1. Introduction
Cotton (
Gossypium spp.) is the most important crop cultivated for natural fibers worldwide and plays a vital role in the global economy [
1]. Cotton fibers are single cells that differentiate from the outer integuments of the cotton plants’ ovule. Four separable biological processes determine the number of fibers present on each ovule (cotton productivity), the final length and the strength of each fiber (fiber quality): fiber initiation; elongation (primary cell wall synthesis); cell wall thickening (secondary cell wall deposition); and maturation. When the cotton flowers are ready to open, the young seeds are still tiny and cluster together in the ovary. The outer epidermal shell of each seed comprises a large number of living plant cells. The fiber initiation stage starts from three days before flowering (anthesis) to three days postanthesis [
2]. At this stage, some of these cells begin to lengthen rapidly. If fertilization occurs, these special cells will continue to elongate and eventually develop into cotton fibers [
3]. After pollination occurs, it requires nearly 50 days for a boll to “open” under optimal conditions. The mature cotton fiber wall is not homogeneous and is made up of four different layers—cuticle, thin primary wall, thick secondary cell wall and lumen. Cotton fiber is comprised mostly of α-cellulose (88.0–96.5%); the other non-cellulosic substances are located either on the outer cuticle and primary cell wall layers or inside the lumens of the fibers [
4].
Surface non-cellulosic substances on cotton fibers serve essential functions during cell growth and development and protect the cells from potential environmental and pathogenic injury. The cuticle is an outer waxy layer containing mostly cutin and pectins. The term ‘cotton waxes’ (0.4–1.2% of the cotton fiber) has been used to encompass all lipid compounds found on the cotton fibers’ surface, including waxes, fats and resins [
4]. Cutin, a lipid biopolymer, is the structural component of the cuticular membrane that wraps the aerial parts of higher plants. It consists mostly of esterified C16 and C18 oxygenated fatty acids and is regarded as the first barrier facing phytopathogens attack [
5].
Pectic substances (0.4–1.2% of the cotton fiber) are the major constituent of plant cells, primary cell walls and middle lamella. Pectin is a complex acidic, colloidal and negatively charged high molecular mass (50−250 kDa) polymer present in the form of magnesium pectate and calcium [
6]. Chemically, pectins are basically branched heteropolysaccharides containing a few hundred to about one thousand building blocks per molecule, with a backbone of galacturonic acid residues, part of which are methyl esterified [
7]. The pectin molecule is a polymer of D-galacturonic acid (GalA) residues joined by α-1, 4 glycosidic linkages. The GalA residues may be esterified or decorated with the side chains of D-xylose, L-arabinose, D-galactose and L-rhamnose molecules. The galacturonic acid’s carboxyl groups are partly esterified by methyl groups and partially or entirely neutralized by sodium, potassium or ammonium ions. Some of the hydroxyl groups on C2 and C3 may be acetylated. Eighty-five percent of the polygalacturonic acid groups are methylated, leading to a highly hydrophobic substance.
Removal of the cotton fibers’ cuticle in the textile industry, i.e., scouring, is accomplished by generic traditional alkaline hydrolysis with hot NaOH. Scouring removes all non-cellulosic materials such as waxes and pectins, together with spinning oils and other impurities of the plant cell cuticle. This is one of the initial steps in processing cotton fibers, done to make the fabrics hydrophilic and wettable for posterior bleaching and dyeing performed under aqueous conditions [
8]. Conventional chemical scouring involves large quantities of water and energy and requires special handling of the strong alkaline effluents [
9]. The pursuit of environmentally friendly alternatives for scouring led many groups to examine enzyme systems for this purpose. Indeed, extracellular lytic enzymes (lipases, cellulases and pectinases) have been considered as potential bio-scouring agents to scour the cotton fabric cuticle’s outer layer and alleviate environmental pollution. These enzymes play an essential role in plant–pathogen interactions by degrading the plant cuticle and tissue and enabling pathogen invasion. Dedicated research efforts made in recent decades examined the implementation of enzymes such as cutinases [
10,
11], pectinases [
12], proteases [
13], xylanases [
14] and lipases for cotton ecofriendly scouring. In many cases, it was found to be as effective as the conventional alkaline treatment at 100 °C, increasing the textile’s wettability and removing its dye.
Indeed, enzymatic scouring is more advantageous than chemical scouring and has several benefits including: low working temperatures (30–40 °C), less quantities of water and energy involved, easy care of the generated wastewater compared to the special handling required by the strong alkaline effluents, and the ability to scour only the cotton in blends containing other components such as wool or polyester. Moreover, the amount of total dissolved solids in textile effluents is greatly reduced. Finally, but no less important, the adoption of bio-scouring techniques led to the improved quality of textile products. The resulting cotton fabrics are less damaged after 20 washings in a domestic washing machine, more abrasion resistant and have a reduced degree of polymerization and a higher fabric hand feeling [
15]. Nowadays, the improvement of cotton products is becoming increasingly important, an aspect that had been the main focal point of cotton research in recent years [
16].
Cutinases (E.C. 3.1.1.74) are serine esterases that belong to the α/β hydrolase family [
5]. The enzymes were categorized between esterases and true lipases since they have a broad substrate range. They can hydrolyze soluble fatty acid esters, triglycerides and insoluble polymers. The role of these hydrolytic enzymes is to degrade the cuticle’s structural component, cutin. Numerous bacterial and fungal cutinases have been characterized. Cutinase was evaluated for use in the cotton bio-scouring, food and chemical industry and for synthetic fibers modification [
5]. This enzyme production and purification method was improved using a high-specificity 4-nitrophenyl (16-methyl sulfide ester) hexadecanoate (pNMSEH) cutinase substrate [
17,
18]. The molecular weight (MW) of most fungal cutinases ranges from 20 to 25 kDa, and they work optimally at neutral or alkaline pH (7.0–9.5). Bacterial cutinases act optimally at 50–60 °C, while most characterized fungal cutinases exhibit temperature optima of 40–45 °C.
Pectin is hydrolyzed by pectinolytic enzymes or pectinases to short polysaccharide fragments. These enzymes are produced mainly by plant pathogens and saprophytes (fungi and bacteria) to degrade plant cell walls. Pectinases include endo-acting hydrolytic enzymes such as pectin lyase (PL: EC 4.2.2.10) and pectin methylesterases (PME or pectin esterase: EC 3.1.1.11). Pectin lyase attacks the glycosidic bond between two carbons containing an ester residue (COOCH
3) or between two carbons containing one ester residue and the other a carboxylic acid residue (COOH). PME catalyzes the methyl group’s removal (de-esterification to produce carboxylic acid residue) from pectin to form pectate (non-methoxylated polygalacturonic acid). Hence, PME catalyzation activity allows the action of polygalacturonases (PG: EC 3.2.1.1.5). These pectinases include two types, which differ in the nature of their action: exo-polygalacturonase acts on the non-reducible end of the chain and disconnects the outermost carbon each time; endo-polygalacturonase and pectate lyase (EC 4.2.2.2) act randomly on the α-1,4-glycosidic bonds at the center of the chain and digest pectate, producing galacturonic acid [
19,
20]. Thus, endo-acting polygalacturonase causes a rapid decrease in viscosity by cutting the long chain into many short chains, while, at the same time, the exo- polygalacturonase and pectate lyase degrade the single long chain from its edges.
Today, the conventional cotton scouring process that involves the use of harsh chemicals is slowly being replaced with an eco-friendly approach using such enzymes [
21]. Alkaline pectinase has been considered the most suitable enzyme for this green technology since the degradation and elimination of pectin facilitates the removal of waxes [
11,
22,
23]. Improved results were achieved when pectinases were used in conjunction with lipases, amylases, cellulases and hemicellulases to discharge sizing agents [
24]. In particular, lipase combined with pectinase resulted in a significant reduction in the time required for cotton fabrics scouring with excellent results [
25].
Despite worldwide scientific efforts and the enhanced progression in developing an enzymatic solution to replace the traditional chemical process of textile materials, there is still an urgent need to deepen our understanding of the candidates’ enzyme combinations for bioprocessing the fibers’ cuticle. In the current research, the additive effect of cutinase and pectinase was investigated for cotton wax and pectin degradation. The combined effect of these enzymes on the scouring of cotton fabrics was studied using evaporative light-scattering reverse-phase high-performance liquid chromatography (ELSD-RP HPLC) and gas chromatography/mass spectrometry (GC-MS) analysis of the reaction components, and measuring changes in the cotton fabrics’ properties.
2. Results
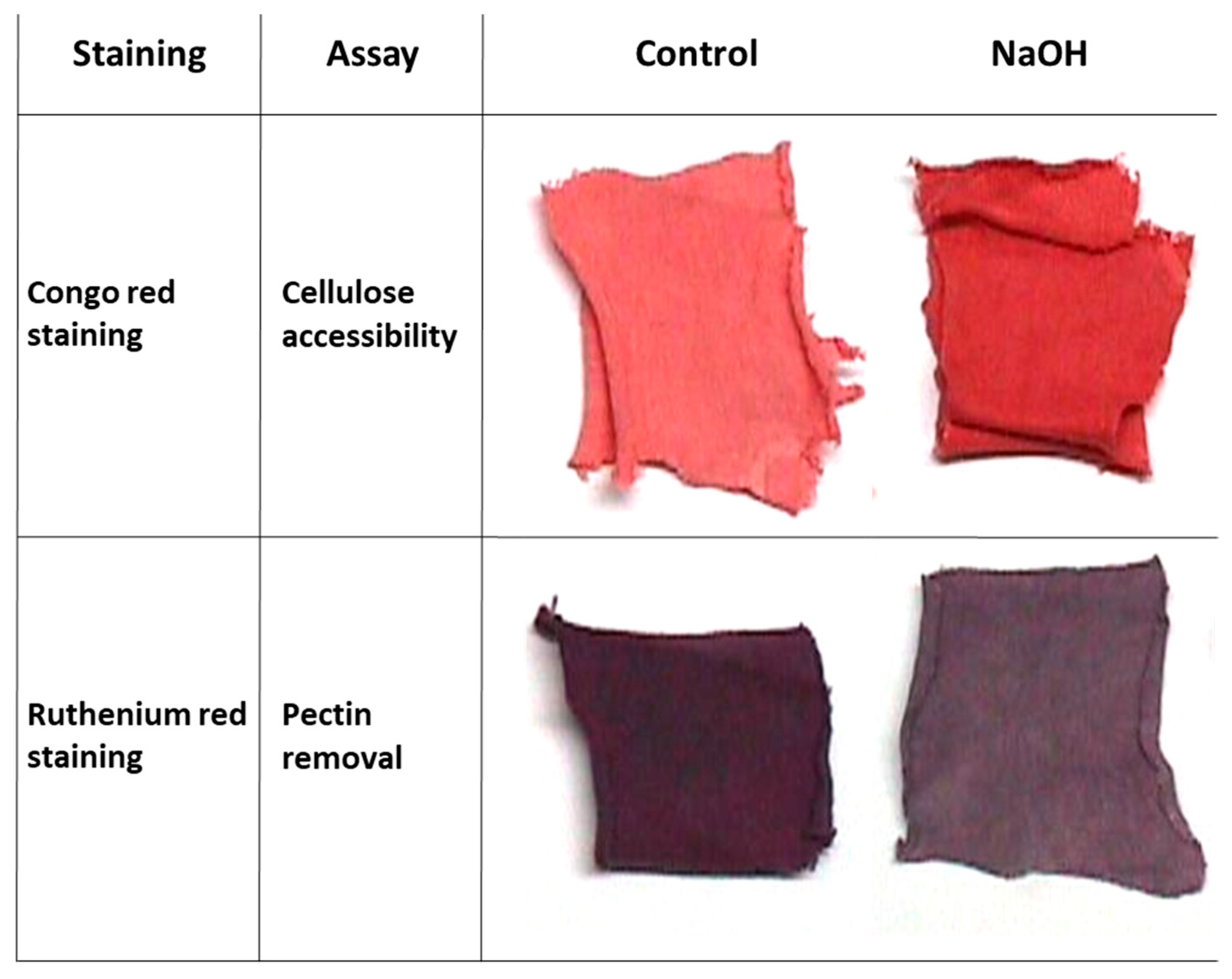
Conventional scouring of cotton fabrics is done with boiling alkaline caustic soda to remove the fibers’ outer layer of non-cellulosic substances. To demonstrate this chemical process outcome on pectin removal and cellulose accessibility, NaOH-treated and untreated (control) cotton fabrics square pieces of 1 g were stained with Congo red (staining of the cellulose layer) and Ruthenium red (staining of the pectin layer). Differences between the sodium hydroxide-treated swatches and the control treatments can be easily observed visually (
Figure 1).
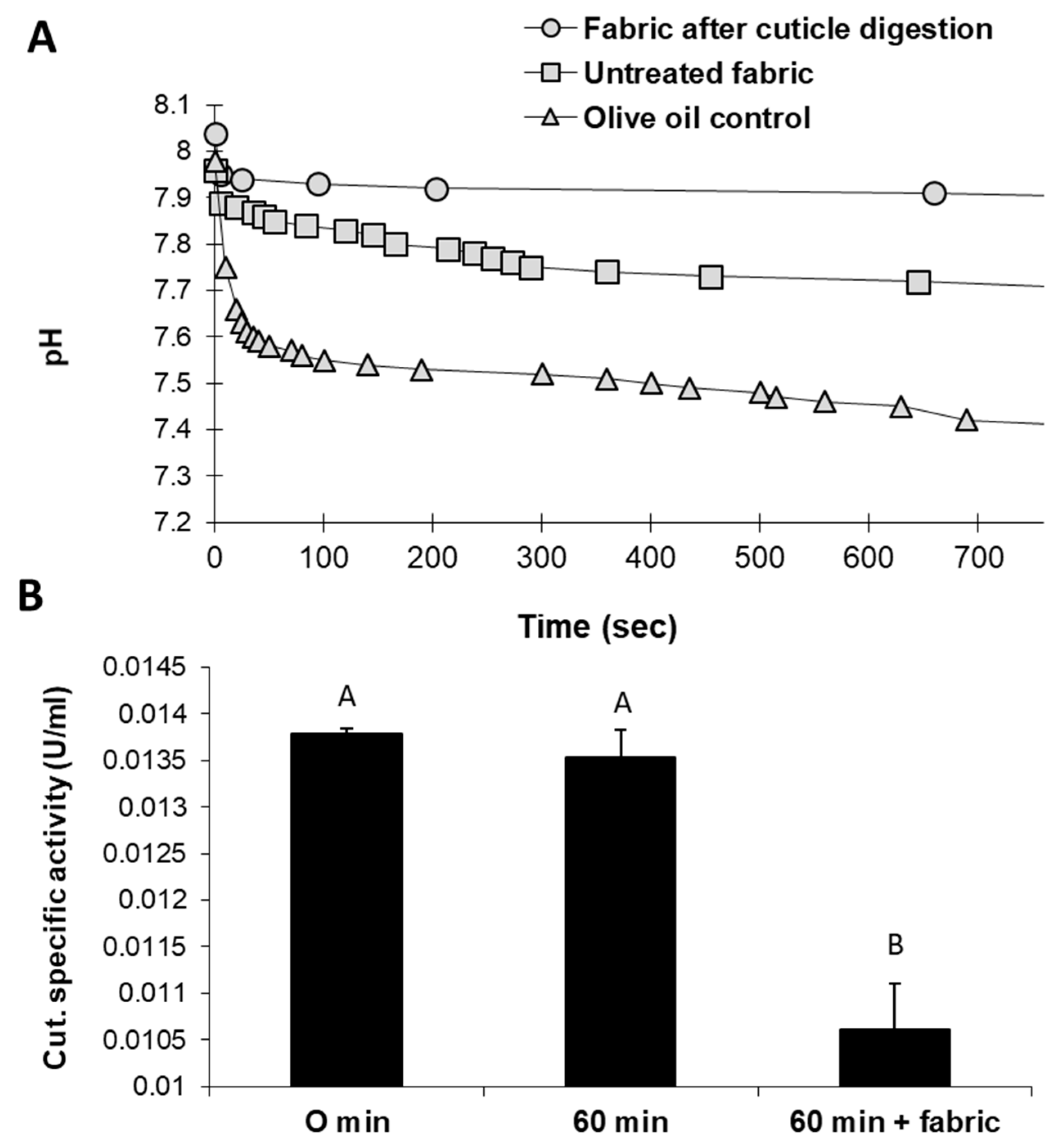
An alternative eco-friendly scouring process substituting NaOH with selective enzymes was studied in a series of experiments. Tracking the changes in the reaction mixture of raw cotton fabrics treated with cutinase revealed intriguing results. Cutinase activity with natural untreated cotton fabrics led to acidification of the reaction solution. These changes resulted from the release of fatty acids—the substrate degradation products. pH level changes were relatively minor when fabrics that underwent generic, traditional alkaline hydrolysis with boiling NaOH were used instead (
Figure 2A). Cutinase activity with raw cotton fabrics was also reflected by decreased cutinase-specific activity after 60 min (
Figure 2B). In this treatment, the enzyme-specific activity decreased significantly (
p < 0.05) by 19% compared to the incubation of cutinase for 60 min without the substrate. It assumes that this reduction in residual cutinase in raw cotton fibers’ presence reflects enhanced enzyme activity.
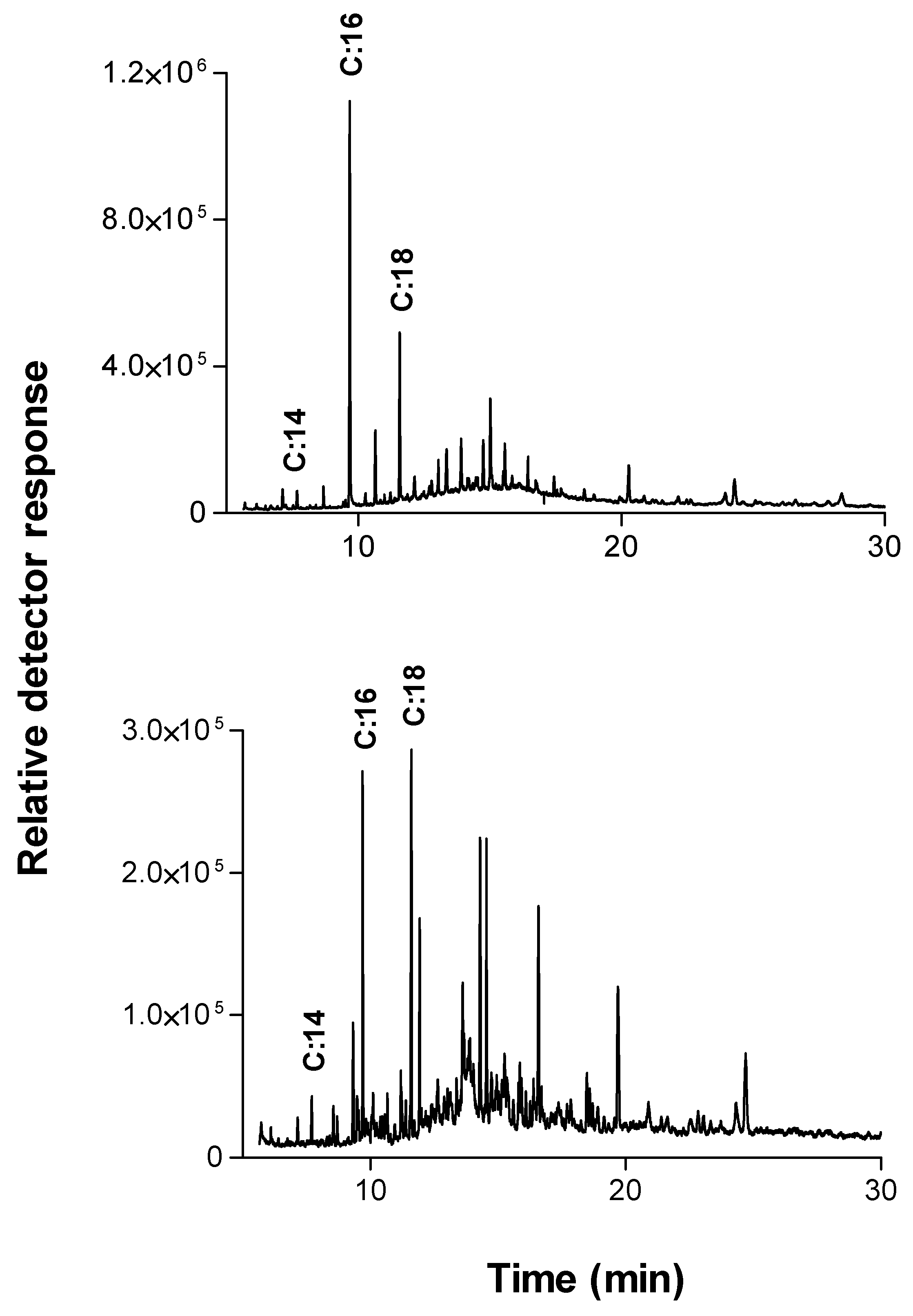
A detailed picture of the raw cotton fabrics’ wax components was obtained using extraction in dichloromethane (in the soxhlet system) overnight followed by GC-MS analysis. The main ingredients found are shown in
Table 1 and
Figure 3A.
The two main components found in the extract verified by GC/MS analysis were saturated fatty acid chains or derivatives of unsaturated fatty acid chains, mainly of C16:0, C18:0. This is in agreement with other reports that n-C:16 and n-C:18 hydroxy fatty acids with hydroxyl groups placed in 1 and 3 positions were recognized as the major lime and apple cutin monomers [
17,
26,
27]. These two components were also found as the dominant products in the extraction of cutinase reaction with raw cotton fabrics (
Figure 3B).
Treating natural cotton fabrics with each of the three enzymes, cutinase, pectin lyase, or polygalacturonase, increased their pectin removal, measured by high D-galacturonic acid concentrations in the reaction mixture (
Figure 4) and other pectin constituents in the reaction fluid (
Figure 5). Tracking the concentration changes over time (up to 60 min) of D-galacturonic in the reaction mixture reveals that after 20 min of incubation, the levels of this substrate remain constant in the polygalacturonase treatment. This is in contrast with the two other enzymes employed, cutinase and pectin lyase, which caused a continuous increase in the substrate’s release. However, the changes caused by these two enzymes were less rapid at the later stages of the reaction than in the first 20 min (
Figure 4). Indeed, polygalacturonase acts on the α1–4 linkages of polygalacturonic acid and required pectin’s conversion to pectate. Thus, this enzyme is limited by the pectate present and the action of other enzymes such as pectin-esterase. On the other hand, cutinase and pectin lyase are not limited and can act on the natural form of pectin.
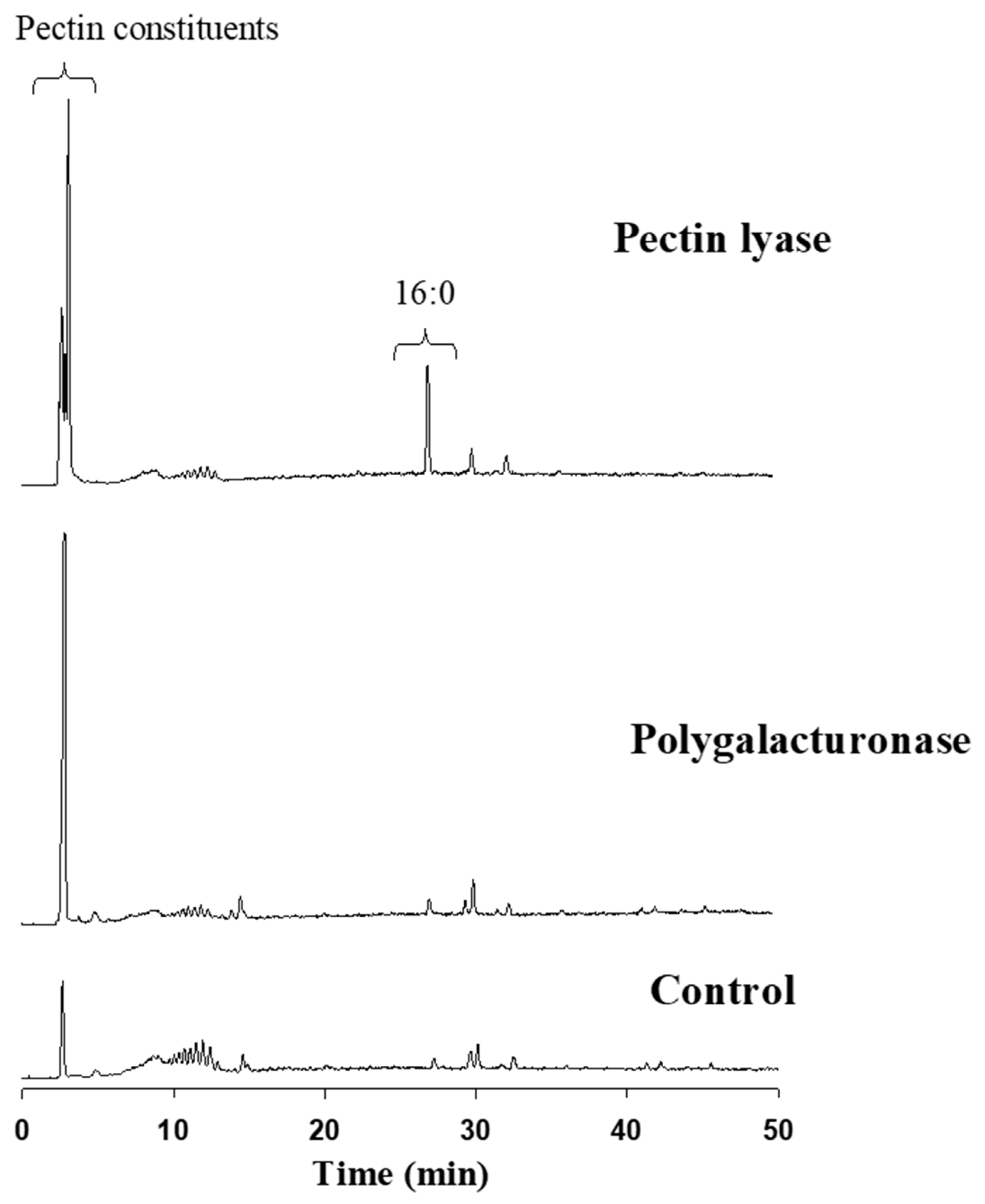
Reverse-phase HPLC-ELSD detected the major constituents released from the cotton fabric as a result of the pectin-degrading enzymes attack. Elution was executed using a gradient system consisting of acetonitrile:tetrahydrofuran:trifluoroacetic acid (0.1% in water). The extent of pectin degradation was increased four-fold as the result of pectin lyase or polygalacturonase compared to the control. This activity was expressed mainly by the release of pectin constituents, observed by a major peak at 2.8–2.9 min (
Figure 5). Interestingly, the pectin lyase attack on the cotton fabrics’ outer layer also resulted in the release of some of the waxy layer components, observed by a significant C:16 peak at ca 28 min. This outcome was not observed in the polygalacturonase reaction fluid.
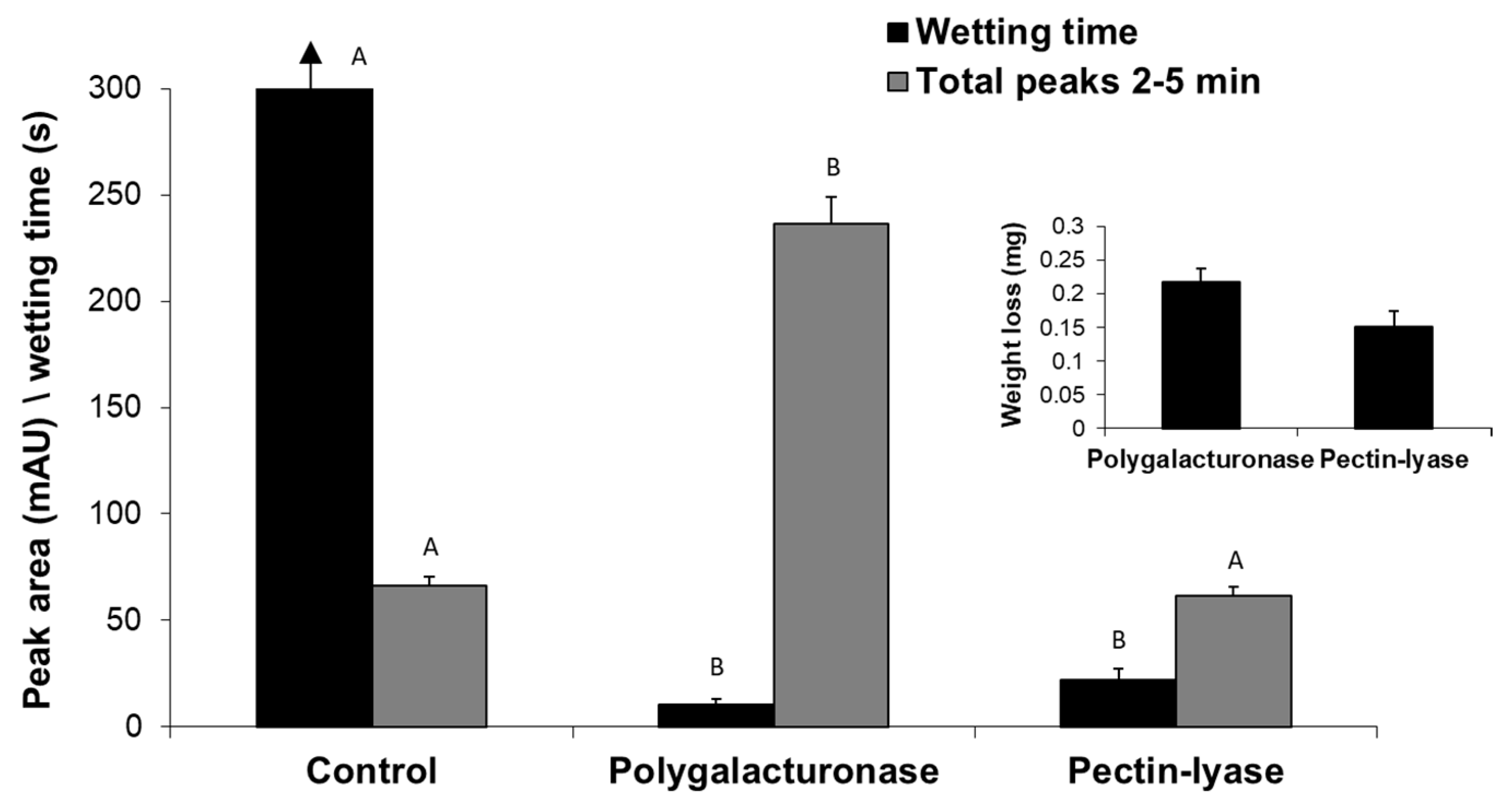
So far, we have described the enzymatic scouring effect measured by changes in the reaction fluid - the appearance of specific hydrolysis products of both the waxy layers and pectin. Still, the goal of bio-scoring is to alter the surface water absorbency properties of cotton fabrics. Indeed, the reaction fluid changes were accompanied by a dramatic decrease (
p < 0.05) in the wetting time of pectin lyase- or polygalacturonase-treated fabrics (
Figure 6). The enzymatic treatment also yielded weight loss of the fabrics and the release of pectin hydrolysis products observed by peaks at 2–5 min. Under these experimental conditions, polygalacturonase achieved a better scouring result than pectin lyase, expressed in a two-fold faster water absorbency time, six-fold higher cutin degradation products (measured by reverse-phase HPLC-ELSD) and 1.4-fold greater weight loss (
Figure 6).
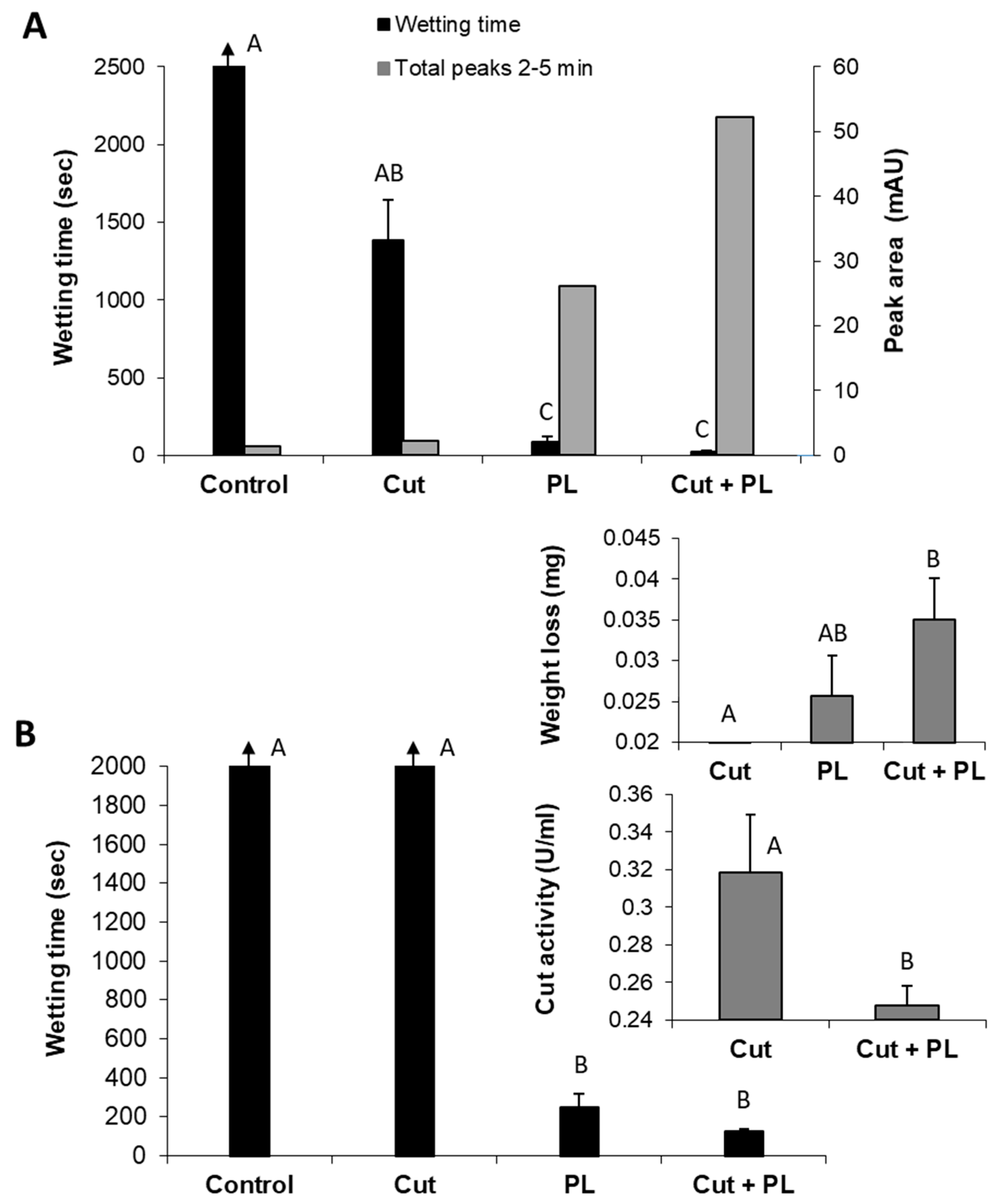
Whereas both polygalacturonase and pectin lyase can effectively scour cotton fabrics, they have different optimal pH values, making attempts to combine them a challenging task. However, pectin lyase and cutinase have similar optimal pH conditions that enable examining their combination for the bio-scouring process. Indeed, the combined treatment of these enzymes resulted in a synergistic effect expressed by high hydrophilicity of the treated cotton knits and the increased appearance of decomposition products (2–5 min peak) in the reaction liquid (
Figure 7A). The combination of these enzymes resulted in a 3.6-fold improvement in the fabrics’ water absorbency (from 87 s to 24 s), in comparison to the pectin lyase alone. Moreover, this enzyme combination with a minute amount of cutinase (1.7 U instead of 80 U, which alone caused an insignificant change), increased the amount of pectin lyase (301 U instead of 200 U), and a shorter reaction time (4 h instead of 24 h), resulted in a two-fold improvement in scouring efficiency (
Figure 7B). However, this combination’s wettability time is still quite long (125 s) and may improve by a longer incubation time. The more intensifying enzymatic reaction (of pectin lyase and cutinase combined) led to increased weight loss (0.02 mg with pectin lyase alone to 0.035 mg in the enzyme mixture) and lower residual cutinase activity (
Figure 7B, insert).
The release of specific hydrolysis products to the reaction fluid by each of the enzymes, pectin lyase (peaks at 2.9 and 4.5 min) and cutinase (peak at 4.1 min), as well as their combination, is shown in
Figure 8. Each enzyme activity separately caused the release of a specific hydrolysis product as expected. However, incubating cotton material with a mixture of both enzymes resulted in a blend of hydrolysis products that is evidently more abundant than combining the sole reactions’ products. This result provides further support for the synergistic effect of cutinase and pectin lyase observed in
Figure 7.
3. Discussion
Enzyme-guided catalysis for bio-scouring in the textile industry is of great interest, accounting for high substrate specificity, less energy consumption, eco-friendly solutions and improved products provided by these proteins. They are used in various desizing, scouring, bleaching, stone washing and dyeing processes [
28].
This work examined the use of enzymes to scour natural textile fabrics. Although cutinase degrades cutin by hydrolyzing ester linkages, whereas polygalacturonase and pectin lyase split the pectin chain’s glycosidic bonds, the final effects of their scouring were similar. Implementation of cutinase or pectinase led to acceptable water absorbency of the treated material, wetting times of 10–25 s, comparable with those reported in other works with lytic enzymes [
20] and alkaline chemical scouring [
11]. The traditional chemical scouring of cotton fibers aims at removing the hydrophobic and non-cellulosic components and producing highly absorbent fibers that can be dyed and finished uniformly. However, these chemicals also attack the cellulose, leading to a reduction in strength and loss of fabric weight. The effect of chemical scouring on the cotton fabrics’ quality (mechanical properties), weight, wetting time, cellulose exposure, color change, and readiness for dyeing and finishing processes carried out afterward is well described in the literature in former studies carried out by us [
11,
23] and others [
8,
20].
Specifically, in this study, the best wettability score of the treated cotton fabric, 10.5 s, in the polygalacturonase treatment or 24 s, achieved by the enzyme mixture of cutinase and pectin lyase, was still higher than that of 8 s by NaOH reported in other studies [
20]. This wetting time was more similar to wettability values received by other studies carried out with enzymes. For example, wettability time was 16.5 s following
Aspergillus aculeatus endo-polygalacturonase bioscouring [
29], 13 s following bioscouring with α-amylase and polygalacturonase enzymes from
Trichoderma harzianum, supplemented with a non-ionic surfactant (Egyptol, 1 g/L) [
30], and 10 s obtained using a mixture of recombinant pectin methylesterase and pectate lyases from
Clostridium thermocellum [
20]. Thus, the wettability process’s effectiveness in this study using mixed enzymes may be considered acceptable compared to chemical treatment.
Still, it should be remembered that the source of the enzymes, their properties, and their titers used may influence the time and effectiveness of the bioscouring. For example, in our previous report, cutinase from
Pseudomonas mandocino achieved a wetting time of 20–30 s after incubation for almost 20 h [
11]. Agrawal et al. [
24] presented a similar result obtained only after 30 min with
Fusarium solani f. pisi cutinase. The combined effect of cutinase and pectate lyase in their study improved the above result even more, achieving almost the same degree of wax removal as solvent extraction (n-hexane for 30 min, 75 °C) within 15 min.
The fabrics’ weight loss results presented here (0.004% in the mixture of cutinase and pectin lyase or 0.02% in the polygalacturonase treatment) are minor compared to the conventional NaOH treatment’s results described in the literature. According to Bristi et al. (2019, [
8]), the cotton fabrics’ weight loss in the textile industry is around 8–12%, which means an excessive loss of fabric weight unnecessarily (the recommended weight loss for caustic scouring is 3–8%). In their experiments, caustic soda scouring resulted in a 6.2% weight loss, while enzymatic scouring with
Scourzyme L. resulted in a 7.5% weight loss. Like in the wetting time measure, the weight loss measure is also influenced by each experiment’s specific settings. Incubation time, temperature, pH, enzyme type, and the titer used, are all may affect the weight loss result. For example, Rajulapati et al. (2020, [
20]) reported that bioscouring with a mixture of recombinant pectin methylesterase and pectate lyases enzymes led to a weight loss of 17.3%, while the untreated cotton fabric (negative control) showed a weight loss of only 0.3%, and the positive control, NaOH treatment, showed a weight loss of 19.1%. In another report, cutinase alone (12.8 U/mL) led to a 4% weight loss after 20 h of incubation, while the positive control, NaOH treatment, resulted in a 14% weight loss [
11].
In the current study, the increase in the fabrics’ wettability and weight loss was attributed to the intensity of the enzymatic hydrolysis of cuticle components, which resulted in the appearance of C:16 and C:18 hydroxy fatty acids in the reaction fluid (based on GC-MS identification). When applied together, cutinase and pectin lyase displayed a synergistic effect, which complies with others’ findings [
24]. Similar extracted products (depicted by a major peak eluted at around 2–5 min), which increased with the treatment’s severity, were measured for both the enzymatic treatment reported here and the previously reported NaOH treatment [
11].
Cotton fibers’ chemical properties are dependent on the plant’s genotype and growth conditions. The fibers are comprised of cellulose (88.0–96.5%), proteins (1.0–1.9%), waxes (0.4–1.2%), pectins (0.4–1.2%), inorganics (0.7–1.6%) and other substances (0.5–0.8) [
4]. Extracellular lytic enzymes, such as lipases, cellulases, pectinases and proteinase, play a pivotal role in phytopathogenic fungi attacks on the host cotton plant. These enzymes degrade the plant’s outer cuticle barrier, enabling pathogen invasion into the inner cellulosic layers. Here, and in previous studies [
10,
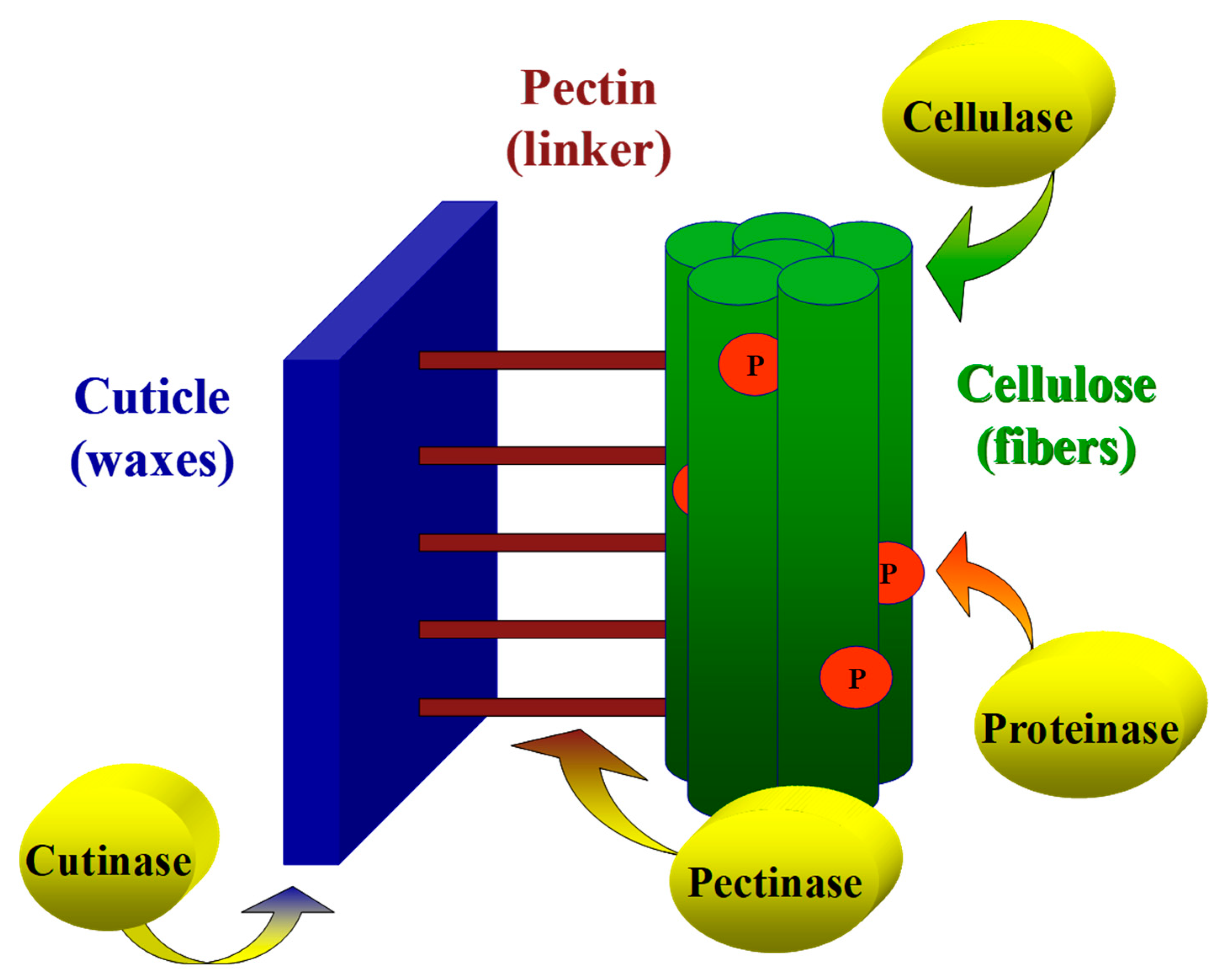
24], it is suggested that the digestion of the cuticle by cutinase results in the enlargement and formation of openings that enable rapid penetration of pectinases into the inner pectinase layer. The combined influence of cutinase and pectinase accelerates the cuticle layer’s removal and enables the subsequent degradation of cellulose in the primary and secondary cell walls. A model that demonstrates the action mechanism of cutinase and pectinase on the cotton fiber cell wall is illustrated in
Figure 9.
Cutinase can improve the wettability of cotton fiber effectively when combined with pectinase, at low temperatures, without the addition of alkali (reviewed by [
31]). However, according to this summary, the cuticle is only partially degraded at relatively low temperatures and can’t be removed completely, leading to the textile’s uneven properties. In contrast, the wax can become soluble and be hydrolyzed entirely at high temperatures (80–90 °C). Still, most cutinases have limited structural stability at those temperatures. Attempts to enhance cutinases’ stability using immobilization technology (see, for example [
32]) led to a disruption of the interaction between cutinase and its substrate. Lately, research studies have aimed at seeking a thermo-stable cutinase that can be used in cotton treatment at hot temperatures. Moreover, cotton fibers also contain the cottonseed coat’s impurities. The cottonseed coat is hard to remove, and its incomplete residues removal during enzymatic scouring can cause difficulty during the subsequent dyeing process. Luckily, the mixture of cutinase with alkaline pectinase or xylanase can improve cottonseed coat degradation during the fabric scouring procedure [
33].
Our results explored the potential of enzyme biotechnology to improve the textile industry scouring step in cotton processing and provide additional support to the results obtained by other researchers [
24]. Following cutinase and pectinase digestion, the fibers’ outer layer constituents found in the reaction medium suggest that a specific degradation of the cutin and pectin layers occurred, with minimal alteration of fiber strength. Indeed, bio-scouring is offered as an adequate procedure for scouring cotton fibers while preserving their natural qualities [
8]. Enzymatic scouring is a repeatable, simple and safe process. Moreover, the use of enzymes is beneficial from an ecological and economic point of view. They reduce water and energy consumption, which ultimately reduces production costs. Nevertheless, much about enzymatic scouring is yet to be discovered. Notwithstanding all the research on enzymatic scouring that has been carried out worldwide in recent decades, it has yet to be applied on an industrial scale [
34]. There is a need for pectinases having stability under alkaline and high-temperature conditions and higher activity. Indeed, dedicated research towards this goal has been gaining momentum in recent years (see, for example [
35,
36]). Such studies are examining attractive biocatalysts alkaline pectinases for the production of wax- and pectin-free fabrics.
4. Materials and Methods
4.1. Chemicals and Enzymes
Cutinase enzyme from Pseudomonas mandocino was obtained from InterSpex Products Inc. (San Mateo, CA, USA), pectin lyase (Pectazyme HF) was received from Rakuto Kasei Ltd. (Yokneam, Israel) and polygalacturonase was obtained from DSM-Gist-Brocades (Heerlen, The Netherlands). Detergents and raw cotton fabrics were kindly supplied by Avco-Chem Ltd. (Tel Aviv, Israel). All chemicals were obtained from Sigma–Aldrich (Rehovot, Israel) unless otherwise indicated.
4.2. Scouring Treatments
4.2.1. Chemical Scouring
Scouring treatments were carried out, as described by [
11]. Raw cotton fabrics were cut into square swatches of approximately 1 gr, dried at 105 °C for 2 h, and weighed precisely using analytical scales. The chemical scouring treatment was performed by boiling dry and weighed fabric samples in 250 mM NaOH solution (1 gr/50 mL fabric-to-liquid ratio) for 30 min, ensuring that the fabrics remained covered during the liquid treatment. The control treatment was conducted by boiling the swatches in double-distilled water (DDW) for the same time.
4.2.2. Enzymatic Scouring
Enzymatic treatment involved the following enzymes: cutinase; pectin lyase; and polygalacturonase [
37] (
Table 2). The treatment was carried out by incubating one dry and weighed swatch of fabric in the reaction solution at a 1 gr cloth/10 mL ratio in 15 mL polycarbonate test tubes (unless otherwise indicated). The reaction mixture contained a non-ionic detergent comprised of a blend of polyoxyethylene fatty alcohols (AvcoPal-AWS, Avco-Chem Ltd., Tel Aviv, Israel) 0.1% (
w/
v), a 100 mM phosphate buffer, pH 8 for cutinase and pectin lyase, and 50 mM acetate buffer, and pH 5 for polygalacturonase and the corresponding enzyme. Enzymatic treatment controls were incubated under the same conditions but without the enzyme. Incubation was performed by placing the test tubes diagonally in a rotary shaker and rotating them at 175 rpm and 37 °C for 20 h. Control tubes were incubated under the same conditions.
Enzyme activity was as indicated in each case. All activities were measured spectrophotometrically. Cutinase activity was tested in an esterase assay based on the degradation of
p-nitrophenyl-butyrate into butyric acid and
p-nitrophenol, which produces a yellow color with a wavelength of 405 nm [
38]. One unit indicates 1 µmol of
p-nitrophenol released/min at 37 °C and pH 8.0. Pectin lyase activity was measured with polygalacturonic acid potassium salt as substrate at 235 nm, as described earlier [
39]. One unit indicates 1 µmol of D-galacturonic acid released/min at 50 °C and pH 7.0. Polygalacturonase activity was also measured with the polygalacturonic acid potassium salt as a substrate. Use of the neocuproine reagent was described previously and reading absorbency was made at 450 nm [
40]. One unit indicates 1 µmol of D-galacturonic acid released/min at 37 °C and pH 5.0.
Following incubation or digestion, the fabric samples were separated from the reaction fluid (squeezed exhaustively using a 50 mL syringe), then rinsed thoroughly with DDW and squeezed. Rinsing and squeezing was repeated three times. The washed samples were then dried at 105 °C and weighed using analytical scales to measure weight loss. The weight loss was calculated by subtracting the final weight from the initial weight. Afterwards, the swatches were used for further water absorbency evaluation. Each treatment comprised at least three independent replicates.
4.3. Staining
To determine the efficiency of the cuticle constituents’ removal for the conventional hot NaOH treatment, a qualitative estimation by staining the treated fabric swatches was applied. For cellulose accessibility, 1% Congo red solution (10 mg/mL in a 20% ethanolic solution) was used for staining the treated fabrics. The removal of pectin substances was confirmed by staining with 1% Ruthenium red (10 mg/mL in DDW). The staining procedure was performed with 1 g fabric swatch/10 mL staining solution for 10 min at 35 °C by shaking (200 rpm). Samples were subsequently washed thoroughly with DDW several times until no color was released.
4.4. Esterase Assay with p-Nitrophenyl Butyrate
Cutinase can be measured spectrophotometrically by following the absorbance change at 405 nm, indicating p-nitrophenol release from
p-nitrophenyl esters of short-chain fatty acids used as a substrate [
38]. This assay is simple, but it does not distinguish between esterases and cutinases. An enzymatic determination of esterase activity can also be made using ethyl butyrate as a substrate and the titration technique, as suggested earlier [
41]. More recently, a cutinase assay based on a specific cutinase substrate, 4-nitrophenyl (16-methyl sulfone ester) hexadecanoate (pNMSEH) was developed [
17,
18]. Since the ethyl butyrate titration assay is time-consuming, and the pNMSEH-assay relies on a substrate that is not commercially available, the
p-nitrophenyl butyrate spectrophotometric assay was chosen for this study. The reaction mixture contains 100 μL of 1 gr/250 mL DDW of the non-ionic detergent Triton
® X-100, 800 μL sodium phosphate buffer (0.1 M, pH = 8), 500 μL 0.1 mM p-nitrophenyl butyrate and 100 μL cutinase enzyme solution in a total volume of 1.5 mL. After vortex mixing the contents, the hydrolysis reaction at 37 °C was followed by measuring absorbance changes at 405 nm with a Shimadzu spectrophotometer (UV-1601). The initial linear rates (up to 20 sec) were used to calculate the rate of reaction (adopted from [
17]). One unit of cutinase (esterase) is defined as the amount of enzyme required to release one micromole p-nitrophenol per ml per min, at the above conditions.
4.5. Lipase Assay for Acidification of the Reaction Solution
The reaction consisted of 1.4 mL bovine serum albumin (BSA) fatty acid-free, buffer phosphate 0.4 M, pH = 8, and cutinase whose activity is calculated as 1 (U/mL) in a bath at 37 °C using a stirrer. The substrate was 1 gr raw untreated cotton swatch, raw cotton fabrics after conventional chemical scouring (see
Section 4.2.1) as a negative control, or 1.4 mL olive oil as a positive control. Changes in the reaction fluid were monitored every 10–15 s at the initial stages and at larger intervals later when the reaction liquid’s acidity changes decreased
. The total reaction time was 12 min. It is expected that the pH drop due to the cutinase activity with raw cotton fibers reflects the release of fatty acids (the substrate degradation products) to the reaction fluid.
4.6. Protein Measurement
Protein concentration determination was done using the Bio-Rad protein assay method according to the manufacturer’s instructions. Briefly, a 200 μL reagent from Bio-Rad (Rishon Le Zion, Israel) and an 800 μL sample were mixed and incubated at room temperature for 10 min. Absorbance was measured at 595 nm. The BSA at concentrations of 0–20 μg/mL was used for the calibration curve.
4.7. Water Absorbency
The treated fabric swatches were evaluated for water repellent ability after washing and drying according to AATCC test method no 27-1977 (AATCC Technical Manual, 1980) by applying a 20 µl water drop. The time (in seconds) between the contact of the water drop deposited onto the fabric surface and its absorbance into the fabric matrix, i.e., the time required for the water’s specular reflection, was recorded as the fabric wetting time. Each fabric swatch was tested in nine (or more) different areas and mean wettability time was determined.
4.8. GC/MS Analysis
GC/MS analysis of the raw fabrics’ extracts was performed after overnight extraction of the fabrics with dichloromethane (DCM) in a Soxhlet apparatus. The hydrophobic ingredients of the extraction fluid were elicitated with chloroform (1:1 v/v) in a separation funnel. The fractionated organic fraction was then evaporated to dryness in a rotary evaporator and kept overnight in a vacuum desiccator. The dried samples after chloroform extraction were silylated in 50 µl dioxane with 50 µl bis-(trimethylsilyl)-trifluoroacetamide (BSTFA) at 60 °C for 30 min. Trimethylsilylated derivatives were separated using a 0.25 mm × 30 m HP5 Phe Me Silicone column on a Hewlett Packard 5972 series gas chromatograph (Palo Alto, CA, USA) with the carrier gas He and detected using a Hewlett Packard 5972 mass selective detector. The column was ramped at 10 °C min−1 from 150 °C to 300 °C and held for 20 min. The detector and injector were set at 300 °C.
4.9. Reverse-Phase (RP) HPLC Analysis
HPLC analysis of the hydrolysis fluid was performed as previously described [
11] in a Hewlett Packard HPLC (HP1100 series, Palo Alto, CA, USA) equipped with UV using a Luna C18 column (25 cm×4.60 mm i.d., 5 m; Phenomenex, Torrance, CA, USA). The UV detector was set at 210 nm. Elution was performed using a gradient system consisting of solvent A (acetonitrile), solvent B (1 mM trifluoroacetic acid solution) and solvent C (tetrahydrofuran) with the following program (in percent
v/
v): initially 50A:25B:25C; linear gradient over 30 min to 60A:20B:20C; linear gradient over 5 min to 60A:5B:35C, then held isocratically for a further 15 min. The flow rate was maintained at 0.85 mL min
−1. All solvents were of far-UV-quality HPLC-grade purity.
4.10. Statistical Analysis
The data were analyzed with the JMP program, 15th edition (SAS Institute Inc., Cary, NC, USA) using the one-way analysis of variance (ANOVA), with post hoc of the Student’s t-test for each pair (without multiple comparisons correction). The significance and confidence level was set at 95%, which indicates p values of less than 0.05 as being statistically significant. The number of repetitions is stated separately for each experiment.