L-Dopa-Decarboxylase (DDC) Is a Positive Prognosticator for Breast Cancer Patients and Epinephrine Regulates Breast Cancer Cell (MCF7 and T47D) Growth In Vitro According to Their Different Expression of Gi- Protein- Coupled Receptors
Abstract
:1. Introduction
2. Results
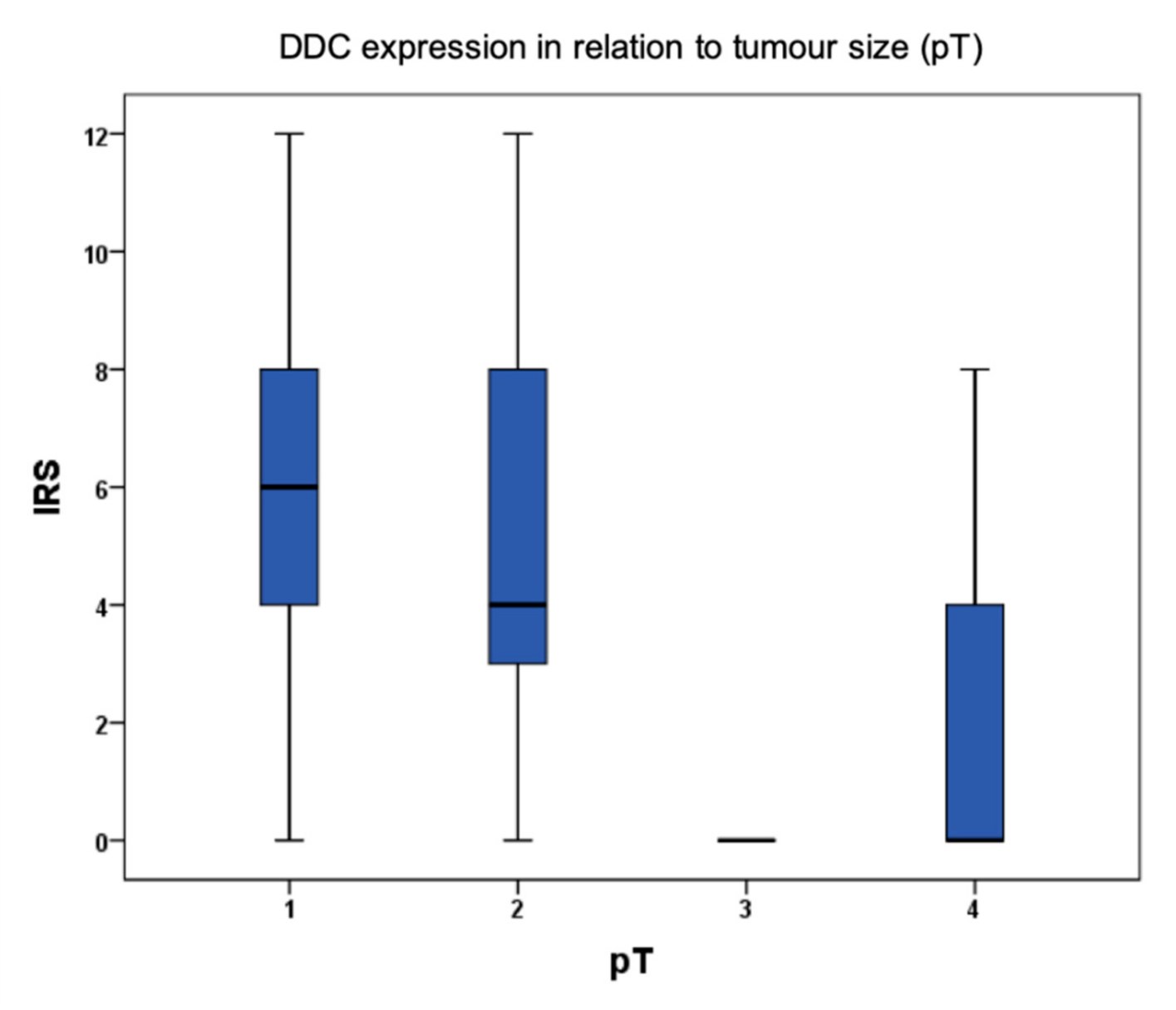
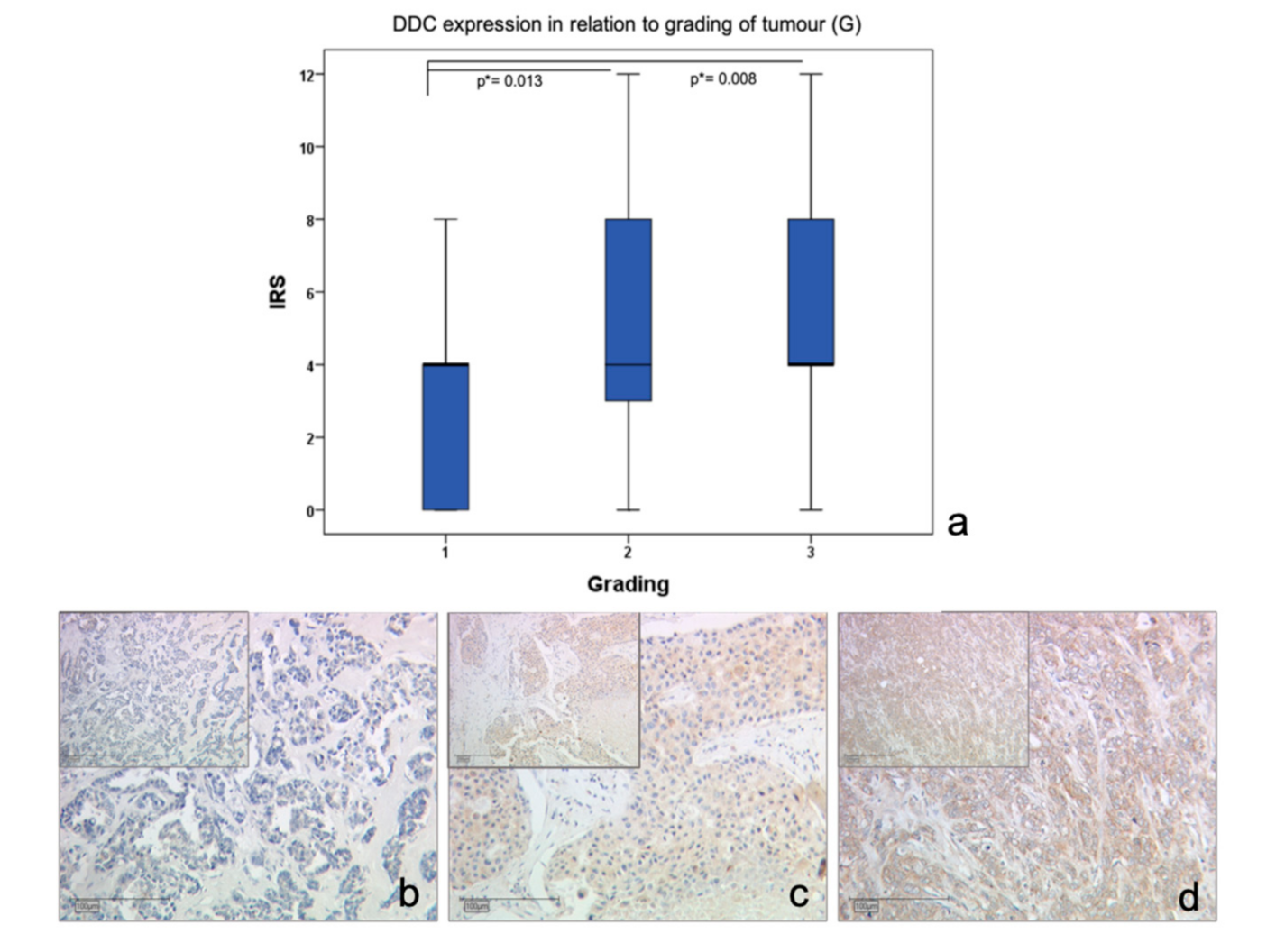
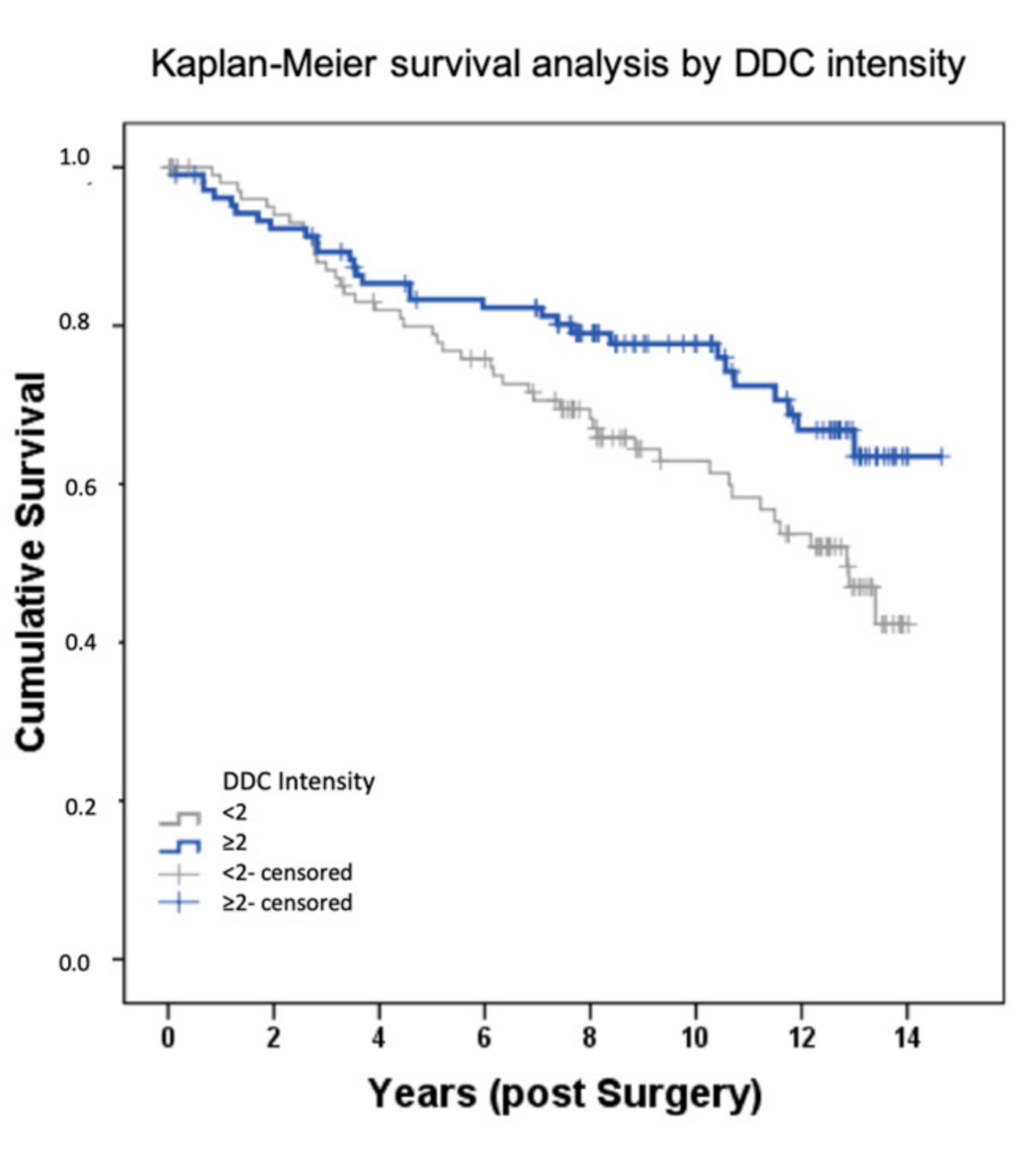
2.1. DDC Protein Expression in Primary Breast Cancer
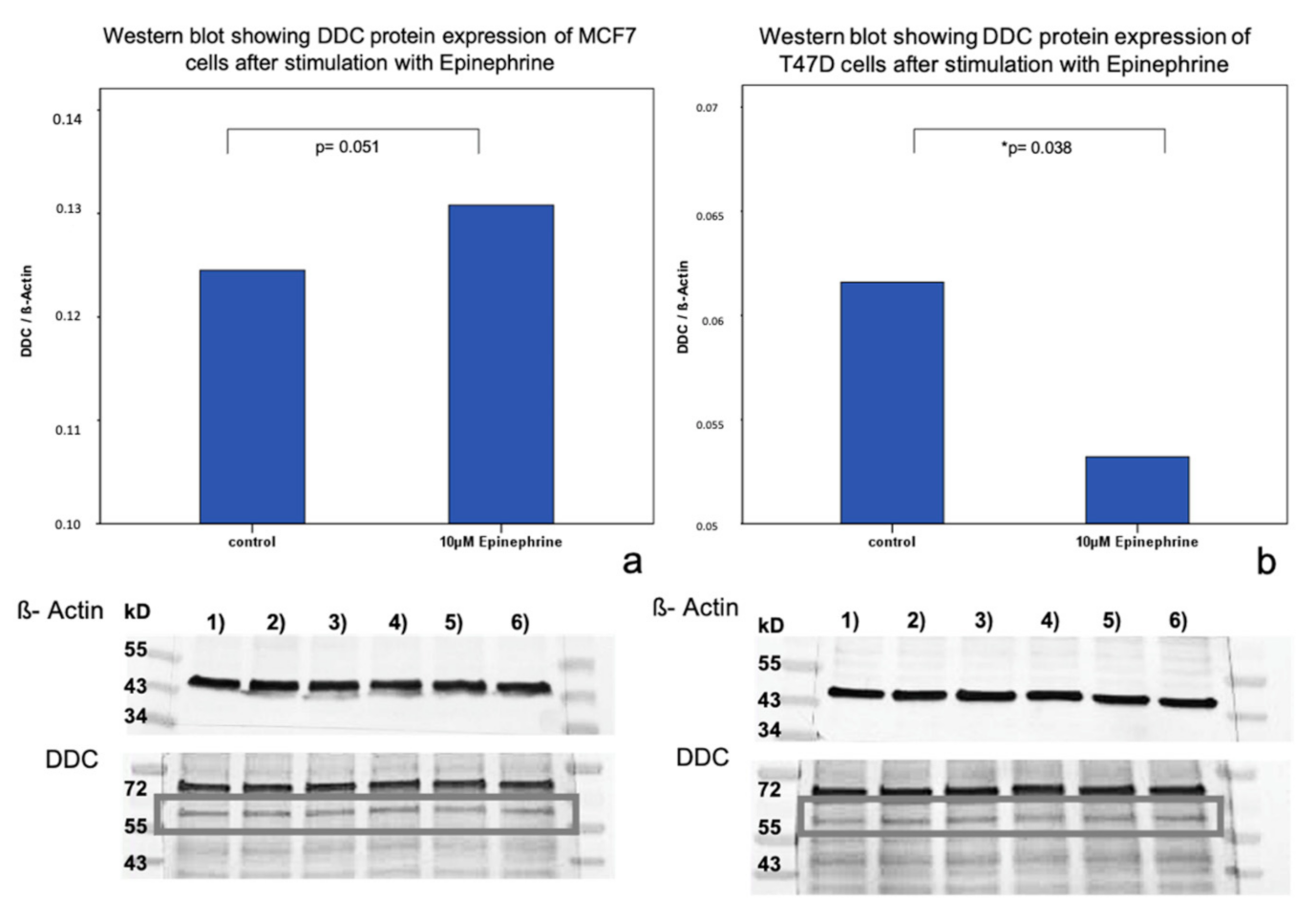
2.2. Influence of Epinephrine on DDC Protein Expression in MCF7 and T47D Breast Cancer Model Cells
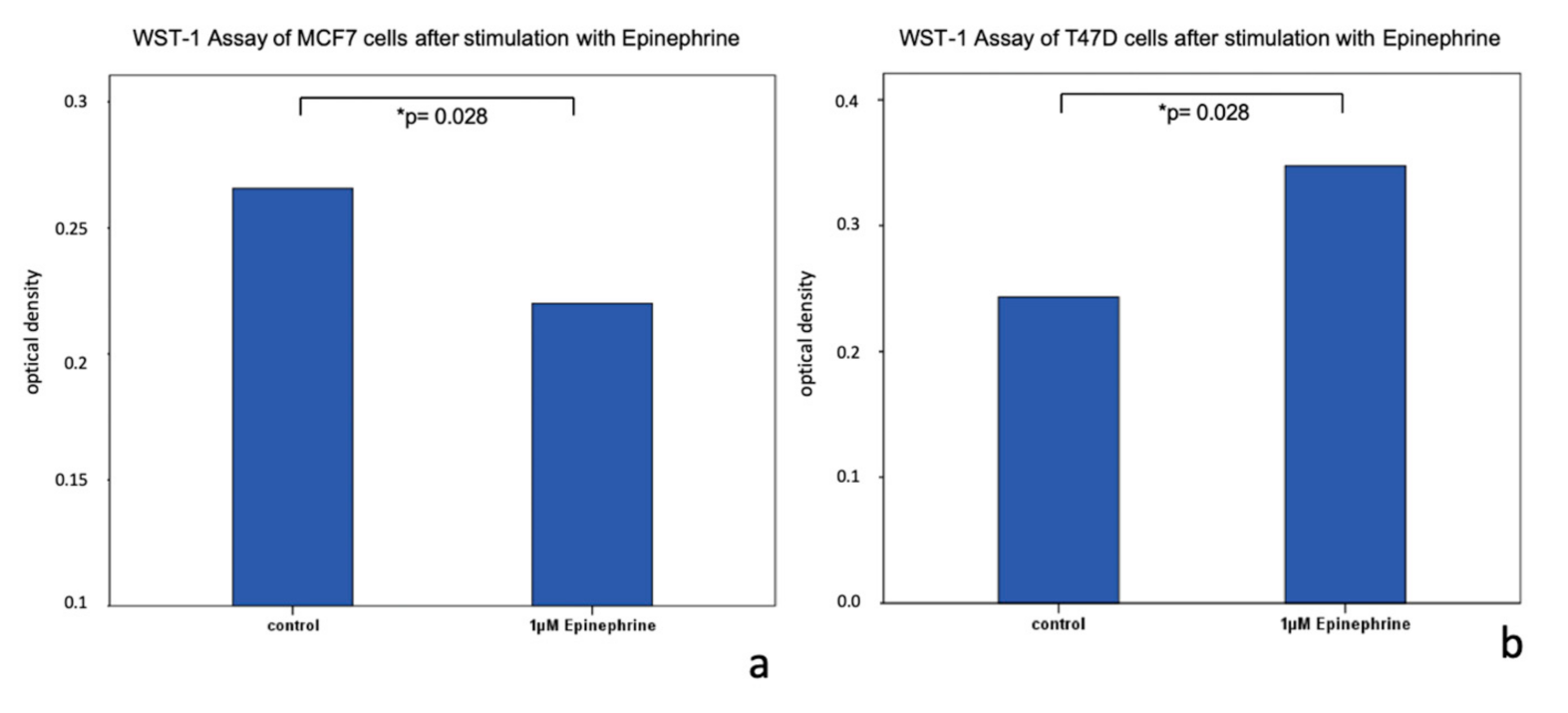
2.3. Viability of MCF7 and T47D Cells after Stimulation with Epinephrine
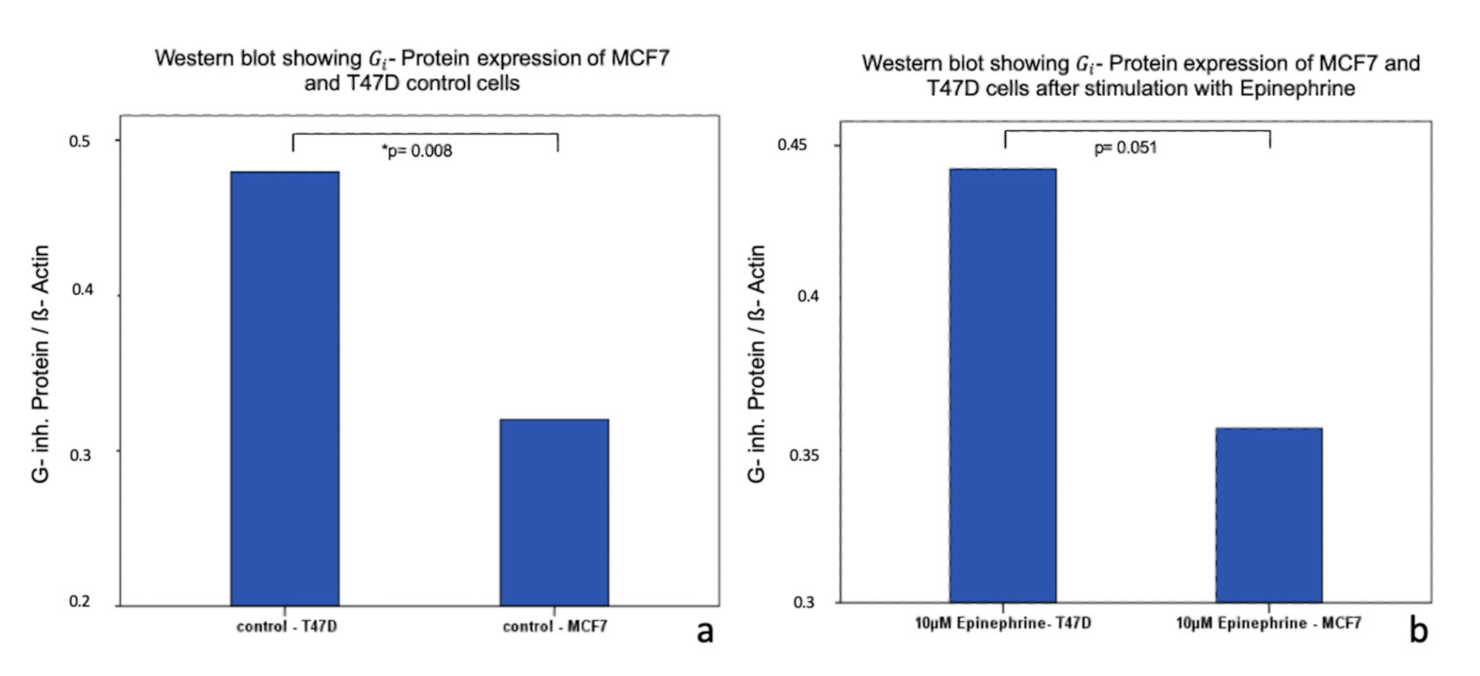
2.4. Influence of Epinephrine on - Protein -Expression in MCF7 and T47D Breast Cancer Model Cells
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Ethical Approval and Informed Consent
4.3. Immunohistochemistry
4.4. Cell Culturing and Cell Stimulation
4.5. Western Blot
4.6. WST-1 Assay
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DDC | L-dopa decarboxylase |
| TAAR1 | trace amine-associated receptor 1 |
| OS | overall survival |
| TAs | trace-amines |
| AR | adrenoreceptors |
| PKA | adenylcylase |
| CREB | cAMP-responsive element binding protein |
| -PcR | - protein coupled receptor |
| ER | estrogen receptor |
| PR | progesterone receptor |
| WST- 1 | water soluble tetrazolium |
| D2R | dopaminergic D2 receptor |
References
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turken, O.; NarIn, Y.; DemIrbas, S.; Onde, M.E.; Sayan, O.; KandemIr, E.G.; Yaylac, I.M.; Ozturk, A. Breast cancer in association with thyroid disorders. Breast Cancer Res. BCR 2003, 5, R110–R113. [Google Scholar] [CrossRef]
- Rasmusson, B.; Feldt-Rasmussen, U.; Hegedus, L.; Perrild, H.; Bech, K.; Hoier-Madsen, M. Thyroid function in patients with breast cancer. Eur. J. Cancer Clin. Oncol. 1987, 23, 553–556. [Google Scholar] [CrossRef]
- Kuijpens, J.L.; Nyklictek, I.; Louwman, M.W.; Weetman, T.A.; Pop, V.J.; Coebergh, J.W. Hypothyroidism might be related to breast cancer in post-menopausal women. Thyroid Off. J. Am. Thyroid Assoc. 2005, 15, 1253–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sogaard, M.; Farkas, D.K.; Ehrenstein, V.; Jorgensen, J.O.; Dekkers, O.M.; Sorensen, H.T. Hypothyroidism and hyperthyroidism and breast cancer risk: A nationwide cohort study. Eur. J. Endocrinol. 2016, 174, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Ditsch, N.; Liebhardt, S.; Von Koch, F.; Lenhard, M.; Vogeser, M.; Spitzweg, C.; Gallwas, J.; Toth, B. Thyroid function in breast cancer patients. Anticancer Res. 2010, 30, 1713–1717. [Google Scholar]
- Hoefig, C.S.; Zucchi, R.; Kohrle, J. Thyronamines and Derivatives: Physiological Relevance, Pharmacological Actions, and Future Research Directions. Thyroid 2016, 26, 1656–1673. [Google Scholar] [CrossRef]
- Harmeier, A.; Obermueller, S.; Meyer, C.A.; Revel, F.G.; Buchy, D.; Chaboz, S.; Dernick, G.; Wettstein, J.G.; Iglesias, A.; Rolink, A.; et al. Trace amine-associated receptor 1 activation silences GSK3beta signaling of TAAR1 and D2R heteromers. Eur. Neuropsychopharmacol. 2015, 25, 2049–2061. [Google Scholar] [CrossRef]
- Brix, K.; Fuhrer, D.; Biebermann, H. Molecules important for thyroid hormone synthesis and action-known facts and future perspectives. Thyroid Res. 2011, 4 (Suppl. 1), S9. [Google Scholar] [CrossRef] [Green Version]
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace amines: Identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971. [Google Scholar] [CrossRef] [Green Version]
- Barak, L.S.; Salahpour, A.; Zhang, X.; Masri, B.; Sotnikova, T.D.; Ramsey, A.J.; Violin, J.D.; Lefkowitz, R.J.; Caron, M.G.; Gainetdinov, R.R. Pharmacological characterization of membrane-expressed human trace amine-associated receptor 1 (TAAR1) by a bioluminescence resonance energy transfer cAMP biosensor. Mol. Pharmacol. 2008, 74, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Tremmel, E.; Hofmann, S.; Kuhn, C.; Heidegger, H.; Heublein, S.; Hermelink, K.; Wuerstlein, R.; Harbeck, N.; Mayr, D.; Mahner, S.; et al. Thyronamine regulation of TAAR1 expression in breast cancer cells and investigation of its influence on viability and migration. Breast Cancer Targets Ther. 2019, 11, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vattai, A.; Akyol, E.; Kuhn, C.; Hofmann, S.; Heidegger, H.; von Koch, F.; Hermelink, K.; Wuerstlein, R.; Harbeck, N.; Mayr, D.; et al. Increased trace amine-associated receptor 1 (TAAR1) expression is associated with a positive survival rate in patients with breast cancer. J. Cancer Res. Clin. Oncol. 2017. [Google Scholar] [CrossRef]
- Vassiliou, A.G.; Fragoulis, E.G.; Vassilacopoulou, D. Detection, purification and identification of an endogenous inhibitor of L-Dopa decarboxylase activity from human placenta. Neurochem. Res. 2009, 34, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Kontos, C.K.; Papadopoulos, I.N.; Fragoulis, E.G.; Scorilas, A. Quantitative expression analysis and prognostic significance of L-DOPA decarboxylase in colorectal adenocarcinoma. Br. J. Cancer 2010, 102, 1384–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wafa, L.A.; Cheng, H.; Plaa, N.; Ghaidi, F.; Fukumoto, T.; Fazli, L.; Gleave, M.E.; Cox, M.E.; Rennie, P.S. Carbidopa abrogates L-dopa decarboxylase coactivation of the androgen receptor and delays prostate tumor progression. Int. J. Cancer 2012, 130, 2835–2844. [Google Scholar] [CrossRef]
- Gratz, M.J.; Stavrou, S.; Kuhn, C.; Hofmann, S.; Hermelink, K.; Heidegger, H.; Hutter, S.; Mayr, D.; Mahner, S.; Jeschke, U.; et al. Dopamine synthesis and dopamine receptor expression are disturbed in recurrent miscarriages. Endocr. Connect. 2018, 7, 727–738. [Google Scholar] [CrossRef]
- Guenter, J.; Lenartowski, R. Molecular characteristic and physiological role of DOPA-decarboxylase. Postępy Higieny i Medycyny Doświadczalnej 2016, 70, 1424–1440. [Google Scholar] [CrossRef]
- Portaro, S.; Gugliandolo, A.; Scionti, D.; Cammaroto, S.; Morabito, R.; Leonardi, S.; Fraggetta, F.; Bramanti, P.; Mazzon, E. When dysphoria is not a primary mental state: A case report of the role of the aromatic L-aminoacid decarboxylase. Medicine 2018, 97, e10953. [Google Scholar] [CrossRef] [PubMed]
- Devos, D.; Lejeune, S.; Cormier-Dequaire, F.; Tahiri, K.; Charbonnier-Beaupel, F.; Rouaix, N.; Duhamel, A.; Sablonniere, B.; Bonnet, A.M.; Bonnet, C.; et al. Dopa-decarboxylase gene polymorphisms affect the motor response to L-dopa in Parkinson’s disease. Park. Relat. Disord. 2014, 20, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Vallar, L.; Muca, C.; Magni, M.; Albert, P.; Bunzow, J.; Meldolesi, J.; Civelli, O. Differential coupling of dopaminergic D2 receptors expressed in different cell types. Stimulation of phosphatidylinositol 4,5-bisphosphate hydrolysis in LtK- fibroblasts, hyperpolarization, and cytosolic-free Ca2+ concentration decrease in GH4C1 cells. J. Biol. Chem. 1990, 265, 10320–10326. [Google Scholar] [PubMed]
- Kaczor, A.A.; Jorg, M.; Capuano, B. The dopamine D2 receptor dimer and its interaction with homobivalent antagonists: Homology modeling, docking and molecular dynamics. J. Mol. Model. 2016, 22, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyland, K.; Clayton, P.T. Aromatic amino acid decarboxylase deficiency in twins. J. Inherit. Metab. Dis. 1990, 13, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Pons, R.; Ford, B.; Chiriboga, C.A.; Clayton, P.T.; Hinton, V.; Hyland, K.; Sharma, R.; De Vivo, D.C. Aromatic L-amino acid decarboxylase deficiency: Clinical features, treatment, and prognosis. Neurology 2004, 62, 1058–1065. [Google Scholar] [CrossRef]
- Sensenbach, W.; Madison, L.; Ochs, L. A comparison of the effects of 1-norepinephrine, synthetic 1-epinephrine, and U.S.P. epinephrine upon cerebral blood flow and metabolism in man. J. Clin. Investig. 1953, 32, 226–232. [Google Scholar] [CrossRef]
- Taylor, B.N.; Cassagnol, M. Alpha Adrenergic Receptors. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2019. [Google Scholar]
- Luthy, I.A.; Bruzzone, A.; Pinero, C.P.; Castillo, L.F.; Chiesa, I.J.; Vazquez, S.M.; Sarappa, M.G. Adrenoceptors: Non Conventional Target for Breast Cancer? Curr. Med. Chem. 2009, 16, 1850–1862. [Google Scholar] [CrossRef]
- Hassan, S.; Karpova, Y.; Flores, A.; D’Agostino, R.; Danhauer, S.C.; Hemal, A.; Kulik, G. A pilot study of blood epinephrine levels and CREB phosphorylation in men undergoing prostate biopsies. Int. Urol. Nephrol. 2014, 46, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Karamouzis, M.V.; Konstantinopoulos, P.A.; Papavassiliou, A.G. Roles of CREB-binding protein (CBP)/p300 in respiratory epithelium tumorigenesis. Cell Res. 2007, 17, 324–332. [Google Scholar] [CrossRef]
- Shaywitz, A.J.; Greenberg, M.E. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu. Rev. Biochem. 1999, 68, 821–861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mantas, I.; Alvarsson, A.; Yoshitake, T.; Shariatgorji, M.; Pereira, M.; Nilsson, A.; Kehr, J.; Andrén, P.E.; Millan, M.J.; et al. Striatal Tyrosine Hydroxylase Is Stimulated via TAAR1 by 3-Iodothyronamine, But Not by Tyramine or β-Phenylethylamine. Front. Pharmacol. 2018, 9, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Tu, K.; Liu, D.; Guo, L.; Chen, Y.; Li, Q.; Maiers, J.L.; Liu, Z.; Shah, V.H.; Dou, C.; et al. p300 Acetyltransferase Is a Cytoplasm-to-Nucleus Shuttle for SMAD2/3 and TAZ Nuclear Transport in Transforming Growth Factor β-Stimulated Hepatic Stellate Cells. Hepatology 2019, 70, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Radde, B.N.; Ivanova, M.M.; Mai, H.X.; Salabei, J.K.; Hill, B.G.; Klinge, C.M. Bioenergetic differences between MCF-7 and T47D breast cancer cells and their regulation by oestradiol and tamoxifen. Biochem. J. 2015, 465, 49–61. [Google Scholar] [CrossRef]
- Kalkhoven, E.; Kwakkenbos-Isbrücker, L.; de Laat, S.W.; van der Saag, P.T.; der Burg, B.v. Synthetic progestins induce proliferation of breast tumor cell lines via the progesterone or estrogen receptor. Mol. Cell. Endocrinol. 1994, 102, 45–52. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Park, S.A.; Park, K.S.; Park, S.; Heo, K.; Seo, Y.K.; Noh, D.Y.; Ryu, S.H.; Suh, P.G. G-protein-coupled receptor 81 promotes a malignant phenotype in breast cancer through angiogenic factor secretion. Oncotarget 2016, 7, 70898–70911. [Google Scholar] [CrossRef] [Green Version]
- Barzegar, E.; Fouladdel, S.; Movahhed, T.K.; Atashpour, S.; Ghahremani, M.H.; Ostad, S.N.; Azizi, E. Effects of berberine on proliferation, cell cycle distribution and apoptosis of human breast cancer T47D and MCF7 cell lines. Iran. J. Basic Med Sci. 2015, 18, 334–342. [Google Scholar]
- Jahanafrooz, Z.; Motameh, N.; Bakhshandeh, B. Comparative Evaluation of Silibinin Effects on Cell Cycling and Apoptosis in Human Breast Cancer MCF-7 and T47D Cell Lines. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 2661–2665. [Google Scholar]
- Gargiulo, L.; May, M.; Rivero, E.M.; Copsel, S.; Lamb, C.; Lydon, J.; Davio, C.; Lanari, C.; Lüthy, I.A.; Bruzzone, A. A Novel Effect of β-Adrenergic Receptor on Mammary Branching Morphogenesis and its Possible Implications in Breast Cancer. J. Mammary Gland Biol. Neoplasia 2017, 22, 43–57. [Google Scholar] [CrossRef]
- King, R.J. A discussion of the roles of oestrogen and progestin in human mammary carcinogenesis. J. Steroid Biochem. Mol. Biol. 1991, 39, 811–818. [Google Scholar] [CrossRef]
- Vera-Badillo, F.E.; Templeton, A.J.; de Gouveia, P.; Diaz-Padilla, I.; Bedard, P.L.; Al-Mubarak, M.; Seruga, B.; Tannock, I.F.; Ocana, A.; Amir, E. Androgen receptor expression and outcomes in early breast cancer: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106, djt319. [Google Scholar] [CrossRef] [PubMed]
- Elebro, K.; Bendahl, P.O.; Jernstrom, H.; Borgquist, S. Androgen receptor expression and breast cancer mortality in a population-based prospective cohort. Breast Cancer Res. Treat. 2017, 165, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Fujii, R.; Hanamura, T.; Suzuki, T.; Gohno, T.; Shibahara, Y.; Niwa, T.; Yamaguchi, Y.; Ohnuki, K.; Kakugawa, Y.; Hirakawa, H.; et al. Increased androgen receptor activity and cell proliferation in aromatase inhibitor-resistant breast carcinoma. J. Steroid Biochem. Mol. Biol. 2014, 144, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Stanislawska-Sachadyn, A.; Sachadyn, P.; Limon, J. Transcriptomic Effects of Estrogen Starvation and Induction in the MCF7 Cells. The Meta-analysis of Microarray Results. Curr. Pharm. Biotechnol. 2015, 17, 161–172. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, F.; Razandi, M.; Pedram, A.; Harvey, B.J.; Levin, E.R. Estrogen modulates metabolic pathway adaptation to available glucose in breast cancer cells. Mol. Endocrinol. 2012, 26, 2058–2070. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.; Zhu, Z.; Yang, C.; Wang, L.; Ding, G.; Jiang, F. Epinephrine increases malignancy of breast cancer through p38 MAPK signaling pathway in depressive disorders. Int. J. Clin. Exp. Pathol. 2019, 12, 1932–1946. [Google Scholar]
- van der Burg, B.; van Selm-Miltenburg, A.J.P.; de Laat, S.W.; van Zoelen, E.J.J. Direct effects of estrogen on c-fos and c-myc protooncogene expression and cellular proliferation in human breast cancer cells. Mol. Cell. Endocrinol. 1989, 64, 223–228. [Google Scholar] [CrossRef]
- Patel, S. Breast cancer: Lesser-known facets and hypotheses. Biomed. Pharmacother. 2018, 98, 499–506. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Ma, Z.; Wang, M.; Shen, W.-J.; Azhar, S.; Guo, Z.; Hu, Z. Feedback inhibition of CREB signaling by p38 MAPK contributes to the negative regulation of steroidogenesis. Reprod. Biol. Endocrinol. RB&E 2017, 15, 19. [Google Scholar] [CrossRef] [Green Version]

| Patient’s Characteristics | n= | |
|---|---|---|
| size | ||
| pT1 | 135 (68%) | |
| pT2 | 66 (29.3%) | |
| pT3 | 1 (0.4%) | |
| pT4 | 5 (2.2%) | |
| grading | ||
| G1 | 16 | |
| G2 | 84 | |
| G3 | 57 | |
| receptor status | ||
| Her2 | positive | 26 |
| negative | 208 | |
| ER | positive | 192 |
| negative | 43 | |
| PR | positive | 141 |
| negative | 94 | |
| luminal | ||
| A | 103 | |
| B | 74 | |
| mean age | ||
| 58.2 years ± 13.3 |
| Antibody | Incubation | Blocking Solution | Blocking Condition |
|---|---|---|---|
| DDC, Polyclonal (Rabbit IgG, LSBio) | 1:300 in PBS 16 h at 4°C | Reagent I (Polymer Kit, Zytomed Systems, Berlin, Germany) | 5 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tremmel, E.; Kuhn, C.; Kaltofen, T.; Vilsmaier, T.; Mayr, D.; Mahner, S.; Ditsch, N.; Jeschke, U.; Vattai, A. L-Dopa-Decarboxylase (DDC) Is a Positive Prognosticator for Breast Cancer Patients and Epinephrine Regulates Breast Cancer Cell (MCF7 and T47D) Growth In Vitro According to Their Different Expression of Gi- Protein- Coupled Receptors. Int. J. Mol. Sci. 2020, 21, 9565. https://doi.org/10.3390/ijms21249565
Tremmel E, Kuhn C, Kaltofen T, Vilsmaier T, Mayr D, Mahner S, Ditsch N, Jeschke U, Vattai A. L-Dopa-Decarboxylase (DDC) Is a Positive Prognosticator for Breast Cancer Patients and Epinephrine Regulates Breast Cancer Cell (MCF7 and T47D) Growth In Vitro According to Their Different Expression of Gi- Protein- Coupled Receptors. International Journal of Molecular Sciences. 2020; 21(24):9565. https://doi.org/10.3390/ijms21249565
Chicago/Turabian StyleTremmel, Eileen, Christina Kuhn, Till Kaltofen, Theresa Vilsmaier, Doris Mayr, Sven Mahner, Nina Ditsch, Udo Jeschke, and Aurelia Vattai. 2020. "L-Dopa-Decarboxylase (DDC) Is a Positive Prognosticator for Breast Cancer Patients and Epinephrine Regulates Breast Cancer Cell (MCF7 and T47D) Growth In Vitro According to Their Different Expression of Gi- Protein- Coupled Receptors" International Journal of Molecular Sciences 21, no. 24: 9565. https://doi.org/10.3390/ijms21249565





