Using Mobiles to Monitor Respiratory Diseases
Abstract
1. Introduction
- It can eliminate the need of regular tests and hence reduce the cost of medical care and consequently provide healthcare for people with low income;
- It can reach patients in even the most remote locations;
- It can increase the reach and efficiency of healthcare;
- It empowers the patients because smartphones can help patients monitor their disease at home. Furthermore, it can be used as a tool for patients to manage appointments, renew prescriptions, or view medical records;
- Doctors are increasingly using smartphones, allowing them to access medical materials. They can also reach patients in rural areas through remote diagnostics and information alerts;
- Remotely monitoring hospital patients or the elderly can free up much needed capacity in hospitals and nursing homes.
2. Background and Related Work
- Forced vital capacity (FVC): The volume of air that can be expired after a maximum inspiration;
- Forced expiratory volume in one second (FEV1): Volume of air expelled in the first second of a forced expiration;
- Forced expiratory flow 25–75% (FEF 25–75): Average expiratory flow rate in the middle part of a forced expiration;
- FEV1/FVC: This is the ratio of the vital capacity that is expired in the first second of maximal expiration;
- Peak expiratory flow (PEF): Maximal expiratory flow rate achieved.
- Dyspnea that is progressive (worsens over time);
- Chronic cough that may be intermittent and may be unproductive;
- Chronic sputum production;
- History of exposure to risk factors such as tobacco smoke, smoke from home cooking, heating fuels, and occupational dusts and chemicals;
- Family history of COPD.
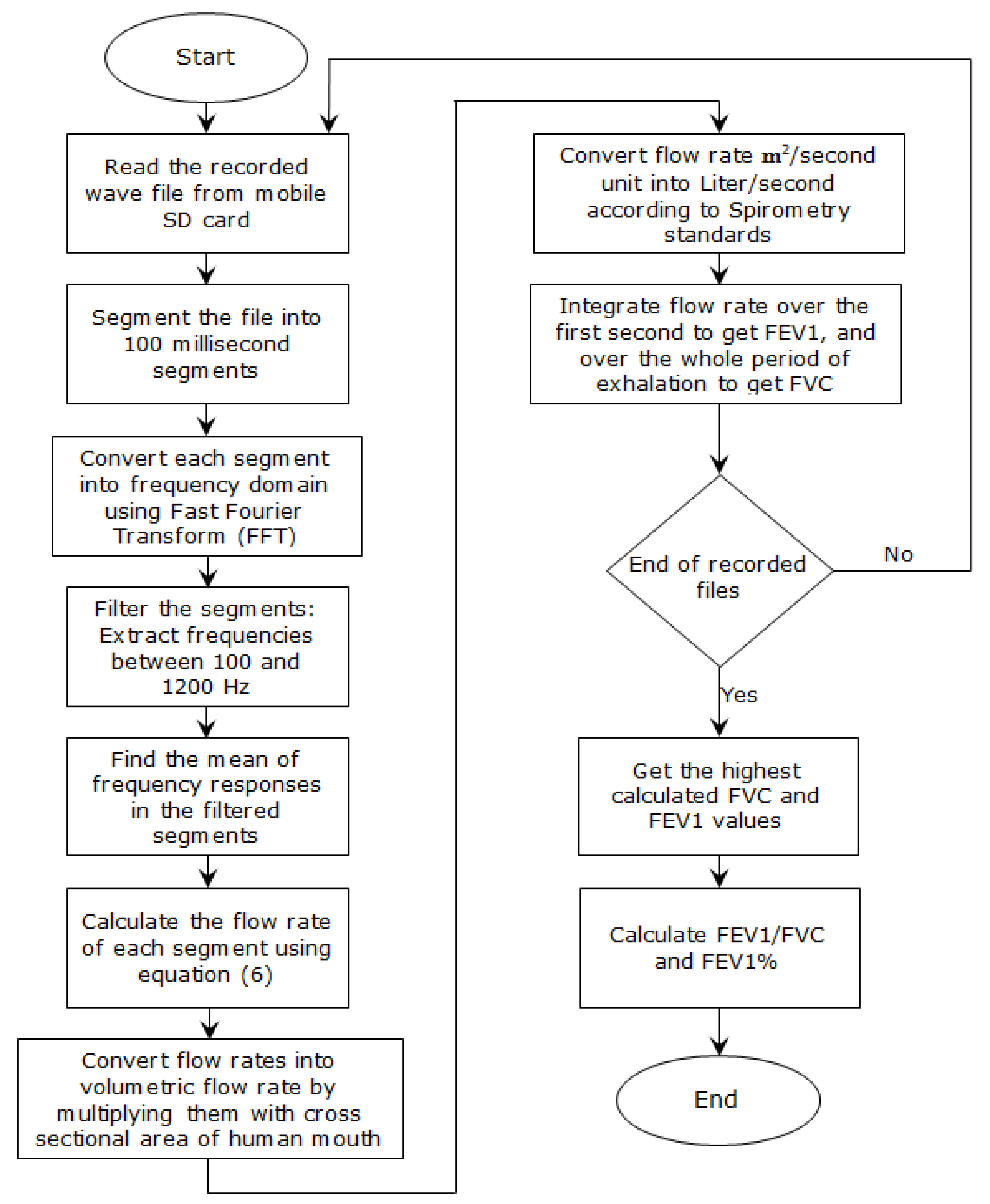
3. Methodology
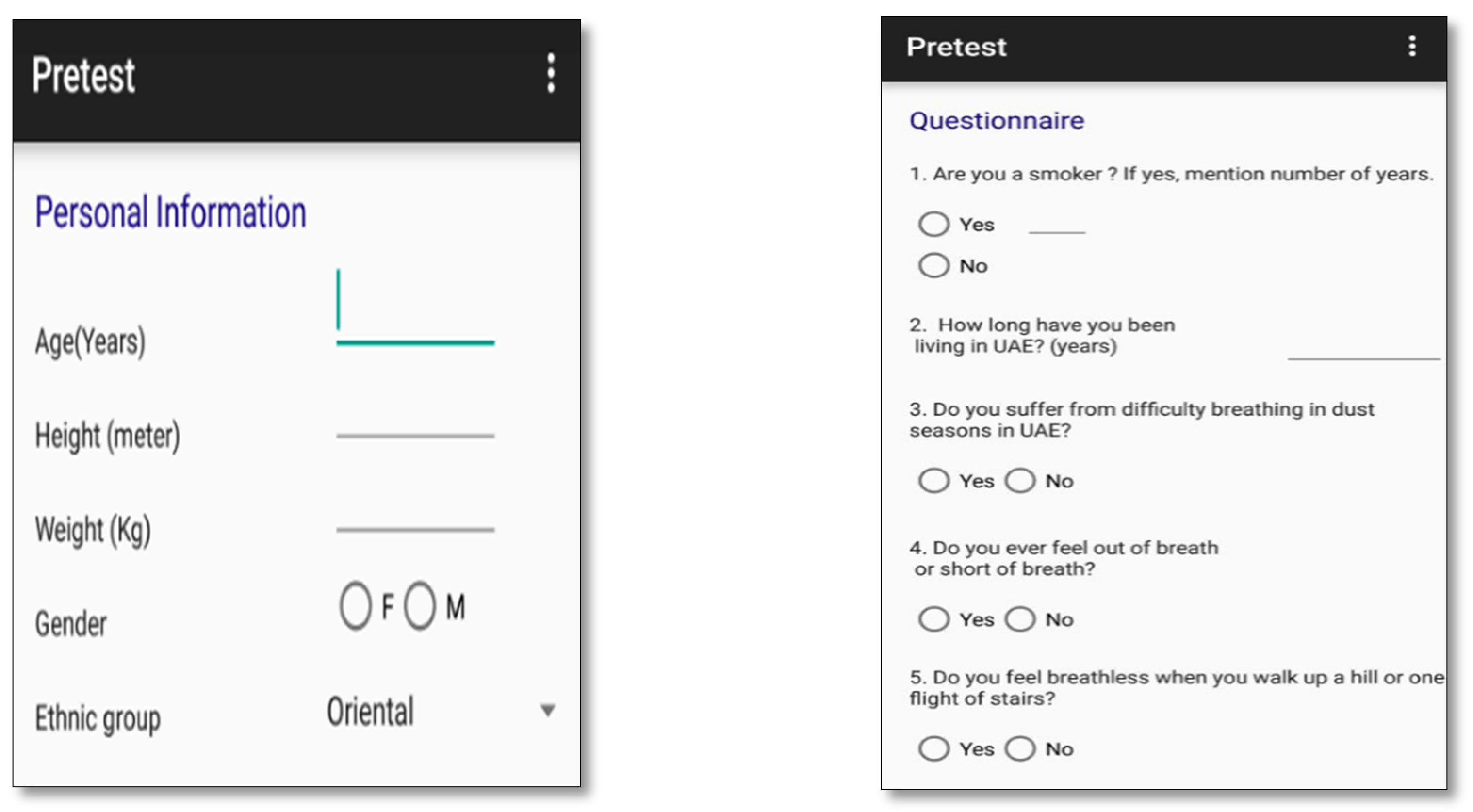
- Developing a “Pretest Activity” tool to be used as a first indicator about the presence of respiratory disease;
- Establishing and using appropriate techniques to extract the required physiological signals (exhalation and oxygen saturation);
- Developing an algorithm to analyze the collected physiological signals (analyze recorded patient exhalations);
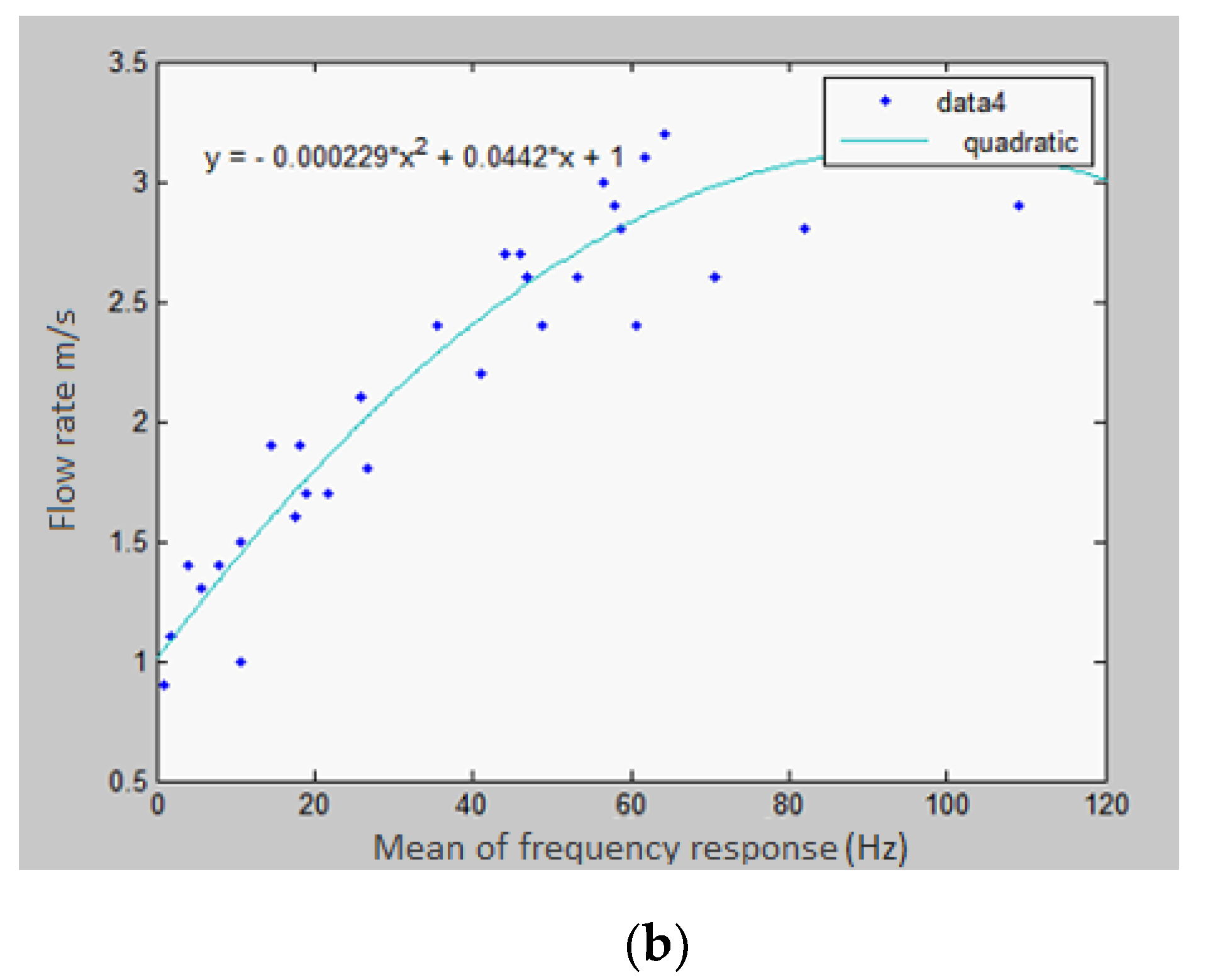
- Developing a model that relates the frequency response of the exhalation recorded by the microphone to the actual flow rate of the exhalation;
- Implementing an Android application that makes use of the developed model to assist in diagnosing whether a patient suffers from COPD or asthma and analyzing the severity of the disease if present;
- Assessing the reliability of the mobile application by using it to examine 25 human subjects and then comparing the results with those obtained using a spirometer.
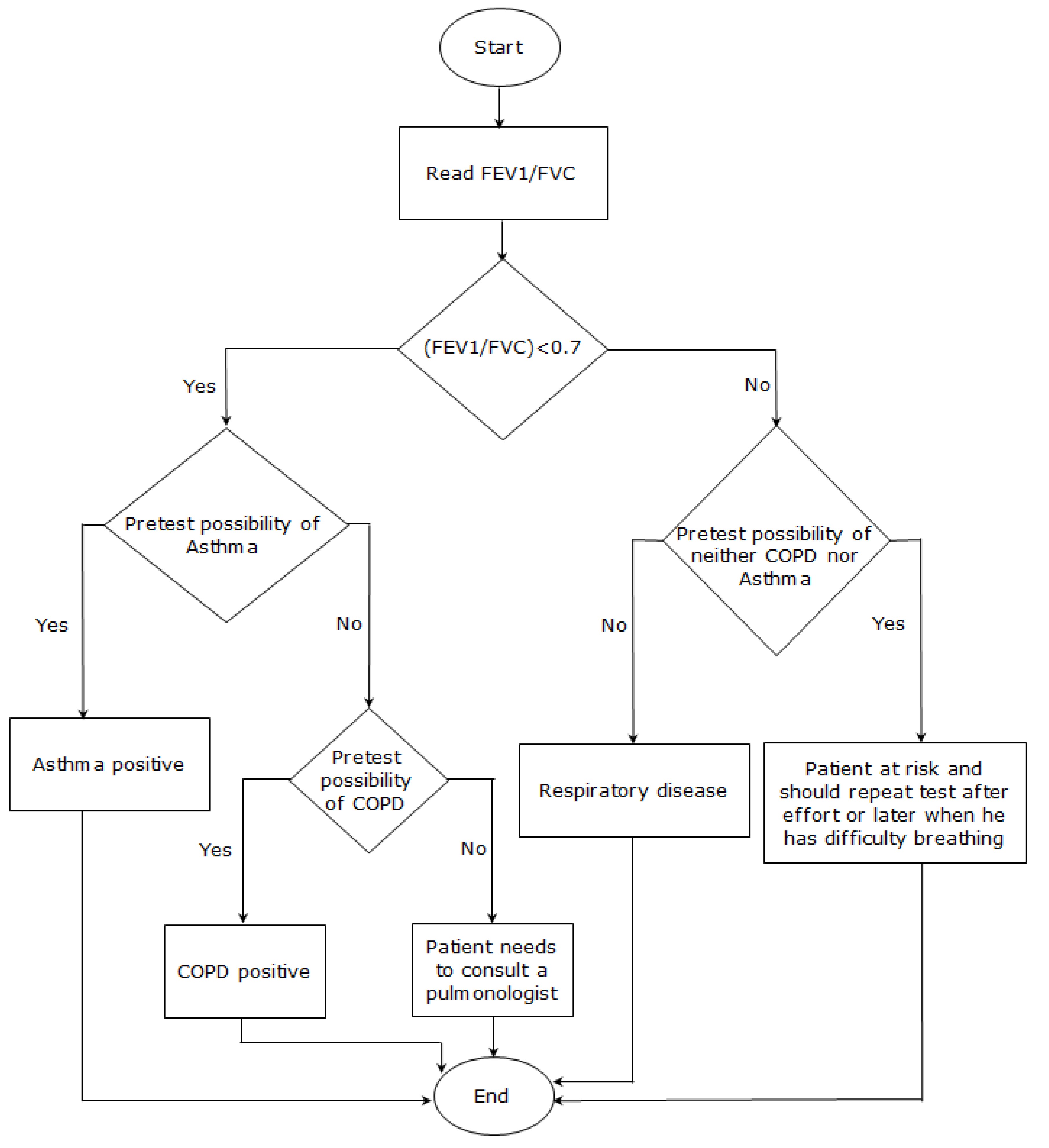
- FEV1/FVC > 0.7 and FEV1% predicted >80% with pretest possibility:
- Patient at risk and may have asthma. Patient should thus repeat the test after exercising or during a period of breathing difficulty in order to confirm the diagnosis.
- FEV1/VFC < 0.7 with pretest possibility:
- Patient has respiratory disease:
- If higher pretest possibility of asthma, then diagnose patient with asthma.
- If higher pretest possibility of COPD, then diagnose patient with COPD.
- SpO2 of 92% or less with FEV1/FVC < 0.7:
- Patient will be notified of impaired respiratory function and possible need of oxygen supplementation.
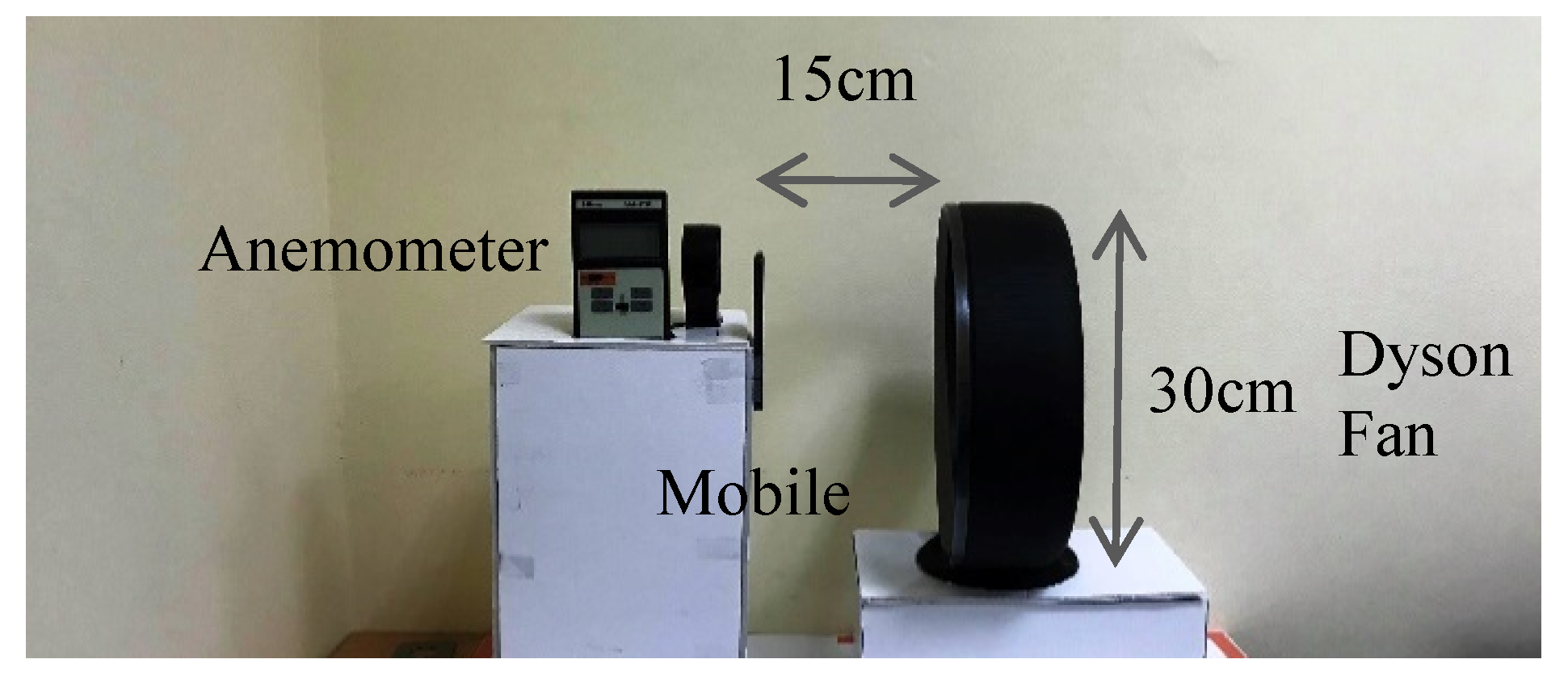
4. Experimental Work
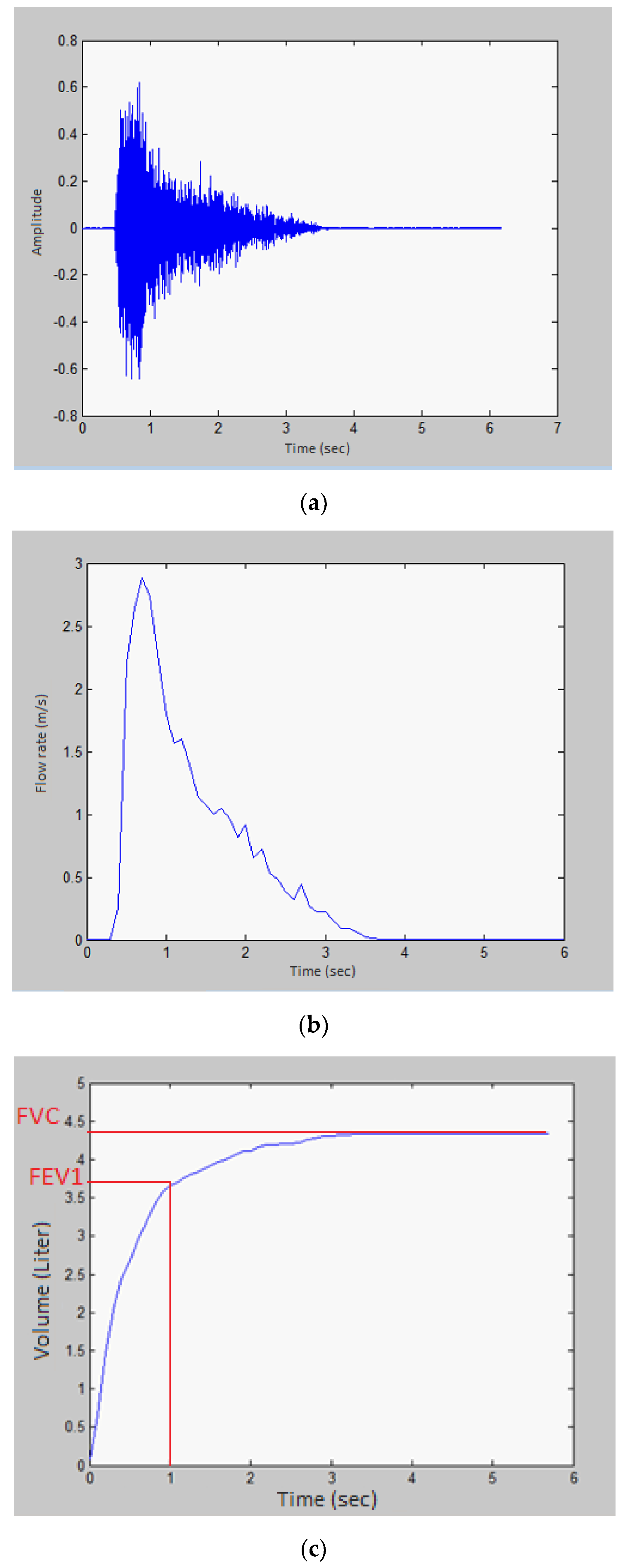
4.1. Time-Domain Analysis
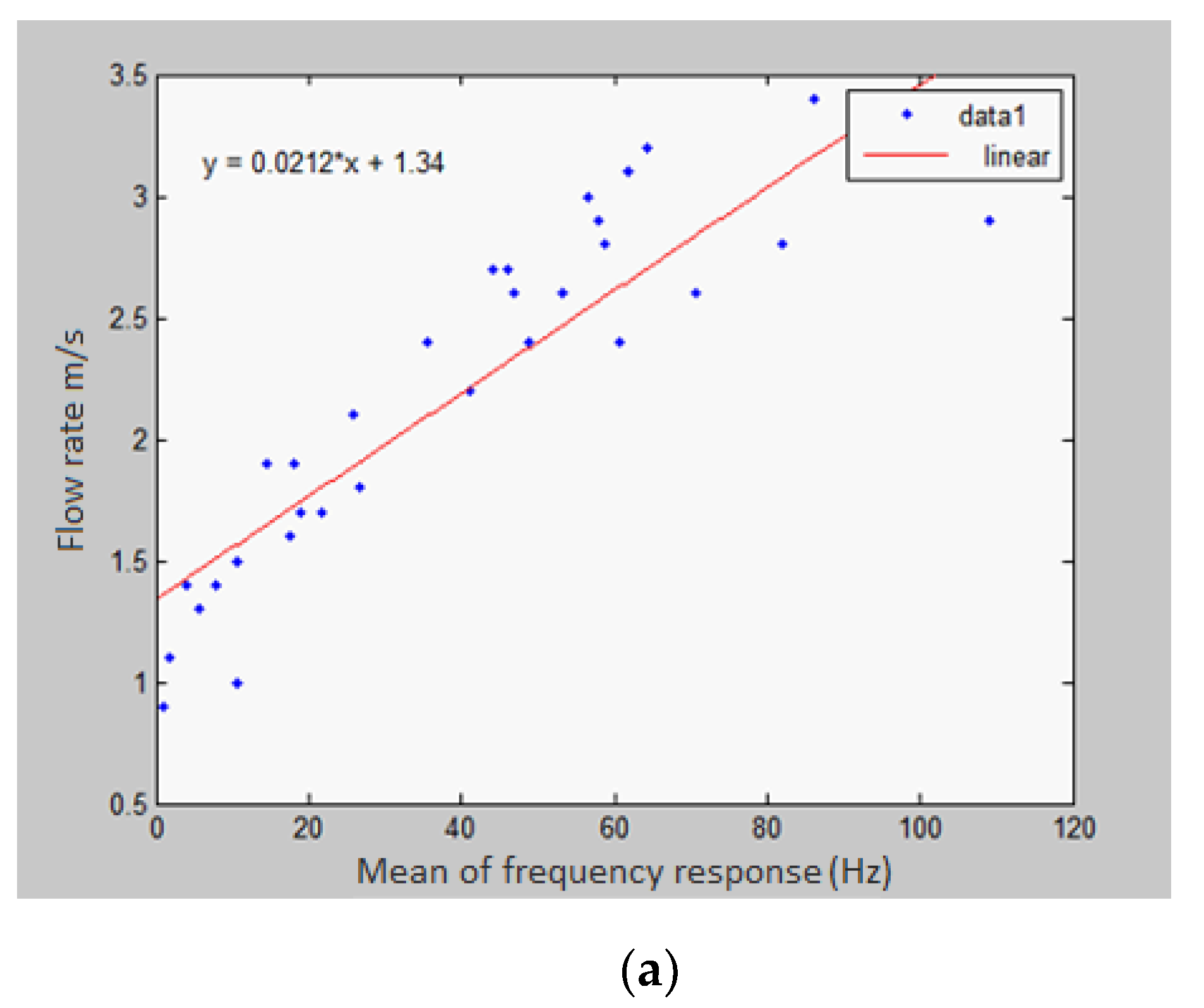
4.2. Frequency-Domain Analysis
5. The Mobile Application
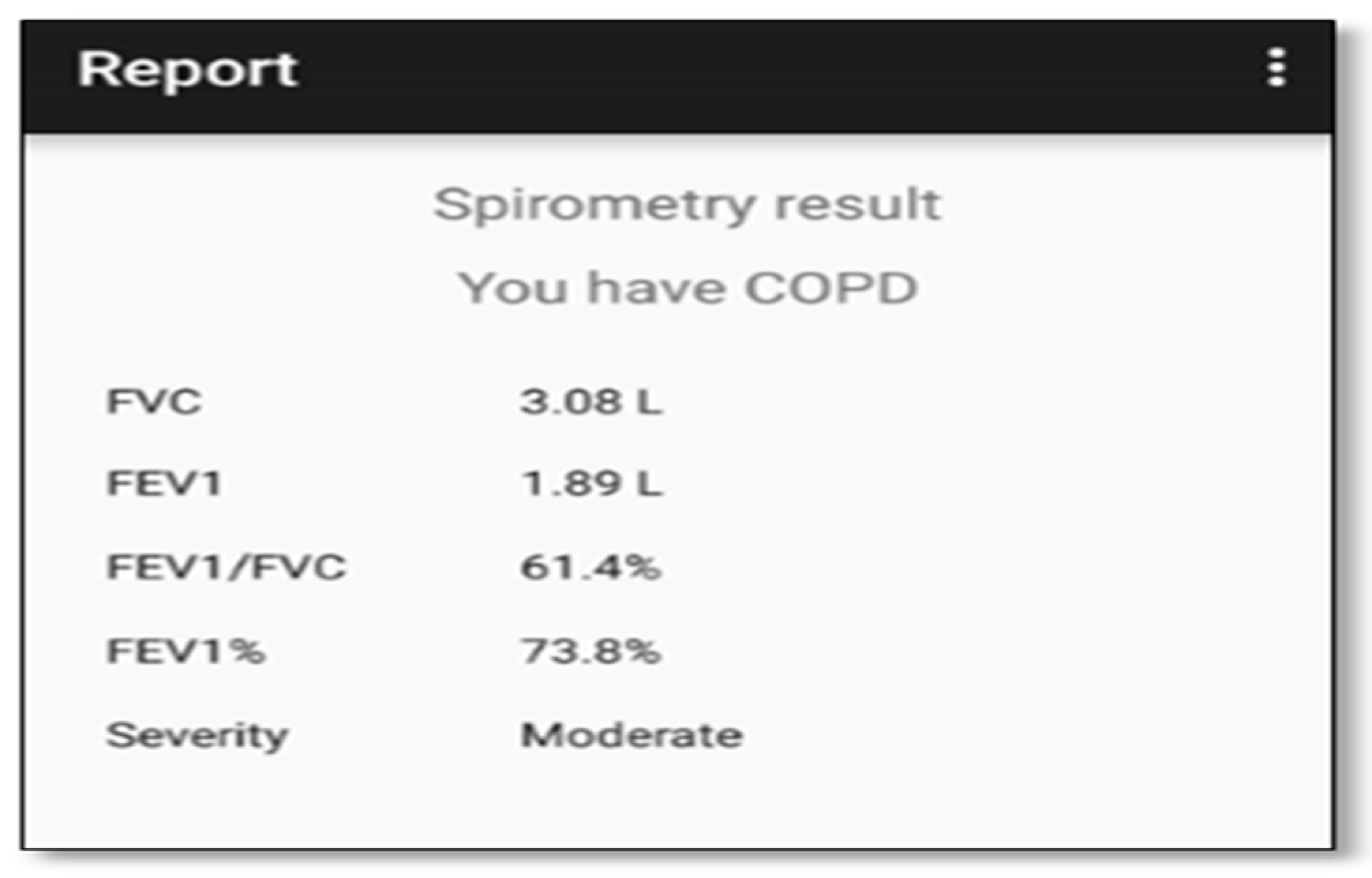
- Spirometry parameters: FVC, FEV1, and FEV1/ FVC ratio;
- Diagnosis result: Whether or not the user has COPD or asthma;
- Disease severity: The level of the disease if the diagnosis is positive. COPD levels are mild, moderate, severe, and very severe, and asthma levels are mild intermittent, mild persistent, moderate persistent, and severe persistent;
- SpO2 warning: Active in case the user suffers from a poor blood oxygenation.
Analysis of Human Exhalations
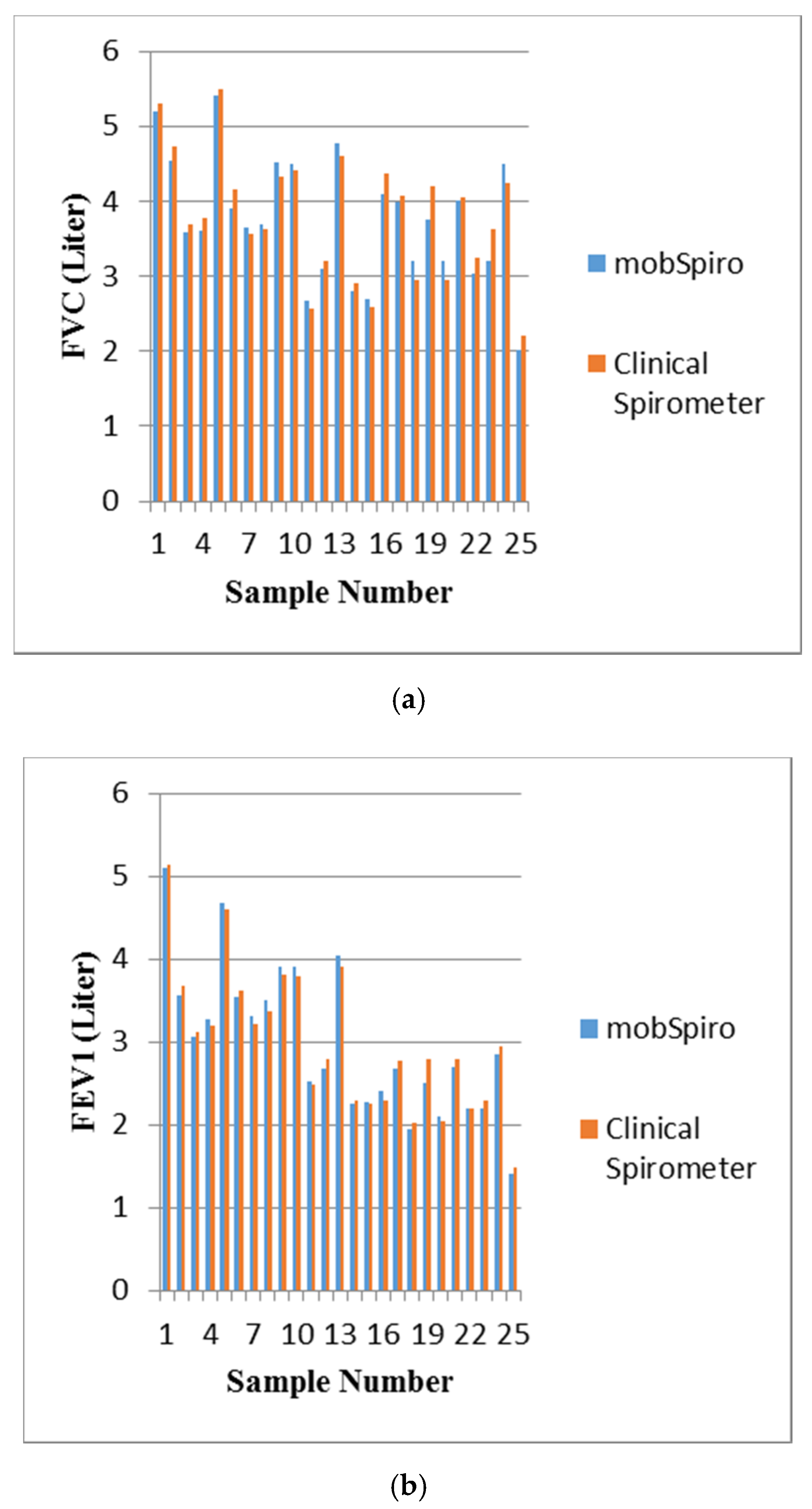
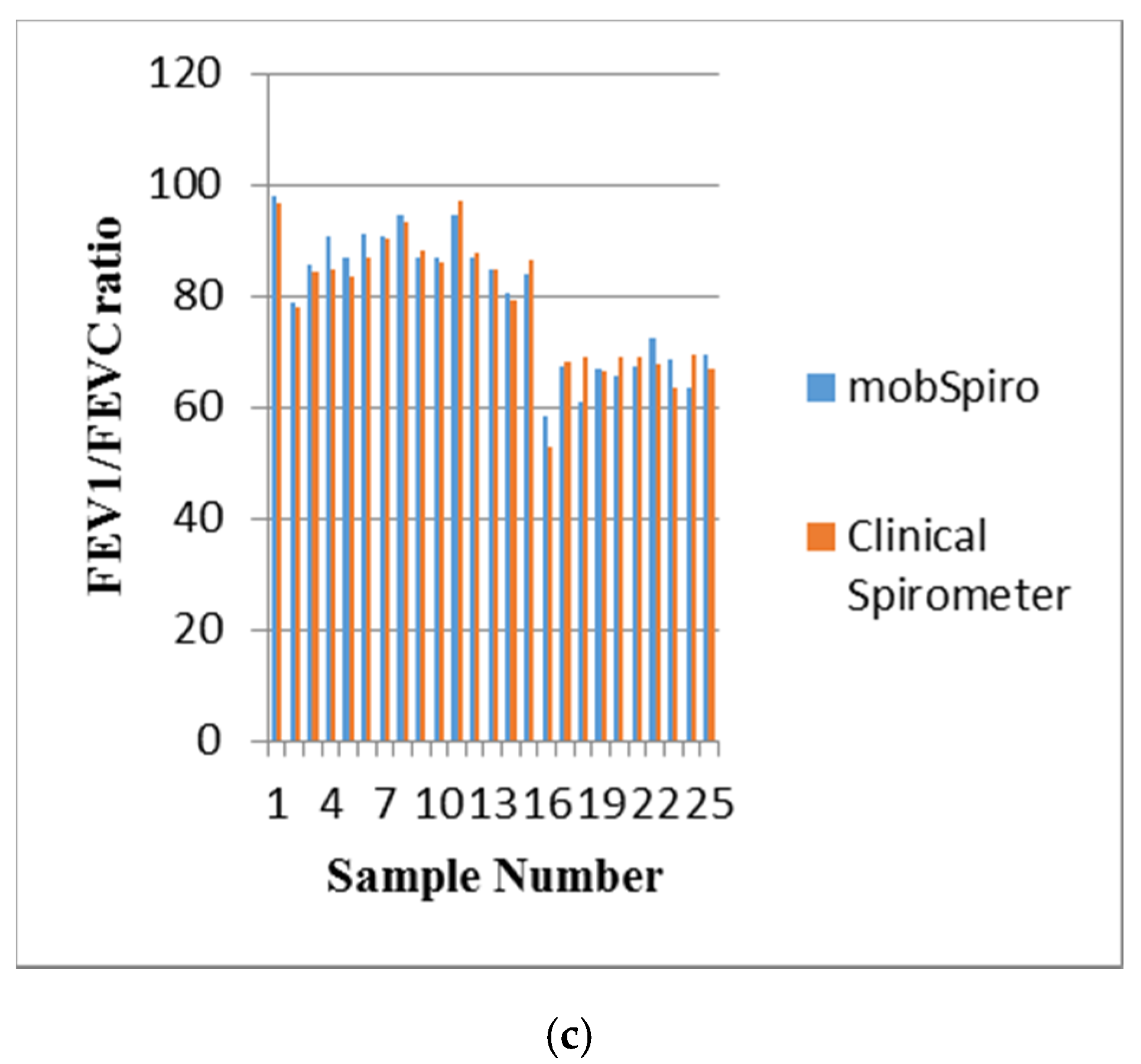
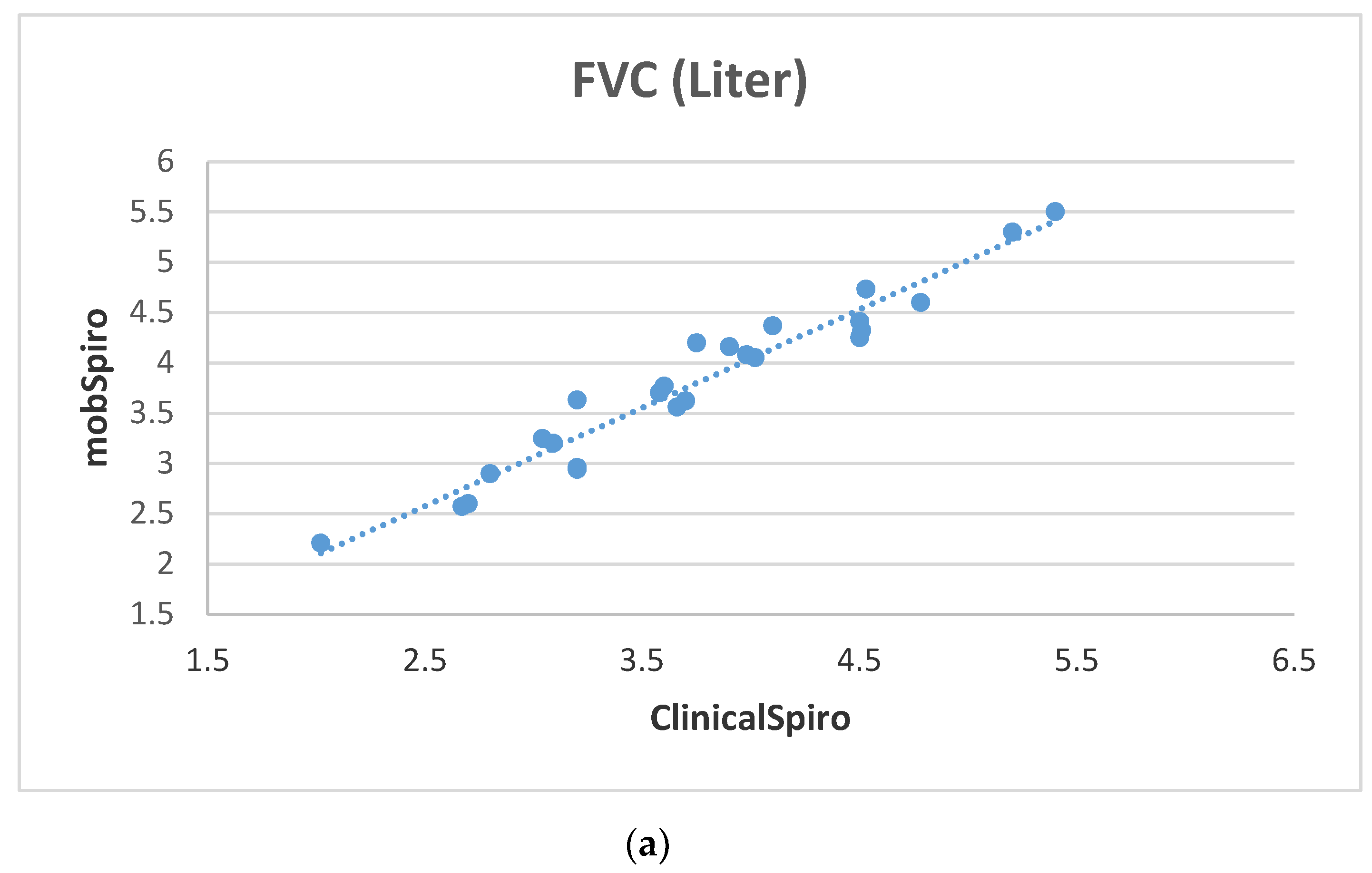
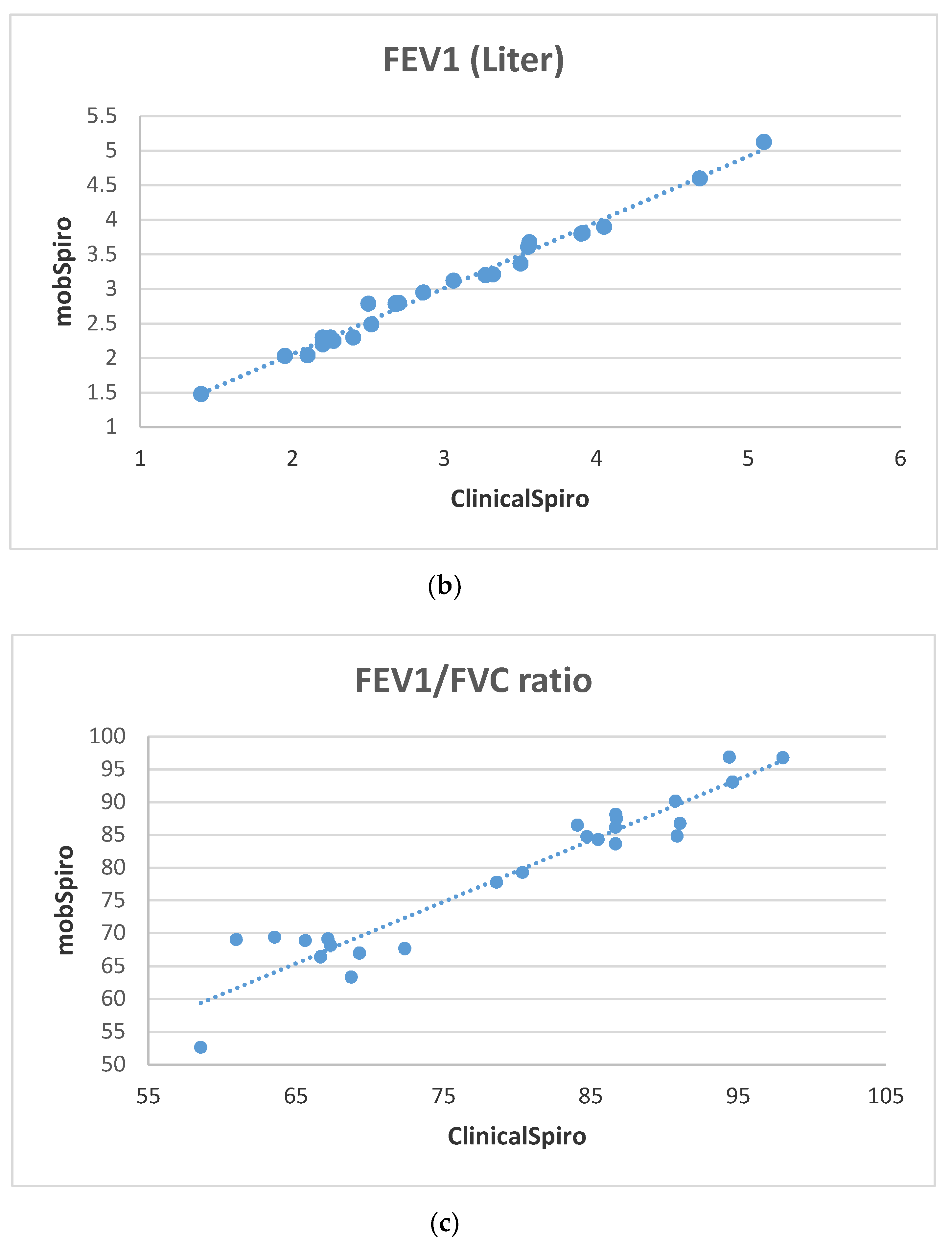
6. Samples Collection and Discussion of Results
- Subjects are asked few questions about disease symptoms and family history of respiratory diseases in order to fill the Pretest Activity;
- Subjects are asked to measure their SpO2 using an external oximeter;
- Subjects are asked to perform spirometry on a handheld spirometer or clinical spirometer (for hospital subjects);
- Subjects are asked to perform spirometry using the mobile application by assuming a comfortable position while sitting down and using the proximity sensor to place the mouth at a distance of 5cm from the mobile microphone followed by a deep inhalation and blowing as hard as possible on the mobile. This activity is repeated three times.
Diagnosis Outcomes
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheng, S.; Mohammed, T.L. Diffuse smoking-related lung disease: Emphysema and interstitial lung disease. Semin. Roentgenol. 2015, 50, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Cavaillès, A.; Brinchault-Rabin, G.; Dixmier, A.; Goupil, F.; Gut-Gobert, C.; Marchand-Adam, S.; Meurice, J.-C.; Morel, H.; Person-Tacnet, C.; Leroyer, C.; et al. Comorbidities of COPD. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2013, 22, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Asthma Cases Continue to Rise in U.S., Affecting Millions U.S. News & World Report. 2012. Available online: http://health.usnews.com/health-news/news/articles/2012/05/15/asthma-cases-continue-to-rise-in-us-affecting-millions (accessed on 7 November 2020).
- Society, A.T. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am. Rev. Respir. Dis. 1987, 136, 225–244. [Google Scholar]
- Coates, A.L.; Graham, B.L.; McFadden, R.G.; McParland, C.; Moosa, D.; Provencher, S.; Road, J.; Canadian Thoracic Society. Spirometery in primary care. Can. Respir. J. 2013, 20, 13–21. [Google Scholar] [CrossRef]
- Wukitsch, M.W.; Petterson, M.M.T.; Tobler, D.R.; Pologe, J.A. Pulse oximetry: Analysis of theory, technology, and practice. J. Clin. Monit. 1988, 4, 290–301. [Google Scholar] [CrossRef]
- Vestbo, J.; Hurd, S.S.; Agusti, A.; Jones, P.; Vogelmeier, C.; Anzueto, A.; Barnes, P.J.; Fabbri, L.M.; Martinez, F.J.; Nishimura, M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 187, 4. [Google Scholar] [CrossRef]
- Lahdenoja, O.; Pankaala, M.; Koivisto, T.; Hurnanen, T.; Iftikhar, Z.; Nieminen, S.; Knuutila, T.; Saraste, A.; Kiviniemi, T.; Vasankari, T.; et al. Atrial fibrillation detection via accelerometer and gyroscope of a smartphone. IEEE J. Biomed. Health Inform. 2018, 22, 1. [Google Scholar] [CrossRef]
- Finkelstein, J.; Wood, J. Mobile eLearning platform for interactive patient education. In Proceedings of the International Conference on Mobile, Hybrid, and On-line Learning, Cancun, Mexico, 1–7 February 2009; pp. 23–27. [Google Scholar]
- Ding, H.; Moodley, Y.; Kanagasingam, Y.; Karunanithi, M. A mobile-health system to manage Chronic Obstructive Pulmonary Disease patients at home. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2012, 2012, 2178–2181. [Google Scholar]
- Abushakra, A.; Faezipour, M. Lung capacity estimation through acoustic signal of breath. In Proceedings of the IEEE 12th International Conference on Bioinformatics & Bioengineering, Larnaca, Cyprus, 11–13 November 2012; pp. 386–391. [Google Scholar]
- Van der Heijden, M.; Lucas, P.J.F.; Lijnse, B.; Heijdra, Y.F.; Schermer, T.R.J. An autonomous mobile system for the management of COPD. J. Biomed. Inform. 2013, 46, 458–469. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, R.; Que, R.Y. A Wireless portable system with microsensors for monitoring respiratory diseases. IEEE Trans. Biomed. Eng. 2012, 59, 3110–3116. [Google Scholar]
- Bellos, C.C.; Papadopoulos, A.; Rosso, R.; Fotiadis, D.I. Identification of COPD patients’ health status using an intelligent system in the CHRONIOUS wearable platform. IEEE J. Biomed. Health Inform. 2014, 18, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.Y.W.; Giani, A.; Shia, V.; Wang, C.; Yan, P.; Yang, A.Y.; Jerrett, M.; Bajcsy, R. A wireless body sensor network for the prevention and management of asthma. In Proceedings of the IEEE International Symposium on Industrial Embedded Systems, Lausanne, Switzerland, 8–10 July 2009; pp. 120–123. [Google Scholar]
- Bumatay, A.; Chan, R.; Lauher, K.; Kwan, A.M.; Stoltz, T.; Delplanque, J.-P.; Kenyon, N.J.; Davis, C.E. Coupled mobile phone platform with peak flow meter enables real-time lung function assessment. IEEE Sens. J. 2012, 12, 685–691. [Google Scholar] [CrossRef]
- Kwan, A.M.; Fung, A.G.; Jansen, P.A.; Schivo, M.; Kenyon, N.J.; Delplanque, J.-P.; Davis, C.E. Personal Lung Function Monitoring Devices for Asthma Patients. IEEE Sens. J. 2015, 15, 2238–2247. [Google Scholar] [CrossRef]
- Bae, W.D.; Alkobaisi, S.; Narayanappa, S.; Liu, C. A mobile data analysis framework for environmental health decision support. In Proceedings of the 9th International Conference on Information Technology: New Generations (ITNG), Las Vegas, NV, USA, 16–18 April 2012; pp. 155–161. [Google Scholar]
- Kumar, K.D. Human health monitoring mobile phone application by using the wireless nanosensor based embedded system. In Proceedings of the International Conference on Information Communication and Embedded Systems (ICICES), Chennai, India, 21–22 February 2013; pp. 889–892. [Google Scholar]
- Bagchi, S.; Chattopadhyay, M. Real-time monitoring of respiratory diseases of distantly located elderly impaired patients. In Proceedings of the 6th International Conference on Sensing Technology (ICST), Kolkata, India, 18–21 December 2012; pp. 146–150. [Google Scholar]
- Larson, E.C.; Goel, M.; Boriello, G.; Heltshe, S.; Rosenfeld, M.; Patel, S. SpiroSmart: Using a microphone to measure lung function on a mobile phone. ACM Conf. Ubiquitous Comput. 2012, 280–289. [Google Scholar] [CrossRef]
- Xu, W.; Huang, M.-C.; Liu, J.J.; Ren, F.; Shen, X.; Liu, X.; Sarrafzadeh, M. mCOPD: Mobile phone based lung function diagnosis and exercise system for COPD. In Proceedings of the 6th International Conference on Pervasive Technologies Related to Assistive Environments, Rhodes, Greece, 29–31 May 2013. [Google Scholar]
- Stein, B. A Mobile Smart Care Platform Home Spirometry by Using the Smartphone Microphone. Master’s Thesis, Leiden University, Leiden, The Netherlands, 2013. [Google Scholar]
- Chamberlain, D.B.; Kodgule, R.; Fletcher, R. A mobile platform for automated screening of asthma and chronic obstructive pulmonary disease. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016, 2016, 5192–5195. [Google Scholar]
- Isaac, N.; Sampath, N.; Gay, V. Modernizing Asthma Management: Personalized Asthma Action Plans Using a Smartphone Application. In Proceedings of the 12th International Symposium on Medical Information and Communication Technology (ISMICT), Sydney, NSW, Australia, 26–28 March 2018; pp. 1–5. [Google Scholar]
- Silva, T.A.; Costa, M.G.F.; Stelmach, R.; Bley, P.K.; Gutierrez, M.A.; Filho, C.F.F.C. Development of a system mobile-based to assist asthma self-management. In Proceedings of the 2018 3rd Biennial South African Biomedical Engineering Conference (SAIBMEC), Stellenbosch, South Africa, 4–6 April 2018; pp. 1–4. [Google Scholar]
- Nathan, V.; Vatanparvar, K.; Rahman, M.; Nemati, E.; Kuang, J. Assessment of Chronic Pulmonary Disease Patients Using Biomarkers from Natural Speech Recorded by Mobile Devices. In Proceedings of the 2019 IEEE 16th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Chicago, IL, USA, 19–22 May 2019; pp. 1–4. [Google Scholar]
- Ding, H.; Karunanithi, M.; Ireland, D.; McCarthy, L.; Hakim, R.; Phillips, K.; Pradhan, R.; Seah, E.; Bowman, R.; Fong, K.; et al. Evaluation of an innovative mobile health programme for the self-management of chronic obstructive pulmonary disease (MH-COPD): Protocol of a randomized controlled trial. BMJ Open 2019, 9, 4. [Google Scholar] [CrossRef]
- Himes, B.; Leszinsky, L.; Walsh, R.; Hepner, H.; Wu, A. Mobile health and inhaler-based monitoring devices for asthma management. J. Allergy Clin. Immunol. Pract. 2019, 7, 2535–2543. [Google Scholar] [CrossRef]
- Park, S.K.; Bang, C.H.; Lee, S.H. Evaluating the effect of a smartphone app-based self-management program for people with COPD: A randomized controlled trial. Appl. Nurs. Res. 2020, 52, 151231. [Google Scholar] [CrossRef]
- Zubaydi, F.; Sagahyroon, A.; Aloul, F.; Mir, H. MobSpiro: Mobile based spirometry for detecting COPD. In Proceedings of the 2017 IEEE 7th Annual Computing and Communication Workshop and Conference (CCWC), Las Vegas, NV, USA, 9–11 January 2017. [Google Scholar]
- Molaie, M.; Moradi, M.H. Heart Sound Localization in Respiratory Sounds Based on Singular Spectrum Analysis and Frequency Features. ETRI J. 2015, 37, 824–832. [Google Scholar] [CrossRef][Green Version]
- Kandaswamy, A.; Kumar, C.S.C.S.; Ramanathan, R.; Jayaraman, S.; Malmurugan, N. Neural classification of lung sounds using wavelet coefficients. Comput. Biol. Med. 2004, 34, 523–537. [Google Scholar] [CrossRef]
- Classification of Asthma. Available online: http://www.webmd.com/asthma/tc/classification-of-asthma-topic-overview (accessed on 7 November 2020).
- AM06 25 cm Desk Fan DYSON. Available online: http://www.dyson.com.sg/fansheaters/fans/desk/am06-10-range/am06-desk-fan-10-iron-blue.aspx (accessed on 7 November 2020).
- AM-4201 Digital Anemometer Lutron. Available online: http://www.lutron.com.tw/ugC_ShowroomItem_Detail.asp?hidKindID=1&hidTypeID=7&hidCatID=&hidShowID=785&hidPrdType=&txtSrhData= (accessed on 7 November 2020).
- Butterworth, S. On the theory of filter amplifiers. Wirel. Eng. 1930, 7, 536–541. [Google Scholar]
- Oliveira, A.; Marques, A. Respiratory sounds in healthy people: A systematic review. Respir. Med. 2014, 108, 550–570. [Google Scholar] [CrossRef]
- Emmanouilidou, D.; Elhilali, M. Characterization of noise contaminations in lung sound recordings. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2013, 2013, 2551–2554. [Google Scholar]
- Mulligan, K.; Adler, A.; Goubran, R. Detecting regional lung properties using audio transfer functions of the respiratory system. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 5697–5700. [Google Scholar]
- Zaeim, H.M.; Scheffer, C.; Blanckenberg, M.; Dellimore, K. Evaluation of the use of frequency response in the diagnosis of pleural effusion on a phantom model of the human lungs. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3418–3421. [Google Scholar]
- SpiroBank II. Available online: http://www.spirometry.com/ENG/Products/SpiroBank2.asp (accessed on 7 November 2020).
- Liistro, G.; Vanwelde, C.; Vincken, W.; Vandevoorde, J. Technical and functional assessment of 10 office spirometers: A multicenter comparative study. Chest 2006, 130, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Lusuardi, M.; de Benedetto, F.; Paggiaro, P.; Sanguinetti, C.M.; Brazzola, G.; Ferri, P.; Donner, C.F. A Randomized controlled trial on office spirometry in Asthma and COPD in standard general practice: Data from spirometry in Asthma and COPD: A comparative evaluation Italian study. Chest 2006, 129, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Degryse, J.-M.; Buffels, J.; Van Dijck, Y.; Decramer, M.; Nemery, B. Accuracy of Office Spirometry Performed by Trained Primary-Care Physicians Using the MIR Spirobank Hand-Held Spirometer. Respiration 2012, 83, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Buffels, J.; Degryse, J.; Heyrman, J.; Decramer, M. DIDASCO Study Office Spirometry Significantly Improves Early Detection of COPD in General Practice. Chest 2004, 125, 1394–1399. [Google Scholar] [CrossRef]
- Spathis, D.; Vlamos, P. Diagnosing asthma and chronic obstructive pulmonary disease with machine learning. Health Inform. J. 2017, 25, 811–827. [Google Scholar] [CrossRef]
- Prosperi, M.; Marinho, S.; Simpson, A.; Custovic, A.; Buchan, I. Predicting phenotypes of asthma and eczema with machine learning. BMC Med. Genom. 2014, 7, S7. [Google Scholar] [CrossRef]
- Zubaidi, F. A Mobile Based Platform for Monitoring Respiratory Diseases. Master’s Thesis, American University of Sharjah, Sharjah, UAE, 2016. [Google Scholar]
- Goto, T.; Camargo, C.A., Jr.; Faridi, M.K.; Yun, B.J.; Hasegawa, K. Machine learning approaches for predicting disposition of asthma and COPD exacerbations in the ED. Am. J. Emerg. Med. 2018, 36, 1650–1654. [Google Scholar] [CrossRef]
- Gurbeta, L.; Badnjević, A.; Maksimovic, M.; Omanović-Mikličanin, E.; Sejdic, E. A telehealth system for automated diagnosis of asthma and chronical obstructive pulmonary disease. J. Am. Med. Inform. Assoc. 2018, 25, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Du, C.; Bai, C.; Song, Y. An Internet of Things based COPD managing system: Its development, challenges and first experiences. Clin. eHealth 2019, 2, 12–15. [Google Scholar] [CrossRef]
- Jung, B. The Future of Connected Asthma and COPD Care: A Stepwise Approach; ONdrugDelivery Magazine: Lewes, UK, 2018; pp. 18–22. [Google Scholar]
| Severity | FEV1% Predicted |
|---|---|
| Mild | >=80 |
| Moderate | >=50 and <80 |
| Severe | >=30 and <50 |
| Very severe | <30 |
| Severity | Age (Years) | Nighttime Awakenings | Interference with Normal Activities | FEV1% Predicted |
|---|---|---|---|---|
| Intermittent | ALL | ≤2 days/week | None | >80% |
| 0–4 | 0 | |||
| >=5 | ≤2x/month | |||
| Mild persistent | All | >2 days/week but not daily | Minor limitation | >80% |
| 0–4 | 1–2x/month | |||
| >=5 | 3–4x/month | |||
| Moderate persistent | All | Daily | Some limitation | 60–80% |
| 0–4 | 3–4x/month | |||
| >=5 | >1x/week but not nightly | |||
| Severe persistent | All | Throughout the day | Extreme limitation | <60% |
| 0–4 | >1x/week | |||
| >=5 | Often 7x/week |
| Sample ID | FEV1/FVC Mobile App. | FEV1 % Mobile App. | Pretest Possibility of COPD | Pretest Possibility of Asthma | Clinical Diagnosis | Mobile App. Diagnosis |
|---|---|---|---|---|---|---|
| Subject 16 | 58.5 | 95.3 | True | False | Mild COPD | Mild COPD |
| Subject 17 | 67.3 | 78.9 | True | False | Moderate COPD | Moderate COPD |
| Subject 18 | 60.9 | 98.5 | True | False | Mild COPD | Mild COPD |
| Subject 19 | 66.6 | 74.4 | True | False | Moderate persistent Asthma | Moderate COPD |
| Subject 20 | 65.6 | 68 | False | True | Moderate persistent Asthma | Moderate persistent Asthma |
| Subject 21 | 67.1 | 60.8 | False | True | Moderate persistent Asthma | Moderate persistent Asthma |
| Subject 22 | 71.2 | 79.1 | False | True | Moderate persistent Asthma | Moderate persistent Asthma |
| Subject 23 | 68.7 | 76.6 | False | True | Moderate persistent Asthma | Moderate persistent Asthma |
| Subject 24 | 63.5 | 82.4 | False | True | Intermittent Asthma | Intermittent Asthma |
| Subject 25 | 69.3 | 87.1 | False | True | Intermittent Asthma | Intermittent Asthma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubaydi, F.; Sagahyroon, A.; Aloul, F.; Mir, H.; Mahboub, B. Using Mobiles to Monitor Respiratory Diseases. Informatics 2020, 7, 56. https://doi.org/10.3390/informatics7040056
Zubaydi F, Sagahyroon A, Aloul F, Mir H, Mahboub B. Using Mobiles to Monitor Respiratory Diseases. Informatics. 2020; 7(4):56. https://doi.org/10.3390/informatics7040056
Chicago/Turabian StyleZubaydi, Fatma, Assim Sagahyroon, Fadi Aloul, Hasan Mir, and Bassam Mahboub. 2020. "Using Mobiles to Monitor Respiratory Diseases" Informatics 7, no. 4: 56. https://doi.org/10.3390/informatics7040056
APA StyleZubaydi, F., Sagahyroon, A., Aloul, F., Mir, H., & Mahboub, B. (2020). Using Mobiles to Monitor Respiratory Diseases. Informatics, 7(4), 56. https://doi.org/10.3390/informatics7040056





