Effectiveness and Safety of Apixaban vs. Warfarin in Venous Thromboembolism Patients with Obesity and Morbid Obesity
Abstract
1. Introduction
2. Experimental Section
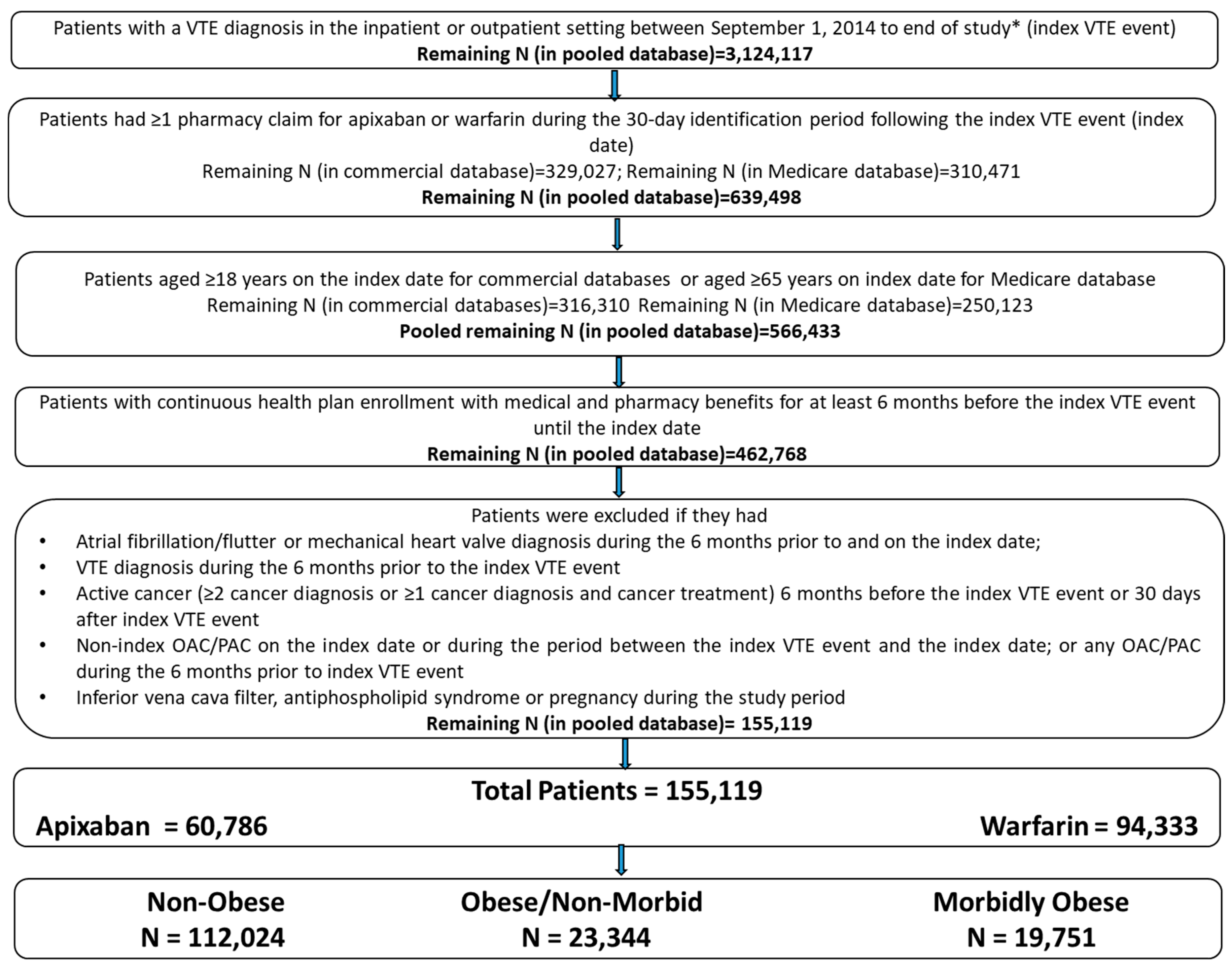
2.1. Data Source and Patient Selection
2.2. Patient Cohorts
- Obese Patients: Obesity was identified based on diagnosis codes that indicated obesity or codes that indicated a BMI of at least 30 (International Classification of Diseases, Ninth Clinical Modification [ICD-9-CM]: 278, V85.3, V85.4; International Classification of Diseases, Tenth Revisions, Clinical Modification [ICD-10-CM]: E66, Z68.3, Z68.4). Obese patients were further classified as morbidly obese and obese/non-morbid.
- Morbidly obese patients were identified based on diagnosis codes that indicated morbid obesity or codes that indicated a BMI of at least 40 (ICD-9-CM: 278.01, V85.4; ICD-10-CM: E66.01, E66.2, Z68.4).
- The remaining obese patients that were not classified as morbidly obese were designated as obese/non-morbid.
- Non-Obese Patients: Patients not classified as obese were categorized as non-obese patients.
2.3. Outcome Measures
2.4. Statistical Methods
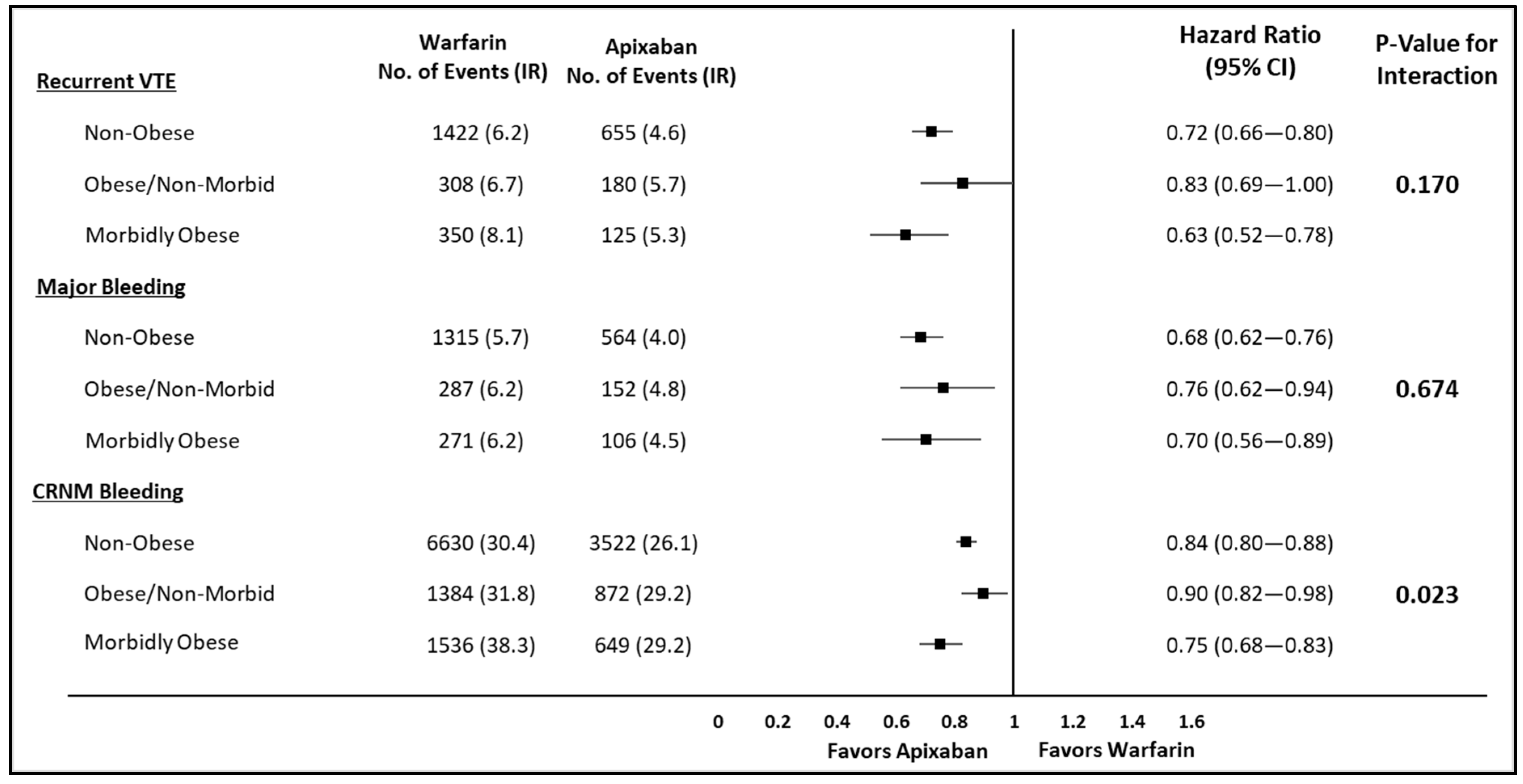
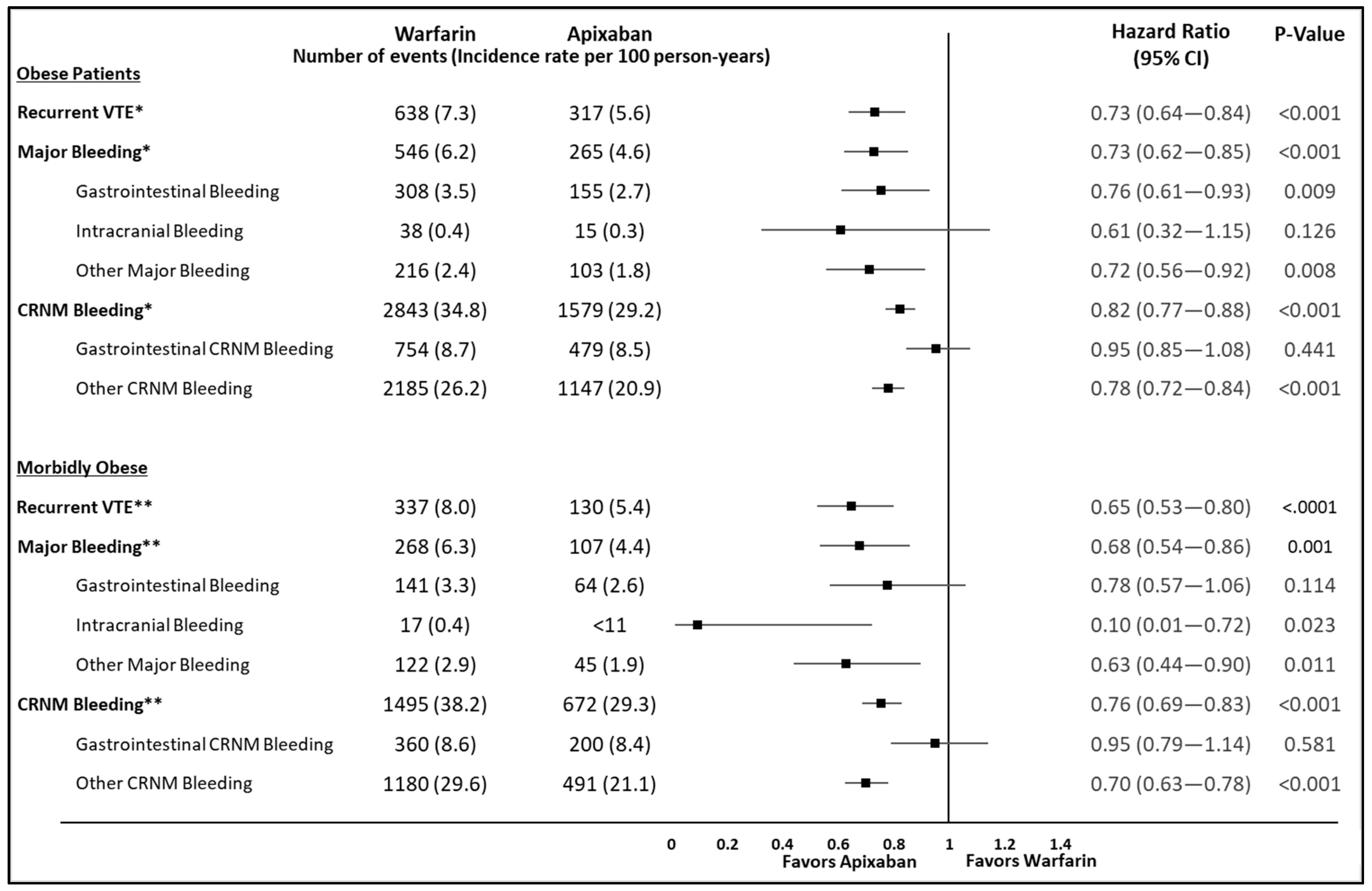
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beckman, M.G.; Hooper, W.C.; Critchley, S.E.; Ortel, T.L. Venous Thromboembolism: A public health concern. Am. J. Prev. Med. 2010, 38 (Suppl. S4), S495–S501. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.E.; Silverstein, R.; Bratzler, D.W.; Heit, J.A.; White, R.H. Surveillance for Deep Vein Thrombosis and Pulmonary Embolism. Am. J. Prev. Med. 2010, 38, S502–S509. [Google Scholar] [CrossRef]
- Moll, S.; Crona, D.J.; Martin, K. Direct oral anticoagulants in extremely obese patients: OK to use? Res. Pr. Thromb. Haemost. 2018, 3, 152–155. [Google Scholar] [CrossRef]
- Yang, G.; De Staercke, C.; Yang, W.C.H.G. The effects of obesity on venous thromboembolism: A review. Open J. Prev. Med. 2012, 2, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Blokhin, I.O.; Lentz, S.R. Mechanisms of thrombosis in obesity. Curr. Opin. Hematol. 2013, 20, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Ornelas, J.; Blaivas, A.; Jimenez, D.; Bounameaux, H.; Huisman, M.; King, C.S.; Morris, T.A.; Sood, N.; et al. Antithrombotic Therapy for VTE Disease. Chest 2016, 149, 315–352. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, M.; Fang, M.C. Assessing bleeding risk in patients taking anticoagulants. J. Thromb. Thrombolysis 2013, 35, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Ogunsua, A.A.; Touray, S.; Lui, J.K.; Ip, T.; Escobar, J.V.; Gore, J. Body mass index predicts major bleeding risks in patients on warfarin. J. Thromb. Thrombolysis 2015, 40, 494–498. [Google Scholar] [CrossRef]
- Lentz, S.R. Thrombosis in the setting of obesity or inflammatory bowel disease. Blood 2016, 128, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- McCaughan, G.J.B.; Favaloro, E.J.; Pasalic, L.; Curnow, J. Anticoagulation at the extremes of body weight: Choices and dosing. Expert Rev. Hematol. 2018, 11, 817–828. [Google Scholar] [CrossRef]
- Martin, K.; Beyer-Westendorf, J.; Davidson, B.L.; Huisman, M.V.; Sandset, P.M.; Moll, S. Use of the direct oral anticoagulants in obese patients: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2016, 14, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Masiukiewicz, U.; Pak, R.; Thompson, J.; Raskob, G.E.; et al. Oral Apixaban for the Treatment of Acute Venous Thromboembolism. N. Engl. J. Med. 2013, 369, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Kearon, C.; Kakkar, A.K.; Mismetti, P.; Schellong, S.; Eriksson, H.; Baanstra, D.; Schnee, J.; Goldhaber, S.Z. Dabigatran versus Warfarin in the Treatment of Acute Venous Thromboembolism. N. Engl. J. Med. 2009, 361, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Buller, H.R.; Décousus, H.; Grosso, M.A.; Mercuri, M.; Middeldorp, S.; Prins, M.H.; Raskob, G.E.; Schellong, S.M.; Schwocho, L.; Segers, A.; et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N. Engl. J. Med. 2013, 369, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Bauersachs, R.; Berkowitz, S.D.; Brenner, B.; Buller, H.R.; Décousus, H.; Gallus, A.S.; Lensing, A.W.; Misselwitz, F.; Prins, M.H.; Raskob, G.E.; et al. Oral Rivaroxaban for Symptomatic Venous Thromboembolism. N. Engl. J. Med. 2010, 363, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Covert, K.; Branam, D.L. Direct-acting oral anticoagulant use at extremes of body weight: Literature review and recommendations. Am. J. Heal. Pharm. 2020, 77, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Weycker, D.; Li, X.; Wygant, G.D.; Lee, T.; Hamilton, M.; Luo, X.; Vo, L.; Mardekian, J.; Pan, X.; Burns, L.; et al. Effectiveness and Safety of Apixaban versus Warfarin as Outpatient Treatment of Venous Thromboembolism in U.S. Clinical Practice. Thromb. Haemost. 2018, 118, 1951–1961. [Google Scholar] [CrossRef]
- Hlavacek, P.; Guo, J.D.; Rosenblatt, L.; Keshishian, A.; Russ, C.; Mardekian, J.; Ferri, M.; Poretta, T.; Yuce, H.; McBane, R. Safety, effectiveness, and health care cost comparisons among elderly patients with venous thromboembolism prescribed warfarin or apixaban in the United States Medicare population. Curr. Med Res. Opin. 2019, 35, 2043–2051. [Google Scholar] [CrossRef]
- Weycker, D.; Wygant, G.D.; Guo, J.D.; Lee, T.; Luo, X.; Rosenblatt, L.; Mardekian, J.; Atwood, M.; Hanau, A.; Cohen, A.T. Bleeding and recurrent VTE with apixaban vs warfarin as outpatient treatment: Time-course and subgroup analyses. Blood Adv. 2020, 4, 432–439. [Google Scholar] [CrossRef]
- Cohen, A.T.; Keshishian, A.; Lee, T.; Wygant, G.; Rosenblatt, L.; Hlavacek, P.; Mardekian, J.; Wiederkehr, D.; Sah, J.; Luo, X. Effectiveness and Safety of Apixaban, Low-Molecular-Weight Heparin, and Warfarin among Venous Thromboembolism Patients with Active Cancer: A U.S. Claims Data Analysis. Thromb. Haemost. 2020. [Google Scholar] [CrossRef]
- Kearon, C.; Ageno, W.; Cannegieter, S.C.; Cosmi, B.; Geersing, G.-J.; Kyrle, P.A. Subcommittees on Control of Anticoagulation, and Predictive and Diagnostic Variables in Thrombotic Disease. Categorization of patients as having provoked or unprovoked venous thromboembolism: Guidance from the SSC of ISTH. J. Thromb. Haemost. 2016, 14, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2014, 33, 1242–1258. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Stuart, E.A. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015, 34, 3661–3679. [Google Scholar] [CrossRef] [PubMed]
- Thoemmes, F.; Ong, A.D. A Primer on Inverse Probability of Treatment Weighting and Marginal Structural Models. Emerg. Adulthood 2015, 4, 40–59. [Google Scholar] [CrossRef]
- Xu, S.; Ross, C.; Raebel, M.A.; Shetterly, S.; Blanchette, C.; Smith, D. Use of Stabilized Inverse Propensity Scores as Weights to Directly Estimate Relative Risk and Its Confidence Intervals. Value Heal. 2010, 13, 273–277. [Google Scholar] [CrossRef]
- Lee, T.; Pan, S.; Byon, W.; Ilyas, B.S. Safety and Efficacy of Apixaban Versus Enoxaparin/Warfarin in Patients with Extremes of Body Weight: Post-Hoc Analysis of the AMPLIFY Trial. Blood 2019, 134, 1152. [Google Scholar] [CrossRef]
- Wysokinski, W.E.; Froehling, D.A.; Houghton, D.E.; McBane, I.R.D.; Vlazny, D.T.; Bott-Kitslaar, D.M.; Kuczmik, W.; Sutkowska, K.; Bator, K.; Hodge, D.O.; et al. Effectiveness and safety of apixaban and rivaroxaban for acute venous thromboembolism therapy in patients with extremes in bodyweight. Eur. J. Haematol. 2020, 105, 484–494. [Google Scholar] [CrossRef]
- Kushnir, M.; Choi, Y.; Eisenberg, R.; Rao, D.; Tolu, S.; Gao, J.; Mowrey, W.; Billett, H.H. Efficacy and safety of direct oral factor Xa inhibitors compared with warfarin in patients with morbid obesity: A single-centre, retrospective analysis of chart data. Lancet Haematol. 2019, 6, e359–e365. [Google Scholar] [CrossRef]
- Choi, Y.; Kushnir, M.; Billett, H.H. Apixaban is safe and effective in morbidly obese patients: A retrospective analy-sis of 390 patients with bmi ≥40. Blood 2017, 130 (Suppl. S1), 1105. [Google Scholar] [CrossRef]
- Coons, J.C.; Albert, L.; Bejjani, A.; Iasella, C.J. Effectiveness and Safety of Direct Oral Anticoagulants versus Warfarin in Obese Patients with Acute Venous Thromboembolism. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 204–210. [Google Scholar] [CrossRef]
- Tamariz, L.; Harkins, T.; Nair, V. Mini-Sentinel Systematic Evaluation of Health Outcome of Interest Definitions for Studies Using Administrative Data Venous Thromboembolism Report. Mini-Sentinel. 2011. Available online: https://www.sentinelinitiative.org/sites/default/files/surveillance-tools/validations-literature/Mini-Sentinel-HOI-Evidence-Review-Venous-Thromboembolism-Report.pdf (accessed on 10 December 2020).
- Jain, R.; Watzker, A.; Luo, X.; Kang, A.L.; Baker, C.L.; Rosenblatt, L.; Mardekian, J.; Menzin, J. Validation of obesity coding among newly treated nonvalvular atrial fibrillation patients using an integrated electronic medical record and claims database. Curr. Med Res. Opin. 2019, 36, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Broder, M.S.; Neary, M.P.; Chang, E.; Cherepanov, D.; Katznelson, L. Treatments, complications, and healthcare utilization associated with acromegaly: A study in two large United States databases. Pituitary 2013, 17, 333–341. [Google Scholar] [CrossRef] [PubMed]
| Non-Obese | Obese/Non-Morbid | STD * | Morbidly Obese | STD * | |
|---|---|---|---|---|---|
| Sample Size | 112,024 | 23,344 | 19,751 | ||
| Age, Mean (SD) | 67.5 (16.5) | 66.2 (14.2) | 8.77 | 62.1 (14.0) | 35.31 |
| Age Categories, n (%) | |||||
| 18–54 | 23,553 (21.0%) | 4737 (20.3%) | 1.81 | 5510 (27.9%) | 16.04 |
| 55–64 | 17,641 (15.7%) | 3915 (16.8%) | 2.77 | 3937 (19.9%) | 10.95 |
| 65–74 | 29,065 (25.9%) | 7890 (33.8%) | 17.22 | 6870 (34.8%) | 19.31 |
| 75–79 | 13,840 (12.4%) | 3129 (13.4%) | 3.13 | 1948 (9.9%) | 7.94 |
| ≥80 | 27,925 (24.9%) | 3673 (15.7%) | 22.99 | 1486 (7.5%) | 48.58 |
| Gender, n (%) | |||||
| Male | 52,410 (46.8%) | 10,482 (44.9%) | 3.78 | 7219 (36.6%) | 20.87 |
| Female | 59,614 (53.2%) | 12,862 (55.1%) | 3.78 | 12,532 (63.4%) | 20.87 |
| Index VTE Setting, n (%) | |||||
| Inpatient | 56,065 (50.0%) | 14,103 (60.4%) | 20.96 | 13,654 (69.1%) | 39.64 |
| Outpatient | 55,959 (50.0%) | 9241 (39.6%) | 20.96 | 6097 (30.9%) | 39.64 |
| Index VTE Event Type, n (%) | |||||
| DVT Only | 67,055 (59.9%) | 12,103 (51.8%) | 16.19 | 8458 (42.8%) | 34.59 |
| PE with or without DVT | 44,969 (40.1%) | 11,241 (48.2%) | 16.19 | 11,293 (57.2%) | 34.59 |
| Index VTE Etiology, n (%) | |||||
| Provoked | 59,790 (53.4%) | 14,437 (61.8%) | 17.21 | 12,978 (65.7%) | 25.33 |
| Unprovoked | 52,234 (46.6%) | 8907 (38.2%) | 17.21 | 6773 (34.3%) | 25.33 |
| Deyo-Charlson Comorbidity Index, Mean (SD) | 2.0 (2.3) | 2.5 (2.5) | 21.89 | 2.9 (2.6) | 36.28 |
| Baseline Comorbidity, n (%) | |||||
| Anemia | 31,341 (28.0%) | 7616 (32.6%) | 10.13 | 6506 (32.9%) | 10.80 |
| Central Venous Catheter | 7925 (7.1%) | 2144 (9.2%) | 7.73 | 2493 (12.6%) | 18.70 |
| ** Hematologic disorders associated with bleeding | 8612 (7.7%) | 2099 (9.0%) | 4.72 | 1710 (8.7%) | 3.54 |
| Ischemic Heart/ Coronary Artery Disease | 26,614 (23.8%) | 6931 (29.7%) | 13.44 | 5705 (28.9%) | 11.66 |
| Dementia | 8584 (7.7%) | 965 (4.1%) | 15.02 | 534 (2.7%) | 22.51 |
| Dyspepsia or Stomach Discomfort | 24,365 (21.7%) | 6049 (25.9%) | 9.78 | 4932 (25.0%) | 7.62 |
| Hyperlipidemia | 49,908 (44.6%) | 13,695 (58.7%) | 28.53 | 10,955 (55.5%) | 21.96 |
| Sleep Apnea | 8597 (7.7%) | 4717 (20.2%) | 36.79 | 7476 (37.9%) | 77.14 |
| *** Thrombophilia | 4214 (3.8%) | 1028 (4.4%) | 3.24 | 848 (4.3%) | 2.70 |
| Varicose Veins | 4357 (3.9%) | 1082 (4.6%) | 3.69 | 1132 (5.7%) | 8.62 |
| Congestive Heart Failure | 16,465 (14.7%) | 4384 (18.8%) | 10.95 | 5055 (25.6%) | 27.42 |
| Diabetes | 28,796 (25.7%) | 8921 (38.2%) | 27.07 | 9614 (48.7%) | 48.93 |
| Hypertension | 72,297 (64.5%) | 18,148 (77.7%) | 29.46 | 16,364 (82.9%) | 42.53 |
| Non-ESRD Renal Disease | 14,803 (13.2%) | 4199 (18.0%) | 13.18 | 3846 (19.5%) | 16.99 |
| End Stage Renal Disease | 2442 (2.2%) | 587 (2.5%) | 2.21 | 541 (2.7%) | 3.61 |
| Chronic Liver Disease | 6917 (6.2%) | 2117 (9.1%) | 10.92 | 1981 (10.0%) | 14.16 |
| Chronic Obstructive Pulmonary Disease | 19,315 (17.2%) | 4560 (19.5%) | 5.92 | 4577 (23.2%) | 14.81 |
| Baseline Bleed | 22,089 (19.7%) | 5418 (23.2%) | 8.51 | 4421 (22.4%) | 6.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, A.; Sah, J.; Lee, T.; Rosenblatt, L.; Hlavacek, P.; Emir, B.; Keshishian, A.; Yuce, H.; Luo, X. Effectiveness and Safety of Apixaban vs. Warfarin in Venous Thromboembolism Patients with Obesity and Morbid Obesity. J. Clin. Med. 2021, 10, 200. https://doi.org/10.3390/jcm10020200
Cohen A, Sah J, Lee T, Rosenblatt L, Hlavacek P, Emir B, Keshishian A, Yuce H, Luo X. Effectiveness and Safety of Apixaban vs. Warfarin in Venous Thromboembolism Patients with Obesity and Morbid Obesity. Journal of Clinical Medicine. 2021; 10(2):200. https://doi.org/10.3390/jcm10020200
Chicago/Turabian StyleCohen, Alexander, Janvi Sah, Theodore Lee, Lisa Rosenblatt, Patrick Hlavacek, Birol Emir, Allison Keshishian, Huseyin Yuce, and Xuemei Luo. 2021. "Effectiveness and Safety of Apixaban vs. Warfarin in Venous Thromboembolism Patients with Obesity and Morbid Obesity" Journal of Clinical Medicine 10, no. 2: 200. https://doi.org/10.3390/jcm10020200
APA StyleCohen, A., Sah, J., Lee, T., Rosenblatt, L., Hlavacek, P., Emir, B., Keshishian, A., Yuce, H., & Luo, X. (2021). Effectiveness and Safety of Apixaban vs. Warfarin in Venous Thromboembolism Patients with Obesity and Morbid Obesity. Journal of Clinical Medicine, 10(2), 200. https://doi.org/10.3390/jcm10020200





