Antiobesity Activity of Elateriospermum tapos Shell Extract in Obesity-Induced Sprague Dawley Rats
Abstract
1. Introduction
2. Results
2.1. Effect of E. tapos Shell Extract on Body Weight and Caloric Intake
2.2. Effect of E. tapos Shell Extract on Organ Weight
2.3. Effect of E. tapos Shell Extract on Lipid Profiles
2.4. Effect of E. tapos Shell Extract on Triglycerides
2.5. Effect of E. tapos Shell Extract on Lipoprotein Lipase (LPL) Activity
2.6. LC–MS Analysis of E. tapos Shell Extract
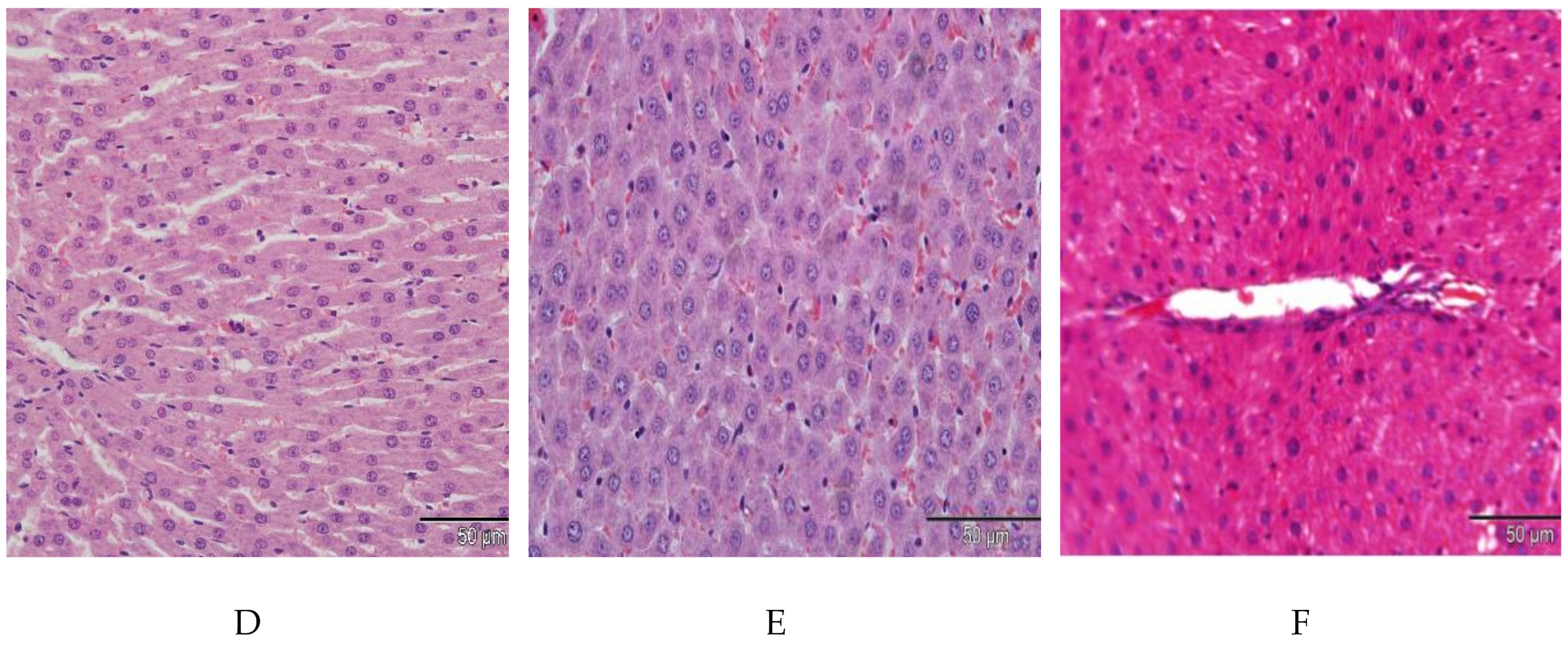
2.7. Effect of E. tapos Shell Extract on Histopathology of Liver
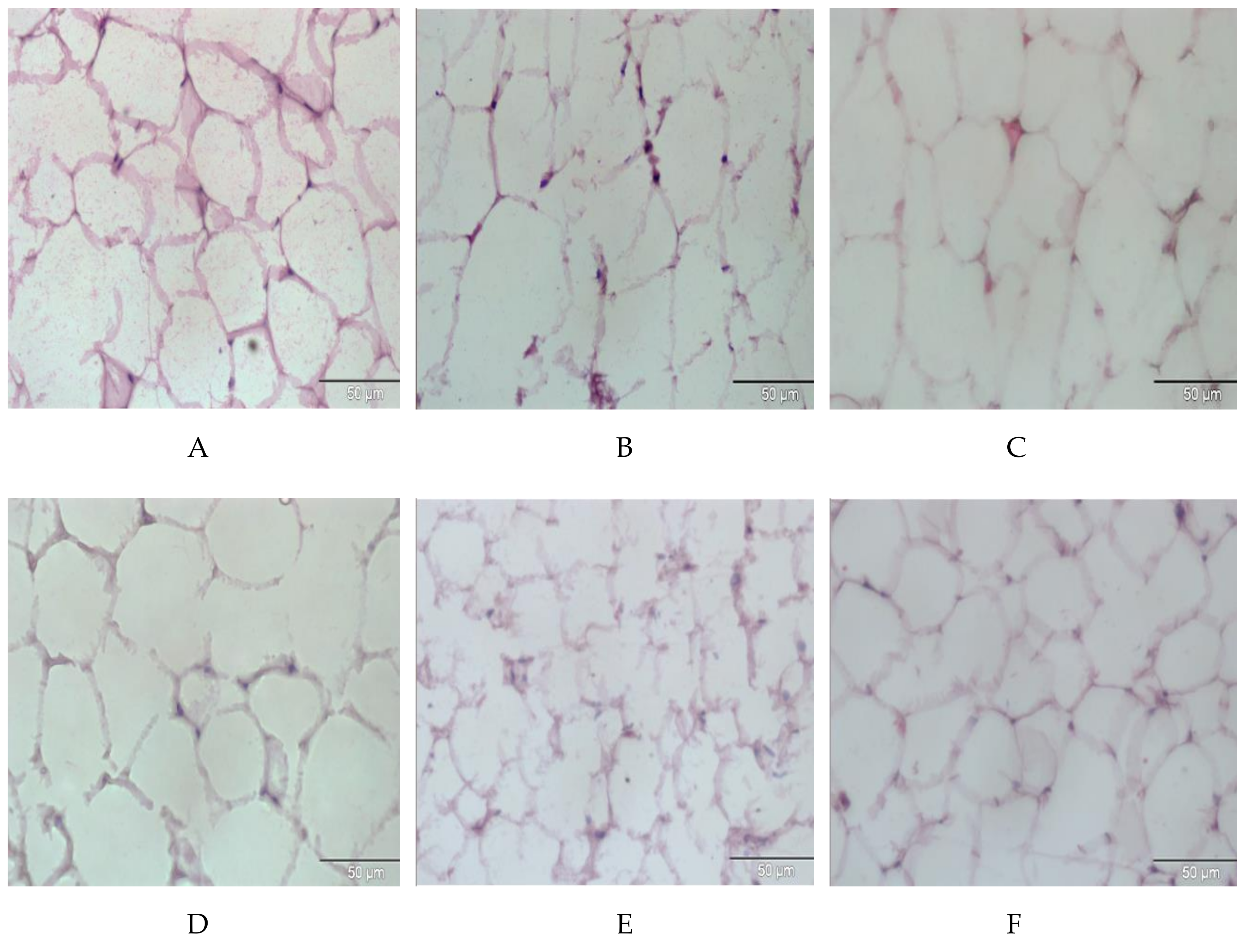
2.8. Effect of E. tapos Shell Extract on Histopathology of Adipose Tissue
3. Discussion
4. Materials and Methods
4.1. Sample, Diets, Chemicals, and Biochemical Analysis
4.1.1. Preparation of E. tapos Shell Extraction
4.1.2. Preparation of High-Fat (HF) Diet
4.1.3. Biochemical Analysis
4.2. Animals
4.3. Measurement of Growth Indicators
4.4. Experimental Design
4.5. Collection of Specimens
4.6. Histopathological Assessment of Liver and Adipose Tissue
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body fatness and cancer—Viewpoint of the IARC working group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Factsheet: Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 1 April 2020).
- Khaserao, S.; Somani, R. Evaluation of anti-obesity activity of solasodine in high fat diet-induced obesity in rat. Int. J. Pharm. Pharm. Sci. 2017, 9, 23. [Google Scholar] [CrossRef][Green Version]
- Felix, H.C.; West, D.S. Effectiveness of weight loss interventions for obese older adults. Am. J. Health Promot. 2013, 27, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Balan:, S.S.; Abidin, A.Z.; Perumal, K.V.; Lotafi, H.A.; Danabala, S.; Manimaran, M.; Shafie, N.H.; Abdullah, M.A.; Jasni, A.S.; Bahari, H. Effect of Elateriospermum tapos extract as coadjuvant in ameliorating maternal obesity on female offspring at weaning. Malays. J. Microsc. 2019, 15, 111–128. [Google Scholar]
- Osada, N.; Takeda, H.; Kawaguchi, H.; Furukawa, A.; Awang, M. Estimation of crown characters and leaf biomass from leaf litter in a Malaysian canopy species, Elateriospermum tapos (Euphorbiaceae). For. Ecol. Manag. 2003, 177, 370–386. [Google Scholar] [CrossRef]
- Ling, S.K.; Fukumori, S.; Tomii, K.; Tanaka, T.; Kouno, I. Isolation, purification and identification of chemical constituents from Elateriospermum tapos. J. Trop. For. Sci. 2006, 18, 81–85. [Google Scholar]
- Chaiittianan, R.; Sutthanut, K.; Rattanathongkom, A. Purple corn silk: A potential anti-obesity agent with inhibition on adipogenesis and induction on lipolysis and apoptosis in adipocytes. J. Ethnopharmacol. 2017, 201, 9–16. [Google Scholar] [CrossRef]
- Nor-Liyana, J.; Siroshini, K.; Nurul-Syahirah, M.; Chang, W.L.; Nurul-Husna, S.; Daryl, J.A.; Khairul-Kamilah, A.K.; Hasnah, B. Phytochemical analysis of Elateriospermum tapos and its inhibitory effects on alpha-amylase, alpha-glucosidase and pancreatic lipase. J. Trop. For. Sci. 2019, 31, 240–248. [Google Scholar]
- Perumal, K.V.; Ja’afar, N.L.; Balan, S.S.; Abidin, A.Z.; Arapoc, D.J.; Shafie, N.H.; Bahari, H. Preventive effect of Elateriospermum tapos seed extract against obese Sprague Dawley rats. Adv. Tradit. Med. 2020, 20, 107–113. [Google Scholar]
- Kim, S.Y.; Oh, M.R.; Kim, M.G.; Chae, H.J.; Chae, S.W. Anti-obesity effects of yerba mate (Ilex Paraguariensis): A randomized, double-blind, placebo-controlled clinical trial. BMC Complement. Altern. Med. 2015, 15, 338. [Google Scholar] [CrossRef]
- Othman, Z.A.; Noordin, L.; Omar, N.; Na, M.Y.; Mohamaed, M. Protective effects of orlistat on lipid profile, cardiac oxidative stress biomarkers and histology in high-fat diet-induced obese rats. Int. Med. J. Malays. 2019, 18, 2. [Google Scholar]
- Shafat, A.; Murray, B.; Rumsey, D. Energy density in cafeteria diet induced hyperphagia in the rat. Appetite 2009, 52, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, N.S.M.; Shafie, N.H.; Ishak, A.H.; Bahari, H. Screening for total phenolic content, total flavonoid content and antioxidant activity of Elateriospermum tapos in aqueous and ethanol extracts. In Proceedings of the 31st Scientific Conference, Kuala Lumpur, Malaysia, 31 May–1 June 2017. [Google Scholar]
- Ling, C.W.; Shafiea, N.H.; Bahari, H. Anti-obesity and anti-diabetic effects of Elateriospermum tapos crude extracts in vitro. In Proceedings of the 31st Scientific Conference, Kuala Lumpur, Malaysia, 31 May–1 June 2017. [Google Scholar]
- Stenman, L.K.; Waget, A.; Garret, C.; Klop, P.; Burcelin, R.; Lahtinen, S. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef. Microb. 2014, 5, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Karimi, G.; Sabran, M.R.; Jamaluddin, R.; Parvaneh, K.; Mohtarrudin, N.; Ahmad, Z.; Khazaai, H.; Khodavandi, A. The anti-obesity effects of Lactobacillus casei strain Shirota vs. Orlistat on high fat diet-induced obese rats. Food Nutr. Res. 2015, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koshy, A.S.; Anila, L.; Vijayalakshmi, N.R. Flavonoids from Garcinia cambogia lower lipid levels in hypercholesterolemic rats. Food Chem. 2001, 72, 289–294. [Google Scholar] [CrossRef]
- Park, H.J.; Jung, U.J.; Cho, S.J.; Jung, H.K.; Shim, S.; Choi, M.S. Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose-and lipid-regulating enzymes in db/db mice. J. Nutr. Biochem. 2013, 24, 419–427. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Dayem, A.D.; Han, J.; Yin, Y.; Kim, K.; Saha, S.S.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 2016, 17, 569. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Mitsuzumi, H.; Sunayama, T.; Yamada, M.; Okada, K.; Kubota, M.; Chaen, H.; Mishima, Y.; Kibata, M. Glucosyl hesperidin lowers serum triglyceride level in hypertriglyceridemic subjects through the improvement of very low-density lipoprotein metabolic abnormality. J. Nutr. Sci. Vitaminol. 2005, 51, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; He, R.R.; Zeng, X.H.; Huang, X.J.; Du, T.L.; Cui, J.C.; Hiroshi, K. Hypotriglyceridemic effects of apple polyphenols extract via up-regulation of lipoprotein lipase in triton WR-1339-induced mice. Chin. J. Integr. Med. 2014, 20, 31–35. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M. Pathology of fatty liver disease. Mod. Pathol. 2007, 20, S40–S48. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.M.; Beaudry, J.L.; Szigiato, A.A.; Trumble, S.J.; Snook, L.A.; Bonen, A.; Giacca, A.; Riddell, M.C. Consumption of a high-fat diet rapidly exacerbates the development of fatty liver disease that occurs with chronically elevated glucocorticoids. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G850–G863. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Liu, L.K.; Chuang, C.M.; Chyau, C.C.; Huang, C.N.; Wang, C.J. Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agric. Food Chem. 2011, 59, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Asterholm, I.W.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014, 20, 103–118. [Google Scholar]
- MacLean, P.S.; Higgins, J.A.; Giles, E.D.; Sherk, V.D.; Jackman, M.R. The role for adipose tissue in weight regain after weight loss. Obes. Rev. 2015, 16, 45–54. [Google Scholar] [CrossRef]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity rat model: A comparison between Wistar and Sprague-Dawley rat. Adipocyte 2016, 5, 11–21. [Google Scholar] [CrossRef]
- Albert, K. Liquid chromatography—Nuclear magnetic resonance spectroscopy. J. Chromatogr. 1999, 856, 199–211. [Google Scholar] [CrossRef]
- Levin, B.E.; Dunn-Meynel, A.A. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R46–R54. [Google Scholar] [CrossRef]


| Group | Body Weight at Death (g) | Caloric Intake (kJ) |
|---|---|---|
| NC | 292.7 ± 37.7 b | 5890.5 ± 3554.5 b |
| PC | 350.0 ± 15.0 a | 9341.9 ± 781.52 a |
| DC | 310.0 ± 24.5 b | 8653.6 ± 834.32 |
| T I | 292.2 ± 41.6 b | 7578.0 ± 983.45 ab |
| T II | 299.2 ± 44.1 ab | 8064.8 ± 914.01 ab |
| T III | 288.0 ± 42.8 b | 7971.6 ± 945.46 a |
| Group | Organ Weight (g) | |||
|---|---|---|---|---|
| Liver (g) | RpWAT (g) | Visceral Fat (g) | Gonadal Fat (g) | |
| NC | 9.7 ± 1.5 ab | 2.2 ± 0.6 b | 2.1 ± 0.4 b | 2.7 ± 0.7 b |
| PC | 11.7 ± 1.7 a | 4.7 ± 1.0 a | 3.3 ± 0.4 a | 4.2 ± 1.0 a |
| DC | 9.0 ± 1.4 b | 3.1 ± 0.6 b | 2.4 ± 0.5 b | 3.5 ± 0.7 ab |
| T I | 9.2 ± 2.0 b | 3.3 ± 1.6 b | 2.1 ± 0.6 b | 3.3 ± 1.0 ab |
| T II | 8.9 ± 1.7 b | 3.9 ± 1.3 a | 2.6 ± 0.9 ab | 3.0 ± 0.5 b |
| T III | 9.0 ± 2.6 b | 4.3 ± 1.3 a | 2.8 ± 0.9 a | 3.6 ± 0.6 ab |
| Group | Lipid Profile (mmol/L) | ||
|---|---|---|---|
| TC | LDL | HDL | |
| NC | 1.38 ± 0.26 | 0.76 ± 0.28 b | 0.39 ± 0.08 |
| PC | 1.63 ± 0.33 | 1.04 ± 0.16 a | 0.35 ± 0.05 |
| DC | 1.58 ± 0.29 | 0.98 ± 0.26 ab | 0.43 ± 0.08 |
| T I | 1.58 ± 0.16 | 0.88 ± 0.21 ab | 0.42 ± 0.10 |
| T II | 1.49 ± 0.17 | 0.88 ± 0.18 ab | 0.40 ± 0.07 |
| T III | 1.48 ± 0.16 | 0.86 ± 0.20 ab | 0.41 ± 0.08 |
| Group | Triglycerides (mmol/L) | ||
|---|---|---|---|
| Plasma | RpWAT | Liver | |
| NC | 1.25 ± 0.20 b | 16.04 ± 1.82 b | 20.82 ± 8.05 |
| PC | 1.77 ± 0.84 a | 26.26 ± 14.29 a | 28.39 ± 8.97 |
| DC | 1.40 ± 0.33 ab | 20.69 ± 7.27 ab | 23.59 ± 8.20 |
| T I | 1.33 ± 0.21 ab | 19.70 ± 3.41 ab | 27.11 ± 11.07 |
| T II | 1.25 ± 0.23 ab | 18.86 ± 1.87 ab | 23.13 ± 4.77 |
| T III | 1.32 ± 0.23 ab | 19.62 ± 3.67 ab | 24.29 ± 4.96 |
| Group | Lipoprotein Lipase | |
|---|---|---|
| Plasma (mU/mL) | RpWAT (mU/g) | |
| NC | 1.25 ± 0.20 b | 16.04 ± 1.82 b |
| PC | 1.77 ± 0.84 a | 26.26 ± 14.29 a |
| DC | 1.40 ± 0.33 ab | 20.69 ± 7.27 ab |
| T I | 1.33 ± 0.21 ab | 19.70 ± 3.41 ab |
| T II | 1.25 ± 0.23 ab | 18.86 ± 1.87 ab |
| T III | 1.32 ± 0.23 ab | 19.62 ± 3.67 ab |
| Name of the Compound | Retention Time (min) | Mass (g/mol) |
|---|---|---|
| 3′4′5-trimethoxyflavone (Flavonoid) | 4.910 | 188.116 |
| Acetyl lysine | 5.987 | 282.1681 |
| 7-methoxychromone | 10.165 | 176.0476 |
| C11 H19 N O2 | 13.845 | 197.1419 |
| C20 H30 N4 O7 S | 18.503 | 470.1832 |
| C23 H44 N4 O4 | 21.636 | 440.3363 |
| Undulatone | 26.527 | 534.2099 |
| Aldosterone 18-glucuronide | 28.334 | 536.2261 |
Sample Availability: Samples of the E. tapos are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, K.V.; Ja’afar, N.L.; Mat Taib, C.N.; Shafie, N.H.; Bahari, H. Antiobesity Activity of Elateriospermum tapos Shell Extract in Obesity-Induced Sprague Dawley Rats. Molecules 2021, 26, 321. https://doi.org/10.3390/molecules26020321
Perumal KV, Ja’afar NL, Mat Taib CN, Shafie NH, Bahari H. Antiobesity Activity of Elateriospermum tapos Shell Extract in Obesity-Induced Sprague Dawley Rats. Molecules. 2021; 26(2):321. https://doi.org/10.3390/molecules26020321
Chicago/Turabian StylePerumal, Kokila Vani, Nor Liyana Ja’afar, Che Norma Mat Taib, Nurul Husna Shafie, and Hasnah Bahari. 2021. "Antiobesity Activity of Elateriospermum tapos Shell Extract in Obesity-Induced Sprague Dawley Rats" Molecules 26, no. 2: 321. https://doi.org/10.3390/molecules26020321
APA StylePerumal, K. V., Ja’afar, N. L., Mat Taib, C. N., Shafie, N. H., & Bahari, H. (2021). Antiobesity Activity of Elateriospermum tapos Shell Extract in Obesity-Induced Sprague Dawley Rats. Molecules, 26(2), 321. https://doi.org/10.3390/molecules26020321






