Geochemical and Mineralogical Characterisation of Historic Zn–Pb Mine Waste, Plombières, East Belgium
Abstract
1. Introduction
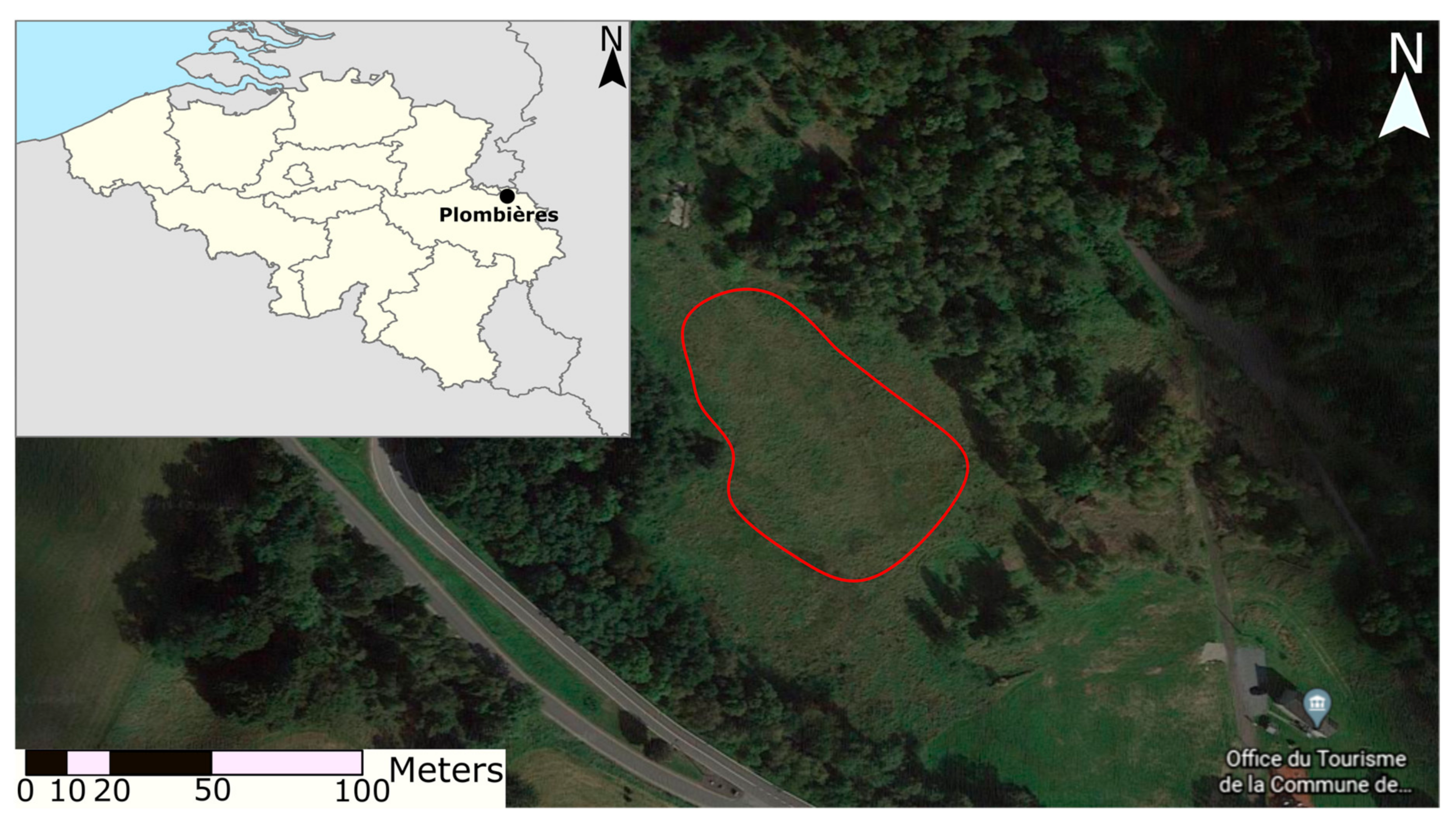
2. Location and History of the Plombières Tailings Pond
2.1. Site Description
2.2. Geological Setting of the Plombières Mineralization
3. Methodology
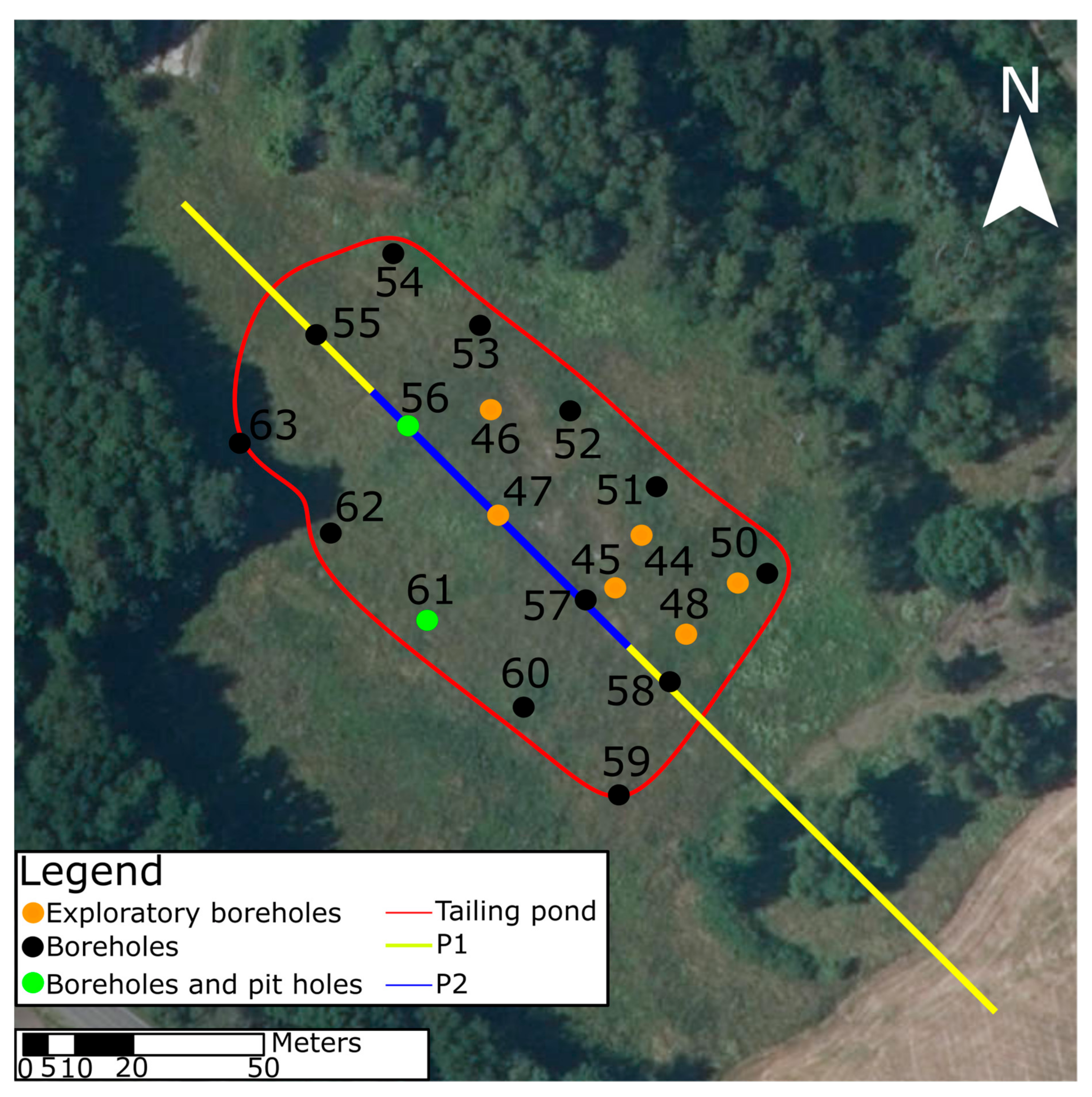
3.1. Sampling and Sample Preparation
3.2. Geophysical Survey
- A long profile—189 m long with a spacing of 3 m between electrodes (P1)
- A short profile—47.25 m long profile with a spacing of 0.75 m between electrodes (P2)
3.3. Laser Diffractometry
3.4. X-Ray Fluorescence
3.5. X-Ray Powder Diffraction
4. Results and Discussion
4.1. Classification of Mine Waste Material Types
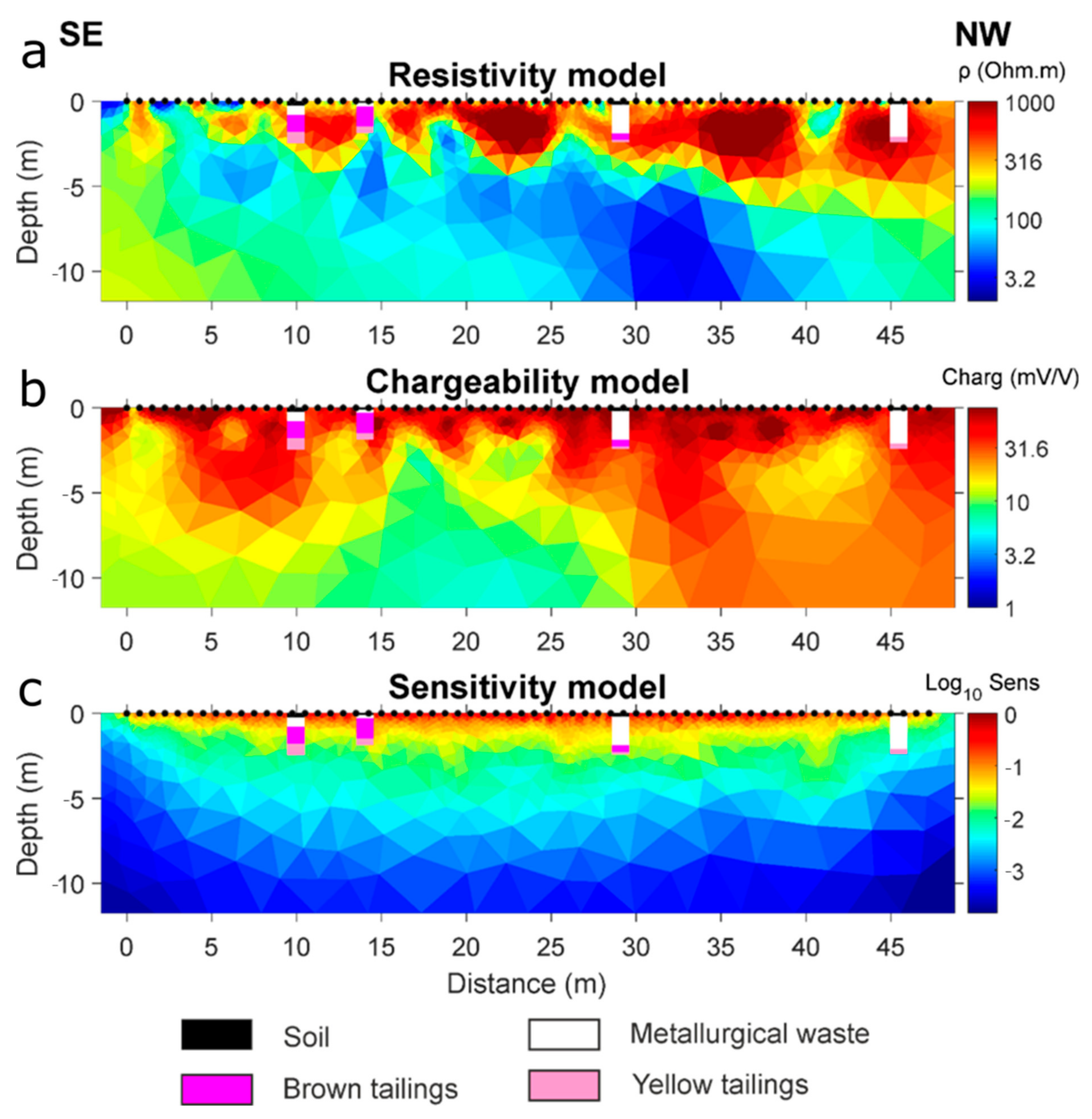
4.2. Geophysical Survey
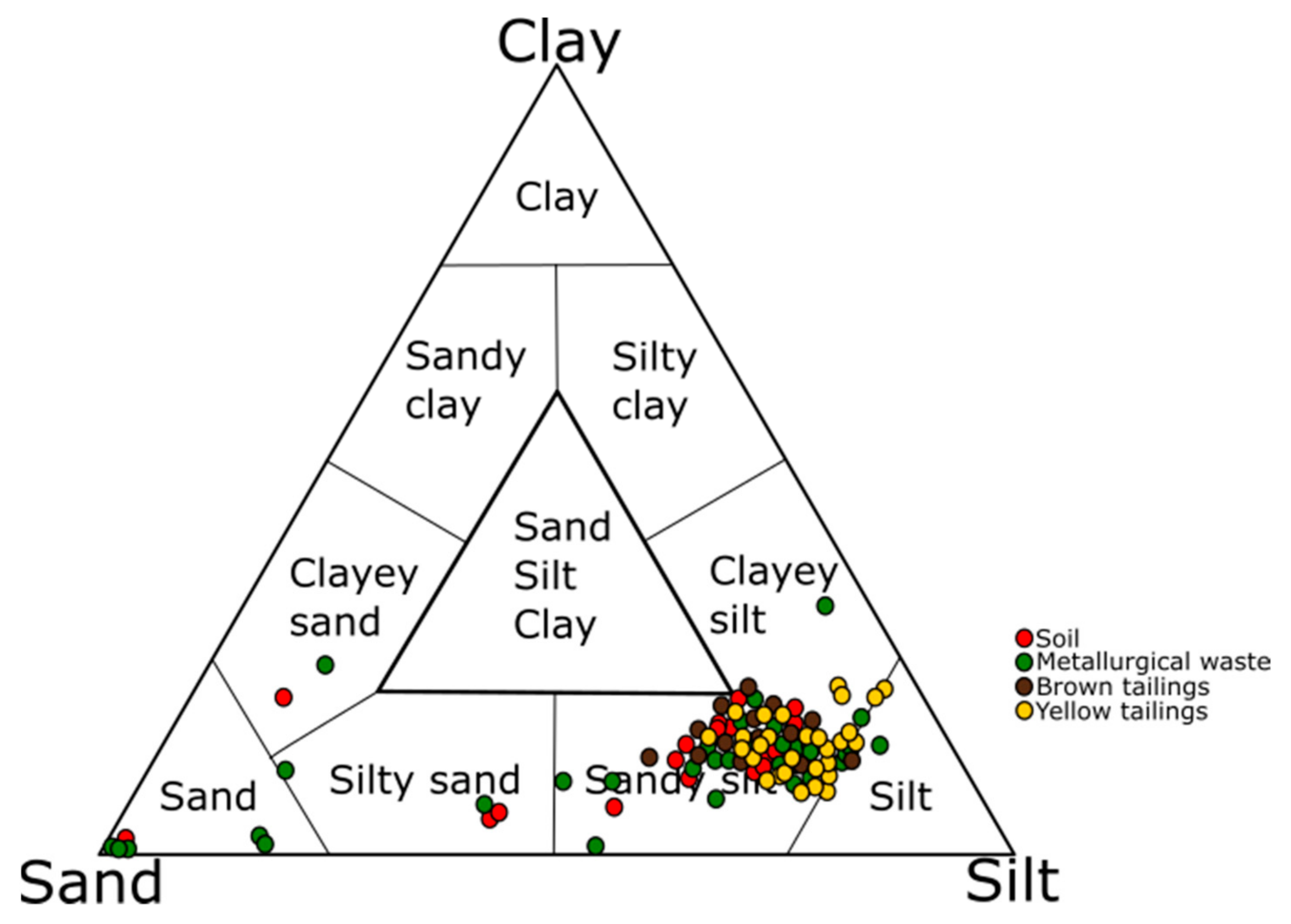
4.3. Grain Size Distribution
4.4. Geochemistry of Plombières Material
4.5. Mineralogy of Plombières Materials
4.6. Lead and Zinc Distribution
4.7. A 3D Model and Resource Estimation of the Plombières Site
4.8. Implications for Application in the Ceramic Industry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Study on the EU’s list of Critical Raw Materials—Final Report; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Ceniceros-Gómez, A.E.; Macías-Macías, K.Y.; De La Cruz-Moreno, J.E.; Gutiérrez-Ruiz, M.E.; Martínez-Jardines, L.G. Characterization of mining tailings in México for the possible recovery of strategic elements. J. South Am. Earth Sci. 2018, 88, 72–79. [Google Scholar] [CrossRef]
- Martinez, J.M.R.; Hidalgo, M.; Rey, J.; Garrido, J.; Kohfahl, C.; Benavente, J.; De Rojas, D.H.F. A multidisciplinary characterization of a tailings pond in the Linares-La Carolina mining district, Spain. J. Geochem. Explor. 2016, 162, 62–71. [Google Scholar] [CrossRef]
- Hudson-Edwards, K.; Jamieson, H.; Lottermoser, B.G. Mine Wastes: Past, Present, Future. Elements 2011, 7, 375–380. [Google Scholar] [CrossRef]
- Ettler, V. Soil contamination near non-ferrous metal smelters: A review. Appl. Geochem. 2016, 64, 56–74. [Google Scholar] [CrossRef]
- Jamieson, H.E.; Walker, S.R.; Parsons, M.B. Mineralogical characterization of mine waste. Appl. Geochem. 2015, 57, 85–105. [Google Scholar] [CrossRef]
- Helser, J.; Cappuyns, V. Trace elements leaching from Pb-Zn mine waste (Plombières, Belgium) and environmental implications. J. Geochem. Explor. 2021, 2020, 106659. [Google Scholar] [CrossRef]
- Environment Agency. Human Health Toxicological Assessment of Contaminants in Soil; (England and Wales); Environment Agency: Bristol, UK, 2009.
- Kuhn, K.; Meima, J.A. Kuhn Characterization and Economic Potential of Historic Tailings from Gravity Separation: Implications from a Mine Waste Dump (Pb-Ag) in the Harz Mountains Mining District, Germany. Minerals 2019, 9, 303. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Mine Wastes: Characterization, Treatment and Environmental Impacts, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2003; p. 400. [Google Scholar]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Iglesias-Godino, F.J.; Corpas-Iglesias, F.A. Development of Porous Asphalt with Bitumen Emulsion, Electric arc Furnace Slag and Cellulose Fibers for Medium Traffic Roads. Minerals 2020, 10, 872. [Google Scholar] [CrossRef]
- Lam, E.J.; Zetola, V.; Ramírez, Y.; Montofré, Í.L.; Pereira, F. Making Paving Stones from Copper Mine Tailings as Aggregates. Int. J. Environ. Res. Public Health 2020, 17, 2448. [Google Scholar] [CrossRef]
- Suárez-Macías, J.; Terrones-Saeta, J.M.; Iglesias-Godino, F.J.; Corpas-Iglesias, F.A. Retention of Contaminants Elements from Tailings from Lead Mine Washing Plants in Ceramics for Bricks. Minerals 2020, 10, 576. [Google Scholar] [CrossRef]
- Büttner, P.; Osbahr, I.; Zimmermann, R.; Leißner, T.; Satge, L.; Gutzmer, J. Recovery potential of flotation tailings assessed by spatial modelling of automated mineralogy data. Miner. Eng. 2018, 116, 143–151. [Google Scholar] [CrossRef]
- Thomassen, B. The Blyklippen lead-zinc mine at Mesters Vig, East Greenland. Geol. Ore 2005, 5, 12. [Google Scholar]
- Sukhoon, P.; Million, T.; Hyeong-Ki, K. The Applications of Mine Tailings to Develop Low-Cost UHPC; International Interactive Symposium on Ultra-High Performance Concrete; Iowa State University Digital Press: Des Moines, IA, USA, 2016. [Google Scholar] [CrossRef]
- Moran, P.; Christoffersen, L.; Gillow, J.; Hay, M. Cemented Tailings Backfill—It’s Better, Now Prove It! In Proceedings of the SME Annual Meeting, Denver, CO, USA, 24–27 February 2013. [Google Scholar]
- Andrews, W.J.; Moreno, C.J.G.; Nairn, R.W. Potential recovery of aluminum, titanium, lead, and zinc from tailings in the abandoned Picher mining district of Oklahoma. Miner. Econ. 2013, 26, 61–69. [Google Scholar] [CrossRef]
- Evdokimova, S.I.; Evdokimova, V.S. Metal Recovery from Old Tailings. J. Min. Sci. 2014, 50, 800–808, © Original Russian Text, Fiziko-Tekhnicheskie Problemy Razrabotki Poleznykh Iskopaemykh; Pleiades Publishing, Ltd.: Russia, 2014; pp. 172–182. [Google Scholar] [CrossRef]
- Mehta, N.; Dino, G.A.; Passarella, I.; Ajmone-Marsan, F.; Rossetti, P.; De Luca, D.A. Assessment of the Possible Reuse of Extractive Waste Coming from Abandoned Mine Sites: Case Study in Gorno, Italy. Sustainability 2020, 12, 2471. [Google Scholar] [CrossRef]
- Pioro, L.; Pioro, I. Reprocessing of metallurgical slag into materials for the building industry. Waste Manag. 2004, 24, 371–379. [Google Scholar] [CrossRef]
- Kucha, H.; Martens, A.; Ottenburgs, R.; De Vos, W.; Viaene, W. Primary minerals of Zn-Pb mining and metallurgical dumps and their environmental behavior at Plombières, Belgium. Environ. Earth Sci. 1996, 27, 1–15. [Google Scholar] [CrossRef]
- Dejonghe, L.; Ladeuze, F.; Jans, D. Atlas des gisementsplombo-zincifères du Synclinorium de Verviers (Est de laBelgique). Mémoire l’Explication Géologique Minières Belgique 1993, 33, 1–483. [Google Scholar]
- Blasenbauer, D.; Bogush, A.; Carvalho, T.; Cleall, P.; Cormio, C.; Guglietta, D.; Fellner, J.; Fernández-Alonso, M.; Heuss-Aßbichler, S.; Huber, F.; et al. Knowledge base to facilitate anthropogenic resource assessment. Deliverable of COST Action Mining the European Anthrothropogenic Resource Assessment. Zenodo 2020. [Google Scholar] [CrossRef]
- Renson, V.; Fagel, N.; Mattielli, N.; Nekrassoff, S.; Streel, M.; De Vleeschouwer, F. Roman road pollution assessed by elemental and lead isotope geochemistry in East Belgium. Appl. Geochem. 2008, 23, 3253–3266. [Google Scholar] [CrossRef]
- Dejonghe, L. Plomb’hier a bonnes mines, Montzen: ASBLE space Culture-Plombières; ASBLE Space Culture: Montzen, Belgium, 2014; Volume 1, p. 12. [Google Scholar]
- Dejonghe, L. Zinc-lead deposits of Belgium. Ore Geol. Rev. 1998, 12, 329–354. [Google Scholar] [CrossRef]
- Blondieau, M.; Polrot, F. Les travaux miniers de schimper, siège sud de la mine du bleyberg (Plombières, Belgique):Plomb, zinc mais aussi argent: Histoire, minéralisations, production d’argent, impact dans le paysage. Geol. Surv. Belg. 2011, 310, 57. [Google Scholar]
- Rainbows, P.S. Trace Metals in the Environment and Living Organisms: The British Isles as a Case Study; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; p. 741. [Google Scholar]
- Heijlen, W.; Muchez, P.; Banks, D.A. Origin and evolution of high-salinity, Zn-Pb mineralizing fluids in the Variscides of Belgium. Miner. Depos. 2001, 36, 165–176. [Google Scholar] [CrossRef]
- Paradis, S.; Hannigan, P.; Dewing, K. Mississippi valley-type lead-zinc deposits. Spec. Publ. Geol. Assoc. Can. Miner. Depos. Div. 2007, 5, 185–204. [Google Scholar]
- Dejonghe, L.; Boni., M. The “calamine-type zinc-lead deposits in Belgium and West Germany: A product of Mesozoic palaeoweathering processes. Geol. Belg. 2005, 8, 3–14. [Google Scholar]
- Muchez, P.; Heijlen, W.; Banks, D.; Blundell Boni, M.; Grandia, F. Extensional tectonics and the timing and formation of basin-hosted deposits in Europe. Ore Geol. Rev. 2005, 27, 241–267. [Google Scholar] [CrossRef]
- Jans, D.; Dejonghe, L. Les gisements plombo-zinciferes de l’est de la belgique. Chron. Rech. Minière 1983, 470, 3–24. [Google Scholar]
- Coppola, V.; Boni, M.; Gilg, H.A.; Balassone, G.; Dejonghe, L. The “calamine” non-sulfide Zn–Pb deposits of Belgium: Petrographical, mineralogical and geochemical characterization. Ore Geol. Rev. 2008, 33, 187–210. [Google Scholar] [CrossRef]
- Abzalov, M.; Newman, C. Sampling of the mineralised tailings dumps ? case study of the Mount Morgan project, central Queensland, Australia. Appl. Earth Sci. 2017, 126, 1–5. [Google Scholar] [CrossRef]
- Service Public de Wallonie. 2019. Available online: geoportail.wallonie.be/walonmap (accessed on 18 August 2020).
- Nikonow, W.; Rammlmair, D.; Furche, M. A multidisciplinary approach considering geochemical reorganization and internal structure of tailings impoundments for metal exploration. Appl. Geochem. 2019, 104, 51–59. [Google Scholar] [CrossRef]
- Lam, E.J.; Carle, R.; González, R.; Montofré, Í.L.; Veloso, E.A.; Bernardo, A.; Álvarez, F.A. A Methodology Based on Magnetic Susceptibility to Characterize Copper Mine Tailings. Minerals 2020, 10, 939. [Google Scholar] [CrossRef]
- Loke, M.H. Tutorial: 2-D and 3-D Electrical Imaging Surveys. Course Notes for USGS Workshop 2-D and 3-D Inversion and Modeling of Surface and Drill hole Resistivity Data; Course Notes for USGS Workshop: Torrs, CT, USA, 2004. [Google Scholar]
- Dahlin, T.; Zhou, B. Multiple-gradient array measurements for multichannel 2D resistivity imaging. Near Surf. Geophys. 2005, 4, 113–123. [Google Scholar] [CrossRef]
- Günther, T.; Rücker, C.; Spitzer, K. Three-dimensional modelling and inversion of dc resistivity data incorporating topography—II. Inversion. Geophys. J. Int. 2006, 166, 506–517. [Google Scholar] [CrossRef]
- Caterina, D.; Beaujean, J.; Robert, T.; Nguyen, F. A comparison study of different image appraisal tools for electrical resistivity tomography. Near Surf. Geophys. 2013, 11, 639–658. [Google Scholar] [CrossRef]
- Oldenburg, D.W.; Li, Y. Estimating depth of investigation in dc resistivity and IP surveys. Geophysics 1999, 64, 403–416. [Google Scholar] [CrossRef]
- Baskaran, R.; Selvakumaran, T.; Subramanian, V. Aerosol test facility for fast reactor safety studies. Indian J. Pure Appl. Phys. 2004, 42, 873–878. [Google Scholar]
- Goossens, D. Techniques to measure grain-size distributions of loamy sediments: A comparative study of ten instruments for wet analysis. Sedimentology 2007, 55, 65–96. [Google Scholar] [CrossRef]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Shepard, F.P. Nomenclature Based on Sand-silt-clay Ratios. J. Sediment. Res. 2003, 24, 151–158. [Google Scholar] [CrossRef]
- Jackson, P.J.; Packham, R.F.; Richards, W.N. The Examination of Organic flocculants and Coagulant Aids 8th Methods of Analysing Proprietary Chemicals Used in Water Supply Treatment Processes. Water Research Centre; Technical Report TR6; Stevenage Laboratory: Stevenage, UK, 1975. [Google Scholar]
- Frederickx, L. The Influence of Measurement Techniques on the Grain Size Distribution of the Boom Clay Formation; SCK-CEN I-0641: Mol, Belgium, 1992. [Google Scholar]
- El Ouahabi, M.; Chêne, G.; Strivay, D.; Auwera, J.V.; Hubert-Ferrari, A. Inter-technique comparison of PIXE and XRF for lake sediments. J. Anal. At. Spectrom. 2018, 33, 883–892. [Google Scholar] [CrossRef]
- Bergmann, J.; Friedel, P.; Kleeberg, R. BGMN—A new fundamental parameter based Rietveld program for laboratory X-ray sources, its use in quantitative analysis and structure investigations. CPD Newsletter, Commission of Powder Diffraction. Int. Union Crystallogr. 1998, 20, 5–8. [Google Scholar]
- Lauwers, M. Geotechnische Studie van een Mijn te Plombières; Departement of Civil Engineering, Section Mining and Geophysical Engineering, KU Leuven: Leuven, Belgium, 1992. [Google Scholar]
- Santini, T.; Banning, N.C. Alkaline tailings as novel soil forming substrates: Reframing perspectives on mining and refining wastes. Hydrometallurgy 2016, 164, 38–47. [Google Scholar] [CrossRef]
- García-Giménez, R.; Jiménez-Ballesta, R. Mine tailings influencing soil contamination by potentially toxic elements. Environ. Earth Sci. 2017, 76, 51. [Google Scholar] [CrossRef]
- Dominy, S.C.; Platten, I.M.; Raine, M.D. Grade and geological continuity in high-nugget effect gold–quartz reefs: Implications for resource estimation and reporting. Appl. Earth Sci. 2003, 112, 239–259. [Google Scholar] [CrossRef]
- Jauniaux, M. Moresnet où l’histoire et la minéralogie se mêlent. Lithorama 2007, 8, 10. [Google Scholar]
- Zhao, P.; Lu, L.; Liu, X.; De La Torre, A.G.; Cheng, X. Error Analysis and Correction for Quantitative Phase Analysis Based on Rietveld-Internal Standard Method: Whether the Minor Phases Can Be Ignored? Crystals 2018, 8, 110. [Google Scholar] [CrossRef]
- Jacobs, J.A.; Lehr, J.H.; Testa, S.M. Acid Mine Drainage, Rock Drainage, and Acid Sulfate Soils: Causes, Assessment, Prediction, Prevention, and Remediation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; p. 504. [Google Scholar]
- Leblanc, M.; Morales, J.A.; Borrego, J.; Elbaz-Poulichet, F. 4,500-year-old mining pollution in southwestern spain: Long-term implications for modern mining pollution. Econ. Geol. 2000, 95, 655–662. [Google Scholar] [CrossRef]
- Pascaud, G.; Leveque, T.; Soubrand, M.; Boussen, S.; Joussein, E.; Dumat, C. Environmental and health risk assessment of Pb, Zn, As and Sb in soccer field soils and sediments from mine tailings: Solid speciation and bioaccessibility. Environ. Sci. Pollut. Res. 2014, 21, 4254–4264. [Google Scholar] [CrossRef]
- Moles, N.; Smyth, D.; Maher, C.; Beattie, E.; Kelly, M. Dispersion of cerussite-rich tailings and plant uptake of heavy metals at historical lead mines near Newtownards, Northern Ireland. Appl. Earth Sci. 2004, 113, 21–30. [Google Scholar] [CrossRef]
- Kicińska, A. Physical and chemical characteristics of slag produced during Pb refining and the environmental risk associated with the storage of slag. Environ. Geochem. Health 2020, 1–19. [Google Scholar] [CrossRef]
- PERC. Reporting Standard. Pan-European Standard for Reporting of Exploration Results, Mineral Resources and Reserves; The Pan-European Reserves and Resources Reporting Committee: Brussels, Belgium; Available online: https://inspire.ec.europa.eu/codelist/ClassificationAndQuantificationFrameworkValue/PERC (accessed on 16 February 2017).
- Liu, Q.; Li, Y.; Zhao, G. The Latest Research Progress of Green Building Materials in Lead and Zinc Tailings. IOP Conf. Ser. Earth Environ. Sci. 2019, 267, 052024. [Google Scholar] [CrossRef]
- Kuranchie, F.A.; Shukla, S.K.; Habibi, D. Utilisation of iron ore mine tailings for the production of geopolymer bricks. Int. J. Mining Reclam. Environ. 2014, 30, 92–114. [Google Scholar] [CrossRef]
- Fontes, W.C.; De Carvalho, J.M.F.; Andrade, L.C.; Segadães, A.M.; Peixoto, R.A. Assessment of the use potential of iron ore tailings in the manufacture of ceramic tiles: From tailings-dams to “brown porcelain”. Constr. Build. Mater. 2019, 206, 111–121. [Google Scholar] [CrossRef]
- Achternbosch, M.; Bräutigam, K.-R.; Gleis, M.; Hartlieb, N.; Kupsch, C.; Richers, U.; Stemmermann, P. Heavy Metals in Cement and Concrete Resulting from the Co-Incineration of Wastes in Cement Kilns with Regard to the Legitimacy of Waste Utilisation, FZKA 6923; Forschungszentrum: Karlsruhe, Germany, 2003; p. 200. [Google Scholar]
- Belgium Legislative on Regulation on Sustainable Management of Material Cycles and Waste Materials (VLAREMA); Decree: Flanders, Belgium; p. 187. Available online: https://navigator.emis.vito.be/mijn-navigator?woId=44696 (accessed on 17 February 2012).
- Dondi, M. Technological and compositional requirements of clay materials for ceramic tiles. In Proceedings of the 12th Int. Clay Conference, Bahía Blanca, Argentina, 22–28 July 2001. [Google Scholar] [CrossRef]
- Dittmann, J.; Willenbacher, N. Microstructural investigations and mechanical properties of macroporous ceramic materials from capillary suspensions. J. Am. Ceram. Soc. 2014, 97, 3787–3792. [Google Scholar] [CrossRef]
- Kambale, K.; Mahajan, A.; Butee, S.P. Effect of grain size on the properties of ceramics. Met. Powder Rep. 2019, 74, 130–136. [Google Scholar] [CrossRef]
- Veiga Simão, F.; Chambart, H.; Vandemeulebroeke, L.; Cappuyns, V. Sustainable use of sulfidic tailing residues in the production of ceramic roof tiles [Abstract]. In Programme and Book of Abstracts of the 13th Conference for Young Scientists in Ceramics (CYSC-2019); Srdić, V.V., Ed.; Faculty of Technology, University of Novi Sad: Novi Sad, Serbia, 2019; pp. 97–98. [Google Scholar]


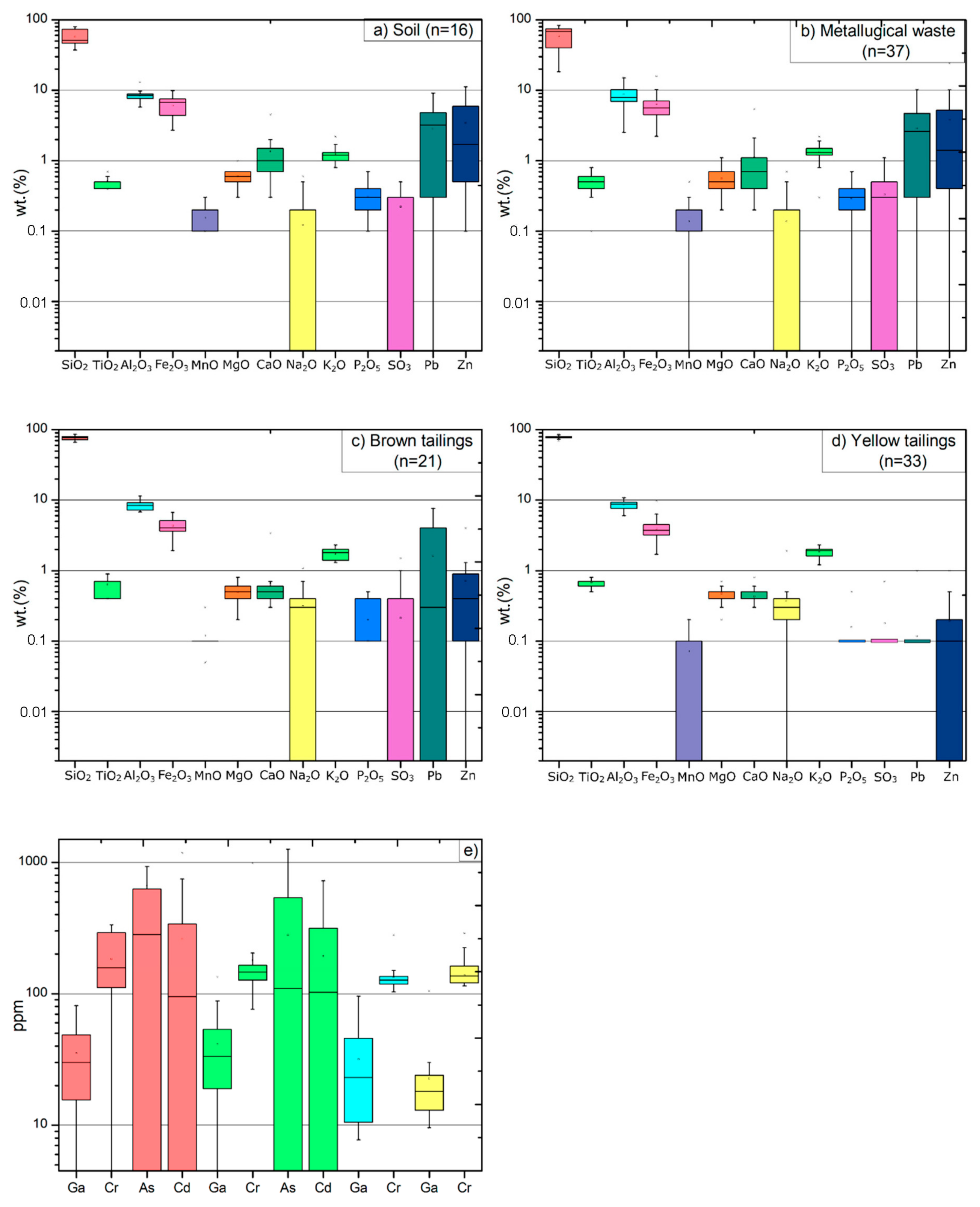



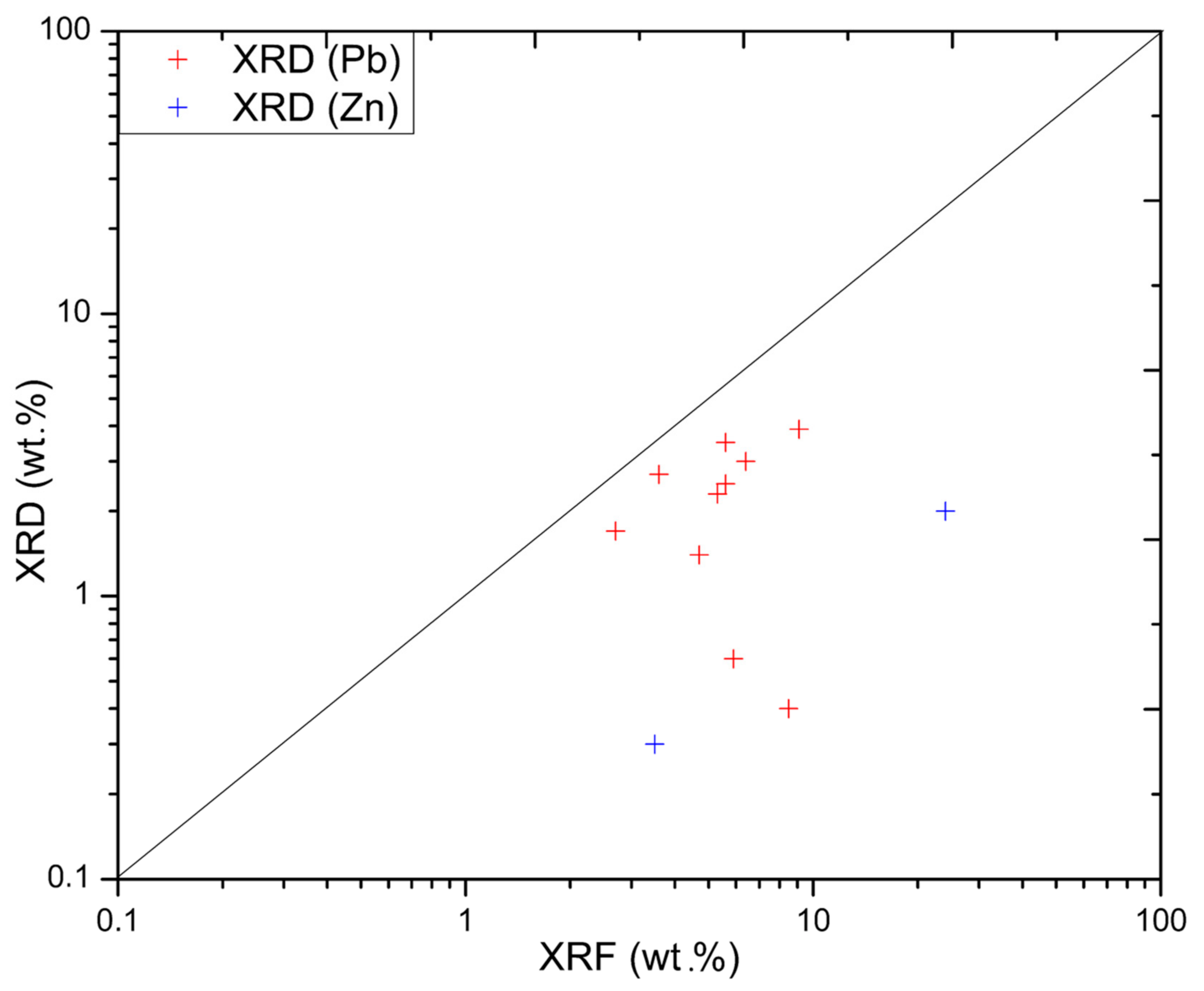
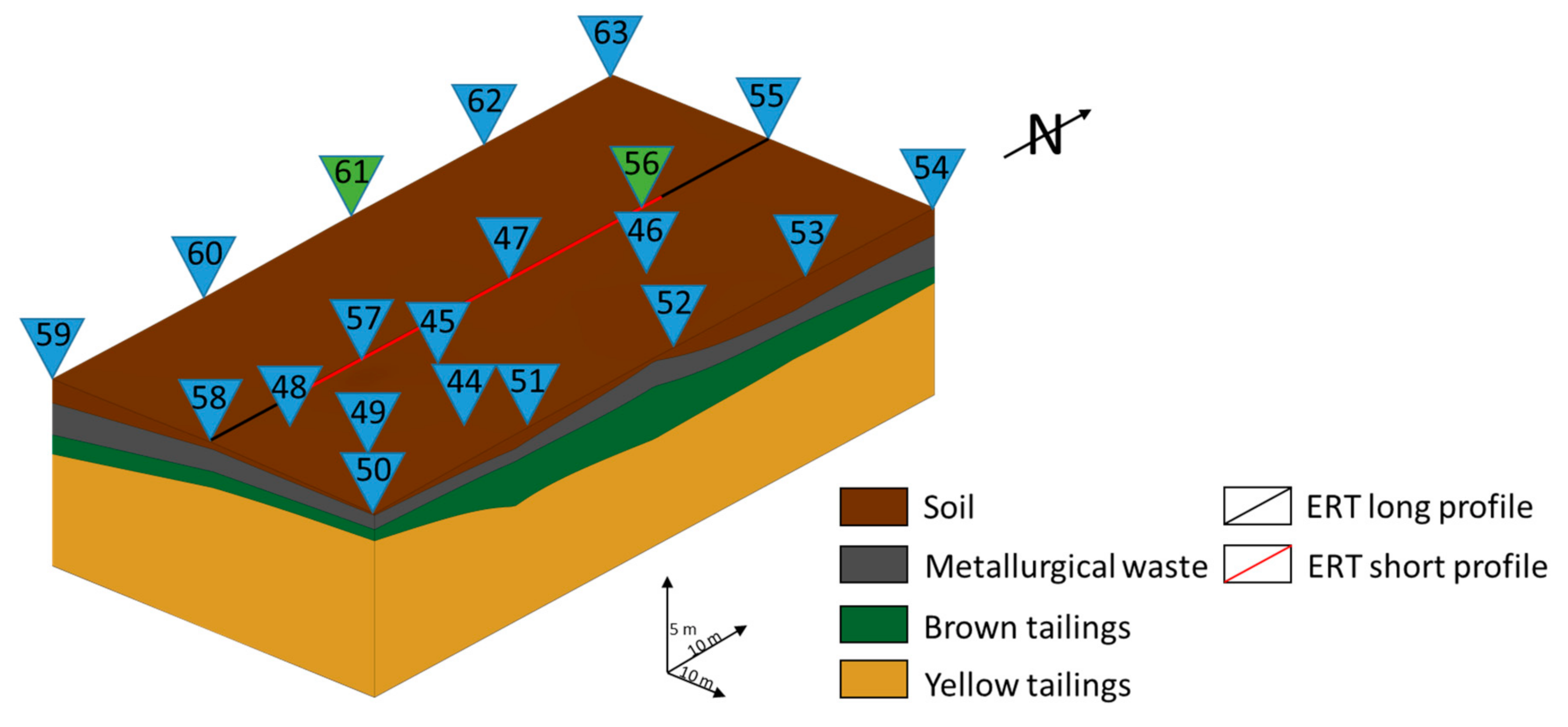
| Material Type | Color | Thickness | Other Properties |
|---|---|---|---|
| Soil | Dark brown | 0.1–0.2 m | Fragments of slag are often present |
| Metallurgical waste | Dark grey to black | 0.6–1.9 m | Fragments of slag, pipes, ceramics and mortar are often present |
| Brown tailings | Dark brown–grey green | 0.4–2.4 m | Often thin layers of weathered ceramics are noticed |
| Yellow tailings | Light brown–yellow–white | 5.0–6.0 m * | - |
| ID | Coordinates | Length (m) | Type | |
|---|---|---|---|---|
| N | E | |||
| 44 | 50°44.080′ | 5°57.763′ | 0.9 | Drill hole |
| 45 | 50°44.076′ | 5°57.759′ | 2.5 | Drill hole |
| 46 | 50°44.098′ | 5°57.735′ | 0.7 | Drill hole |
| 47 | 50°44.082′ | 5°57.744′ | 2.4 | Drill hole |
| 48 | 50°44.070′ | 5°57.771′ | 1.5 | Drill hole |
| 49 | 50°44.076′ | 5°57.777′ | 1.0 | Drill hole |
| 50 | 50°44.077′ | 5°57.780′ | 1.0 | Drill hole |
| 51 | 50°44.085′ | 5°57.767′ | 3.5 | Drill hole |
| 52 | 50° 44.092′ | 5°57.754′ | 1.0 | Drill hole |
| 53 | 50°44.099′ | 5°57.740′ | 3.5 | Drill + pit hole |
| 54 | 50°44.104′ | 5°57.727′ | 1.0 | Drill hole |
| 55 | 50°44.095′ | 5°57.719′ | 3.5 | Drill hole |
| 56 | 50°44.087′ | 5°57.732′ | 2.4 | Drill hole |
| 57 | 50°44.075′ | 5°57.756′ | 2.4 | Drill hole |
| 58 | 50°44.065′ | 5°57.769′ | 1.9 | Drill hole |
| 59 | 50°44.057′ | 5°57.762′ | 0.9 | Drill hole |
| 60 | 50° 44.065′ | 5°57.750′ | 1.0 | Drill hole |
| 61 | 50°44.071′ | 5°57.737′ | 3.0 | Drill hole |
| 62 | 50°44.080′ | 5°57.723′ | 2.0 | Drill + Pit hole |
| 63 | 50°44.087′ | 5°57.708′ | 1.0 | Drill hole |
| Material | Area (m2) | Thickness (m) | Volume (m3) | Density (t/m3) | Tonnage (ton) | Pb (wt.%) | Zn (wt.%) | Pb (wt.%) | Zn (wt.%) | Tonnage (t) | Pb (t) | Zn (t) | Pb (t) | Zn (t) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MW | 3200 | 1.7 | 5440 | 1.3 | 7072 | 2.8 | 4.7 | 2.4 | 3.5 | 12,192 | 198.0 | 332.4 | 279.9 | 373.3 |
| BT | 3200 | 0.8 | 2560 | 2.0 | 5120 | 1.6 | 0.8 | 81.9 | 41.0 | |||||
| YT | 3200 | 5 | 16,000 | 2.0 | 32,000 | 0.2 | 0.2 | 0.2 | 0.2 | 32,000 | 64.0 | 64.0 | - | - |
| - | MW + BT + YT | - | - | 0.9 | 1.3 | 44,192 | - | - | 343.9 | 437.3 | ||||
| Metal | VLAREMA, 2012 (ppm) | Metallurgical Waste (ppm) | Brown Tailings (ppm) | Yellow Tailings (ppm) |
|---|---|---|---|---|
| As | 250 | 220 | <10 | <10 |
| Cd | 10 | 176 | <10 | <10 |
| Cr | 1250 | 160 | 126 | 139 |
| Pb | 1250 | 28,000 | 19,768 | 1725 |
| Zn | 1250 | 33,000 | 7528 | 1912 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bevandić, S.; Blannin, R.; Vander Auwera, J.; Delmelle, N.; Caterina, D.; Nguyen, F.; Muchez, P. Geochemical and Mineralogical Characterisation of Historic Zn–Pb Mine Waste, Plombières, East Belgium. Minerals 2021, 11, 28. https://doi.org/10.3390/min11010028
Bevandić S, Blannin R, Vander Auwera J, Delmelle N, Caterina D, Nguyen F, Muchez P. Geochemical and Mineralogical Characterisation of Historic Zn–Pb Mine Waste, Plombières, East Belgium. Minerals. 2021; 11(1):28. https://doi.org/10.3390/min11010028
Chicago/Turabian StyleBevandić, Srećko, Rosie Blannin, Jacqueline Vander Auwera, Nicolas Delmelle, David Caterina, Frederic Nguyen, and Philippe Muchez. 2021. "Geochemical and Mineralogical Characterisation of Historic Zn–Pb Mine Waste, Plombières, East Belgium" Minerals 11, no. 1: 28. https://doi.org/10.3390/min11010028
APA StyleBevandić, S., Blannin, R., Vander Auwera, J., Delmelle, N., Caterina, D., Nguyen, F., & Muchez, P. (2021). Geochemical and Mineralogical Characterisation of Historic Zn–Pb Mine Waste, Plombières, East Belgium. Minerals, 11(1), 28. https://doi.org/10.3390/min11010028





