Reciprocal Common Garden Altitudinal Transplants Reveal Potential Negative Impacts of Climate Change on Abies religiosa Populations in the Monarch Butterfly Biosphere Reserve Overwintering Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.1.1. Seed Collection Sites
2.1.2. Provenance Test Sites in Common Gardens
2.2. Production of Plants in Nursery
2.3. Test of Provenances in Common Gardens in the Field
2.4. Measurements
2.5. Estimation of Aerial Biomass
2.6. Climatic Data
2.7. Statistical Analysis
2.7.1. Mixed Model
2.7.2. Selection of Variables to Fit the Best Mixed Model
- (1)
- Five climatic variables were selected that best described the climate of the provenance, estimating the Spearman correlations between the values of the climatic variables and the average value per provenance across sites of the response variables, and selecting those with the highest |r| value.
- (2)
- The climatic variables for the climatic transfer distance were selected by fitting a reduced mixed model, eliminating from the model (Equation (2)) the term of climate of the provenance (Cj) and its respective interaction (Dij × Cj). The five climatic variables selected were those for which the model obtained the lowest (and thus the best) value of the Akaike information criterion (AIC) and, in addition, that necessarily presented the estimated parameter of the quadratic term both negative and significant, in order to ensure that it was biologically sound [15,16].
- (3)
- Subsequently, 5 × 5 = 25 full “competing” models were run, which included all of the possible combinations of the five variables of the climate of provenance and the five variables of climatic transfer distance preselected in the previous two steps. The best model was selected based on the AIC value.
3. Results
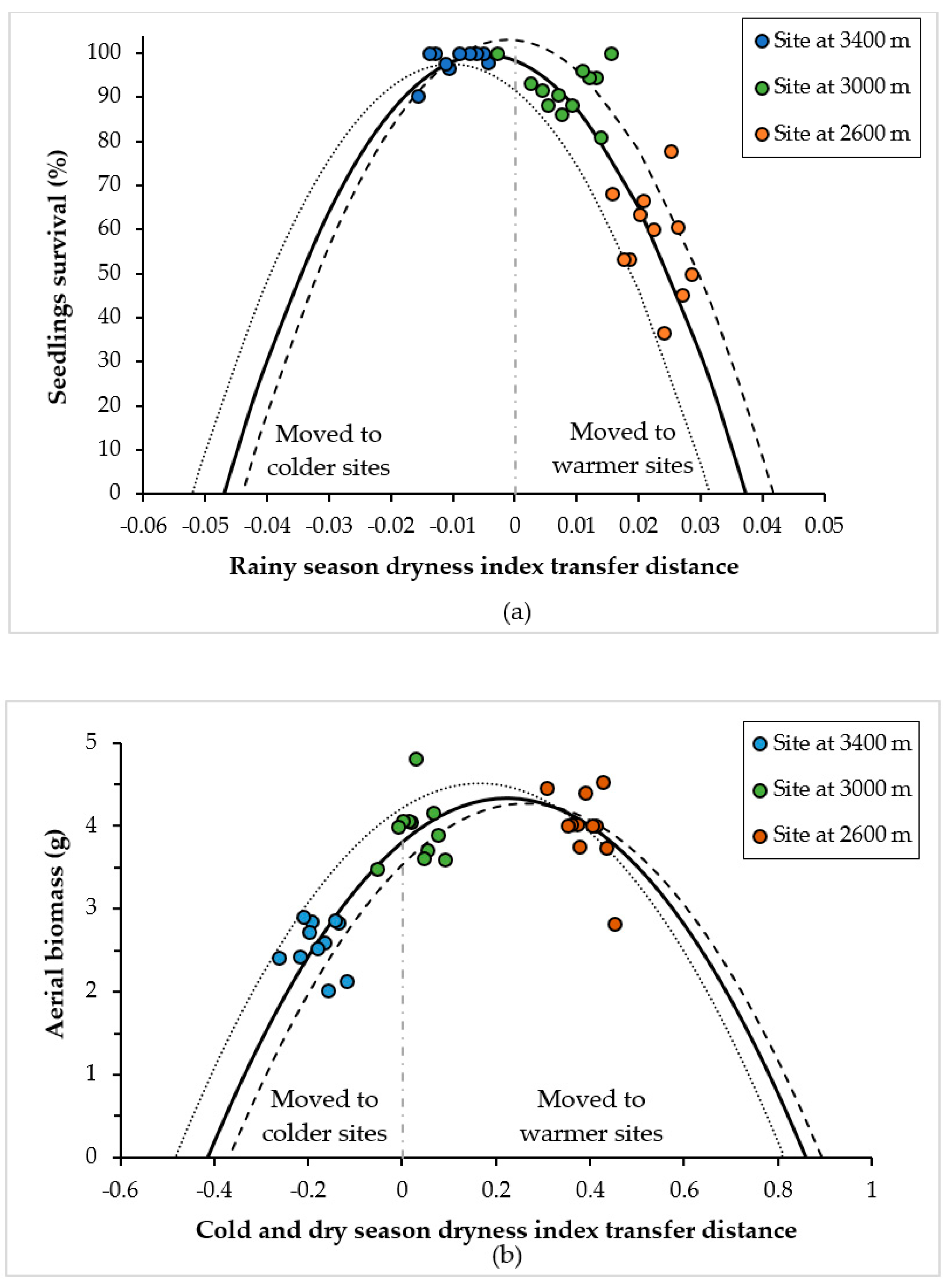
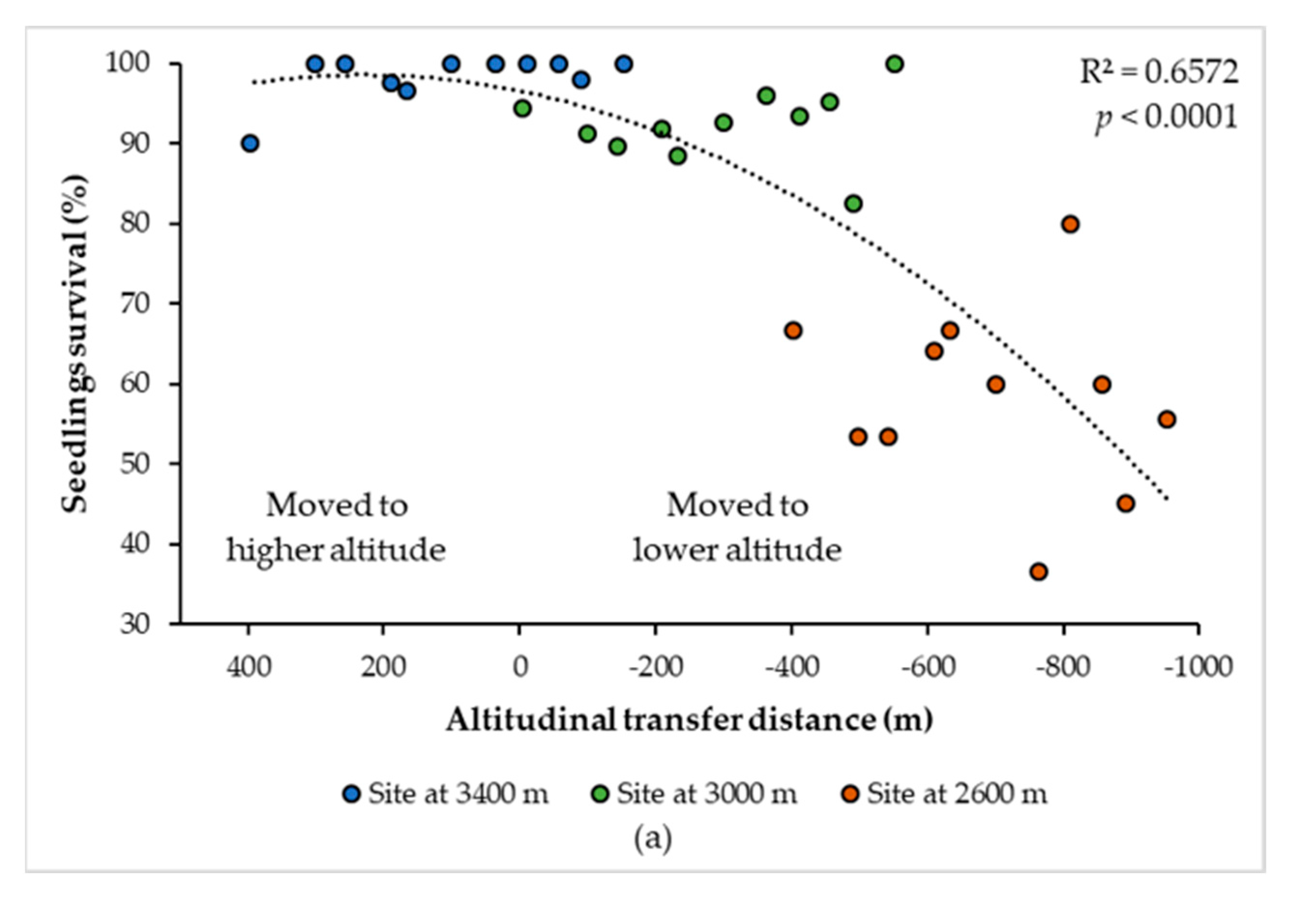
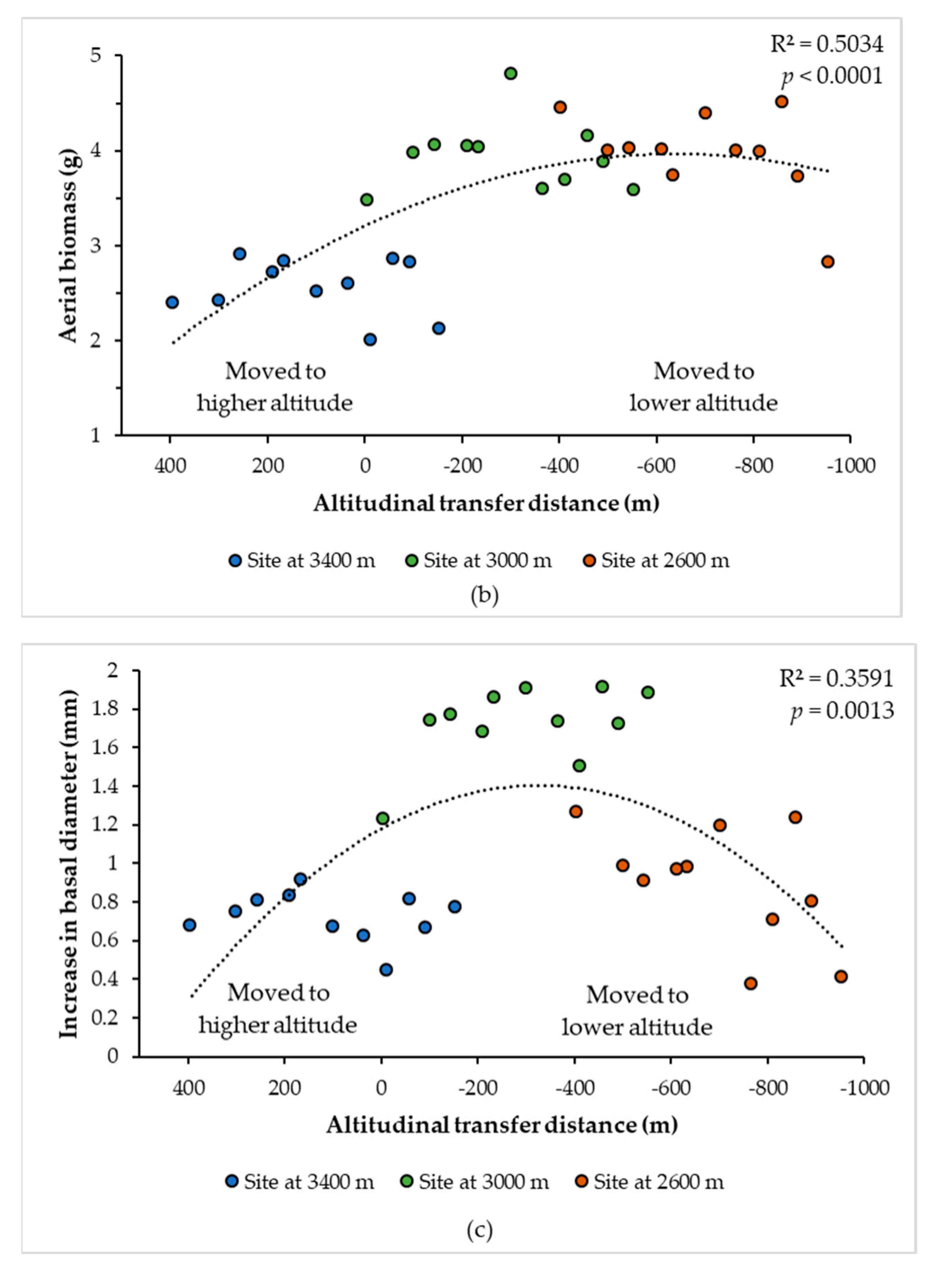
3.1. Climatic Variables That Best Explained the Climatic Transfer Distance Response Function
3.2. Curves of Response to the Climatic and Altitudinal Transfer Distance
4. Discussion
Implications for Management of Abies religiosa Inside the MBBR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, G.; Shevliakova, E.; Artaxo, P.; De Noblet-Ducoudré, N.; Houghton, R.; House, J.; Kitajima, K.; Lennard, C.; Popp, A.; Sirin, A.; et al. Land–Climate Interactions. In Climate Change and Land: An Ipcc Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019; pp. 1–186. [Google Scholar]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Rehfeldt, G.E.; Leites, L.P.; Joyce, D.G.; Weiskittel, A.R. Role of population genetics in guiding ecological responses to climate. Glob. Chang. Biol. 2017, 24, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Matias, L. Changes in the distribution limits of tree species as a consequence of climate variations. Ecosistemas 2012, 21, 91–96. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, R.I.; Fady, B.; Vendramin, G.G.; Dawson, I.K.; Fleming, R.A.; Sáenz-Romero, C.; Lindig-Cisneros, R.A.; Murdock, T.; Vinceti, B.; Navarro, C.M.; et al. The role of forest genetic resources in responding to biotic and abiotic factors in the context of anthropogenic climate change. For. Ecol. Manag. 2014, 333, 76–87. [Google Scholar] [CrossRef]
- Rubin-Aguirre, A.; Sáenz-Romero, C.; Lindig-Cisneros, R.; del-Rio-Mora, A.; Tena-Morelos, C.; Campos-Bolaños, R.; del-Val, E. Bark beetle pests in an altitudinal gradient of a Mexican managed forest. For. Ecol. Manag. 2015, 343, 73–79. [Google Scholar] [CrossRef]
- del-Val, E.; Sáenz-Romero, C. Bark-beetles (Coleoptera: Curculionidae) and climate change: Current situation and perspectives for temperate forests. TIP Rev. Espec. Cienc. Químico Biológicas 2017, 20, 53–60. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; López-García, J. Detection of trees damaged by pests in Abies religiosa forests in the Monarch Butterfly Biosphere reserve using infrared aerial photography. Investig. Geográficas 2017, 1–12. [Google Scholar] [CrossRef]
- Morales-Rangel, A.; Cambrón-Sandoval, V.H.; Soto-Correa, J.C.; Jones, R.W.; Obregón-Zúñiga, J.A. Temperature effect in populations of Dendroctonus frontalis Zimmerman and Dendroctonus mexicanus Hopkins (Coleoptera: Curculionidae: Scolytinae) under a climate change scenario in Sierra Gorda queretana. Acta Zoológica Mex. 2018, 34, 1–8. [Google Scholar] [CrossRef]
- Flores-Nieves, P.; López-López, M.Á.; Ángeles-Pérez, G.; de la Isla-Serrano, M.d.L.; Calva-Vásquez, G. Biomass estimation and distribution models of Abies religiosa (Kunth) Schltdl. et Cham. In decline. Rev. Mex. Cienc. For. 2011, 2, 9–20. [Google Scholar]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Mátyás, C.; Vendramin, G.G.; Fady, B. Forests at the limit: Evolutionary–genetic consequences of environmental changes at the receding (xeric) edge of distribution. Report from a research workshop. Ann. For. Sci. 2009, 66, 800. [Google Scholar] [CrossRef]
- Mátyás, C. Forecasts needed for retreating forests. Nature 2010, 464, 1271. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, G.E.; Wykoff, W.R.; Ying, C.C. Physiologic plasticity, evolution, and impacts of a changing climate on Pinus contorta. Clim. Chang. 2001, 50, 355–376. [Google Scholar]
- Leites, L.P.; Rehfeldt, G.E.; Robinson, A.P.; Crookston, N.L.; Jaquish, B. Possibilities and limitations of using historic provenance tests to infer forest species growth responses to climate change. Nat. Resour. Model. 2012, 25, 409–433. [Google Scholar] [CrossRef]
- Rehfeldt, G.E. Ecological Genetics of Pinus contorta from the Rocky Mountains (USA): A Synthesis. Silvae Genet. 1988, 37, 3–4. [Google Scholar]
- Rehfeldt, G. A model of genetic variation for Pinus ponderosa in the Inland Northwest (USA): Applications in gene resource management. Can. J. For. Res. 1991, 21, 1491–1500. [Google Scholar] [CrossRef]
- Ruiz-Talonia, L.; Sánchez-Vargas, N.; Bayuelo-Jiménez, J.; Lara-Cabrera, S.; Sáenz-Romero, C. Altitudinal genetic variation among native Pinus patula provenances: Performance in two locations, seed zone delineation and adaptation to climate change. Silvae Genet. 2014, 63, 139–148. [Google Scholar] [CrossRef]
- Ortiz-Bibian, M.; Blanco-García, A.; Lindig-Cisneros, R.; Gómez-Romero, M.; Castellanos-Acuña, D.; Herrerías-Diego, Y.; Sánchez-Vargas, N.; Sáenz-Romero, C. Genetic variation in Abies religiosa for quantitative traits and delineation of elevational and climatic zoning for maintaining monarch butterfly overwintering sites in Mexico, considering climatic change. Silvae Genet. 2017, 66, 14–23. [Google Scholar] [CrossRef]
- Zobel, B.; Talbert, J. Applied Forest Tree Improvement; John Wiley & Sons: North Carolina State Univ., NC, USA, 1984. [Google Scholar]
- Viveros-Viveros, H.; Sáenz-Romero, C.; López-Upton, J.; Vargas-Hernández, J.J. Growth and frost damage variation among Pinus pseudostrobus, P. montezumae and P. hartwegii tested in Michoacán, México. For. Ecol. Manag. 2007, 253, 81–88. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Tapia-Olivares, B. Genetic variation in frost damage and seed zone delineation within an altitudinal transect of Pinus devoniana (P. michoacana) in Mexico. Silvae Genet. 2008, 57, 165–170. [Google Scholar] [CrossRef]
- Loya-Rebollar, E.; Sáenz-Romero, C.; Lindig-Cisneros, R.; Lobit, P.; Villegas-Moreno, J.; Sánchez-Vargas, N. Clinal variation in Pinus hartwegii populations and its application for adaptation to climate change. Silvae Genet. 2013, 62, 86–95. [Google Scholar] [CrossRef]
- Oberhauser, K.; Peterson, A.T. Modeling current and future potential wintering distributions of eastern North American monarch butterflies. Proc. Natl. Acad. Sci. USA 2003, 100, 14063–14068. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Rehfeldt, G.E.; Duval, P.; Lindig-Cisneros, R.A. Abies religiosa habitat prediction in climatic change scenarios and implications for monarch butterfly conservation in Mexico. For. Ecol. Manag. 2012, 275, 98–106. [Google Scholar] [CrossRef]
- de Pascual Pola, C.N.; Musálem, M.Á.; Ortega-Alcalá, J. Study of some characteristics of cones and seeds of Abies religiosa (HBK) Schl. et Cham. Agrociencia 2003, 37, 521–531. Available online: http://www.redalyc.org/articulo.oa?id=30237509 (accessed on 10 August 2020).
- Ortiz-Bibian, M.A.; Castellanos-Acuña, D.; Gómez-Romero, M.; Lindig-Cisneros, R.; Silva-Farías, M.Á.; Sáenz-Romero, C. Variation among Abies religiosa (H.B.K.) Schl. et Cham populations along an altitudinal gradient. I. Seed germination capacity. Rev. Fitotec. Mex. 2019, 42, 301–308. [Google Scholar]
- Guzmán-Aguilar, G.; Carbajal-Navarro, A.; Sáenz-Romero, C.; Herrerías-Diego, Y.; López-Toledo, L.; Blanco-García, A. Abies religiosa seedling limitations for passive restoration practices at the Monarch Butterfly Biosphere Reserve in Mexico. Front. Ecol. Evol. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Gómez-Pineda, E.; Sáenz-Romero, C.; Ortega-Rodríguez, J.M.; Blanco-García, A.; Madrigal-Sánchez, X.; Lindig-Cisneros, R.; López-Toledo, L.; Pedraza-Santos, M.E.; Rehfeldt, G.E. Suitable climatic habitat changes for Mexican conifers along altitudinal gradients under climatic change scenarios. Ecol. Appl. 2020, 30, e02041. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, J.; Gégout, J.-C.; Marquet, P.; De Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Jump, A.S.; Mátyás, C.; Peñuelas, J. The altitude-for-latitude disparity in the range retractions of woody species. Trends Ecol. Evol. 2009, 24, 694–701. [Google Scholar] [CrossRef]
- Carbajal-Navarro, A.; Navarro-Miranda, E.; Blanco-García, A.; Cruzado-Vargas, A.L.; Gómez-Pineda, E.; Zamora-Sánchez, C.; Pineda-García, F.; O’Neill, G.; Gómez-Romero, M.; Lindig-Cisneros, R.; et al. Ecological restoration of Abies religiosa forests using nurse plants and assisted migration in the Monarch Butterfly Biosphere Reserve, Mexico. Front. Ecol. Evol. 2019, 7, 421. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Rehfeldt, G.E.; Crookston, N.L.; Duval, P.; St-Amant, R.; Beaulieu, J.; Richardson, B.A. Spline models of contemporary, 2030, 2060 and 2090 climates for Mexico and their use in understanding climate-change impacts on the vegetation. Clim. Chang. 2010, 102, 595–623. [Google Scholar] [CrossRef]
- Vargas, A.L.C. Variation of quantitative Characters between Procedures of Abies religiosa Originated in the Altitudinal Gradient in the Reserve of the Biosphere of the Monarch Butterfly: Stage of Vivero. Master’s Thesis, Universidad Autónoma Chapingo, Texcoco de Mora, Estado de México, México, 2017. Available online: http://repositorio.chapingo.edu.mx:8080/bitstream/handle/20.500.12098/425/mccf-cval_17.pdf?sequence=1&isAllowed=y (accessed on 15 August 2020).
- Sánchez, C.Z. Variación Genética en Abies religiosa (Kunth) Schltdl. et Cham. a lo Largo de un Gradiente Altitudinal en Respuesta al Estrés por Sequía. Master’s Thesis, Universidad Michoacana de San Nicolás de Hidalgo, Morelia, Michoacán, México, 2020. Available online: http://bibliotecavirtual.dgb.umich.mx:8083/xmlui/bitstream/handle/DGB_UMICH/1912/IIAF-M-2020-0195.pdf?sequence=1&isAllowed=y (accessed on 15 August 2020).
- Sáenz-Ceja, J.E.; Pérez-Salicrup, D.R. Modification of fire regimes inferred from the age structure of two conifer species in a tropical montane forest, Mexico. Forests 2020, 11, 1193. [Google Scholar] [CrossRef]
- Flores-Martínez, J.J.; Martínez-Pacheco, A.; Rendón-Salinas, E.; Rickards, J.; Sarkar, S.; Sánchez-Cordero, V. Recent forest cover loss in the core zones of the Monarch Butterfly Biosphere Reserve in Mexico. Front. Environ. Sci. 2019, 7, 167. [Google Scholar] [CrossRef]
- Prieto-Ruíz, J.A.; Bustamante-García, V.; Muñoz-Flores, H.J.; Álvarez-Zagoya, R. Análisis de conos y semillas en coníferas. In Técnicas en el Manejo Sustentable de los Recursos Naturales; Ocañas, F.G., González, J.A.G., Mendoza, H.V., Parra, A.C., Eds.; Facultad de Ciencias Forestales, Universidad Autónoma de Nuevo León: Monterrey, Mexico, 2014; pp. 27–44. [Google Scholar]
- Research on Forest Climate Change: Potential Effects of Global Warming on Forests and Plant Climate Relationships in Western North America and Mexico. Available online: http://charcoal.cnre.vt.edu/climate/ (accessed on 1 November 2020).
- Arriola, P.; Flores, G.; Gijón, H.; Pineda, O.; Jacob, C.; de Pascual, P.N. Producción de Planta de Abies Religiosa (Kunth) Schltdl. & Cham. en Vivero; Folleto Técnico Núm 19; CENID-COMEF, INIFAP: Ciudad de México, Mexico, 2015. [Google Scholar]
- Martinez-Luna, J.E.; Carrillo-Anzures, F.; Acosta-Mireles, M.; Sánchez, M.E.R.; Perez-Miranda, R. Allometric equations to estimate carbon in seedlings of Pinus hartwegii Lindl. Rev. Mex. Cienc. For. 2020, 11, 11. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT (Version 9.4) Computer Software; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- SAS Institute. Base SAS 9.4 Procedures Guide, 5th ed.; SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Sáenz-Romero, C.; Rehfeldt, G.E.; Ortega-Rodríguez, J.M.; Marín-Togo, M.C.; Madrigal-Sánchez, X. Pinus leiophylla suitable habitat for 1961–1990 and future climate. Bot. Sci. 2015, 93, 709–718. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Lamy, J.B.; Ducousso, A.; Musch, B.; Ehrenmann, F.; Delzon, S.; Cavers, S.; Chałupka, W.; Dağdaş, S.; Hansen, J.K.; et al. Adaptive and plastic responses of Quercus petraea populations to climate across Europe. Glob. Chang. Biol. 2017, 23, 2831–2847. [Google Scholar] [CrossRef]
- Mátyás, C. Modeling climate change effects with provenance test data. Tree Physiol. 1994, 14, 797–804. [Google Scholar] [CrossRef]
- Leites, L.P.; Robinson, A.P.; Rehfeldt, G.E.; Marshall, J.D.; Crookston, N.L. Height-growth response to climatic changes differs among populations of Douglas-fir: A novel analysis of historic data. Ecol. Appl. 2012, 22, 154–165. [Google Scholar] [CrossRef]
- Miranda, R.P.; Sánchez, F.M.; Hernández, A.G.; Padilla, V.J.A. Distribution of Abies religiosa (Kunth) Schltdl. et. Cham. and Pinus montezumae Lamb. in the face of climate change. Rev. Mex. Cienc. For. 2014, 5, 18–33. [Google Scholar]
- Islas-Báez, A.; Pérez-Miranda, R.; González-Hernández, A.; Romero-Sánchez, M.E.; Velasco-Bautista, E. Risk habitat of the monarch butterfly (Danaus plexippus) by climate change scenarios. Ra Ximhai 2015, 11, 49–59. [Google Scholar]
- Sánchez-Velásquez, L.R.; Pineda-López, M.R.; Ibarra-Zavaleta, S.P.; López-Serrano, Y. Fir forest demography using matrix projections: Anomaly precipitation due to climatic change decrease population viability. For. Ecol. Manag. 2021, 482, 118845. [Google Scholar] [CrossRef]



| Altitude (m a.s.l) | Lat. N | Long. W | MAT (°C) | MAP (mm) | MTCM (°C) | ADI Index | WDSDI Index | CDSDI Index | RSDI Index |
|---|---|---|---|---|---|---|---|---|---|
| 3552 | 19.564 | 100.229 | 8.5 | 1107 | 6.2 | 0.034 | 0.152 | 0.160 | 0.029 |
| 3491 | 19.567 | 100.233 | 8.8 | 1094 | 6.5 | 0.035 | 0.161 | 0.176 | 0.030 |
| 3457 | 19.571 | 100.235 | 9.0 | 1089 | 6.7 | 0.036 | 0.165 | 0.184 | 0.031 |
| 3411 | 19.573 | 100.237 | 9.3 | 1079 | 7.0 | 0.038 | 0.173 | 0.199 | 0.032 |
| 3364 | 19.575 | 100.234 | 9.5 | 1065 | 7.2 | 0.039 | 0.183 | 0.206 | 0.033 |
| 3300 | 19.579 | 100.231 | 9.9 | 1048 | 7.5 | 0.041 | 0.191 | 0.222 | 0.035 |
| 3233 | 19.580 | 100.224 | 10.2 | 1029 | 7.8 | 0.043 | 0.201 | 0.233 | 0.036 |
| 3210 | 19.581 | 100.220 | 10.3 | 1022 | 8.0 | 0.044 | 0.204 | 0.238 | 0.037 |
| 3143 | 19.581 | 100.214 | 10.6 | 1001 | 8.3 | 0.046 | 0.216 | 0.250 | 0.039 |
| 3099 | 19.586 | 100.214 | 10.8 | 987 | 8.5 | 0.047 | 0.226 | 0.260 | 0.040 |
| 3003 | 19.595 | 100.210 | 11.3 | 969 | 8.9 | 0.050 | 0.253 | 0.303 | 0.041 |
| Code | Unit | Definition |
|---|---|---|
| MAT | °C | Mean annual temperature |
| MAP | mm | Mean annual precipitation |
| GSP | mm | Growing season precipitation (total precipitation from April–September) |
| WDSP | mm | Warm and dry season (total precipitation March–May) |
| RSP | mm | Rainy season (total precipitation June–October) |
| CDSP | mm | Cold and dry season (total precipitation November–February) |
| MTCM | °C | Mean temperature in the coldest month |
| MMIN | °C | Mean minimum temperature in the coldest month |
| MTWM | °C | Mean temperature in the warmest month |
| MMAX | °C | Mean maximum temperature in the warmest month |
| DD5 | °C | Degree-days > 5 °C |
| WDSDD5 | °C | Warm and dry season (March–May degree days > 5 °C) |
| RSDD5 | °C | Rainy season (June–October degree days > 5 °C) |
| CDSDD5 | °C | Cold and dry season (November–February degree days > 5 °C) |
| ADI | index | Annual dryness index () |
| GSDI | index | Growing season dryness index () |
| WDSDI | index | Warm and dry season (March–May) dryness index ( |
| RSDI | index | Rainy season (June–October) dryness index () |
| CDSDI | index | Cold and dry season (November–February) dryness index ( |
| Site | Altitude (m a.s.l.) | Lat. N | Long. W | MAT (°C) | MAP (mm) | MTCM (°C) | ADI Index | WDSDI Index | CDSDI Index | RSDI Index |
|---|---|---|---|---|---|---|---|---|---|---|
| Llano Grande 1 | 3400 | 19.57 | 100.23 | 9.3 | 1076 | 7.0 | 0.026 | 0.198 | 0.041 | 0.026 |
| La Mesa 2 | 3000 | 19.58 | 100.18 | 11.3 | 951 | 8.9 | 0.056 | 0.537 | 0.251 | 0.044 |
| Tlalpujahua 3 | 2600 | 19.80 | 100.16 | 12.8 | 906 | 9.6 | 0.078 | 0.930 | 0.611 | 0.057 |
| Parameter or Source of Variation | Survival | Biomass | Increase in Basal Diameter | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fixed Effects | Estimate | p | Estimate | p | Estimate | p | |||
| Akaike Information Criterion | 1651.1 | --- | 2397.2 | --- | 1917.3 | --- | |||
| Intercept | 119.7 | 0.0115 | 2.4 | 0.0478 | 0.081 | 0.8824 | |||
| Climate at seed source | |||||||||
| Warm and dry season dryness index (WDSDI) | −111.3 | 0.0941 | 6.8 | 0.0198 | --- | --- | |||
| Mean temperature in the coldest month (MTCM) | --- | --- | --- | --- | 0.19 | 0.0036 | |||
| Climate transfer distance | |||||||||
| Rainy season dryness index (RSDI) | 1426.8 | 0.3542 | --- | --- | --- | --- | |||
| Cold and dry season dryness index (CDSDI) | --- | --- | 8.7 | 0.0002 | --- | --- | |||
| Annual dryness index (ADI) | --- | --- | --- | --- | 143.5 | <0.0001 | |||
| (Climate transfer distance)2 | |||||||||
| RSDI2 | −55,955 | 0.0112 | --- | --- | --- | --- | |||
| CDSDI2 | --- | --- | −10.6 | <0.0001 | --- | --- | |||
| ADI2 | --- | --- | --- | --- | −1291.2 | <0.0001 | |||
| Interaction climate seed source × Climate transfer distance | −10,202 | 0.1273 | −20.6 | 0.0395 | −14.7 | <0.0001 | |||
| Random Effects | Variance | % * | p | Variance | % * | p | Variance | % * | p |
| Site | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| Population | 1.5 | 0.34 | 0.4412 | 0.042 | 3.10 | 0.1217 | 0.010 | 1.4 | 0.1364 |
| Block (Site) | 91.2 | 21.02 | 0.0252 | 0.097 | 7.19 | 0.0196 | 0.108 | 15.04 | 0.0095 |
| Site × Population | 0 | 0 | 1 | 0.019 | 1.43 | 0.2036 | 0 | 0 | 1 |
| Error | 341.0 | 78.64 | <0.0001 | 1.195 | 88.28 | <0.0001 | 0.598 | 83.55 | <0.0001 |
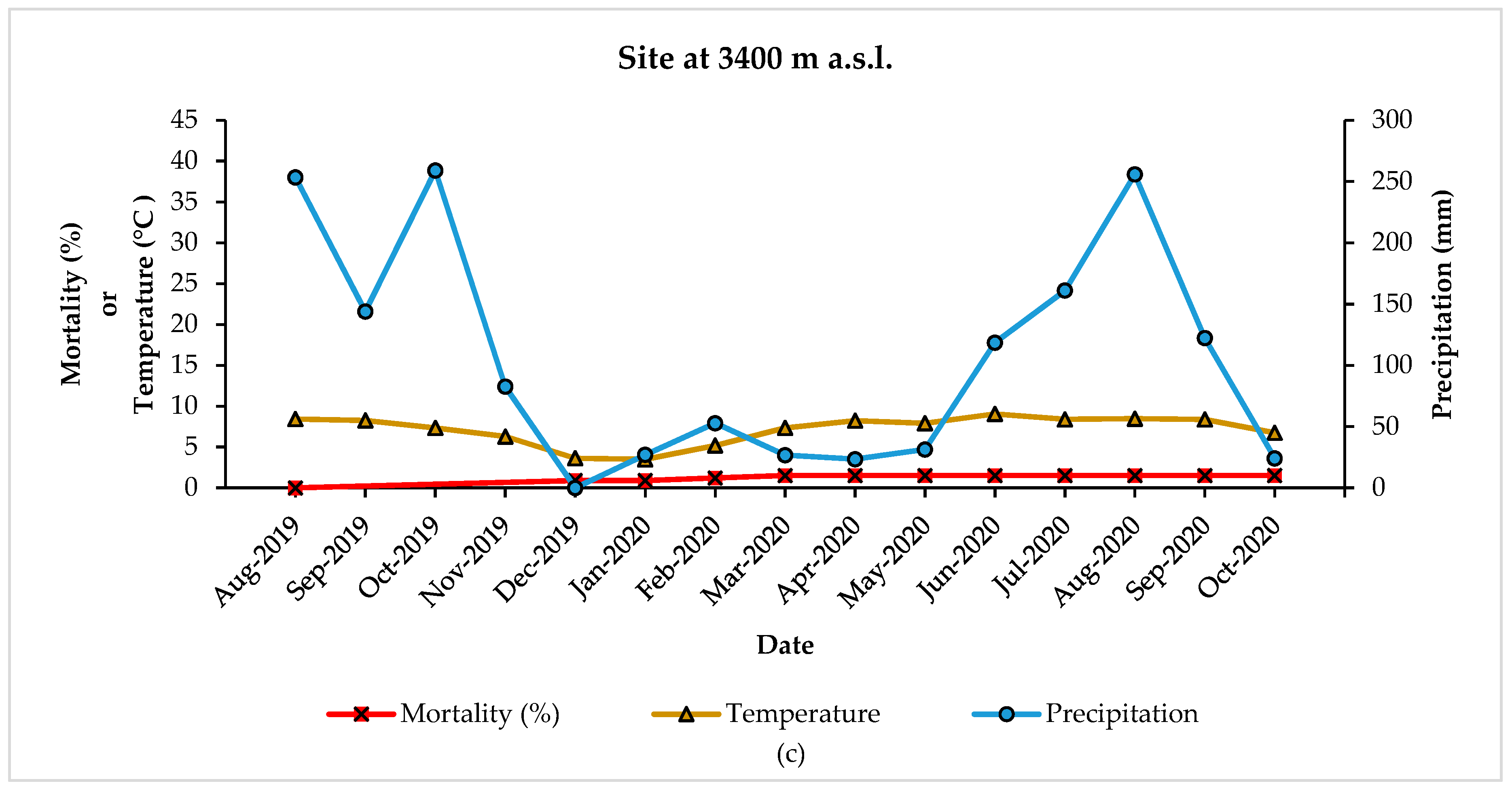
| Climatic Variable | Reference Period 1961–1990 | December 2019–November 2020 | Difference |
|---|---|---|---|
| MAT | 11.13 °C | 11.71 °C | +0.58 °C |
| MAP | 980 mm | 952.7 mm | −27.3 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruzado-Vargas, A.L.; Blanco-García, A.; Lindig-Cisneros, R.; Gómez-Romero, M.; Lopez-Toledo, L.; de la Barrera, E.; Sáenz-Romero, C. Reciprocal Common Garden Altitudinal Transplants Reveal Potential Negative Impacts of Climate Change on Abies religiosa Populations in the Monarch Butterfly Biosphere Reserve Overwintering Sites. Forests 2021, 12, 69. https://doi.org/10.3390/f12010069
Cruzado-Vargas AL, Blanco-García A, Lindig-Cisneros R, Gómez-Romero M, Lopez-Toledo L, de la Barrera E, Sáenz-Romero C. Reciprocal Common Garden Altitudinal Transplants Reveal Potential Negative Impacts of Climate Change on Abies religiosa Populations in the Monarch Butterfly Biosphere Reserve Overwintering Sites. Forests. 2021; 12(1):69. https://doi.org/10.3390/f12010069
Chicago/Turabian StyleCruzado-Vargas, Ana Laura, Arnulfo Blanco-García, Roberto Lindig-Cisneros, Mariela Gómez-Romero, Leonel Lopez-Toledo, Erick de la Barrera, and Cuauhtémoc Sáenz-Romero. 2021. "Reciprocal Common Garden Altitudinal Transplants Reveal Potential Negative Impacts of Climate Change on Abies religiosa Populations in the Monarch Butterfly Biosphere Reserve Overwintering Sites" Forests 12, no. 1: 69. https://doi.org/10.3390/f12010069
APA StyleCruzado-Vargas, A. L., Blanco-García, A., Lindig-Cisneros, R., Gómez-Romero, M., Lopez-Toledo, L., de la Barrera, E., & Sáenz-Romero, C. (2021). Reciprocal Common Garden Altitudinal Transplants Reveal Potential Negative Impacts of Climate Change on Abies religiosa Populations in the Monarch Butterfly Biosphere Reserve Overwintering Sites. Forests, 12(1), 69. https://doi.org/10.3390/f12010069






