Current Challenges and Innovative Developments in Hydroxyapatite-Based Coatings on Metallic Materials for Bone Implantation: A Review
Abstract
:1. Introduction
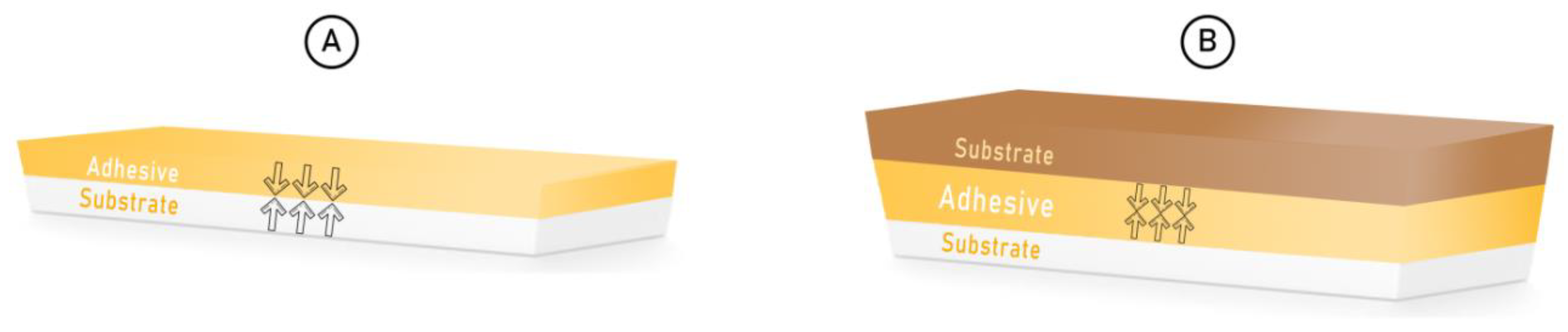
2. Theory of Adhesive Bond
Theory behind Adhesion
3. Existing Challenges in Coating for Metallic Biomaterials
4. Coating Methods
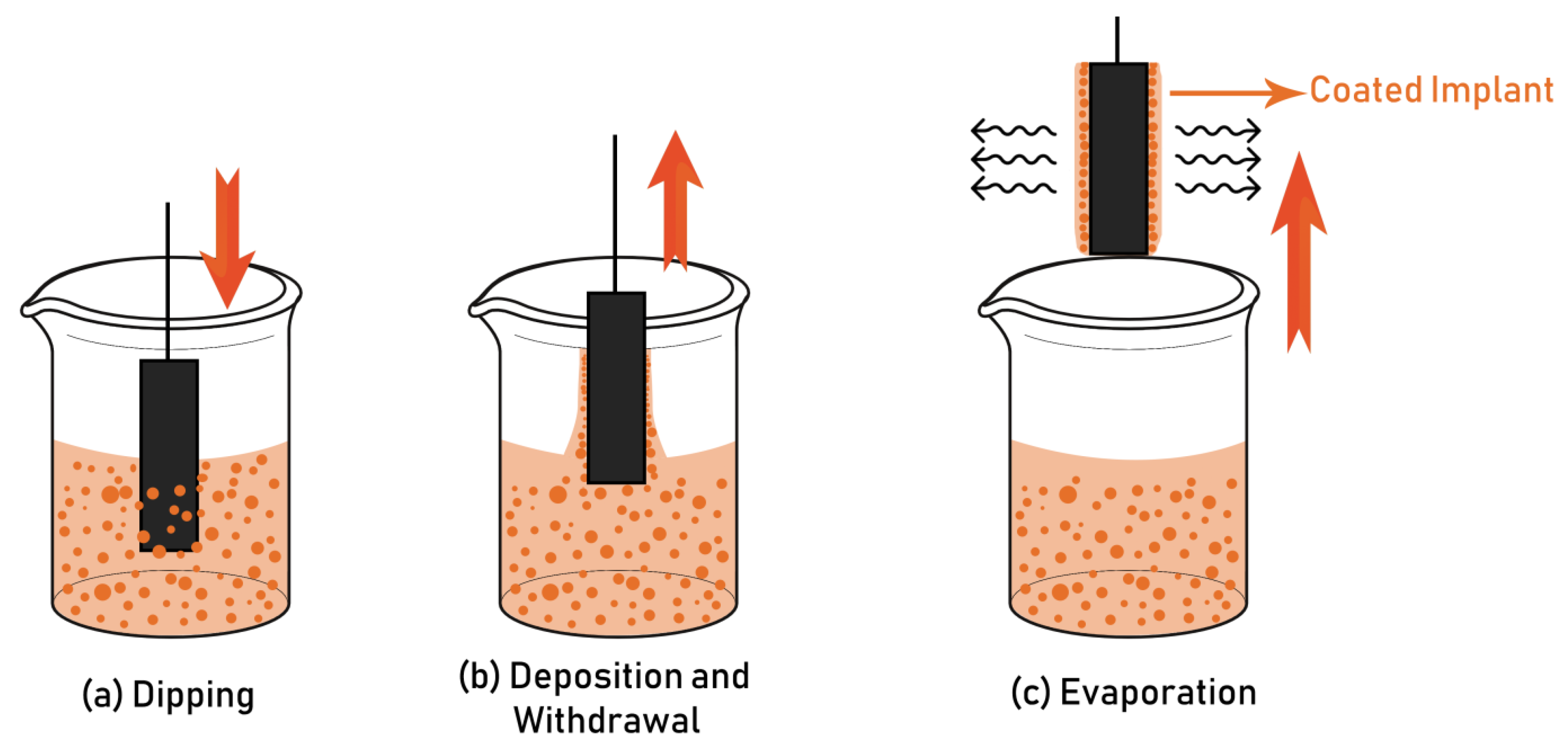
4.1. Sol-Gel and Dip Coating
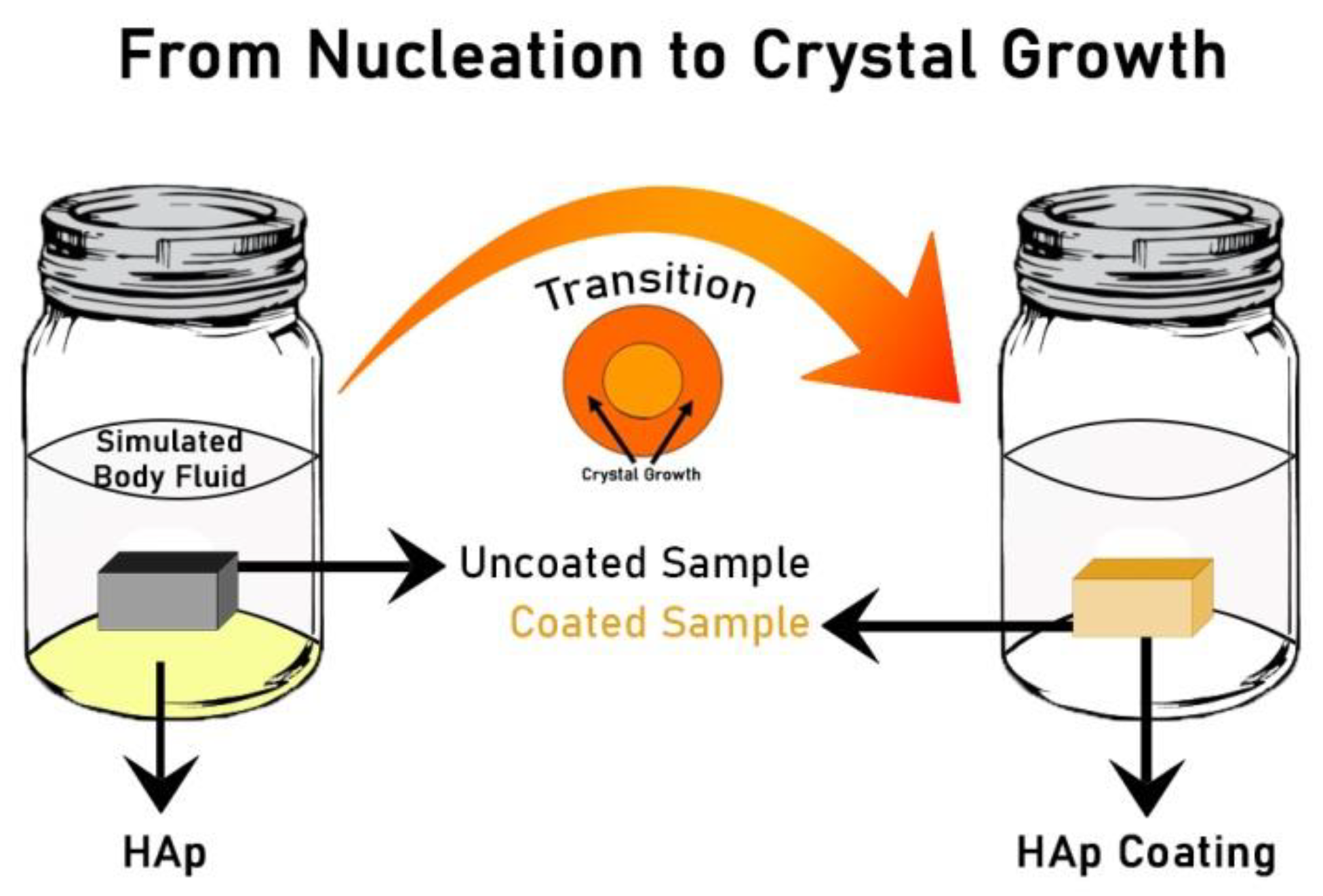
4.2. Biomimetic Deposition
4.3. Chemical Vapor Deposition
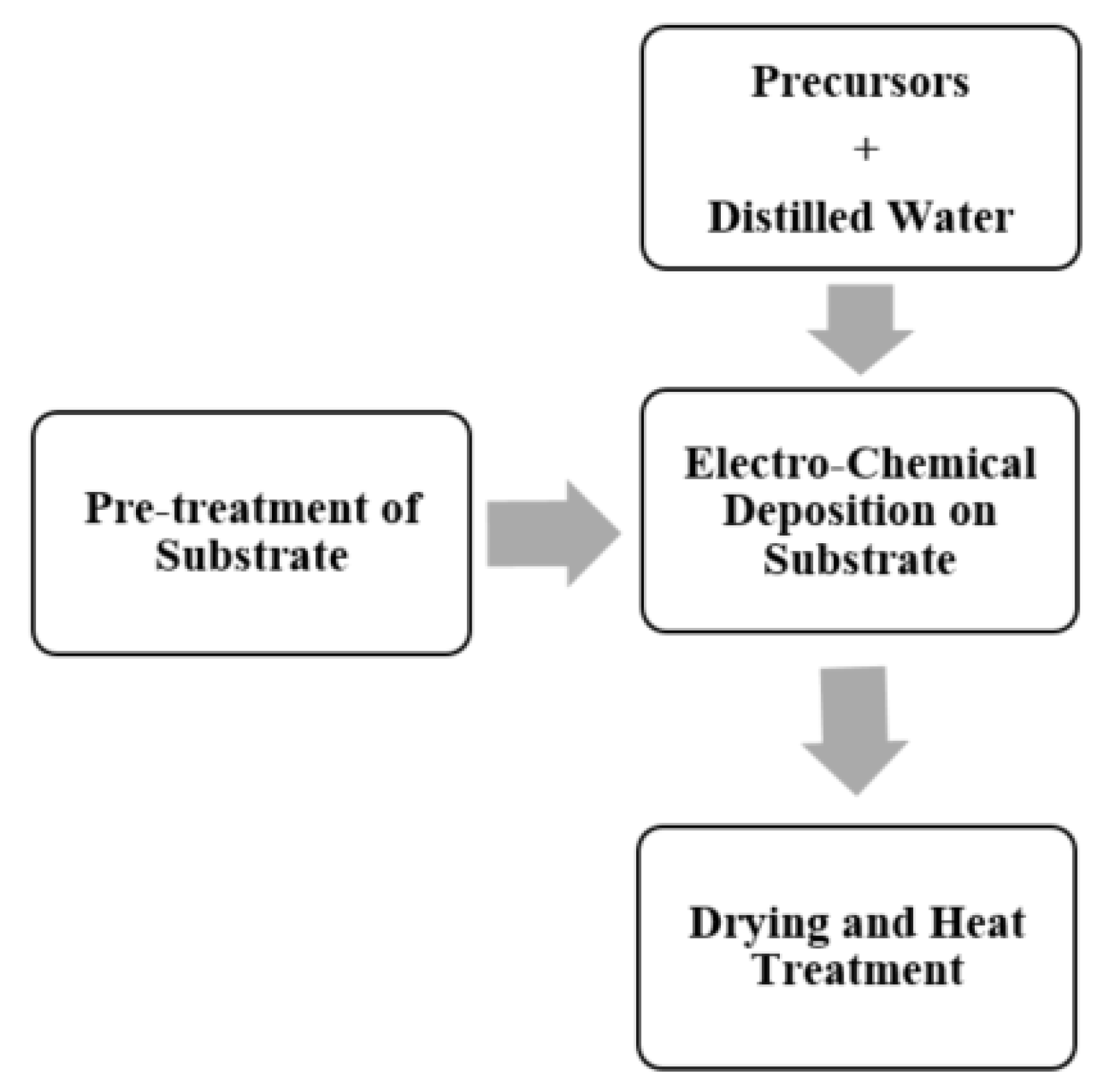
4.4. Electro-Chemical Deposition
4.5. Thermal Spraying
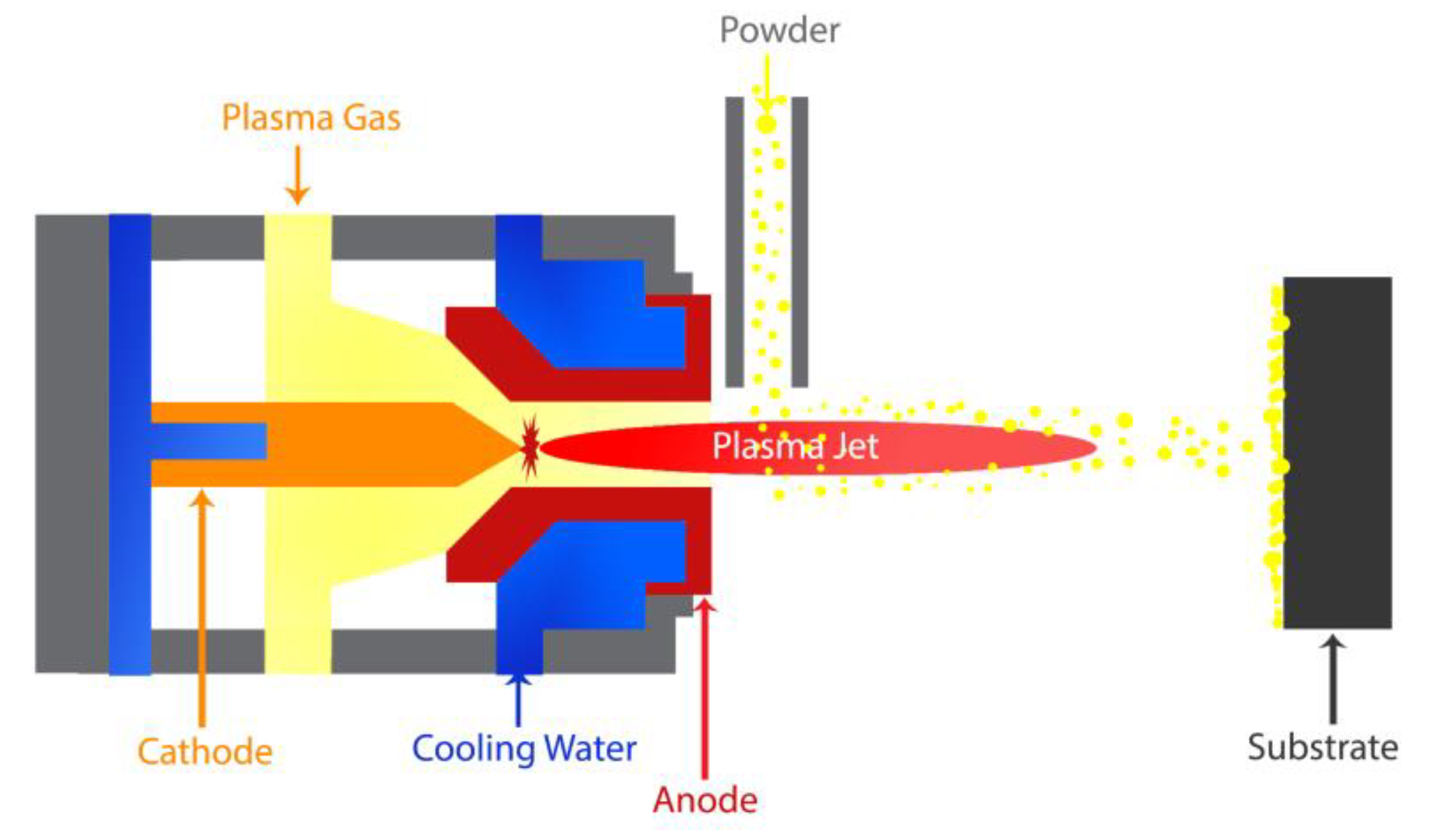
4.5.1. Plasma Spraying
4.5.2. High-Velocity Suspension Flame Spraying (HVSFS)
4.5.3. Pulsed Laser Deposition
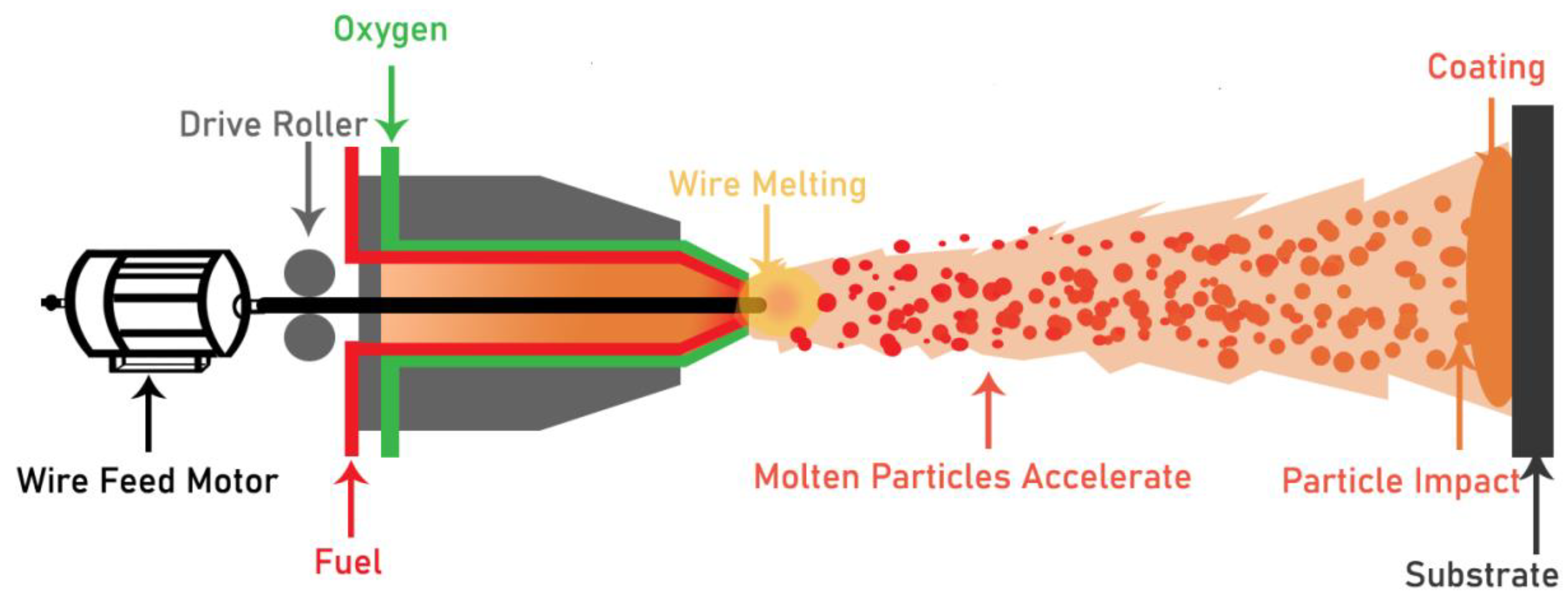
4.5.4. Flame Spray Coating
5. Innovative Methods of Coating
6. Conclusions and Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols | |
| HAp | Hydroxyapatite |
| FDA | The food and drug administration (USA) |
| RF | Radiofrequency |
| MAO | Micro-arc oxidation |
| CVD | Chemical vapor deposition |
| PLD | Pulsed laser deposition |
| HVSFS | High-velocity suspension flame spraying |
| PCL | Poly-(ε-caprolactone) |
| EPD | Electro-phoretic deposition |
| ELD | Electrolytic deposition |
| SBF | Simulated body fluid |
| FS | Flame spraying |
| SWNT | Single-walled nano-tubes |
| SLPM | Standard liters per minute |
| SCFH | Standard cubic feet per hour |
| SHS | Super-high-speed |
References
- Nasab, M.B.; Hassan, M.R.; Sahari, B. Metallic biomaterials of knee and hip: A review. Trends Biomater. Artif. Organs 2010, 24, 69–82. [Google Scholar]
- Maier, P.; Hort, N. Magnesium Alloys for Biomedical Applications; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2020. [Google Scholar]
- Hanawa, T. In vivo metallic biomaterials and surface modification. Mater. Sci. Eng. 1999, 267, 260–266. [Google Scholar] [CrossRef]
- Zhang, S. Hydroxyapatite Coatings for Biomedical Applications; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Priyadarshini, B.; Rama, M.; Chetan; Vijayalakshmi, U. Bioactive coating as a surface modification technique for biocompatible metallic implants: A review. J. Asian Ceram. Soc. 2019, 7, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Han, G.; Zheng, X.; Chen, G.; Zhu, P. Characterization and biocompatibility study of hydroxyapatite coating on the surface of titanium alloy. Surf. Coat. Technol. 2019, 375, 645–651. [Google Scholar] [CrossRef]
- Arcos, D.; Vallet-Regí, M. Substituted hydroxyapatite coatings of bone implants. J. Mater. Chem. B 2020, 8, 1781–1800. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Transcations A 2002, 33, 477. [Google Scholar] [CrossRef]
- Sridhar, T. Nanobioceramic coatings for biomedical applications. Mater. Technol. 2010, 25, 184–195. [Google Scholar] [CrossRef]
- Francis, M.D.; Webb, N.C. Hydroxyapatite formation from a hydrated calcium monohydrogen phosphate precursor. Calcif. Tissue Res. 1970, 6, 335–342. [Google Scholar] [CrossRef]
- Grafts, I.B.; Substitutes, B. Three-dimensionally engineered hydroxyapatite ceramics with interconnected pores as a bone substitute and tissue engineering scaffold. In Biomaterials in Orthopedics; Marcel Dekker, Inc.: New York, NY, USA, 2004. [Google Scholar]
- Martin, R.; Brown, P. Mechanical properties of hydroxyapatite formed at physiological temperature. J. Mater. Sci. Mater. Med. 1995, 6, 138–143. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis–a review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Fabrication, properties and applications of dense hydroxyapatite: A review. J. Funct. Biomater. 2015, 6, 1099–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Chen, H.; Yuan, B.; Zhou, Y.; Min, L.; Xiao, Z.; Zhu, X.; Tu, C.; Zhang, X. Electrochemical Deposition of Nanostructured Hydroxyapatite Coating on Titanium with Enhanced Early Stage Osteogenic Activity and Osseointegration. Int. J. Nanomed. 2020, 15, 6605. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Awasthi, A.; Saxena, K.K. Metallic implants with properties and latest production techniques: A review. Adv. Mater. Process. Technol. 2020, 6, 405–440. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Awasthi, S.; Pandey, S.K.; Arunan, E.; Srivastava, C. A Review on Hydroxyapatite Coatings for Biomedical Application: Experimental and Theoretical Perspectives. J. Mater. Chem. B 2020. [Google Scholar] [CrossRef]
- Song, Y.; Shan, D.; Han, E.J. Electrodeposition of hydroxyapatite coating on AZ91D magnesium alloy for biomaterial application. Mater. Lett. 2008, 62, 3276–3279. [Google Scholar] [CrossRef]
- Zhong, Z.; Qin, J.; Ma, J. Cellulose acetate/hydroxyapatite/chitosan coatings for improved corrosion resistance and bioactivity. Mater. Sci. Eng. C 2015, 49, 251–255. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A. A critical review of decades of research on calcium-phosphate-based coatings: How far are we from their widespread clinical application? Curr. Opin. Biomed. Eng. 2019, 10, 35–44. [Google Scholar] [CrossRef]
- Cizek, J.; Matejicek, J. Medicine Meets Thermal Spray Technology: A Review of Patents. J. Therm. Spray Technol. 2018, 27, 1251–1279. [Google Scholar] [CrossRef] [Green Version]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Lacefield, W. Hydroxyapatite coatings. Ann. New York Acad. Sci. 1988, 523, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, A. Bio-functional nano-coatings on metallic biomaterials. Mater. Sci. Eng. C 2015, 55, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.; Cheng, M.; Tang, S.; Yu, S.; Liao, K.; Tan, C.; Khor, K.; Cheang, P. Tensile properties, tension–tension fatigue and biological response of polyetheretherketone–hydroxyapatite composites for load-bearing orthopedic implants. Biomaterials 2003, 24, 2245–2250. [Google Scholar] [CrossRef]
- Roy, M.; Krishna, B.V.; Bandyopadhyay, A.; Bose, S. Laser processing of bioactive tricalcium phosphate coating on titanium for load-bearing implants. Acta Biomater. 2008, 4, 324–333. [Google Scholar] [CrossRef]
- Kannan, M. Hydroxyapatite coating on biodegradable magnesium and magnesium-based alloys. In Hydroxyapatite (HAp) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 289–306. [Google Scholar]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Störmer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable magnesium–hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef] [Green Version]
- Kezhi, L.; Qian, G.; Leilei, Z.; Yulei, Z.; Shoujie, L.; Kebing, G.; Shaoxian, L. Synthesis and characterization of Si-substituted hydroxyapatite bioactive coating for SiC-coated carbon/carbon composites. Ceram. Int. 2017, 43, 1410–1414. [Google Scholar] [CrossRef]
- Ballarre, J.; López, D.A.; Schreiner, W.H.; Durán, A.; Ceré, S.M. Protective hybrid sol–gel coatings containing bioactive particles on surgical grade stainless steel: Surface characterization. Appl. Surf. Sci. 2007, 253, 7260–7264. [Google Scholar] [CrossRef]
- Ballarre, J.; Manjubala, I.; Schreiner, W.H.; Orellano, J.C.; Fratzl, P.; Ceré, S. Improving the osteointegration and bone–implant interface by incorporation of bioactive particles in sol–gel coatings of stainless steel implants. Acta Biomater. 2010, 6, 1601–1609. [Google Scholar] [CrossRef]
- Ballarre, J.; Seltzer, R.; Mendoza, E.; Orellano, J.C.; Mai, Y.-W.; García, C.; Ceré, S. Morphologic and nanomechanical characterization of bone tissue growth around bioactive sol–gel coatings containing wollastonite particles applied on stainless steel implants. Mater. Sci. Eng. C 2011, 31, 545–552. [Google Scholar] [CrossRef]
- Hsieh, M.-F.; Perng, L.-H.; Chin, T.-S. Hydroxyapatite coating on Ti6Al4V alloy using a sol–gel derived precursor. Mater. Chem. Phys. 2002, 74, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.-M.; Yang, Q.; Troczynski, T. Sol–gel hydroxyapatite coatings on stainless steel substrates. Biomaterials 2002, 23, 691–698. [Google Scholar] [CrossRef]
- Stoch, A.; Jastrze, W.; Długoń, E.; Lejda, W.; Trybalska, B.; Stoch, G.; Adamczyk, A. Sol–gel derived hydroxyapatite coatings on titanium and its alloy Ti6Al4V. J. Mol. Struct. 2005, 744, 633–640. [Google Scholar] [CrossRef]
- Wang, X.; Cai, S.; Liu, T.; Ren, M.; Huang, K.; Zhang, R.; Zhao, H. Fabrication and corrosion resistance of calcium phosphate glass-ceramic coated Mg alloy via a PEG assisted sol–gel method. Ceram. Int. 2014, 40, 3389–3398. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Tkalčec, E.; Kwokal, A.; Piljac, J. An in vitro study of Ti and Ti-alloys coated with sol–gel derived hydroxyapatite coatings. Surf. Coat. Technol. 2003, 165, 40–50. [Google Scholar] [CrossRef]
- García, C.; Ceré, S.; Durán, A. Bioactive coatings prepared by sol–gel on stainless steel 316L. J. Non-Cryst. Solids 2004, 348, 218–224. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhu, S.; Luo, E.; Li, J.; Feng, G.; Liao, Y.; Hu, J. The effect of strontium-substituted hydroxyapatite coating on implant fixation in ovariectomized rats. Biomaterials 2010, 31, 9006–9014. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, R.; Zhang, X. Synthesis and characterization of sol–gel hydroxyapatite coatings deposited on porous NiTi alloys. J. Alloy. Compd. 2011, 509, 4643–4648. [Google Scholar] [CrossRef]
- Bryington, M.S.; Hayashi, M.; Kozai, Y.; VanDeWeghe, S.; Andersson, M.; Wennerberg, A.; Jimbo, R. The influence of nano hydroxyapatite coating on osseointegration after extended healing periods. Dent. Mater. 2013, 29, 514–520. [Google Scholar] [CrossRef]
- Rojaee, R.; Fathi, M.; Raeissi, K. Controlling the degradation rate of AZ91 magnesium alloy via sol–gel derived nanostructured hydroxyapatite coating. Mater. Sci. Eng. C 2013, 33, 3817–3825. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Lamanna, G. TiO2/PCL Hybrid Layers Prepared via Sol-Gel Dip Coating for the Surface Modification of Titanium Implants: Characterization and Bioactivity Evaluation. Appl. Mech. Mater. 2015, 760, 353–358. [Google Scholar] [CrossRef]
- Fu, T.; Sun, J.-M.; Alajmi, Z.; Wu, F. Sol-gel preparation, corrosion resistance and hydrophilicity of Ta-containing TiO2 films on Ti6Al4V alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 471–476. [Google Scholar] [CrossRef]
- Zhao, H.; Cai, S.; Niu, S.; Zhang, R.; Wu, X.; Xu, G.; Ding, Z. The influence of alkali pretreatments of AZ31 magnesium alloys on bonding of bioglass–ceramic coatings and corrosion resistance for biomedical applications. Ceram. Int. 2015, 41, 4590–4600. [Google Scholar] [CrossRef]
- Ma, M.; Ye, W.; Wang, X.X. Effect of supersaturation on the morphology of hydroxyapatite crystals deposited by electrochemical deposition on titanium. Mater. Lett. 2008, 62, 3875–3877. [Google Scholar] [CrossRef]
- Wang, H.; Eliaz, N.; Xiang, Z.; Hsu, H.-P.; Spector, M.; Hobbs, L.W. Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy. Biomater. 2006, 27, 4192–4203. [Google Scholar] [CrossRef]
- Qiu, D.; Wang, A.; Yin, Y. Characterization and corrosion behavior of hydroxyapatite/zirconia composite coating on NiTi fabricated by electrochemical deposition. Appl. Surf. Sci. 2010, 257, 1774–1778. [Google Scholar] [CrossRef]
- Wang, L.-N.; Luo, J.-L. Preparation of hydroxyapatite coating on CoCrMo implant using an effective electrochemically-assisted deposition pretreatment. Mater. Charact. 2011, 62, 1076–1086. [Google Scholar] [CrossRef]
- Qiu, D.; Yang, L.; Yin, Y.; Wang, A. Preparation and characterization of hydroxyapatite/titania composite coating on NiTi alloy by electrochemical deposition. Surf. Coat. Technol. 2011, 205, 3280–3284. [Google Scholar] [CrossRef]
- Rad, A.T.; Solati-Hashjin, M.; Abu Osman, N.A.; Faghihi, S. Improved bio-physical performance of hydroxyapatite coatings obtained by electrophoretic deposition at dynamic voltage. Ceram. Int. 2014, 40, 12681–12691. [Google Scholar] [CrossRef] [Green Version]
- Rojaee, R.; Fathi, M.; Raeissi, K.; Sharifnabi, A. Biodegradation assessment of nanostructured fluoridated hydroxyapatite coatings on biomedical grade magnesium alloy. Ceram. Int. 2014, 40, 15149–15158. [Google Scholar] [CrossRef]
- Sun, G.; Ma, J.; Zhang, S. Electrophoretic deposition of zinc-substituted hydroxyapatite coatings. Mater. Sci. Eng. C 2014, 39, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Boanini, E.; Bracci, B.; Facchini, A.; Panzavolta, S.; Segatti, F.; Sturba, L. Nanocrystalline hydroxyapatite coatings on titanium: A new fast biomimetic method. Biomaterials 2005, 26, 4085–4089. [Google Scholar] [CrossRef] [PubMed]
- Schrooten, J.; Helsen, J. Adhesion of bioactive glass coating to Ti6Al4V oral implant. Biomaterials 2000, 21, 1461–1469. [Google Scholar] [CrossRef]
- Yang, Y.; Ong, J.L.; Tian, J. Deposition of highly adhesive ZrO(2) coating on Ti and CoCrMo implant materials using plasma spraying. Biomaterials 2003, 24, 619–627. [Google Scholar] [CrossRef]
- Yang, Y.; Chou, B. Bonding strength investigation of plasma-sprayed HA coatings on alumina substrate with porcelain intermediate layer. Mater. Chem. Phys. 2007, 104, 312–319. [Google Scholar] [CrossRef]
- Grandfield, K.; Palmquist, A.; Goncalves, S.; Taylor, A.; Taylor, M.; Emanuelsson, L.; Thomsen, P.; Engqvist, H. Free form fabricated features on CoCr implants with and without hydroxyapatite coating in vivo: A comparative study of bone contact and bone growth induction. J. Mater. Sci. Mater. Electron. 2011, 22, 899–906. [Google Scholar] [CrossRef]
- Vencl, A.; Arostegui, S.; Favaro, G.; Zivic, F.; Mrdak, M.; Mitrović, S.; Popovic, V. Evaluation of adhesion/cohesion bond strength of the thick plasma spray coatings by scratch testing on coatings cross-sections. Tribol. Int. 2011, 44, 1281–1288. [Google Scholar] [CrossRef]
- Hung, K.-Y.; Lo, S.-C.; Shih, C.-S.; Yang, Y.-C.; Feng, H.-P.; Lin, Y.-C. Titanium surface modified by hydroxyapatite coating for dental implants. Surf. Coat. Technol. 2013, 231, 337–345. [Google Scholar] [CrossRef]
- Latifi, A.; Imani, M.; Khorasani, M.T.; Joupari, M.D. Plasma surface oxidation of 316L stainless steel for improving adhesion strength of silicone rubber coating to metal substrate. Appl. Surf. Sci. 2014, 320, 471–481. [Google Scholar] [CrossRef]
- Hameed, P.; Gopal, V.; Bjorklund, S.; Ganvir, A.; Sen, D.; Markocsan, N.; Manivasagam, G. Axial suspension plasma spraying: An ultimate technique to tailor Ti6Al4V surface with HAp for orthopaedic applications. Colloids Surf. B: Biointerfaces 2019, 173, 806–815. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.-H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process?an alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Rauch, J.; Bolelli, G.; Killinger, A.; Gadow, R.; Cannillo, V.; Lusvarghi, L. Advances in High Velocity Suspension Flame Spraying (HVSFS). Surf. Coat. Technol. 2009, 203, 2131–2138. [Google Scholar] [CrossRef]
- Bolelli, G.; Bellucci, D.; Cannillo, V.; Lusvarghi, L.; Sola, A.; Stiegler, N.; Müller, P.; Killinger, A.; Gadow, R.; Altomare, L.; et al. Suspension thermal spraying of hydroxyapatite: Microstructure and in vitro behaviour. Mater. Sci. Eng. C 2014, 34, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Bolelli, G.; Bellucci, D.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Müller, P.; Sola, A. Comparison between Suspension Plasma Sprayed and High Velocity Suspension Flame Sprayed bioactive coatings. Surf. Coat. Technol. 2015, 280, 232–249. [Google Scholar] [CrossRef]
- Clèries, L.; Martínez, E.; Fernández-Pradas, J.; Sardin, G.; Esteve, J.; Morenza, J. Mechanical properties of calcium phosphate coatings deposited by laser ablation. Biomaterials 2000, 21, 967–971. [Google Scholar] [CrossRef]
- Fernández-Pradas, J.; Clèries, L.; Martínez, E.; Sardin, G.; Esteve, J.; Morenza, J. Influence of thickness on the properties of hydroxyapatite coatings deposited by KrF laser ablation. Biomaterials 2001, 22, 2171–2175. [Google Scholar] [CrossRef]
- Khandelwal, H.; Singh, G.; Agrawal, K.; Prakash, S.; Agarwal, R. Characterization of hydroxyapatite coating by pulse laser deposition technique on stainless steel 316 L by varying laser energy. Appl. Surf. Sci. 2013, 265, 30–35. [Google Scholar] [CrossRef]
- Li, J.; Liao, H.; Hermansson, L. Sintering of partially-stabilized zirconia and partially-stabilized zirconia—hydroxyapatite composites by hot isostatic pressing and pressureless sintering. Biomaterials 1996, 17, 1787–1790. [Google Scholar] [CrossRef]
- Onoki, T.; Hashida, T. New method for hydroxyapatite coating of titanium by the hydrothermal hot isostatic pressing technique. Surf. Coat. Technol. 2006, 200, 6801–6807. [Google Scholar] [CrossRef]
- Das, B.; Bandyopadhyay, P.; Nath, A.K. An investigation on corrosion resistance and mechanical properties of laser remelted flame sprayed coating. Adv. Mater. Process. Technol. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Monsalve, M.; Lopez, E.; Ageorges, H.; Vargas, F. Bioactivity and mechanical properties of bioactive glass coatings fabricated by flame spraying. Surf. Coat. Technol. 2015, 268, 142–146. [Google Scholar] [CrossRef]
- Surmenev, R.A. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf. Coat. Technol. 2012, 206, 2035–2056. [Google Scholar] [CrossRef]
- Qu, J.; Ouyang, L.; Kuo, C.-C.; Martin, D. Stiffness, strength and adhesion characterization of electrochemically deposited conjugated polymer films. Acta Biomater. 2016, 31, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Say, Y.; Aksakal, B.; Dikici, B. Effect of hydroxyapatite/SiO 2 hybride coatings on surface morphology and corrosion resistance of REX-734 alloy. Ceram. Int. 2016, 42, 10151–10158. [Google Scholar] [CrossRef]
- Fomin, A.; Fomina, M.; Koshuro, V.; Rodionov, I.; Zakharevich, A.; Skaptsov, A. Structure and mechanical properties of hydroxyapatite coatings produced on titanium using plasma spraying with induction preheating. Ceram. Int. 2017, 43, 11189–11196. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.; Hamouda, A.M.; Goh, B.; Yoon, G.H. Ti/TiN/HA coating on Ti–6Al–4V for biomedical applications. Ceram. Int. 2015, 41, 14447–14457. [Google Scholar] [CrossRef]
- Packham, D.E. Surface energy, surface topography and adhesion. Int. J. Adhes. Adhes. 2003, 23, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhang, X.; Zhang, H.; Qiao, H.; Zhang, X.; Jia, T.; Han, S.; Gao, Y.; Xiao, H.; Yang, H.J. Fabrication of silver- and strontium-doped hydroxyapatite/TiO2 nanotube bilayer coatings for enhancing bactericidal effect and osteoinductivity. Ceram. Int. 2017, 43, 992–1007. [Google Scholar] [CrossRef]
- Mahjoubi, H.; Buck, E.; Manimunda, P.; Farivar, R.; Chromik, R.; Murshed, M.; Cerruti, M. Surface phosphonation enhances hydroxyapatite coating adhesion on polyetheretherketone and its osseointegration potential. Acta Biomater. 2017, 47, 149–158. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; Von Der Mark, K.; Park, J. Engineering biocompatible implant surfaces: Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Tao, Y.; Ke, G.; Xie, Y.; Chen, Y.; Shi, S.; Guo, H. Adhesion strength and nucleation thermodynamics of four metals (Al, Cu, Ti, Zr) on AlN substrates. Appl. Surf. Sci. 2015, 357, 8–13. [Google Scholar] [CrossRef]
- Huang, Y.; Hao, M.; Nian, X.; Qiao, H.; Zhang, X.; Zhang, X.; Song, G.; Guo, J.; Pang, X.; Zhang, H. Strontium and copper co-substituted hydroxyapatite-based coatings with improved antibacterial activity and cytocompatibility fabricated by electrodeposition. Ceram. Int. 2016, 42, 11876–11888. [Google Scholar] [CrossRef]
- Zhao, Z.; Du, L.; Tao, Y.; Li, Q.; Luo, L. Enhancing the adhesion strength of micro electroforming layer by ultrasonic agitation method and the application. Ultrason. Sonochemistry 2016, 33, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Hannink, G.; Arts, J.C. Bioresorbability, porosity and mechanical strength of bone substitutes: What is optimal for bone regeneration? Injury 2011, 42, S22–S25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mediaswanti, K.; Wen, C.; Ivanova, E.; Berndt, C.; Malherbe, F.; Pham, V.; Wang, J. A review on bioactive porous metallic biomaterials. J. Biomim. Biomater. Tissue Eng. 2013, 18, 1–8. [Google Scholar]
- Berndt, C.C.; Hasan, F.; Tietz, U.; Schmitz, K.-P. A Review of Hydroxyapatite Coatings Manufactured by Thermal Spray. In Springer Series in Biomaterials Science and Engineering; Springer Science and Business Media LLC: Larkspur, CA, USA, 2014; pp. 267–329. [Google Scholar]
- Ma, J.; Wang, C.; Ban, C.; Chen, C.; Zhang, H. Pulsed laser deposition of magnesium-containing bioactive glass film on porous Ti–6Al–4V substrate pretreated by micro-arc oxidation. Vacuum 2016, 125, 48–55. [Google Scholar] [CrossRef]
- Almeida, R.M.; Gama, A.; Vueva, Y. Bioactive sol–gel scaffolds with dual porosity for tissue engineering. J. Sol.-Gel Sci. Technol. 2011, 57, 336–342. [Google Scholar] [CrossRef]
- MI, Z.R.; Shuib, S.; Hassan, A.; Shorki, A.; Ibrahim, M.M. Problem of Stress Shielding and Improvement to the Hip Implat Designs: A Review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar]
- Bugbee, W.D.; Sychterz, C.J.; Engh, C.A. Bone Remodeling Around Cementless Hip Implants. South. Med. J. 1996, 89, 1036–1040. [Google Scholar] [CrossRef]
- Bellucci, D.; Veronesi, E.; Strusi, V.; Petrachi, T.; Murgia, A.; Mastrolia, I.; Dominici, M.; Cannillo, V. Human Mesenchymal Stem Cell Combined with a New Strontium-Enriched Bioactive Glass: An ex-vivo Model for Bone Regeneration. Materials 2019, 12, 3633. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Q.; Golden, T.D. Electrochemical study of hydroxyapatite coatings on stainless steel substrates. Thin Solid Film. 2009, 518, 55–60. [Google Scholar] [CrossRef]
- Suwanprateeb, J.; Suvannapruk, W.; Chokevivat, W.; Kiertkrittikhoon, S.; Jaruwangsanti, N.; Tienboon, P. Bioactivity of a sol–gel-derived hydroxyapatite coating on titanium implants in vitro and in vivo. Asian Biomed. 2018, 12, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Xianting, Z.; Yongsheng, W.; Kui, C.; Wenjian, W. Adhesion strength of sol–gel derived fluoridated hydroxyapatite coatings. Surf. Coat. Technol. 2006, 200, 6350–6354. [Google Scholar] [CrossRef]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [Green Version]
- Costa, D.O.; Dixon, S.J.; Rizkalla, A.S. One- and Three-Dimensional Growth of Hydroxyapatite Nanowires during Sol–Gel–Hydrothermal Synthesis. Acs Appl. Mater. Interfaces 2012, 4, 1490–1499. [Google Scholar] [CrossRef]
- Liu, D.-M.; Troczynski, T.; Tseng, W.J. Water-based sol–gel synthesis of hydroxyapatite: Process development. Biomaterials 2001, 22, 1721–1730. [Google Scholar] [CrossRef]
- Cardoso, D.A.; Jansen, J.A.; Leeuwenburgh, S.C.G. Synthesis and application of nanostructured calcium phosphate ceramics for bone regeneration. J. Biomed. Mater. Res. Part. B: Appl. Biomater. 2012, 100, 2316–2326. [Google Scholar] [CrossRef]
- Choi, A.H.; Ben-Nissan, B. Sol-gel production of bioactive nanocoatings for medical applications. Part II: Current research and development. Nanomedicine 2007, 2, 51–61. [Google Scholar] [CrossRef]
- Davar, F.; Shayan, N. Preparation of zirconia-magnesia nanocomposite powders and coating by a sucrose mediated sol-gel method and investigation of its corrosion behavior. Ceram. Int. 2017, 43, 3384–3392. [Google Scholar] [CrossRef]
- Shadanbaz, S.; Dias, G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: A review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef]
- Qu, H.; Wei, M. Improvement of bonding strength between biomimetic apatite coating and substrate. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2008, 84, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Bakan, F.; Laçin, O.; Saraç, H. A novel low temperature sol–gel synthesis process for thermally stable nano crystalline hydroxyapatite. Powder Technol. 2013, 233, 295–302. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Ferrara, C.; Mustarelli, P. Silica–polyethylene glycol hybrids synthesized by sol–gel: Biocompatibility improvement of titanium implants by coating. Mater. Sci. Eng. C 2015, 55, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, H. Fabrication and characterization of thin nano-hydroxyapatite coatings on titanium. Surf. Coat. Technol. 2004, 185, 268–274. [Google Scholar] [CrossRef]
- Motealleh, A.; Eqtesadi, S.; Perera, F.H.; Pajares, A.; Guiberteau, F.; González, P.M. Understanding the role of dip-coating process parameters in the mechanical performance of polymer-coated bioglass robocast scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 64, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.J.A.b. Biomedical coatings on magnesium alloys–a review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Yusoff, M.F.M.; Kadir, M.R.A.; Iqbal, N.; Hassan, M.A.; Hussain, R. Dipcoating of poly (ε-caprolactone)/hydroxyapatite composite coating on Ti6Al4V for enhanced corrosion protection. Surf. Coat. Technol. 2014, 245, 102–107. [Google Scholar] [CrossRef]
- Yuan, J.; Zhao, K.; Cai, T.; Gao, Z.; Yang, L.; He, D. One-step dip-coating of uniform γ-Al 2 O 3 layers on cordierite honeycombs and its environmental applications. Ceram. Int. 2016, 42, 14384–14390. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Giovanardi, R.; Veronesi, P. Corrosion behavior and mechanical properties of bioactive sol-gel coatings on titanium implants. Mater. Sci. Eng. C 2014, 43, 375–382. [Google Scholar] [CrossRef]
- Gray, J.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloy Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Dinda, G.; Shin, J.; Mazumder, J. Pulsed laser deposition of hydroxyapatite thin films on Ti–6Al–4V: Effect of heat treatment on structure and properties. Acta Biomater. 2009, 5, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Fujibayashi, S.; Yamaguchi, S.; Yamamoto, K.; Otsuki, B.; Takemoto, M.; Tsukanaka, M.; Kizuki, T.; Matsushita, T.; Kokubo, T.; et al. Bioactivity of sol–gel-derived TiO2 coating on polyetheretherketone: In vitro and in vivo studies. Acta Biomater. 2016, 35, 305–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-W.; Koh, Y.-H.; Li, L.-H.; Lee, S.; Kim, H.-E. Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol–gel method. Biomaterials 2004, 25, 2533–2538. [Google Scholar] [CrossRef] [PubMed]
- Usinskas, P.; Stankeviciute, Z.; Beganskiene, A.; Kareiva, A. Sol-gel derived porous and hydrophilic calcium hydroxyapatite coating on modified titanium substrate. Surf. Coat. Technol. 2016, 307, 935–940. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Mali, S.A.; Nune, K.; Misra, R.D.K. Biomimetic nanostructured hydroxyapatite coatings on metallic implant materials. Mater. Technol. 2016, 31, 782–790. [Google Scholar] [CrossRef]
- Escada, A.L.; Machado, J.P.B.; Schneider, S.G.; Alves-Rezende, M.C.R.; Claro, A.P.R.A. Biomimetic calcium phosphate coating on Ti-7.5Mo alloy for dental application. J. Mater. Sci. Mater. Electron. 2011, 22, 2457–2465. [Google Scholar] [CrossRef]
- Stigter, M.; De Groot, K.; Layrolle, P. Incorporation of tobramycin into biomimetic hydroxyapatite coating on titanium. Biomaterials 2002, 23, 4143–4153. [Google Scholar] [CrossRef]
- Bharati, S.; Sinha, M.K.; Basu, D. Hydroxyapatite coating by biomimetic method on titanium alloy using concentrated SBF. Bull. Mater. Sci. 2005, 28, 617–621. [Google Scholar] [CrossRef]
- Arrés, M.; Salama, M.; Rechena, D.; Paradiso, P.; Reis, L.; Alves, M.M.; Rego, A.M.B.D.; Carmezim, M.J.; Vaz, M.F.; Deus, A.; et al. Surface and mechanical properties of a nanostructured citrate hydroxyapatite coating on pure titanium. J. Mech. Behav. Biomed. Mater. 2020, 108, 103794. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Barrère, F.; Van Blitterswijk, C.; Groot, K.; Layrolle, P. Biomimetic Hydroxyapatite Coating on Metal Implants. J. Am. Ceram. Soc. 2004, 85, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Luan, B. Formation of hydroxyapatite coating using novel chemo-biomimetic method. J. Mater. Sci. Mater. Electron. 2008, 19, 3211–3220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, M.; Ting, O.P.; Yee, T.S.; Pushparajan, S.; Swaminathan, D.; Kutty, M.G. Biomimetic Coating of Modified Titanium Surfaces with Hydroxyapatite Using Simulated Body Fluid. Adv. Mater. Sci. Eng. 2015, 2015, 407379. [Google Scholar] [CrossRef] [Green Version]
- Gunputh, U.F.; Le, H. A Review of In-Situ Grown Nanocomposite Coatings for Titanium Alloy Implants. J. Compos. Sci. 2020, 4, 41. [Google Scholar] [CrossRef] [Green Version]
- Goto, T.; Katsui, H. Chemical vapor deposition of Ca–P–O film coating. In Interface Oral Health Science 2014; Springer: New York, NY, USA, 2015; pp. 103–115. [Google Scholar]
- Cabañas, M.V.; Vallet-Regí, M. Calcium phosphate coatings deposited by aerosol chemical vapour deposition. J. Mater. Chem. 2003, 13, 1104–1107. [Google Scholar] [CrossRef]
- Gao, Y. Synthesis and Characterization of Calcium Phosphate Coatings by Metalorganic Chemical Vapor Deposition. In Proceedings of the MRS Proceedings; Cambridge University Press (CUP): Cambridge, UK, 1998; Volume 550, p. 550. [Google Scholar]
- Darr, J.; Guo, Z.X.; Raman, V.; Bououdina, M.; Rehman, I.U. Metal organic chemical vapour deposition (MOCVD) of bone mineral like carbonated hydroxyapatite coatingsElectronic supplementary information (ESI) available: Experimental data. Chem. Commun. 2004, 696. [Google Scholar] [CrossRef]
- Sato, M.; Tu, R.; Goto, T.; Ueda, K.; Narushima, T. Hydroxyapatite Formation on CaTiO3 Film Prepared by Metal-Organic Chemical Vapor Deposition. Mater. Trans. 2007, 48, 1505–1510. [Google Scholar] [CrossRef]
- Tsutsumi, H.; Niinomi, M.; Nakai, M.; Gozawa, T.; Akahori, T.; Saito, K.; Tu, R.; Goto, T. Fabrication of Hydroxyapatite Film on Ti-29Nb-13Ta-4.6Zr Using a MOCVD Technique. Mater. Trans. 2010, 51, 2277–2283. [Google Scholar] [CrossRef] [Green Version]
- Zhitomirsky, I. Cathodic electrodeposition of ceramic and organoceramic materials. Fundam. Asp. 2002, 97, 279–317. [Google Scholar]
- Li, T.-T.; Ling, L.; Lin, M.-C.; Peng, H.-K.; Ren, H.-T.; Lou, C.-W.; Lin, J.-H. Recent advances in multifunctional hydroxyapatite coating by electrochemical deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Sobolev, A.; Valkov, A.; Kossenko, A.; Wolicki, I.; Zinigrad, M.; Borodianskiy, K. Bioactive Coating on Ti Alloy with High Osseointegration and Antibacterial Ag Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39534–39544. [Google Scholar] [CrossRef] [PubMed]
- Nuswantoro, N.F.; Budiman, I.; Septiawarman, A.; Tjong, D.H.; Manjas, M. Gunawarman Effect of Applied Voltage and Coating Time on Nano Hydroxyapatite Coating on Titanium Alloy Ti6Al4V Using Electrophoretic Deposition for Orthopaedic Implant Application. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 547, p. 012004. [Google Scholar]
- Fadli, A.; Komalasari; Indriyani, I. Coating Hydroxyapatite on 316L Stainless Steel Using Electroforesis Deposition Method. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1351, p. 012015. [Google Scholar]
- He, D.-H.; Wang, P.; Liu, P.; Liu, X.; Ma, F.-C.; Zhao, J. HA coating fabricated by electrochemical deposition on modified Ti6Al4V alloy. Surf. Coat. Technol. 2016, 301, 6–12. [Google Scholar] [CrossRef]
- Eliaz, N.; Shmueli, S.; Shur, I.; Benayahu, D.; Aronov, D.; Rosenman, G. The effect of surface treatment on the surface texture and contact angle of electrochemically deposited hydroxyapatite coating and on its interaction with bone-forming cells. Acta Biomater. 2009, 5, 3178–3191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Y.; Tao, J.; Pang, Y.-C.; Wang, W.; Wang, T. Electrochemical deposition of hydroxyapatite coatings on titanium. Trans. Nonferrous Met. Soc. China 2006, 16, 633–637. [Google Scholar] [CrossRef]
- Eliaz, N.; Sridhar, T.; Mudali, U.K.; Raj, B. Electrochemical and electrophoretic deposition of hydroxyapatite for orthopaedic applications. Surf. Eng. 2005, 21, 238–242. [Google Scholar] [CrossRef]
- Li, T.-T.; Ling, L.; Lin, M.-C.; Jiang, Q.; Lin, J.; Lin, J.; Lou, C. Properties and Mechanism of Hydroxyapatite Coating Prepared by Electrodeposition on a Braid for Biodegradable Bone Scaffolds. Nanomaterials 2019, 9, 679. [Google Scholar] [CrossRef] [Green Version]
- Isa, N.N.C.; Mohd, Y.; Yury, N. Electrochemical Deposition and Characterization of Hydroxyapatite (HAp) on Titanium Substrate. Apcbee Procedia 2012, 3, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Parcharoen, Y.; Kajitvichyanukul, P.; Sirivisoot, S.; Termsuksawad, P. Hydroxyapatite electrodeposition on anodized titanium nanotubes for orthopedic applications. Appl. Surf. Sci. 2014, 311, 54–61. [Google Scholar] [CrossRef]
- Li’Nan, J.; Chenghao, L.; Naibao, H.; Feng, D.; Lixia, W. Formation and Characterization of Hydroxyapatite Coating Prepared by Pulsed Electrochemical Deposition. Rare Met. Mater. Eng. 2015, 44, 592–598. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Vladescu, A.; Dinu, M.; Vranceanu, D.M. Influence of deposition temperature on the properties of hydroxyapatite obtained by electrochemical assisted deposition. Ceram. Int. 2018, 44, 669–677. [Google Scholar] [CrossRef]
- Kwok, C.; Wong, P.; Cheng, F.; Man, H. Characterization and corrosion behavior of hydroxyapatite coatings on Ti6Al4V fabricated by electrophoretic deposition. Appl. Surf. Sci. 2009, 255, 6736–6744. [Google Scholar] [CrossRef]
- Yang, G.-L.; He, F.-M.; Hu, J.-A.; Wang, X.-X.; Zhao, S.-F. Biomechanical Comparison of Biomimetically and Electrochemically Deposited Hydroxyapatite–Coated Porous Titanium Implants. J. Oral Maxillofac. Surg. 2010, 68, 420–427. [Google Scholar] [CrossRef]
- Ayu, H.M.; Izman, S.; Daud, R.; Krishnamurithy, G.; Shah, A.; Tomadi, S.; Salwani, M.S. Surface Modification on CoCrMo Alloy to Improve the Adhesion Strength of Hydroxyapatite Coating. Procedia Eng. 2017, 184, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Catauro, M.; Bollino, F.; Giovanardi, R.; Veronesi, P. Modification of Ti6Al4V implant surfaces by biocompatible TiO2 /PCL hybrid layers prepared via sol–gel dip coating: Structural characterization, mechanical and corrosion behavior. Mater. Sci. Eng. C 2017, 74, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Mahfuz, H.; Rondinone, A.J.; Leventouri, T. Improvement of the fracture toughness of hydroxyapatite (HAp) by incorporation of carboxyl functionalized single walled carbon nanotubes (CfSWCNTs) and nylon. Mater. Sci. Eng. C 2016, 60, 204–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manonmani, R.; Vinodhini, S.P.; Venkatachalapathy, B.; Sridhar, T.M. Electrochemical, mechanical and osseointegration evaluation of NBPC-coated 316L SS by EPD. Surf. Eng. 2017, 34, 511–519. [Google Scholar] [CrossRef]
- Supriadi, S.; Putri, S.L.; Ramadhan, R.; Suharno, B. Alkali-Heat Treatment of Ti-6Al-4V to Hydroxyapatite Coating Using Electrophoretic Method. Key Eng. Mater. 2020, 846, 175–180. [Google Scholar] [CrossRef]
- Rath, P.C.; Besra, L.; Singh, B.P.; Bhattacharjee, S. Titania/hydroxyapatite bi-layer coating on Ti metal by electrophoretic deposition: Characterization and corrosion studies. Ceram. Int. 2012, 38, 3209–3216. [Google Scholar] [CrossRef]
- Albayrak, O.; El-Atwani, O.; Altintas, S. Hydroxyapatite coating on titanium substrate by electrophoretic deposition method: Effects of titanium dioxide inner layer on adhesion strength and hydroxyapatite decomposition. Surf. Coat. Technol. 2008, 202, 2482–2487. [Google Scholar] [CrossRef]
- Gadow, R.; Killinger, A.; Stiegler, N. Hydroxyapatite coatings for biomedical applications deposited by different thermal spray techniques. Surf. Coat. Technol. 2010, 205, 1157–1164. [Google Scholar] [CrossRef]
- Heimann, R.B. Structure, properties, and biomedical performance of osteoconductive bioceramic coatings. Surf. Coat. Technol. 2013, 233, 27–38. [Google Scholar] [CrossRef]
- Saadati, A.; Hesarikia, H.; Nourani, M.R.; Taheri, R.A. Electrophoretic deposition of hydroxyapatite coating on biodegradable Mg–4Zn–4Sn–0.6 Ca–0.5 Mn alloy. Surf. Eng. 2019, 36, 908–918. [Google Scholar] [CrossRef]
- Santos, M.; Santos, C.; Carmezim, M.J. Production of bioactive hydroxyapatite coating by coblast process for orthopedic implants. In Proceedings of the 2019 IEEE 6th Portuguese Meeting on Bioengineering (ENBENG); Institute of Electrical and Electronics Engineers (IEEE), Lisbon, Portugal, 22–23 February 2019; pp. 1–4. [Google Scholar]
- Morks, M.F. Fabrication and characterization of plasma-sprayed HA / SiO2 coatings for biomedical application. J. Mech. Behav. Biomed. Mater. 2008, 1, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Fauchais, P.; Vardelle, A. Innovative and emerging processes in plasma spraying: From micro- to nano-structured coatings. J. Phys. D Appl. Phys. 2011, 44. [Google Scholar] [CrossRef]
- Ročňáková, I.; Slámečka, K.; Montufar, E.; Remešová, M.; Dyčková, L.; Břínek, A.; Jech, D.; Dvořák, K.; Čelko, L.; Kaiser, J. Deposition of hydroxyapatite and tricalcium phosphate coatings by suspension plasma spraying: Effects of torch speed. J. Eur. Ceram. Soc. 2018, 38, 5489–5496. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Singh, V.; Seal, S.; Agarwal, A. Aluminum composite reinforced with multiwalled carbon nanotubes from plasma spraying of spray dried powders. Surf. Coat. Technol. 2009, 203, 1544–1554. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Ardhaoui, M.; Benyounis, K.; Looney, L.; Stokes, J.T. Plasma sprayed hydroxyapatite coatings: Understanding process relationships using design of experiment analysis. Surf. Coat. Technol. 2015, 283, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Fielding, G.A.; Roy, M.; Bandyopadhyay, A.; Bose, S. Antibacterial and biological characteristics of silver containing and strontium doped plasma sprayed hydroxyapatite coatings. Acta Biomater. 2012, 8, 3144–3152. [Google Scholar] [CrossRef] [Green Version]
- Vahabzadeh, S.; Roy, M.; Bandyopadhyay, A.; Bose, S. Phase stability and biological property evaluation of plasma sprayed hydroxyapatite coatings for orthopedic and dental applications. Acta Biomater. 2015, 17, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Singh, S.; Prakash, S. Role of Post Heat Treatment of Plasma Sprayed Pure and Al2O3-TiO2 Reinforced Hydroxyapatite Coating on the Microstructure and Mechanical Properties. J. Miner. Mater. Charact. Eng. 2010, 9, 1059–1069. [Google Scholar] [CrossRef]
- Popa, M.V.; Moreno, J.M.C.; Popa, M.; Vasilescu, E.; Drob, P.; Vasilescu, C.; Drob, S.I.J.S.; Technology, C. Electrochemical deposition of bioactive coatings on Ti and Ti–6Al–4V surfaces. Surf. Coat. Technol. 2011, 205, 4776–4783. [Google Scholar] [CrossRef]
- Chen, Y.M.; Lu, Y.P.; Li, M.S. Surface changes of plasma sprayed hydroxyapatite coatings before and after heat treatment. Surf. Eng. 2006, 22, 462–467. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mate. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef]
- Roy, M.; Balla, V.K.; Bose, S.; Bandyopadhyay, A. Comparison of Tantalum and Hydroxyapatite Coatings on Titanium for Applications in Load Bearing Implants. Adv. Eng. Mater. 2010, 12, B637–B641. [Google Scholar] [CrossRef]
- Ke, D.; Vu, A.A.; Bandyopadhyay, A.; Bose, S. Compositionally graded doped hydroxyapatite coating on titanium using laser and plasma spray deposition for bone implants. Acta Biomater. 2019, 84, 414–423. [Google Scholar] [CrossRef]
- Singh, G.; Singh, S.; Prakash, S. Surface characterization of plasma sprayed pure and reinforced hydroxyapatite coating on Ti6Al4V alloy. Surf. Coat. Technol. 2011, 205, 4814–4820. [Google Scholar] [CrossRef]
- Li, H.; Khor, K.; Cheang, P. Titanium dioxide reinforced hydroxyapatite coatings deposited by high velocity oxy-fuel (HVOF) spray. Biomaterials 2002, 23, 85–91. [Google Scholar] [CrossRef]
- Bolelli, G.; Giovanardi, R.; Lusvarghi, L.; Manfredini, T. Corrosion resistance of HVOF-sprayed coatings for hard chrome replacement. Corros. Sci. 2006, 48, 3375–3397. [Google Scholar] [CrossRef]
- Ban, Z.-G.; Shaw, L.L. Characterization of Thermal Sprayed Nanostructured WC-Co Coatings Derived From Nanocrystalline WC-18wt.%Co Powders. J. Spray Technol. 2003, 12, 112–119. [Google Scholar] [CrossRef]
- Visai, L.; De Nardo, L.; Punta, C.; Melone, L.; Cigada, A.; Imbriani, M.; Arciola, C.R. Titanium Oxide Antibacterial Surfaces in Biomedical Devices. Int. J. Artif. Organs 2011, 34, 929–946. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Pala, Z.; Voisey, K.; Hussain, T. Gas and liquid-fuelled HVOF spraying of Ni50Cr coating: Microstructure and high temperature oxidation. Surf. Coat. Technol. 2017, 318, 224–232. [Google Scholar] [CrossRef]
- Heimann, R.B.; Lehmann, H.D. Deposition, Structure, Properties and Biological Function of Plasma-Sprayed Bioceramic Coatings. Bioceram. Coat. Med. Implant. 2015, 6, 253–308. [Google Scholar]
- Yao, H.-L.; Wang, H.-T.; Bai, X.-B.; Ji, G.-C.; Chen, Q.-Y. Improvement in mechanical properties of nano-structured HA/TiO2 multilayer coatings deposited by high velocity suspension flame spraying (HVSFS). Surf. Coat. Technol. 2018, 342, 94–104. [Google Scholar] [CrossRef]
- Cotell, C.M.; Chrisey, D.B.; Grabowski, K.S. Pulsed Laser Deposition of Biocompatible Thin Films: Calcium Hydroxylapatrte and Other Calcium Phosphates. MRS Proc. 1991, 252, 549. [Google Scholar] [CrossRef]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Yang, Y.; Chang, E. Influence of residual stress on bonding strength and fracture of plasma-sprayed hydroxyapatite coatings on Ti–6Al–4V substrate. Biomaterials 2001, 22, 1827–1836. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, M.; Chen, C.; Liu, J. The role of the pressure in pulsed laser deposition of bioactive glass films. J. Non-Cryst. Solids 2008, 354, 4000–4004. [Google Scholar] [CrossRef]
- Xiong, J.; Li, Y.; Hodgson, P.D.; Wen, C. Nanohydroxyapatite coating on a titanium–niobium alloy by a hydrothermal process. Acta Biomater. 2010, 6, 1584–1590. [Google Scholar] [CrossRef]
- Ninomiya, J.T.; Struve, J.A.; Stelloh, C.T.; Toth, J.M.; Crosby, K.E. Effects of hydroxyapatite participate debris on the production of cytokines and proteases in human fibroblasts. J. Orthop. Res. 2001, 19, 621–628. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Coelho, P.G.; Kang, B.S.; Sul, Y.T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef] [PubMed]
- JONES, J.R. Scaffolds for tissue engineering. In Biomaterials, Artificial Organs and Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2005; pp. 201–214. [Google Scholar]
- Nelea, V.; Morosanu, C.; Iliescu, M.; Mihailescu, I. Hydroxyapatite thin films grown by pulsed laser deposition and radio-frequency magnetron sputtering: Comparative study. Appl. Surf. Sci. 2004, 228, 346–356. [Google Scholar] [CrossRef]
- Johnson, S. Pulsed laser deposition of hydroxyapatite thin films. Mater. Sci. Eng. C 2007, 27, 484–494. [Google Scholar]
- Carradò, A. Nano-crystalline pulsed laser deposition hydroxyapatite thin films on Ti substrate for biomedical application. J. Coat. Technol. Res. 2011, 8, 749–755. [Google Scholar] [CrossRef]
- Rau, J.V.; Cacciotti, I.; Laureti, S.; Fosca, M.; Varvaro, G.; Latini, A. Bioactive, nanostructured Si-substituted hydroxyapatite coatings on titanium prepared by pulsed laser deposition. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2015, 103, 1621–1631. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Lin, G.S.; Wang, J.-Y.; Cheng, C.-S.; Yang, Y.-C.; Lee, B.-S.; Tung, K.-L. Synthesis and characterization of porous hydroxyapatite coatings deposited on titanium by flame spraying. Surf. Coat. Technol. 2018, 349, 357–363. [Google Scholar] [CrossRef]
- Guo, D.; Li, F.; Wang, J.; Sun, J. Effects of post-coating processing on structure and erosive wear characteristics of flame and plasma spray coatings. Surf. Coat. Technol. 1995, 73, 73–78. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Chen, C.-C.; Wang, J.-B.; Wang, Y.-C.; Lin, F.-H. Flame sprayed zinc doped hydroxyapatite coating with antibacterial and biocompatible properties. Ceram. Int. 2017, 43, S829–S835. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A. Hydroxyapatite/polymer composite flame-sprayed coatings for orthopedic applications. J. Biomater. Sci. Polym. Ed. 2002, 13, 977–990. [Google Scholar] [CrossRef]
- Antala, N.; Rathod, P. A Review of the Coating Characteristics Achieved Employing Thermal Flame-spray Coating Method. Int. J. Eng. Technol. Manag. Appl. Sci. 2017, 5. [Google Scholar]
- Zheng, X.; Huang, M.; Ding, C. Bond strength of plasma-sprayed hydroxyapatite/Ti composite coatings. Biomaterials 2000, 21, 841–849. [Google Scholar] [CrossRef]
- Mumith, A.; Cheong, V.S.; Fromme, P.; Coathup, M.J.; Blunn, G.W. The effect of strontium and silicon substituted hydroxyapatite electrochemical coatings on bone ingrowth and osseointegration of selective laser sintered porous metal implants. PLoS ONE 2020, 15, e0227232. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Siddiqui, M.A.; Liu, H.; Kolawole, S.K.; Zhang, J.; Zhang, S.; Ren, L.; Yang, K. Mechanical, Biological, and Antibacterial Characteristics of Plasma-Sprayed (Sr, Zn) Substituted Hydroxyapatite Coating. ACS Biomater. Sci. Eng. 2020, 6, 1355–1366. [Google Scholar] [CrossRef]
- Zieliński, A.; Antoniuk, P.; Krzysztofowicz, K. Nanotubular oxide layers and hydroxyapatite coatings on ‘Ti–13Zr–13Nb’alloy. Surf. Eng. 2014, 30, 643–649. [Google Scholar] [CrossRef]
- Vu, A.A.; Robertson, S.F.; Ke, D.; Bandyopadhyay, A.; Bose, S. Mechanical and biological properties of ZnO, SiO2, and Ag2O doped plasma sprayed hydroxyapatite coating for orthopaedic and dental applications. Acta Biomater. 2019, 92, 325–335. [Google Scholar] [CrossRef]
- Furko, M.; Havasi, V.; Kónya, Z.; Grünewald, A.; Detsch, R.; Boccaccini, A.R.; Balázsi, C. Development and characterization of multi-element doped hydroxyapatite bioceramic coatings on metallic implants for orthopedic applications. Bol. De La Soc. Española De Ceram. Y Vidr. 2018, 57, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Kurzweg, H.; Heimann, R.; Troczynski, T.; Wayman, M. Development of plasma-sprayed bioceramic coatings with bond coats based on titania and zirconia. Biomaterials 1998, 19, 1507–1511. [Google Scholar] [CrossRef]
- Jung, U.-W.; Hwang, J.-W.; Choi, D.-Y.; Hu, K.-S.; Kwon, M.-K.; Choi, S.-H.; Kim, H.-J. Surface characteristics of a novel hydroxyapatite-coated dental implant. J. Periodontal Implant. Sci. 2012, 42, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Liang, C.; Huang, N.; Duan, F.; Wang, L. Formation of Hydroxyapatite Produced by Microarc Oxidation Coupled with Sol-gel Technology. Mater. Manuf. Process. 2014, 29, 1085–1094. [Google Scholar] [CrossRef]
- Li, L.-H.; Kim, H.-W.; Lee, S.-H.; Kong, Y.-M.; Kim, H.-E. Biocompatibility of titanium implants modified by microarc oxidation and hydroxyapatite coating. J. Biomed. Mater. Res. Part. A 2005, 73, 48–54. [Google Scholar] [CrossRef]
- Jin, Y.-Z.; Zheng, G.-B.; Jang, H.L.; Lee, K.M.; Lee, J.H. Whitlockite Promotes Bone Healing in Rabbit Ilium Defect Model. J. Med. Biol. Eng. 2019, 39, 944–951. [Google Scholar] [CrossRef]
- Batool, S.; Liaqat, U.; Hussain, Z.; Sohail, M. Synthesis, Characterization and Process Optimization of Bone Whitlockite. Nanomaterials 2020, 10, 1856. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Qi, C.; Chen, Y.-X.; Zhu, Y.-J.; Sun, T.-W.; Chen, F.; Zhang, C. Comparative study of porous hydroxyapatite/chitosan and whitlockite/chitosan scaffolds for bone regeneration in calvarial defects. Int. J. Nanomed. 2017, 12, 2673–2687. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Metals | Density (g/cm3) | Elastic Modulus (Gpa) | Advantage | Disadvantage |
|---|---|---|---|---|
| Stainless steel 316 L | 8 | 193 |
|
|
| Titanium (Ti-6Al-4V) | 4.4 | 110 |
|
|
| Co-Cr alloys | 9.2 | 210 |
|
|
| Mg | 1.74 | 41–45 |
|
|
| Methods | Coating Layer Thickness | Pros | Cons | References |
|---|---|---|---|---|
| Sol-Gel | <1 μm |
|
| [32,33,34,35,36,37,38] |
| Dip coating | 0.05–15 mm |
|
| [39,40,41,42,43,44,45,46,47] |
| Electro-chemical deposition | 0.05–0.5 mm |
|
| [48,49,50,51] |
| Electro-phoretic deposition | 0.1–2.0 mm |
|
| [52,53,54,55] |
| Bio-mimetic coating | <30 μm |
|
| [56] |
| Plasma spraying | <20 μm |
|
| [57,58,59,60,61,62,63,64] |
| Sputter coating | 0.5–3 μm |
|
| [59,65] |
| High-velocity suspension flame spraying (HVSFS) | ≤50 μm |
|
| [66,67,68] |
| Pulsed laser deposition | 0.05–5 μm |
|
| [69,70,71] |
| Hot iso-static pressing | 0.2–2.0 mm |
|
| [72,73] |
| Flame spraying | 100–250 μm |
|
| [74,75] |
| Sr.No | Precursor for Sols and Other Materials | Solvent | Implant Metal | Operating Conditions | Outcome | Year | References |
|---|---|---|---|---|---|---|---|
| 1 | Triethylphosphite and calcium nitrate | Water | Stainless steel 316 L |
|
| 2002 | [36] |
| 2 | Titanium propoxide, Di-ethanolamine, Calcium nitrate, tetrahydrate and Triethylphosphite | Water and ethanol | Titanium |
|
| 2004 | [119] |
| 3 | Calcium nitrate tetra-hydrate, Tri-ammonium phosphate tri-hydrate and Gelatine | Water | Titanium |
|
| 2005 | [37] |
| 4 | Calcium Nitrate, Strontium Nitrate and Phosphoruspenta oxide | Ethanol | Titanium |
|
| 2010 | [41] |
| 5 | Triethylphosphite and Calcium nitrate | Water, Acetone, Ethanol | Nickel-Titanium Alloy |
|
| 2011 | [42] |
| 6 | Calcium nitrate Tetra hydrate, phosphorous penta oxide | Ethanol | Magnesium AZ91 |
|
| 2013 | [44] |
| 7 | HAp Nano-particle | - | Titanium |
|
| 2013 | [43] |
| 8 | Titanium isopropoxide, Calcium acetate monohydrate,1,2-ethandiol, poly vinyl alcohol, Triethanol amine and ortho phosphoric acid | Water | Titanium |
|
| 2016 | [120] |
| Sr.No | Coating Material | Solvent | Implant Metal | Operating Conditions | Outcome | Year | References |
|---|---|---|---|---|---|---|---|
| 1 | Calcium phosphate, Tobramycin | Water | Titanium alloy |
|
| 2002 | [124] |
| 2 | Calcium phosphate | Water, Human blood plasma (HBP), Simulated body fluid (SBF) | Titanium and tantalum |
|
| 2004 | [127] |
| 3 | Calcium phosphate, CaO–SiO2 based glass | Water, Simulated body fluid (SBF) | Titanium |
|
| 2005 | [125] |
| 4 | Sodium hydroxide, Calcium phosphate, | Water | Titanium |
|
| 2008 | [128] |
| 5 | Hydroxyapatite and tri-calcium phosphate | Water | Titanium alloy |
|
| 2015 | [129] |
| Sr.No | Precursor | Carrier Gas | Implant Metal | Operating Conditions | Outcome | Year | References |
|---|---|---|---|---|---|---|---|
| 1 | Calcium diketonate and tri-methyl phosphate | Oxygen | Titanium |
|
| 1998 | [133] |
| 2 | Fluorine-containing carbonated hydroxyapatite, 2,2,6,6,-tetramethylheptane-3,5-dione | Argon | Titanium |
|
| 2004 | [134] |
| 3 | Calcium dipivaloylmethanate and Titanium di(i-propoxy)bis(dipivaloylmethanate) | Argon | Titanium |
|
| 2007 | [135] |
| 4 | Bis-dipivaloylmethanocalcium and Triphenyl Phosphate | Argon | Titanium |
|
| 2010 | [136] |
| Sr.No | Electrolyte and Other Chemicals | Solvent | Implant Metal | Operating Conditions | Outcome | Year | References |
|---|---|---|---|---|---|---|---|
| 1 | Calcium nitrate, Ammonium di hydrogen phosphate, Sodiumnitrate, Hydrogen peroxide, Zirconium oxide | Water, Ammonia, Nitric acid | Nickel-Titanium |
|
| 2010 | [50] |
| 2 | Calcium nitrate and Sodium hydrogen phosphate and Tris-hydroxy-methyl-amino-methane | De-ionized water | Cobalt-Chromium-Molybdenum |
|
| 2011 | [51] |
| 3 | Calcium chloride, Ammonium di hydrogen phosphate, Sodium hydroxide | Distilled water | Titanium |
|
| 2012 | [147] |
| 4 | Calcium nitrate, Ammonium di hydrogen phosphate, Titanium nano tubes | Distilled water | Titanium |
|
| 2014 | [148] |
| 5 | Calcium nitrate, Ammonium di hydrogen phosphate | Distilled water | Magnesium |
|
| 2015 | [149] |
| 6 | Calcium nitrate, Ammonium di hydrogen phosphate | Distilled water | Ti6Al4V Alloy |
|
| 2016 | [142] |
| 7 | Calcium nitrate, Ammonium di hydrogen phosphate | Ultra purewater | Pure titanium |
|
| 2018 | [150] |
| Sr.No | Raw Materials | Metallic Implant | Process Conditions | Outcome | Year | References |
|---|---|---|---|---|---|---|
| 1 | HAp, Al2O3 | Titanium |
|
| 2015 | [168] |
| 2 | HAp, Al2O3 | Titanium |
|
| 2017 | [169] |
| 3 | HAp, Al2O3,Tri-CalciumPhosphate | Steel |
|
| 2018 | [166] |
| Sr.No | Coating Materials | Metallic Implant | Solvent | Process Conditions | Outcome | Year | References |
|---|---|---|---|---|---|---|---|
| 1 | HAp | Titanium | Water or Di-ethylene glycol (DEG) |
|
| 2011 | [158] |
| 2 | HAp | - | Water or Di-ethylene glycol |
|
| 2015 | [68] |
| 3 | HAp/TiO2 | 316 L Stainless Steel | Water and Iso-propanol |
|
| 2018 | [184] |
| Sr.No | Coatings | Metallic Implant | Process Parameters | Outcomes | Year | References |
|---|---|---|---|---|---|---|
| 1 | HAp | Titanium |
|
| 2004 | [193] |
| 2 | HAp | Silicon(100) and Titanium |
|
| 2005 | [194] |
| 3 | HAp | Titanium |
|
| 2009 | [117] |
| 4 | HAp | Titanium |
|
| 2011 | [195] |
| 5 | HAp and Silicon | Titanium |
|
| 2014 | [196] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beig, B.; Liaqat, U.; Niazi, M.F.K.; Douna, I.; Zahoor, M.; Niazi, M.B.K. Current Challenges and Innovative Developments in Hydroxyapatite-Based Coatings on Metallic Materials for Bone Implantation: A Review. Coatings 2020, 10, 1249. https://doi.org/10.3390/coatings10121249
Beig B, Liaqat U, Niazi MFK, Douna I, Zahoor M, Niazi MBK. Current Challenges and Innovative Developments in Hydroxyapatite-Based Coatings on Metallic Materials for Bone Implantation: A Review. Coatings. 2020; 10(12):1249. https://doi.org/10.3390/coatings10121249
Chicago/Turabian StyleBeig, Bilal, Usman Liaqat, Muhammad Farooq Khan Niazi, Inamullah Douna, Muhammad Zahoor, and Muhammad Bilal Khan Niazi. 2020. "Current Challenges and Innovative Developments in Hydroxyapatite-Based Coatings on Metallic Materials for Bone Implantation: A Review" Coatings 10, no. 12: 1249. https://doi.org/10.3390/coatings10121249
APA StyleBeig, B., Liaqat, U., Niazi, M. F. K., Douna, I., Zahoor, M., & Niazi, M. B. K. (2020). Current Challenges and Innovative Developments in Hydroxyapatite-Based Coatings on Metallic Materials for Bone Implantation: A Review. Coatings, 10(12), 1249. https://doi.org/10.3390/coatings10121249







