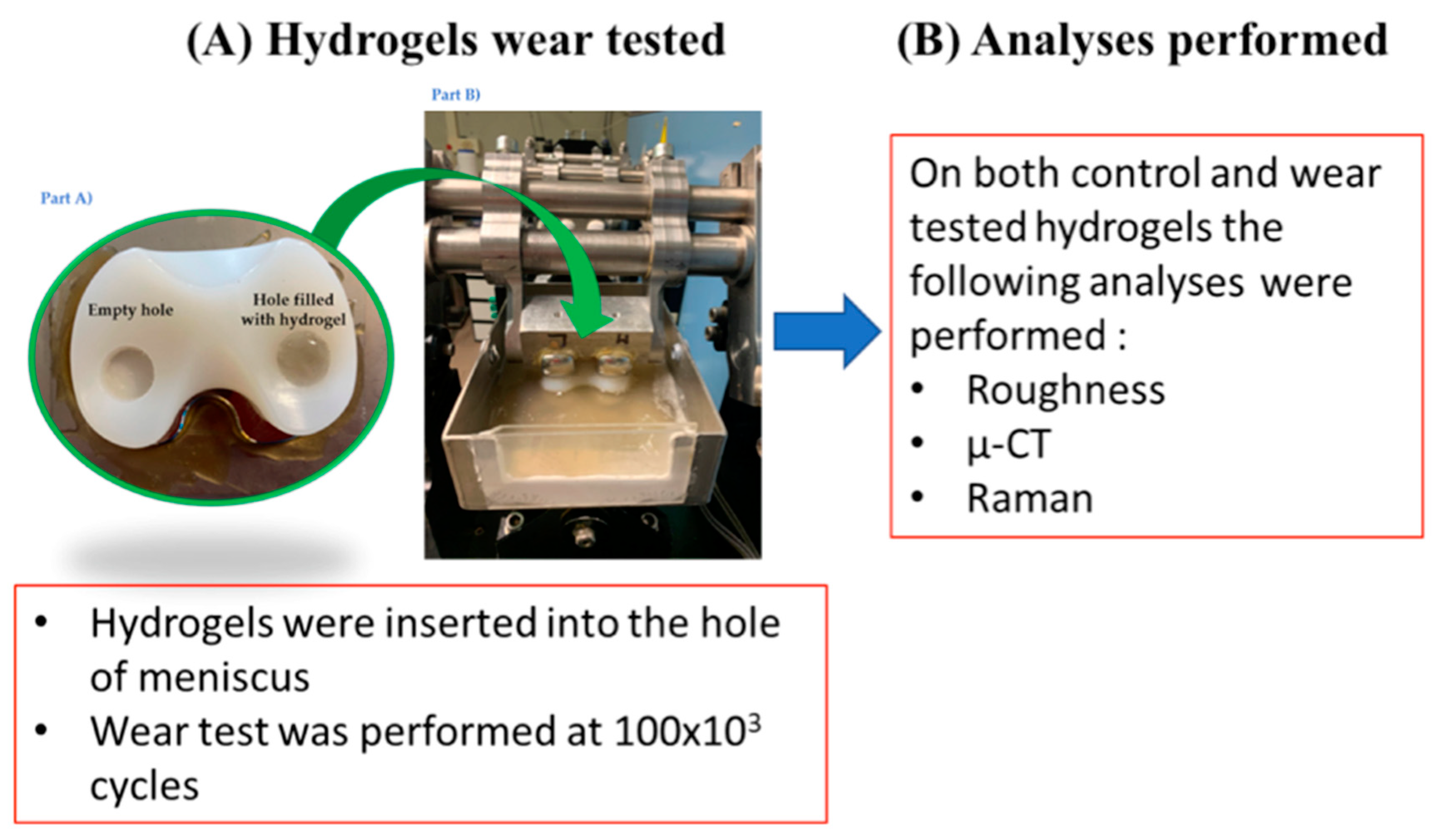
2.1. Test Specimens
- Four hydrogels for each batch were used for the mechanical test (test specimens).
- One hydrogel for each batch was used as control specimen.
2.2. Experimental Wear Study Protocol
- flexion/extension angle oscillating between 0 (neutral) and 58° (flexion) synchronously with the load,
- anterior/posterior translation oscillating between 0 (neutral) and 5.2 mm (posterior),
- intra/extra rotation oscillating between 2.1 and 5.7°.
2.3. Roughness Measurements
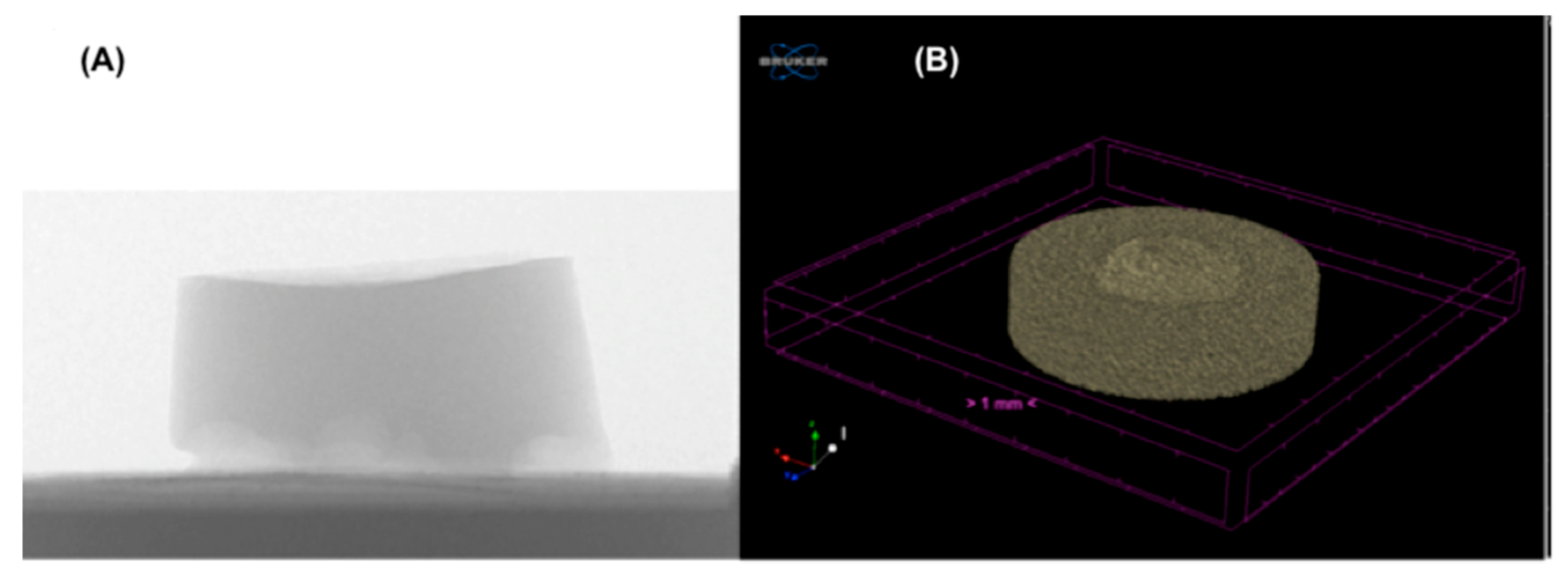
2.4. CT Characterization
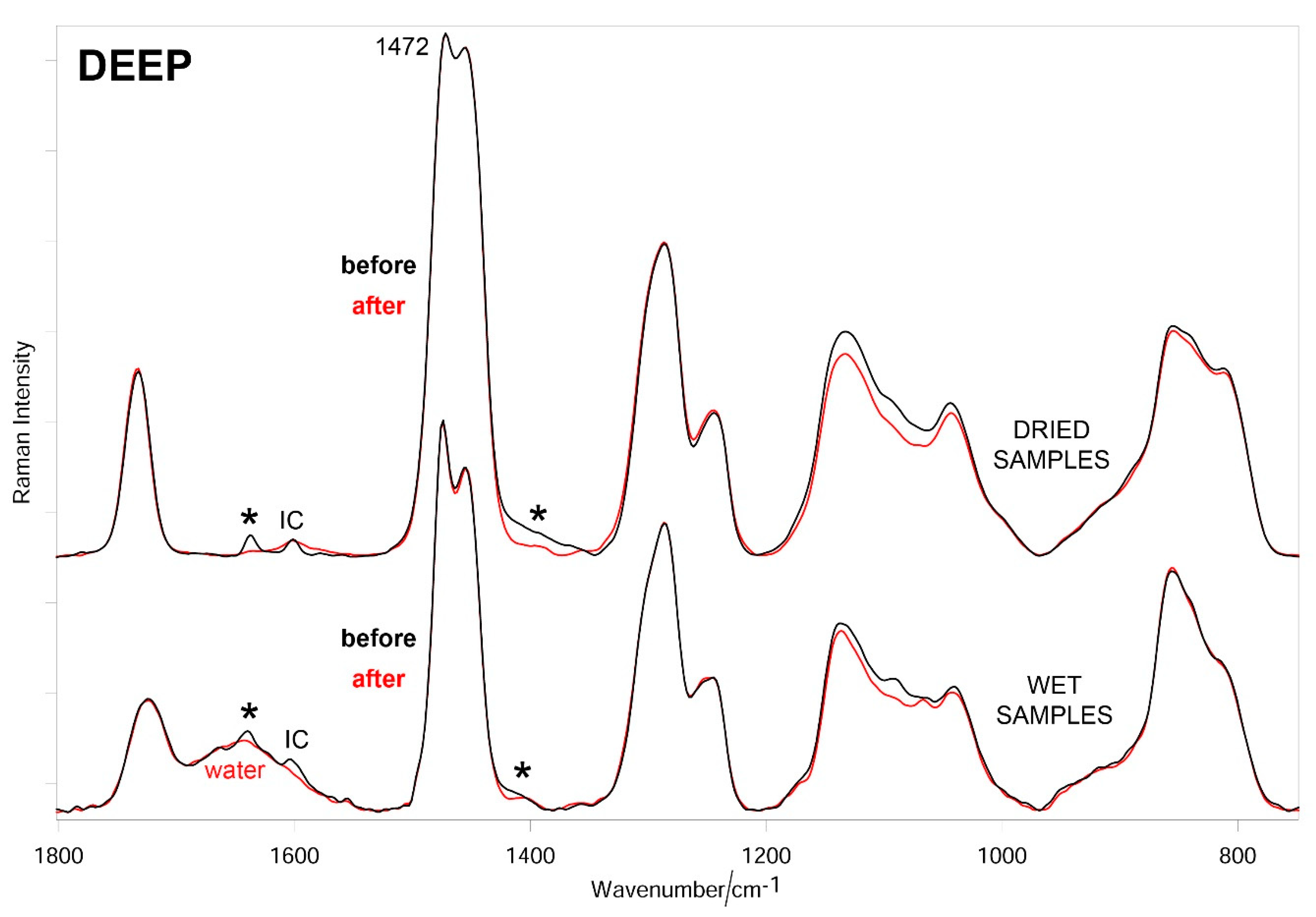
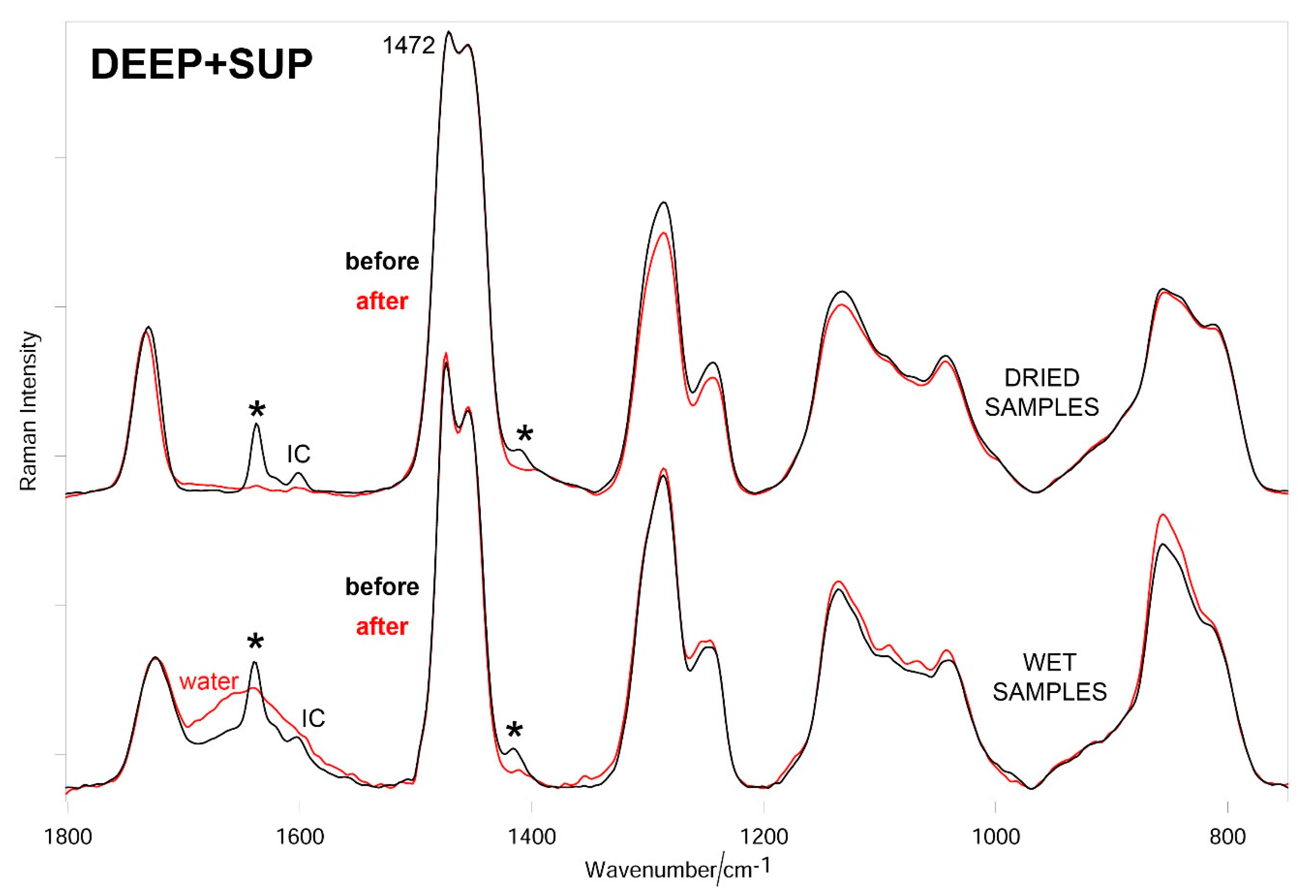
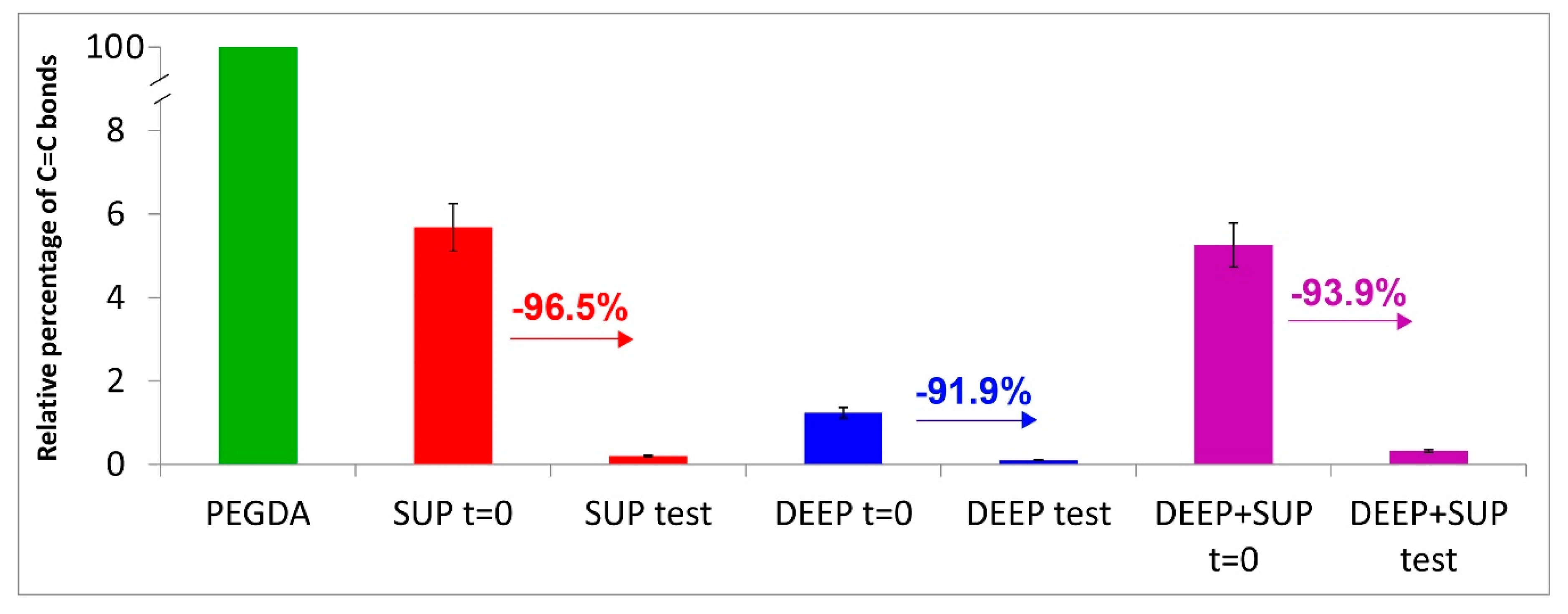
2.5. Raman Characterization
3. Results
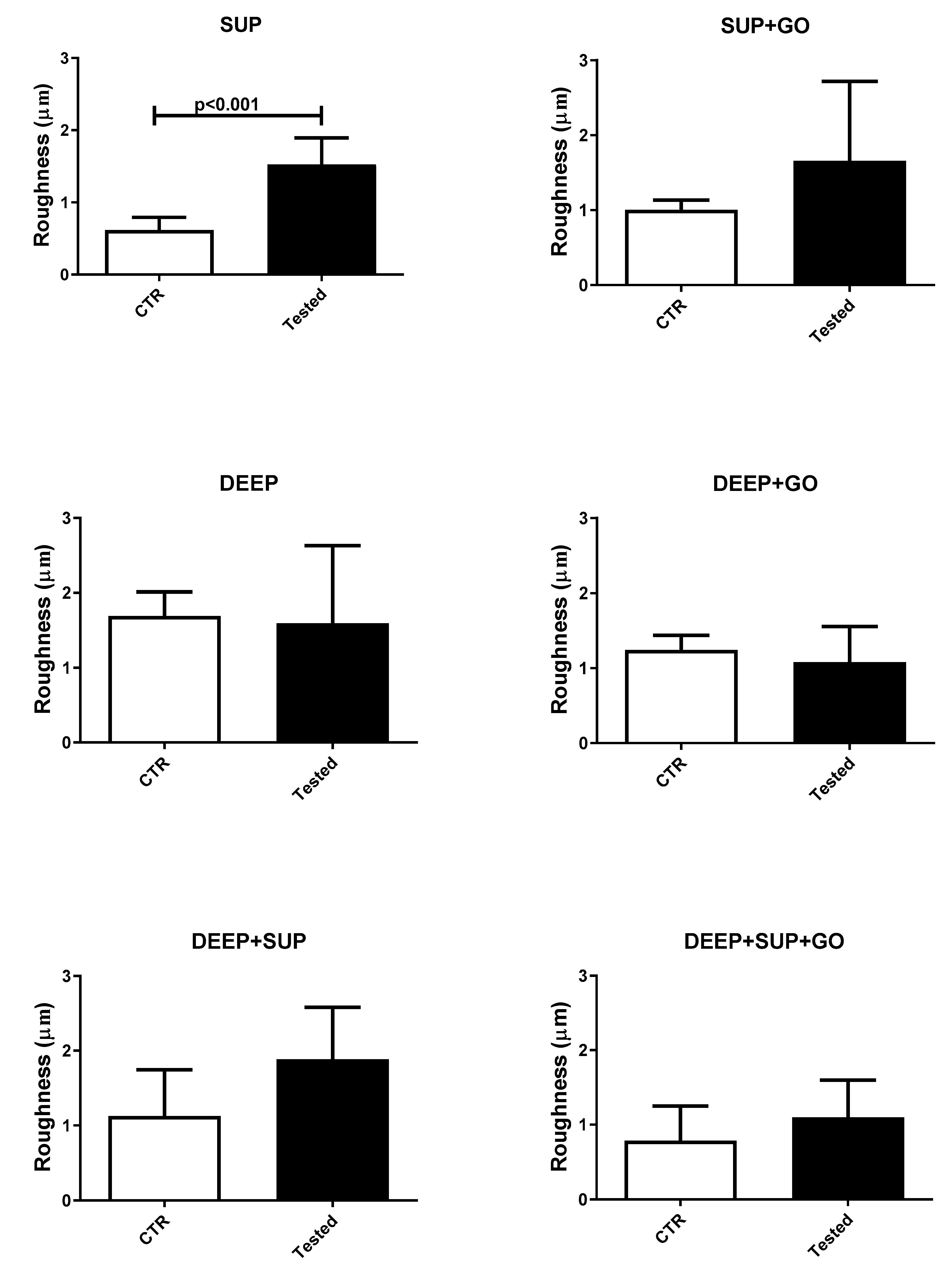
3.1. Roughness Results
3.2. CT Results
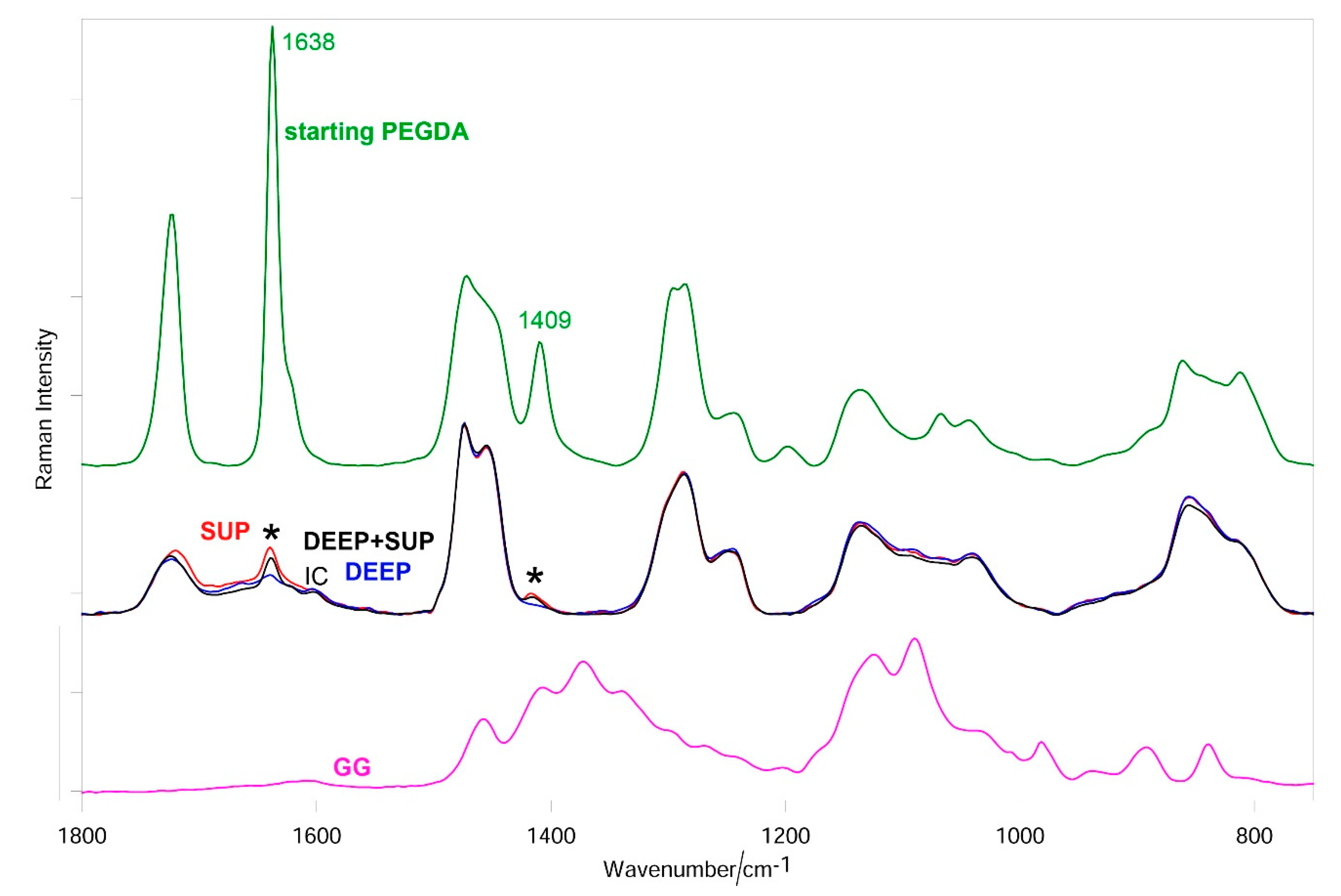
3.3. Raman Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GG | gellan gum |
| PEGDA | poly (ethylene glycol) diacrylate |
| GO | graphene oxide |
| DMEM | Dulbecco′s modified Eagle′s medium |
| MgCl2 | magnesium chloride |
| SUP | superior layer |
| DEEP | deep layer |
| DEEP | deep layer without graphene oxide |
| SUP | superior layer without graphene oxide |
| DEEP + SUP | deep and superior layers both without graphene oxide |
| DEEP + GO | graphene oxide-embedded deep layer |
| SUP + GO | graphene oxide-embedded superior layer |
| DEEP + SUP + GO | deep and superior layers both with embedded graphene oxide |
References
- Nazari, G. Knee osteoarthritis. J. Physiother. 2017, 63, 188. [Google Scholar] [CrossRef] [PubMed]
- Song, E.K.; Seon, J.K.; Moon, J.Y.; Ji-Hyoun, Y.; Kinov, P. The Evolution of Modern Total Knee Prostheses; InTech: Rijeka, Croatia, 2013; Volume 10, p. 54343. [Google Scholar]
- Unknown Development of a New Generation of Knee Prostheses with Enhanced Lifespan Features Using Advanced Computational Biomechanics|TOTAL.KNEE Project|FP7|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/303861/it (accessed on 20 December 2020).
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Antons, J.; Marascio, M.G.M.; Nohava, J.; Martin, R.; Applegate, L.A.; Bourban, P.E.; Pioletti, D. Zone-dependent mechanical properties of human articular cartilage obtained by indentation measurements. J. Mater. Sci. Mater. Med. 2018, 29, 57. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Wu, J.P.; Kirk, T.B.; Carrino, J.A.; Xiang, C.; Xu, J. High-resolution measurements of the multilayerultra-structure of articular cartilage and their translational potential. Arthritis Res. Ther. 2014, 16, 205. [Google Scholar] [CrossRef] [Green Version]
- Beddoes, C.M.; Whitehouse, M.R.; Briscoe, W.H.; Su, B. Hydrogels as a Replacement Material for Damaged Articular Hyaline Cartilage. Materials 2016, 9, 443. [Google Scholar] [CrossRef] [Green Version]
- Walter, S.G.; Ossendorff, R.; Schildberg, F.A. Articular cartilage regeneration and tissue engineering models: A systematic review. Arch. Orthop. Trauma Surg. 2018, 139, 305–316. [Google Scholar] [CrossRef]
- Hurley, E.T.; Murawski, C.D.; Paul, J.; Marangon, A.; Prado, M.P.; Xu, X.; Hangody, L.; Kennedy, J.G.; Ackermann, J.; Adams, S.B.; et al. Osteochondral Autograft: Proceedings of the International Consensus Meeting on Cartilage Repair of the Ankle. Foot Ankle Int. 2018, 39, 28S–34S. [Google Scholar] [CrossRef]
- Means, A.K.; Grunlan, M.A. Modern Strategies to Achieve Tissue-Mimetic, Mechanically Robust Hydrogels. ACS Macro Lett. 2019, 8, 705–713. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Liang, Y.; Ren, L.; Yu, Z.; Zhang, Z.; Qiuc, F.; Rena, L. Design and fabrication of nanofibrillated cellulose-containing bilayer hydrogel actuators with temperature and near infrared laser responses. J. Mater. Chem. B 2018, 8, 1260–1271. [Google Scholar] [CrossRef]
- Ji, S.; Almeida, E.; Guvendiren, M. 3D bioprinting of complex channels within cell-laden hydrogels. Acta Biomater. 2019, 95, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, M.A.; Swindle-Reilly, K.E.; Ravi, N. Hydrogels for intraocular lenses and other ophthalmic prostheses. In Biomedical Hydrogels: Biochemistry, Manufacture and Medical Applications; Woodhead Publishing: Cambridge, UK, 2011; ISBN 9781845695903. [Google Scholar]
- Koehler, J.; Brandl, F.P.; Goepferich, A. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Cao, R.; Huang, Z.; Varghese, T.; Nabi, G. Tissue mimicking materials for the detection of prostate cancer using shear wave elastography: A validation study. Med. Phys. 2013, 40, 022903. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified Gellan Gum hydrogels with tunable physical and mechanical properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Lee, H.J.; An, H.; Lee, K.Y. Alginate hydrogels modified with low molecular weight hyaluronate for cartilage regeneration. Carbohydr. Polym. 2017, 162, 100–107. [Google Scholar] [CrossRef]
- Trucco, D.; Vannozzi, L.; Teblum, E.; Telkhozhayeva, M.; Nessim, G.D.; Affatato, S.; Al-Haddad, H.; Lisignoli, G.; Ricotti, L. Graphene Oxide-doped Gellan Gum-PEGDA bilayered hydrogel mimicking the mechanical and lubrication properties of articular cartilage. Adv. Healthc. Mater. (under review).
- Grillini, L.; Affatato, S. How to Measure Wear following Total Hip Arthroplasty. HIP Int. 2013, 23, 233–242. [Google Scholar] [CrossRef]
- Parrilli, A.; Falcioni, S.; Fini, M.; Affatato, S. Is micro-computed tomography useful for wear assessment of ceramic femoral heads? A preliminary evaluation of volume measurements. J. Appl. Biomater. Funct. Mater. 2016, 14, e483–e489. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.-S.; Qin, Y.-X.; Cheung, W.-H.; Qin, L.; Salmon, P. Micro-CT 3D Image Analysis Techniques for Orthopedic Applications: Metal Implant-to-Bone Contact Surface and Porosity of Biomaterials. In A Practical Manual for Musculoskeletal Research; World Scientific: Singapore, 2008; pp. 583–603. [Google Scholar] [CrossRef]
- Liang, H.; Bu, Y.; Zhang, J.; Cao, Z.; Liang, A. Graphene Oxide Film as Solid Lubricant. ACS Appl. Mater. Interfaces 2013, 5, 6369–6375. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Li, T.; Gong, D.; Fanglin, X.; Wang, M. Preparation and tribological properties of grapheneoxide/nano-MoS2hybrid as multidimensionalassembly used in the polyimide nanocomposites. RSC Adv. 2017, 7, 6323–6335. [Google Scholar] [CrossRef] [Green Version]
- Marciano, O.; Gonen, S.; Levy, N.; Teblum, E.; Yemini, R.; Nessim, G.D.; Ruthstein, S.; Elbaz, L. Modulation of Oxygen Content in Graphene Surfaces Using Temperature Programmed Reductive Annealing: Electron Paramagnetic Resonance (EPR) and Electrochemical Study. Langmuir 2016, 32, 11672–11680. [Google Scholar] [CrossRef] [PubMed]
- Affatato, S.; Leardini, W.; Rocchi, M.; Toni, A.; Viceconti, M. Investigation on Wear of Knee Prostheses Under Fixed Kinematic Conditions. Artif. Organs 2008, 32, 13–18. [Google Scholar] [CrossRef]
- Taddei, P.; Modena, E.; Grupp, T.M.; Affatato, S. Mobile or fixed unicompartmental knee prostheses? In-vitro wear assessments to solve this dilemma. J. Mech. Behav. Biomed. Mater. 2011, 4, 1936–1946. [Google Scholar] [CrossRef]
- Abdel-Jaber, S.; Belvedere, C.; Leardini, A.; Affatato, S. Wear simulation of total knee prostheses using load and kinematics waveforms from stair climbing. J. Biomech. 2015, 48, 3830–3836. [Google Scholar] [CrossRef]
- Affatato, S. Displacement or Force Control Knee Simulators? Variations in Kinematics and in Wear. Artif. Organs 2016, 40, 195–201. [Google Scholar] [CrossRef]
- Affatato, S.; Zanini, F.; Carmignato, S. Micro X-Ray Computed Tomography Mass Loss Assessment of Different UHMWPE: A Hip Joint Simulator Study on Standard vs. Cross-Linked Polyethylene. PLoS ONE 2017, 12, e0170263. [Google Scholar] [CrossRef] [Green Version]
- Zanini, F.; Carmignato, S.; Savio, E.; Affatato, S. Uncertainty determination for X-ray computed tomography wear assessment of polyethylene hip joint prostheses. Precis. Eng. 2018, 52, 477–483. [Google Scholar] [CrossRef]
- Bäckström, S.; Benavente, J.; Berg, R.W.; Stibius, K.; Larsen, M.S.; Bohr, H.; Nielsen, C. Tailoring Properties of Biocompatible PEG-DMA Hydrogels with UV Light. Mater. Sci. Appl. 2012, 3, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Ermeydan, M.A. Modification of spruce wood by UV-crosslinked PEG hydrogels inside wood cell walls. React. Funct. Polym. 2018, 131, 100–106. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Pizarro, G.D.C.; Sarabia-Vallejos, M.A.; Terraza, C.A.; López-Cabaña, Z.E. In situ-preparation and characterization of silver-HEMA/PEGDA hydrogel matrix nanocomposites: Silver inclusion studies into hydrogel matrix. Arab. J. Chem. 2019, 12, 1413–1423. [Google Scholar] [CrossRef] [Green Version]
- Nuki, G. Osteoarthritis: A problem of joint failure. Zeitschrift fur Rheumatologie 1999, 58, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Kean, W.F.; Kean, R.; Buchanan, W.W. Osteoarthritis: Symptoms, signs and source of pain. Inflammopharmacology 2004, 12, 3–31. [Google Scholar] [CrossRef]
- Kurtz, S.; Ong, K.; Lau, E.C.; Mowat, F.; Halpern, M. Projections of Primary and Revision Hip and Knee Arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg.-Am. Vol. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Bozic, K.J. Impact of the Economic Downturn on Total Joint Replacement Demand in the United States. J. Bone Jt. Surg.-Am. Vol. 2014, 96, 624–630. [Google Scholar] [CrossRef]
- Chang, K.-Y.; Cheng, L.-W.; Ho, G.-H.; Huang, Y.-P.; Lee, Y.-D. Fabrication and characterization of poly(γ-glutamic acid)-graft-chondroitin sulfate/polycaprolactone porous scaffolds for cartilage tissue engineering. Acta Biomater. 2009, 5, 1937–1947. [Google Scholar] [CrossRef]
- Lee, J.M.; Sultan, T.; Kim, S.H.; Kumar, V.; Yeon, Y.K.; Lee, O.J.; HumParkae, C. Artificial Auricular Cartilage Using Silk Fibroin and Polyvinyl Alcohol Hydrogel. Int. J. Mol. Sci. 2017, 18, 1707. [Google Scholar] [CrossRef] [Green Version]
- Stillman, Z.S.; Jarai, B.M.; Raman, N.; Patel, P.; Fromen, C.A. Degradation profiles of poly(ethylene glycol)diacrylate (PEGDA)-based hydrogel nanoparticles. Polym. Chem. 2020, 11, 568–580. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Piwowarczyk, W.; Pamula, E.; Liskova, J.; Schaubroeck, D.; Leeuwenburgh, S.C.G.; Brackman, G.; Balcaen, L.; Detsch, R.; Declercq, H.; et al. Injectable self-gelling composites for bone tissue engineering based on gellan gum hydrogel enriched with different bioglasses. Biomed. Mater. 2014, 9, 045014. [Google Scholar] [CrossRef] [PubMed]
- Serafim, A.; Cecoltan, S.; Olaret, E.; Dragusin, D.-M.; Vasile, E.; Popescu, V.; Mastalier, B.S.M.; Iovu, H.; Stancu, I.C. Bioinspired Hydrogel Coating Based on Methacryloyl Gelatin Bioactivates Polypropylene Meshes for Abdominal Wall Repair. Polymers (Basel) 2020, 12, 1677. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Jana, S.; Das, A.; Chakraborty, A.; Chowdhury, A.R.; Datta, P. Bioprinting of radiopaque constructs for tissue engineering and understanding degradation behavior by use of Micro-CT. Bioact. Mater. 2020, 5, 569–576. [Google Scholar] [CrossRef] [PubMed]



| Materials Content | Study Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| SUP | SUP + GO | DEEP | DEEP + GO | DEEP + SUP | DEEP + SUP + GO | |||
| GG | 1.5% | 1.5% | 1.5% | 1.5% | 1.5% | 1.5% | 1.5% | 1.5% |
| PEGDA | 10% | 10% | 15% | 15% | 15% | 10% | 15% | 10% |
| GO | - | 0.01% | - | 0.01% | - | - | 0.01% | 0.01% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Affatato, S.; Trucco, D.; Taddei, P.; Vannozzi, L.; Ricotti, L.; Nessim, G.D.; Lisignoli, G. Wear Behavior Characterization of Hydrogels Constructs for Cartilage Tissue Replacement. Materials 2021, 14, 428. https://doi.org/10.3390/ma14020428
Affatato S, Trucco D, Taddei P, Vannozzi L, Ricotti L, Nessim GD, Lisignoli G. Wear Behavior Characterization of Hydrogels Constructs for Cartilage Tissue Replacement. Materials. 2021; 14(2):428. https://doi.org/10.3390/ma14020428
Chicago/Turabian StyleAffatato, Saverio, Diego Trucco, Paola Taddei, Lorenzo Vannozzi, Leonardo Ricotti, Gilbert Daniel Nessim, and Gina Lisignoli. 2021. "Wear Behavior Characterization of Hydrogels Constructs for Cartilage Tissue Replacement" Materials 14, no. 2: 428. https://doi.org/10.3390/ma14020428
APA StyleAffatato, S., Trucco, D., Taddei, P., Vannozzi, L., Ricotti, L., Nessim, G. D., & Lisignoli, G. (2021). Wear Behavior Characterization of Hydrogels Constructs for Cartilage Tissue Replacement. Materials, 14(2), 428. https://doi.org/10.3390/ma14020428








