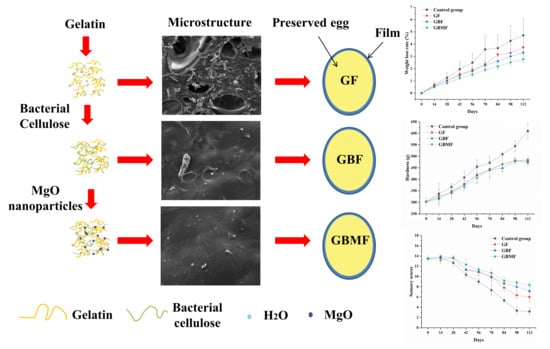
Gelatin-Based Nanocomposite Film with Bacterial Cellulose–MgO Nanoparticles and Its Application in Packaging of Preserved Eggs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Dissolution of BC
2.2.2. Preparation of Film Solutions and Films
2.2.3. Determination of Film Solution Characteristics
Rheological Properties
Particle Size and Zeta Potential
2.2.4. Determination of Film Properties
Microstructure
Water Contact Angle
Mechanical Properties
Water Vapor Permeability (WVP)
2.2.5. Determination of the Quality of Preserved Eggs
Pickling and Coating of Preserved Eggs
Weight Loss Rate
pH
Texture Characteristics
TVB-N
Color
Sensory Evaluation
2.2.6. Statistics and Analysis
3. Results and Discussion
3.1. The Properties of Film Solutions
3.1.1. Rheological Properties of the Film Solutions
3.1.2. Particle Size and Zeta Potential of the Film Solutions
3.2. The Properties of Films
3.2.1. Microstructure of the Films
3.2.2. Water Contact Angle of the Films
3.2.3. Mechanical Properties of the Films
3.2.4. WVP of the Films
3.3. The Preservation Effect of Films on Preserved Eggs
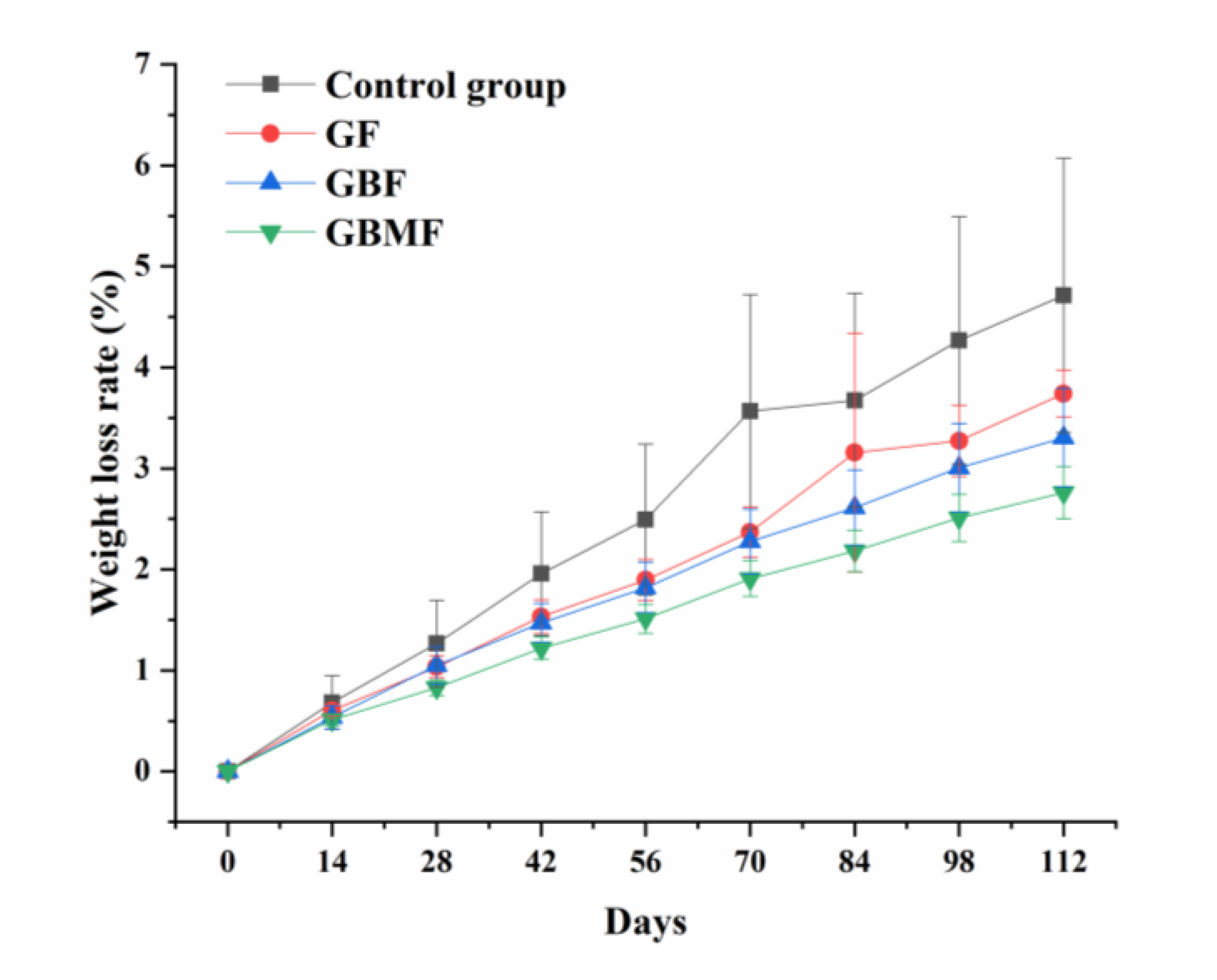
3.3.1. The Changes in the Weight Loss Rate of Preserved Eggs
3.3.2. The Changes in the pH of Preserved Eggs
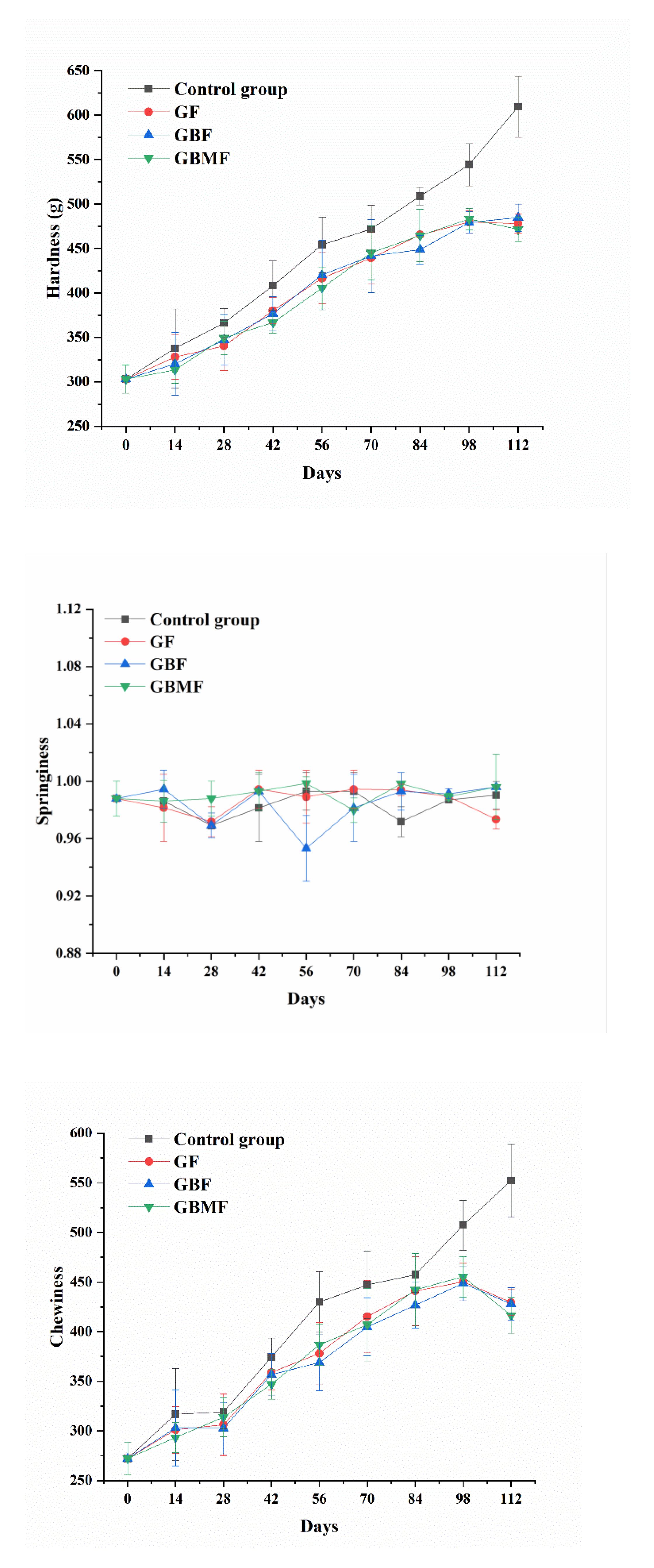
3.3.3. The Changes of Textural Characteristics of Preserved Eggs
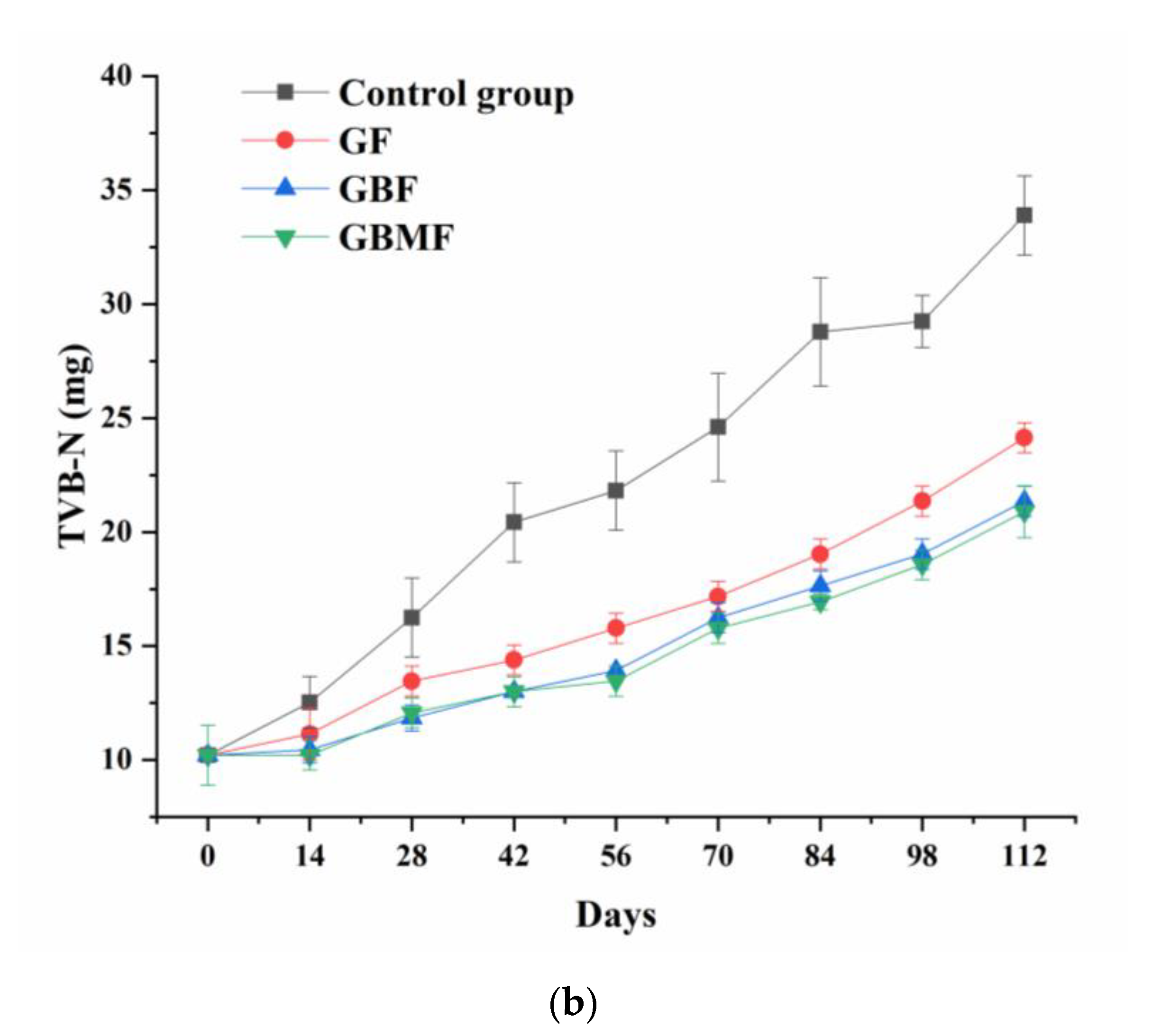
3.3.4. The Changes in the Content of TVB-N in Preserved Eggs
3.3.5. The Changes in the Color of Preserved Eggs
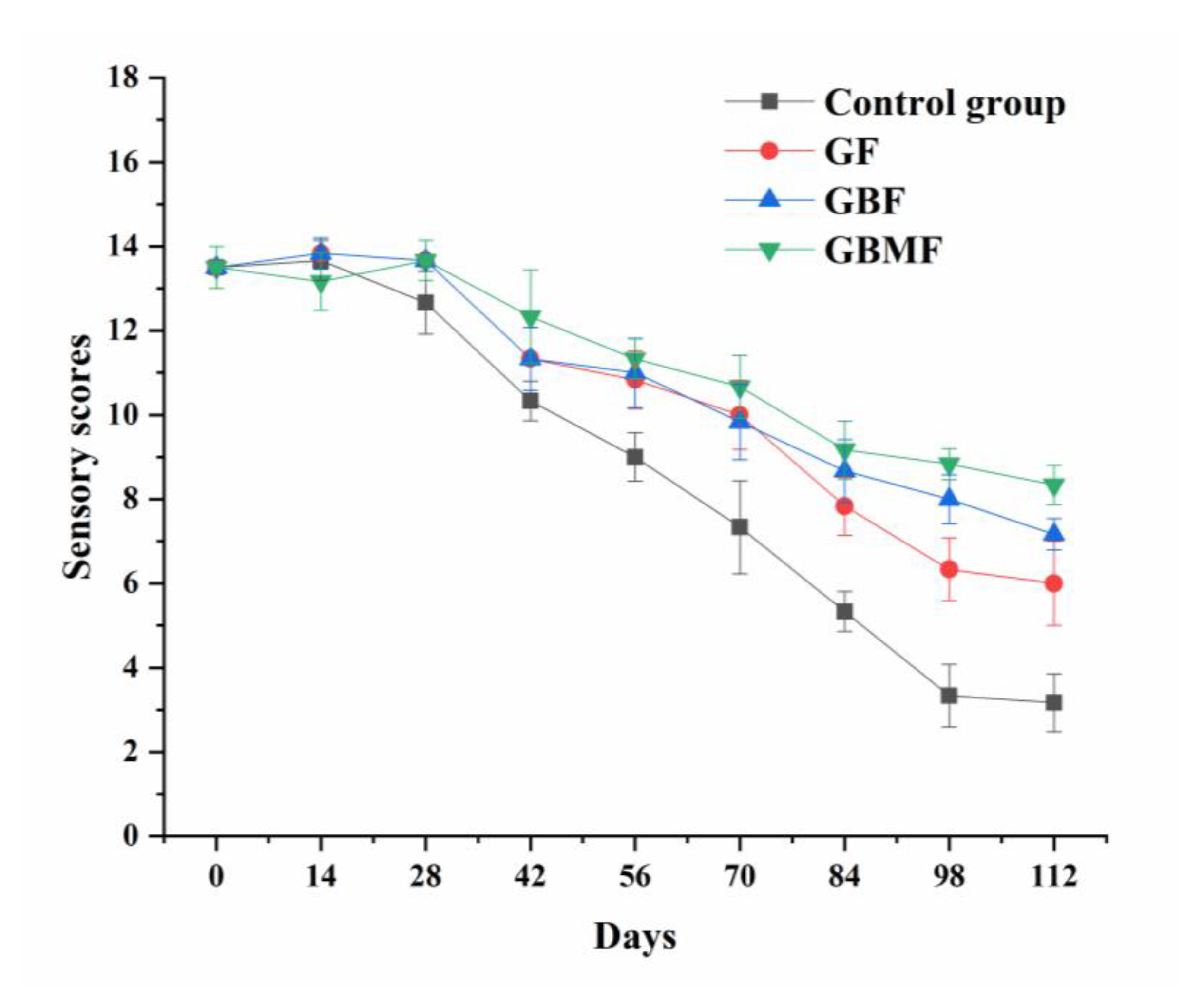
3.3.6. The Changes in Sensory Evaluation of Preserved Eggs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, M. Egg and Egg Product Processing; In Chinese; China Agriculture Press: Beijing, China, 2007. [Google Scholar]
- Tu, Y.; Zhao, Y.; Wu, N.; Yao, Y.; Xu, M.; Du, H.Y.; Tu, Y.G. The anti-inflammatory activity of peptides from simulated gastrointestinal digestion of preserved egg white in DSS-induced mouse colitis. Food Funct. 2018, 9, 6444–6454. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, Y.; Yao, Y.; Xu, M.; Du, H.; Wu, N.; Tu, Y. Isolation and identification of peptides from simulated gastrointestinal digestion of preserved egg white and their anti-inflammatory activity in TNF-α-induced Caco-2 cells. J. Nutr. Biochem. 2019, 63, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Giosafatto, C.V.L.; Al-Asmar, A.; D’Angelo, A.; Roviello, V.; Esposito, M.; Mariniello, L. Preparation and characterization of bioplastics from grass pea flour cast in the presence of microbial transglutaminase. Coatings 2018, 8, 435. [Google Scholar] [CrossRef] [Green Version]
- Santos, T.M.; Filho, M.D.S.M.S.; Caceres, C.A.; Rosa, M.D.F.; Morais, J.P.S.; Pinto, A.M.; Azeredo, H.M. Fish gelatin films as affected by cellulose whiskers and sonication. Food Hydrocoll. 2014, 41, 113–118. [Google Scholar] [CrossRef]
- Alfaro, A.D.T.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2014, 7, 33–44. [Google Scholar] [CrossRef]
- Sobral, P.J.D.A.; Menegalli, F.; Hubinger, M.; Roques, M. Mechanical, water vapor barrier and thermal properties of gelatin based edible films. Food Hydrocoll. 2001, 15, 423–432. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Deng, L.; Zou, L.; Feng, F.; Zhang, H. Hydrophobic Ethylcellulose/Gelatin Nanofibers Containing Zinc Oxide Nanoparticles for Antimicrobial Packaging. J. Agric. Food Chem. 2018, 66, 9498–9506. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Jeong, S.I.; Lee, S.E.; Yang, H.; Jin, Y.-H.; Park, C.-S.; Park, Y.S. Toxicologic evaluation of bacterial synthesized cellulose in endothelial cells and animals. Mol. Cell. Toxicol. 2010, 6, 370–377. [Google Scholar] [CrossRef]
- Espitia, P.J.; Otoni, C.G. Nanotechnology and Edible Films for Food Packaging Applications, Bio-based Materials for Food Packaging; Springer: Berlin/Heidelberg, Germany, 2018; pp. 125–145. [Google Scholar]
- Pakseresht, S.; Alogaili, A.W.M.; Akbulut, H.; Placha, D.; Pazdziora, E.; Klushina, D.; Konvičková, Z.; Kratošová, G.; Holešová, S.; Martynková, G.S. Silver/Chitosan Antimicrobial Nanocomposites Coating for Medical Devices: Comparison of Nanofiller Effect Prepared via Chemical Reduction and Biosynthesis. J. Nanosci. Nanotechnol. 2019, 19, 2938–2942. [Google Scholar] [CrossRef] [PubMed]
- Fortunati, E.; Peltzer, M.A.; Armentano, I.; Jiménez, A.; Kenny, J.M. Combined effects of cellulose nanocrystals and silver nanoparticles on the barrier and migration properties of PLA nano-biocomposites. J. Food Eng. 2013, 118, 117–124. [Google Scholar] [CrossRef]
- Qi, H.; Yang, Q.; Zhang, L.; Liebert, T.; Heinze, T. The dissolution of cellulose in NaOH-based aqueous system by two-step process. Cellulose 2011, 18, 237–245. [Google Scholar] [CrossRef]
- Handa, A.; Takahashi, K.; Kuroda, N.; Froning, G.W. Heat-induced Egg White Gels as Affected by pH. J. Food Sci. 1998, 63, 403–407. [Google Scholar] [CrossRef]
- Chinese Standard. GB 1040.3-2006, Plastics-Determination of Tensile Properties—Part 3: Test Conditions for Film and Sheets; The Standard Press of PR China: Beijing, China, 2006. [Google Scholar]
- Tu, Y.-G.; Zhao, Y.; Xu, M.; Li, X.; Du, H.-Y. Simultaneous Determination of 20 Inorganic Elements in Preserved Egg Prepared with Different Metal Ions by ICP-AES. Food Anal. Methods 2012, 6, 667–676. [Google Scholar] [CrossRef]
- Luo, W.; Xue, H.; Xiong, C.; Li, J.; Tu, Y.; Zhao, Y. Effects of temperature on quality of preserved eggs during storage. Poult. Sci. 2020, 99, 3144–3157. [Google Scholar] [CrossRef]
- Chinese Standard. GB/T 5009.47-2003, Analytical Methods for Hygienic Standards of Eggs and Egg Products; The Standard Press of PR China: Beijing, China, 2003. [Google Scholar]
- Chinese Standard. GB 5009.228-2016 Determination of Volatile Basic Nitrogen in Food; The Standard Press of PR China: Beijing, China, 2016. [Google Scholar]
- Chinese Standard. GB 2749-2015, Eggs and Egg Products; The Standard Press of PR China: Beijing, China, 2015. [Google Scholar]
- Ghasemlou, M.; Khodaiyan, F.; Oromiehie, A. Rheological and structural characterisation of film-forming solutions and biodegradable edible film made from kefiran as affected by various plasticizer types. Int. J. Biol. Macromol. 2011, 49, 814–821. [Google Scholar] [CrossRef]
- Lu, J.; Luo, Z. Effect of glutamic acid on rheological properties of tapioca starch paste. Food Sci. 2012, 33, 11–14. [Google Scholar]
- Wang, Z.; Liang, J.; Jiang, L.; Li, Y.; Wang, J.; Zhang, H.; Li, D.; Han, F.; Li, Q.; Wang, R.; et al. Effect of the interaction between myofibrillar protein and heat-induced soy protein isolates on gel properties. CyTA J. Food 2015, 1–8. [Google Scholar] [CrossRef]
- Augustine, R.; Malik, H.N.; Singhal, D.K.; Mukherjee, A.; Malakar, D.; Kalarikkal, N.; Thomas, S. Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J. Polym. Res. 2014, 21, 1–17. [Google Scholar] [CrossRef]
- Atares, L.; Bonilla, J.; Chiralt, A. Characterization of sodium caseinate-based edible films incorporated with cinnamon or ginger essential oils. J. Food Eng. 2010, 100, 678–687. [Google Scholar] [CrossRef]
- Yu, J.; Kim, H.-J.; Go, M.-R.; Bae, S.-H.; Choi, S.-J. ZnO Interactions with Biomatrices: Effect of Particle Size on ZnO-Protein Corona. Nanomaterials 2017, 7, 377. [Google Scholar] [CrossRef] [Green Version]
- Matusiak, J.; Grządka, E.; Bastrzyk, A. Stability, adsorption and electrokinetic properties of the chitosan/silica system. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 245–252. [Google Scholar] [CrossRef]
- Sagis, L.M.C.; Veerman, C.; Ganzevles, R.; Ramaekers, M.; Bolder, S.G.; Van Der Linden, E. Mesoscopic structure and viscoelastic properties of β-lactoglobulin gels at low pH and low ionic strength. Food Hydrocoll. 2002, 16, 207–213. [Google Scholar] [CrossRef]
- Veerman, C.; Sagis, L.M.C.; Heck, J.; Van Der Linden, E. Mesostructure of fibrillar bovine serum albumin gels. Int. J. Biol. Macromol. 2003, 31, 139–146. [Google Scholar] [CrossRef]
- Weijers, M.; Van De Velde, F.; Stijnman, A.; Van De Pijpekamp, A.; Visschers, R.W. Structure and rheological properties of acid-induced egg white protein gels. Food Hydrocoll. 2006, 20, 146–159. [Google Scholar] [CrossRef]
- Shah, N.K.; Brodsky, B.; Kirkpatrick, A.; Ramshaw, J.A. Structural consequences of D-amino acids in collagen triple-helical peptides. Biopolymers 1999, 49, 297–302. [Google Scholar] [CrossRef]
- Guo, L.; Colby, R.H.; Lusignan, C.P.; Whitesides, T.H. Kinetics of Triple Helix Formation in Semidilute Gelatin Solutions. Macromolecules 2003, 36, 9999–10008. [Google Scholar] [CrossRef]
- Natu, M.V.; De Sousa, H.C.; Gil, M.H. Effects of drug solubility, state and loading on controlled release in bicomponent electrospun fibers. Int. J. Pharm. 2010, 397, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, M.; Alboofetileh, M.; Rezaei, M.; Behrooz, R. Comparing physico-mechanical and thermal properties of alginate nanocomposite films reinforced with organic and/or inorganic nanofillers. Food Hydrocoll. 2013, 32, 416–424. [Google Scholar] [CrossRef]
- Anitha, S.; Brabu, B.; Thiruvadigal, D.J.; Gopalakrishnan, C.; Natarajan, T. Optical, bactericidal and water repellent properties of electrospun nano-composite membranes of cellulose acetate and ZnO. Carbohydr. Polym. 2013, 97, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Miao, J. Gelatin and its application in science and technology. Gelatin Sci. Technol. 2009, 29, 28–49. [Google Scholar]
- Rezaei, M.; Motamedzadegan, A. The Effect of Plasticizers on Mechanical Properties and Water Vapor Permeability of Gelatin-Based Edible Films Containing Clay Nanoparticles. World J. Nano Sci. Eng. 2015, 5, 178–193. [Google Scholar] [CrossRef] [Green Version]
- Valencia, G.A.; Lourenço, R.V.; Bittante, A.M.Q.B.; Sobral, P.J.D.A. Physical and morphological properties of nanocomposite films based on gelatin and Laponite. Appl. Clay Sci. 2016, 124, 260–266. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Tu, Y.; Zhao, Y.; Luo, X.; Wang, J.; Wang, M. Changes in gel characteristics of egg white under strong alkali treatment. Food Hydrocoll. 2015, 45, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, A.; Chen, M.; Ockerman, H.W.; Chen, J. Effect of different alkali treatments on the chemical composition, physical properties, and microstructure of pidan white. J. Food Sci. Technol. 2013, 52, 2264–2271. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Zhao, Y.; Yao, Y.; Wu, N.; Xu, M.; Du, H.; Tu, Y. Relationship between protein structure changes and in vitro digestion of preserved egg white during pickling. Int. J. Biol. Macromol. 2019, 138, 116–124. [Google Scholar] [CrossRef]
- Ganasen, P.; Benjakul, S. Effects of green tea and chinese tea on the composition and physical properties of pidan white. J. Food Process. Preserv. 2011, 35, 907–916. [Google Scholar] [CrossRef]
- Ganasen, P.; Benjakul, S. Chemical composition, physical properties and microstructure of pidan white as affected by different divalent and monovalent cations. J. Food Biochem. 2011, 35, 1528–1537. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Li, J.; Xu, M.; Shao, Y.; Tu, Y. Changes of microstructure characteristics and intermolecular interactions of preserved egg white gel during pickling. Food Chem. 2016, 203, 323–330. [Google Scholar] [CrossRef]
- Ganesan, P.; Benjakul, S. Effect of glucose treatment on texture and colour of pidan white during storage. J. Food Sci. Technol. 2011, 51, 729–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Index | 3 Points | 2 Points | 1 Points | 0 Points |
|---|---|---|---|---|
| Color | Brown transparent | Yellow transparent | Dark brown opaque | Brown-black opacity |
| Appearance | Full and complete form | Slightly defective | Large defect | Severe defect or liquefaction |
| Texture | Elasticity | Hardness increases | Hardness is very high | Shrinkage |
| Flavor | Rich fragrance | The fragrance fades | No fragrance fades | Mildew taste heavier |
| Taste | Normal, odorless | Taste light | A slight odor | Heavy odor |
| Samples | Particle Size(μm) | Zeta Potential(mv) |
|---|---|---|
| GF | 20.37 ± 1.67 a | 8.01 ± 0.45 a |
| GBF | 63.40 ± 1.51 b | 23.73 ± 1.36 c |
| GBMF | 69.40 ± 0.45 c | 16.87 ± 0.48 b |
| Samples | EM (MPa) | TS (MPa) | EB (%) | WVP (g mm/m2 d kPa) |
|---|---|---|---|---|
| GF | 50.11 ± 4.04 a | 3.20 ± 0.31 a | 102.07 ± 4.81 a | 3.54 ± 0.10 a |
| GBF | 0.85 ± 0.49 b | 1.13 ± 0.12 b | 186.04 ± 11.18 b | 2.86 ± 0.09 b |
| GBMF | 1.25 ± 0.42 b | 0.71 ± 0.08 b | 131.49 ± 11.82 a | 2.84 ± 0.1 b |
| Group | 0 d | 14 d | 28 d | 42 d | 56 d | 70 d | 84 d | 98 d | 112 d | |
|---|---|---|---|---|---|---|---|---|---|---|
| L* | Control | 32.38 ± 1.56 a | 27.82 ± 0.64 b | 26.07 ± 0.49 c | 24.43 ± 1.43 cd | 23.95 ± 1.31 cd | 23.70 ± 0.88 d | 22.86 ± 0.70 d | 22.90 ± 0.38 d | 23.30 ± 0.58 d |
| GF | 32.38 ± 1.56 a | 27.49 ±1.31 b | 25.36 ± 0.72 c | 23.40 ± 0.51 d | 22.94 ± 0.96 d | 22.34 ± 1.10 d | 21.97 ± 0.50 d | 20.72 ± 0.74 de | 19.87 ± 0.68 e | |
| GBF | 32.38 ± 1.56 a | 28.14 ± 1.29 b | 25.30 ± 1.02 c | 23.30 ± 0.51 c | 22.25 ± 1.78 c d | 21.86 ± 0.82 d | 20.40 ± 0.25 d | 20.72 ± 20.72 d | 20.27 ± 0.52 d | |
| GBMF | 32.38 ± 1.56 a | 29.10 ± 0.66 b | 25.90 ± 0.61 c | 23.68 ± 0.44 d | 23.49 ± 1.16 d | 22.91 ± 1.05 de | 21.70 ± 0.47 ef | 20.68 ± 0.92 ef | 20.25 ± 0.81 f | |
| a* | Control | 6.50 ± 1.48 b | 12.26 ± 2.91 a | 10.55 ± 2.2 ab | 10.01 ± 1.79 ab | 10.25 ± 2.67 ab | 9.56 ± 1.18 a | 8.9 ± 2.59 ab | 9.62 ± 1.07 a | 10.2 ± 0.92 a |
| GF | 6.50 ± 1.48 def | 12.56 ± 1.80 a | 11.4 ± 2.40 abde | 8.39 ± 1.04 bcde | 12.08 ± 1.81 ac | 10.88 ± 1.85 abc | 7.10 ± 2.63 cdef | 5.99 ± 2 ef | 4.17 ± 1.29 f | |
| GBF | 6.50 ± 1.48 b | 14.20 ± 1.27 a | 7.38 ± 1.67 b | 6.95 ± 0.94 bc | 12.39 ± 4.00 ab | 8.91 ± 1.08 b | 8.24 ± 2.05 b | 6.21 ± 1.68 b | 4.16 ± 1.57 c | |
| GBMF | 6.50 ± 1.48 b | 12.06 ± 1.76 a | 7.49 ± 2.22 b | 7.78 ± 1.46 b | 13.26 ± 2.08 a | 9.74 ± 2.82 ab | 4.55 ± 2.48 b | 5.30 ± 1.9 b | 3.85 ± 2.23 b | |
| b* | Control | 16.30 ± 2.46 a | 11.10 ± 1.50 b | 7.85 ± 0.98 c | 6.76 ± 1.71 cd | 6.2 ± 2.02 cde | 3.31 ± 0.91 e | 4.66 ± 1.5 de | 4.20 ± 0.79 e | 3.25 ± 1.97 e |
| GF | 16.30 ± 2.46 a | 11.14 ± 1.99 b | 7.42 ± 1.38 c | 5.54 ± 0.84 cd | 6.39 ± 1.10 cd | 5.04 ± 1.91 cde | 2.90 ± 2.67 def | 1.61 ± 0.91 ef | 0.55 ± 0.66 f | |
| GBF | 16.30 ± 2.46 a | 15.33 ± 2.06 a | 4.52 ± 1.52 b | 4.61 ± 1.03 b | 7.42 ± 2.95 b | 3.11 ± 1 b | 3.89 ± 0.89 b | 1.74 ± 0.91 c | 0.72 ± 1.16 c | |
| GBMF | 16.30 ± 2.46 a | 12.48 ± 3.6 ab | 4.10 ± 1.19 cd | 4.83 ± 0.94 c | 8.32 ± 1.7 b | 3.72 ± 1.83 cde | 1.37 ± 1.85 def | 0.53 ± 1.27 ef | −0.74 ± 1.65 f | |
| Group | 0 d | 14 d | 28 d | 42 d | 56 d | 70 d | 84 d | 98 d | 112 d | |
|---|---|---|---|---|---|---|---|---|---|---|
| L* | Control | 30.51 ± 1.81 ab | 27.34 ± 1.24 b | 30.26 ± 1.39 ab | 30.19 ± 2.87 ab | 29.32 ± 1.86 ab | 28.83 ± 0.62 ab | 30.41 ± 1.04 a | 31.02 ± 1.41 a | 31.21 ± 1.57 a |
| GF | 30.51 ± 1.81 a | 28.36 ± 2.2 a | 30.74 ± 2.36 a | 30.11 ± 1.12 a | 26.27 ± 1.80 a | 28.08 ± 0.64 a | 29.66 ± 1.44 a | 30.16 ± 1.13 a | 29.56 ± 0.5 a | |
| GBF | 30.51 ± 1.81 ab | 28.82 ± 0.67 b | 31.50 ± 1.27 ab | 29.24 ± 0.93 b | 27.5 ± 0.95 b | 29.06 ± 2.05 b | 29.87 ± 1.54 b | 32.44 ± 0.19 a | 28.58 ± 1.21 b | |
| GBMF | 30.51 ± 1.81 a | 28.80 ± 1.29 a | 31.42 ± 2.22 a | 30.52 ± 2.92 a | 28.66 ± 1.4 a | 28.95 ± 1.79 a | 28.65 ± 2.16 a | 29.14 ± 1.53 a | 28.80 ± 0.89 a | |
| a* | Control | −4.37 ± 0.29 c | −4.31 ± 0.58 c | −3.38 ± 0.20 bc | −3.29 ± 0.27 abc | −3.21 ± 0.30 ab | −3.21 ± 0.21 ab | −2.81 ± 0.51 ab | −2.65 ± 0.38 a | −2.94 ± 0.25 ab |
| GF | −4.37 ± 0.29 c | −4.41 ± 0.59 c | −3.15 ± 0.16 ab | −3.31 ± 0.3 b | −2.68 ± 0.26 a | −3.70 ± 0.27 bc | −3.15 ± 0.45 ab | −3.22 ± 0.28 a | −2.87 ± 0.24 a | |
| GBF | −4.37 ± 0.29 c | −3.74 ± 1.20 ab | −3.07 ± 0.3 ab | −2.97 ± 0.21 ab | −3.14 ± 0.38 ab | −3.25 ± 0.25 b | −3.02 ± 0.36 ab | −2.79 ± 0.03 ab | −2.68 ± 0.21 a | |
| GBMF | −4.37 ± 0.29 d | −3.39 ± 0.38 bc | −2.87 ± 0.21 ab | −3.17 ± 0.26 abc | −3.27 ± 0.55 abc | −3.62 ± 0.18 c | −2.83 ± 0.32 ab | −2.93 ± 0.27 ab | −2.76 ± 0.14 a | |
| b* | Control | 3.20 ± 0.71 c | 3.30 ± 1.3 bc | 4.66 ± 0.93 abc | 5.15 ± 1.75 abc | 4.57 ± 1.03 b | 2.90 ± 0.58 c | 5.24 ± 0.88 ab | 6.05 ± 0.66 a | 5.14 ± 1.58 abc |
| GF | 3.20 ± 0.71 ab | 5.05 ± 1.60 a | 4.03 ± 1.40 ab | 4.81 ± 1.21 a | 4.38 ± 0.66 a | 2.75 ± 0.41 b | 4.34 ± 0.59 a | 3.44 ± 0.36 ab | 4.67 ± 0.52 a | |
| GBF | 3.20 ± 0.71 c | 6.47 ± 1.71 a | 4.56 ± 1.29 abc | 4.82 ± 0.88 abc | 5.21 ± 0.65 ab | 4.06 ± 1.2 bc | 5.30 ± 0.57 a | 5.00 ± 0.14 a | 4.22 ± 0.42 b | |
| GBMF | 3.20 ± 0.71 ab | 4.90 ± 0.98 a | 3.60 ± 1.03 ab | 5.26 ± 1.64 a | 4.12 ± 0.48 ab | 2.55 ± 1.09 b | 4.58 ± 1.29 ab | 2.55 ± 1.36 b | 4.10 ± 1.26 ab | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Luo, W.; Tu, Y.; Zhao, Y. Gelatin-Based Nanocomposite Film with Bacterial Cellulose–MgO Nanoparticles and Its Application in Packaging of Preserved Eggs. Coatings 2021, 11, 39. https://doi.org/10.3390/coatings11010039
Wang Y, Luo W, Tu Y, Zhao Y. Gelatin-Based Nanocomposite Film with Bacterial Cellulose–MgO Nanoparticles and Its Application in Packaging of Preserved Eggs. Coatings. 2021; 11(1):39. https://doi.org/10.3390/coatings11010039
Chicago/Turabian StyleWang, Yuting, Wenxiang Luo, Yonggang Tu, and Yan Zhao. 2021. "Gelatin-Based Nanocomposite Film with Bacterial Cellulose–MgO Nanoparticles and Its Application in Packaging of Preserved Eggs" Coatings 11, no. 1: 39. https://doi.org/10.3390/coatings11010039