1. Introduction
The effects of stress are inevitable while we live. All physical, chemical, biological and mental stimuli can be classed as stress, whether these involve eustress or distress. Moderate stress stimulates the sympathetic nervous system and promotes an improvement in judgment and behavioral abilities. However, excessive and chronic stress adversely burden the mind and body, and are concerned as risk factors causing disorders such as depression. Depression has a duration of more than a week with symptoms of the mind and body such as anxiety, hopeless feelings, reduced thinking ability, decreased interest and motivation, anorexia, and insomnia. Recently, the number of patients has increased in the world. Depression has a high risk of causing suicide impulses, and prevention, early diagnosis and treatment are important.
Suppression/elimination of stress is thought to be important for reducing the risk of developing depression. In recent years, various kinds of foods have drawn attention due to their stress relaxation effects. Sesame seeds have been popular as healthy foods since ancient times in Japan, and their imports are the second largest following the US and Europe [
1]. Sesame contains antioxidants called sesame lignans [
2]. It has been reported that active oxygen is eliminated by ingestion of vitamin E (α-tocopherol) and sesame lignan (sesamin, episesamin) which are abundantly present in sesame. These compounds are, therefore, thought to relieve fatigue and oxidative stress [
3]. Sesame has also been suggested to have various effects such as antithrombotic effects [
4], amelioration of lipid metabolism disorders [
5], and protection of dopaminergic neurons [
6].
As mentioned above, many studies have been reported on the effect of consuming sesame. However, there are few studies on the effect of the scent of sesame on the living body. The unique fragrance ingredients of roasted sesame seeds are composed of several molecules, such as pyrazine compounds with a fragrant aroma, furan compounds which possess a burning scent, etc. [
7]. Pyrazines are also commonly found in the roasted aroma of coffee and tea [
8]. It has been reported that coffee bean volatiles have anxiolytic and hypnotic effects in mice [
9]. We previously demonstrated alterations in the expression of genes and proteins in the brain after stress, and suggested that the aroma of coffee beans has anti-stress effects on the brain [
10]. Briefly, among the differentially expressed genes and proteins between the stress and stress with coffee group, NGFR (nerve growth factor receptor), trkC (tyrosine kinase receptor type 3), GIR (glucocorticoid-induced receptor), thiol-specific antioxidant protein, and heat shock 70 kDa protein 5 were identified as anti-oxidant or antistress factors.
The aim of the present study is to clarify the effect of sesame oil aroma on behavior and stress-responsive biomarkers (stress markers) in the blood and brain. Water immersion stress was applied to mice, which is thought to be suitable for preparing model animals before development of depression (undiagnosed condition). For behavioral analysis, we conducted an elevated plus maze test that is suitable for evaluating anxiety-related behaviors. We measured corticosterone—named as the stress hormone—levels in the serum and searched for stress marker candidates in the brain. Kruppel-like factor-4 (
Klf-4) and dual-specificity phosphatase-1 (
Dusp-1) are known to be negative regulators of the mitogen-activated protein kinase (MAPK) cascade, and the function of this cascade decreases in depression [
11]. In this study, we focused on the expression
Klf-4 and
Dusp-1 genes in the hippocampus and striatum.
3. Discussion
In the present study, we measured the body weight of mice before and after the 24 h loading of water immersion stress, and found that the rate of change in body weight after stress application was significantly decreased (
Figure 2), indicating that stress sufficiently affected the living body. Glucocorticoid secretion is a key endocrine response to stress. Previous reports have suggested that glucocorticoid secreted due to acute stress has a lipolytic effect (lipolysis) and enhances the mitochondrial utilization of sugars, lipids and amino acids. Weight loss due to stress application is a supporting result of previous reports [
12].
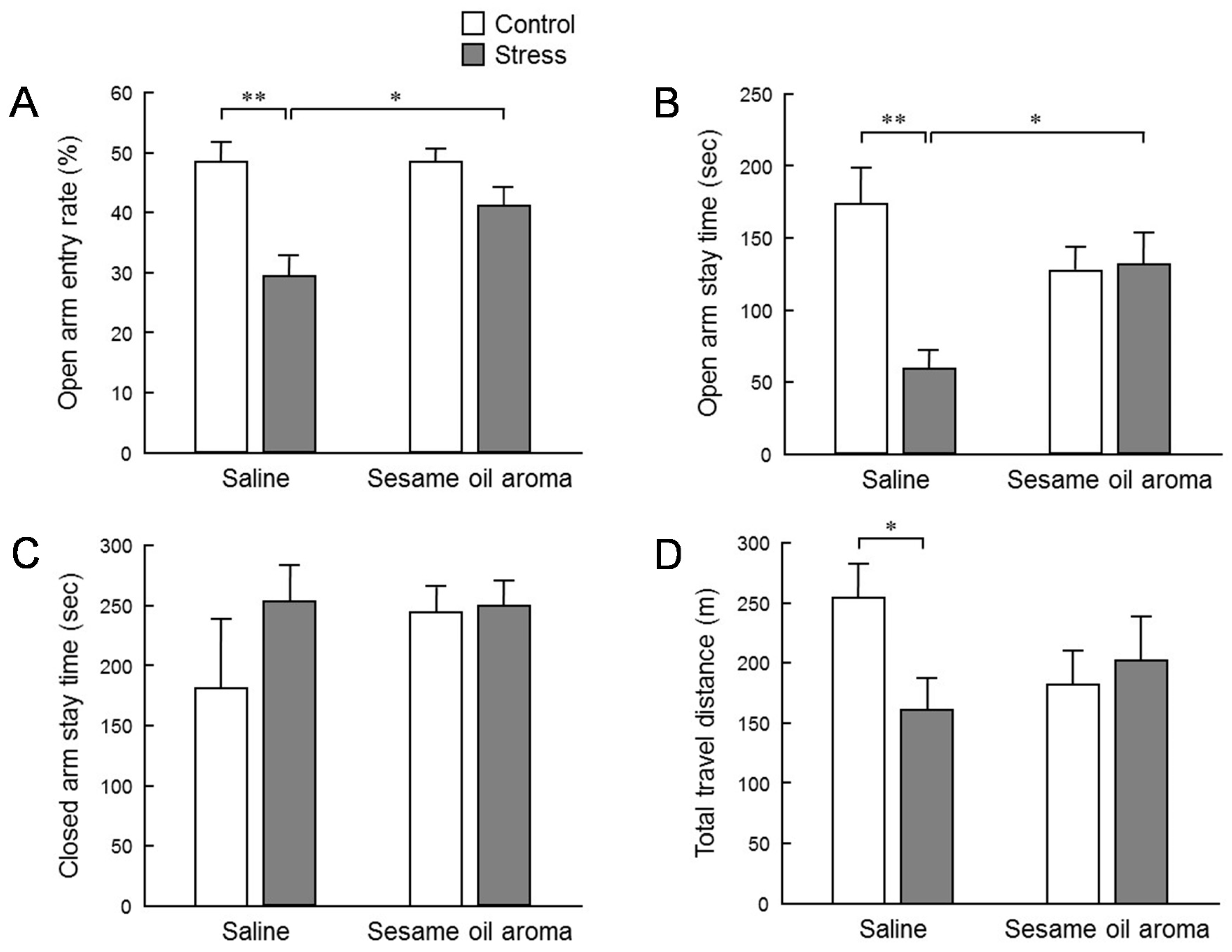
To evaluate the effect of stress on behavior, we conducted an elevated plus maze test (
Figure 3). The stress significantly lowered the penetration rate to the open arm, the staying time at the open arms, and the total moving distance. The declining rate of entry into the open arm and staying time indicates that anxiety-like behavior was enhanced. In addition, the total migration distance is an indicator of spontaneous motor activity. Thus, these results suggest that the water immersion stress load in this study had an influence in suppressing the subsequent spontaneous motor activities. Inhalation of sesame oil scent significantly restored the penetration rate to the open arm and staying time at the open arm which were decreased by stress. These behavioral changes strongly suggest the possibility that the aroma of sesame oil suppresses anxiety induced by stress. On the other hand, the effect of stress on the total migration distance was not significantly suppressed by the scent of the sesame oil. However, the total migration distance in stress/sesame group was higher than the stress/saline group. Therefore, it is conceivable that the aroma component of sesame oil acts in the direction of increasing locomotor activity. Additionally, although it was not statistically significant, it is interesting that the behavior of control mice was also reduced by the scent of sesame oil. It is possible that the aroma components of sesame oil may contain some factor that suppresses spontaneous motor activity. As stated in the introduction, an anti-anxiety-like stress-reducing effect is recognized in coffee which has a fragrant component which is partially common to sesame seeds [
9]. It is necessary to clarify in the future whether or not the fragrant component of sesame oil that suppressed spontaneous movement in this study is the same as the volatile component of coffee which produces a relaxing effect.
From the GC-MS analysis of sesame oil aroma (
Figure 1), it was found that heterocyclic amines and 2-methoxyphenol were common ingredients present in coffee [
13]. The compound 2-methoxyphenol is used in traditional dental pulp sedation and it has been reported that 2-methoxyphenol is a potent scavenger of reactive oxygen radicals [
14]. Furthermore, it has been reported that alkylpyrazine derivatives demonstrated a sleep prolongation effect. In particular, the duration of pentobarbital-induced sleep in mice was dose-dependently increased by 2,5-dimethylpyrazine, and the gamma-aminobutyric acid level in brains of mice was increased by the administration of 2,5-dimethylpyrazine [
15]. The sedative and central inhibitory effect of these compounds may be involved in the stress relieving effect of the sesame oil aroma.
Sesame is rarely sniffed intentionally as is the case with an aroma oil. Generally we are most likely to be exposed to the scent of sesame in our daily life in the form of the fragrance of sesame itself, or when we eat dishes using sesame or sesame oil. Sesame seeds contain abundant antioxidants, and it has been clarified that removal of active oxygen alleviates oxidative stress [
3]. The elevated plus maze test in this study suggested that the scent of sesame oil may have anxiolytic effects. Therefore, sesame seeds may be excellent anti-stress foods, having stress-suppressing effects not only after digestion but also in the process of sensing the aroma during eating.
Stress is transmitted to the hypothalamus via the cerebral cortex, and activates the “hypothalamus-sympathetic-adrenal medullary system (SAM)” axis and “hypothalamus-pituitary anterior lobe-adrenal cortex (HPA)” axis. When the HPA system is activated, glucocorticoids, such as cortisol and corticosterone, are released into the blood, which causes various physiological changes, such as increments in blood pressure, gluconeogenesis, cardiac contractility, cardiac output, and suppression of inflammation. However, hypersecretion of glucocorticoid is controlled via glucocorticoid receptors localized in the hippocampus, hypothalamus and pituitary gland (negative feedback). If glucocorticoid secretion persists due to excessive stress exposure, the hippocampal neurons are impaired and the glucocorticoid receptor decreases [
16], which impairs hippocampal atrophy and the negative feedback mechanism, consequently causing depression-like behaviors [
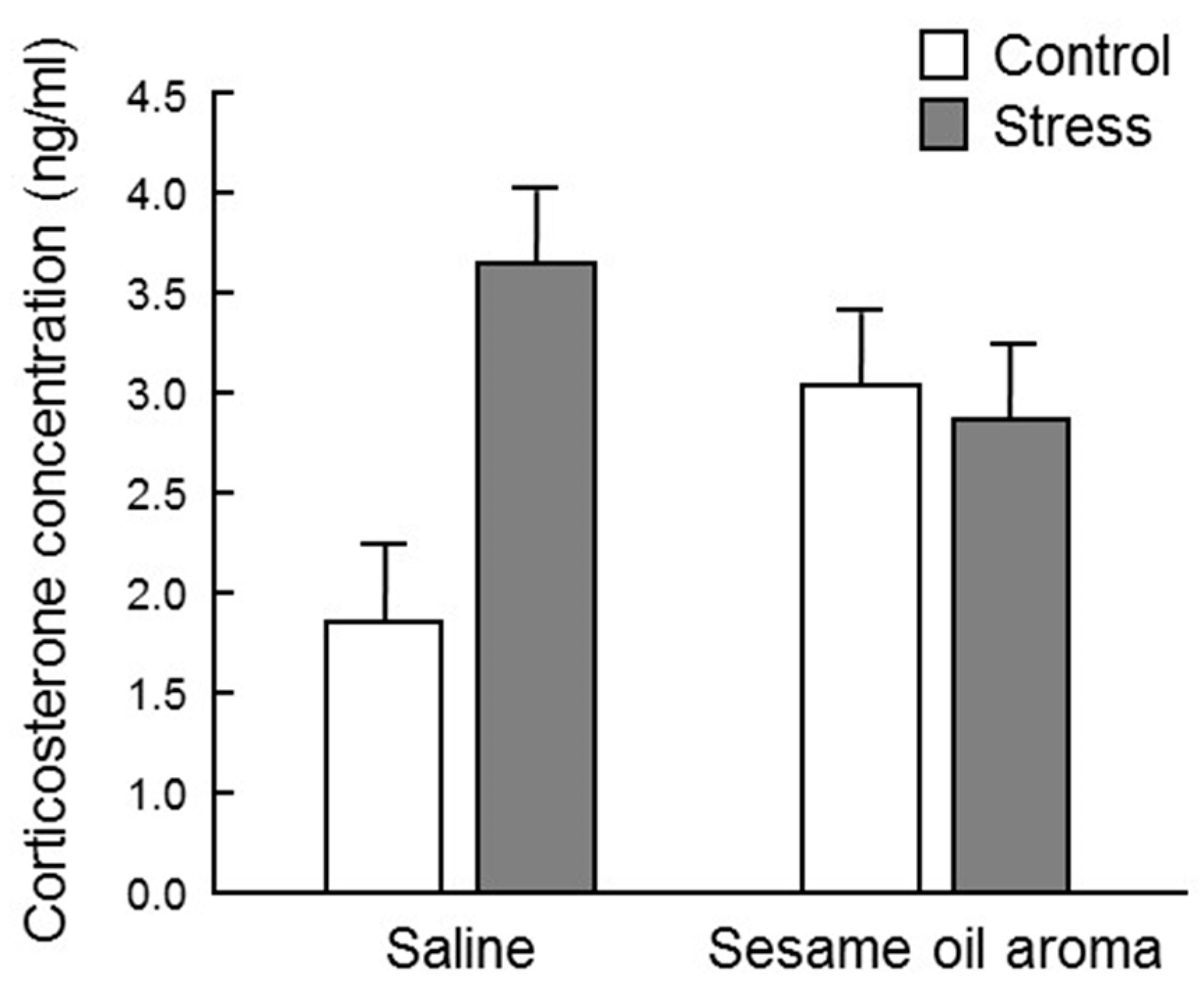
17]. In this study, the serum corticosterone concentration was highly variable among individuals, and barely any significant difference was observed among the four groups (
Figure 4). However, the stress/saline group increased to about twice as much as the control/saline, which supports the idea that corticosterone is one of the stress response biomarkers. Since the corticosterone level in the control/sesame group was higher than the control/saline group, the scent of sesame seems to be stressful to the mouse itself. It is necessary to clarify whether stress due to scent is eustress or distress. However, the fact that the scent of sesame oil lowered the concentration of corticosterone under water immersion stress suggests that sesame may suppress the effect of distress. Moreover, although statistical significance was not observed, the scent of sesame oil showed a tendency to suppress the effect of water immersion stress. Regarding these effects of sesame oil, it is possible that significant differences may be observed by investigating the change after a stronger stress stimulus than water immersion stress.
We searched for stress marker candidates showing alterations in expression levels in both the brain and blood due to stress. In this study, we focused on
Klf-4 and
Dusp-1.
Klf-4 is one of the four factors used for making induced pluripotent stem (iPS) cells, also called Yamanaka factor. It plays certain roles as a tumor suppressor for many kinds of cancers. However, the p53 tumor suppressor is inhibited by
Klf-4 in breast cancer, so
Klf-4 also functions as a tumor inducing factor [
18]. It is also known that
Klf-4 affects various mechanisms of cells by inhibiting the MAPK pathway. In the present study, the stress caused a significant increase in
Klf-4 expression in the striatum and an increasing tendency in the hippocampus. These levels were significantly attenuated by the scent of sesame oil (
Figure 6). Behavioral results suggest that the mouse used in this study was depressed by water immersion stress, and the MAPK pathway is considered to be showing reduced function. The odor component of sesame oil may inhibit the expression of
Klf-4 to repair the MAPK pathway.
Dusp-1 is a member of a class of enzymes that remove phosphate groups from proteins. It is a major negative regulator of the MAPK cascade, an important signaling pathway involved in neuronal function [
19]. It is known that the expression level of
Dusp-1 is elevated in the hippocampus of rats experiencing chronical stress [
20] and decreased by treatment with antidepressants [
21]. The induction of
Dusp-1 is not only a direct result of stress but also an important negative regulator of MAPK that contributes to the development of depressive symptoms. Previously, the significance of altered
Dusp-1 was tested in rodent models of depression and it was demonstrated that increased central
Dusp-1 expression, as a result of stress or viral-mediated gene transfer, causes depressive behaviors. Conversely, chronic antidepressant treatment normalizes the stress-induced
Dusp-1 expression and behavior, and mice lacking
Dusp-1 are resilient to stress [
22]. Therefore,
Dusp-1 is representative of promising new drug targets for the treatment of depression and other mood disorders. We observed that the scent of sesame oil significantly inhibited the expression level of
Dusp-1 in both the hippocampus and striatum of the control and stress groups (
Figure 7).
In the present study, Dusp-1 expression in the hippocampus was not increased due to water immersion stress, but we found an increasing trend in the striatum. The intensity of water immersion stress in this study may be too weak to cause a significant increase in Dusp-1 expression. However, suppression of Dusp-1 expression under normal and stress conditions suggests that the scent of sesame may suppress stress, not only in water immersion stress, but also in normal environment.
In this study, a significant anxious-like behavior was counteracted by the scent of sesame oil. Changes in serum corticosterone levels due to stress were greatly varied by individuals and alterations were not statistically significant. However, its levels were increased by stress, and tended to be suppressed by the scent of sesame. The expression level of Klf-4 in the striatum was significantly increased by stress, which was significantly inhibited by the sesame oil aroma. Similar changes were found in Klf-4 expression in the hippocampus. Dusp-1 expression in the striatum and hippocampus showed alterations similar to those of Klf-4. However, sesame oil significantly inhibited the expression levels of Dusp-1 in both regions, regardless of the presence or absence of stress exposure.
Our results suggest that the aroma component of sesame oil has a stress-suppressing effect in behavior via alterations in the expression of Klf-4 and Dusp-1 in the brain. Further studies on the interaction of different kinds of stress could be necessary. The present study is the first step to link the changes in the level of expression of intracerebral factors and in behavior due to the scent of sesame.
4. Materials and Methods
4.1. Materials
Aromatic sesame oil used in this study was purchased from Kuki Sangyo Co., Ltd. (Mie, Japan). This oil was produced by pressing and extracting from white sesame from Guatemala, followed by a cooling process.
4.2. Volatile Compound Analysis
Samples (1 g) were placed in a glass vial (10 mL) and heated at 40 °C for 5 min. Released volatiles were adsorbed on SPME fiber polydimethylsiloxane/carboxen/divinylbenzene (PDMS/Carboxen/DVB, Sigma-Aldrich Corp., St. Louis, MO, USA) at 40 °C for 20 min. The volatiles adsorbed on SPME were separated using an Agilent 7890B gas chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA) with a fused silica capillary column (DB-WAX, 30 m × 0.25 mm × 0.25 μm; Agilent Technologies Inc.). Volatiles isolated were analyzed using an Agilent 5977A mass spectrometer (Agilent Technologies Inc.). Operating conditions were as follows: injector temperature, 250 °C; helium flow rate, 1 mL/min; oven temperature, 35 °C for 5 min and then programmed to increase at 5 °C/min to 120 °C, and increased at 15 °C/min to 220 °C and held for 6 min. Mass spectra were obtained by EI ionization at 70 eV over 29 to 290 mass units, with an ion source temperature of 230 °C. Volatile compounds were identified by comparison of their mass spectra similarities with standard compounds and the NIST 02 spectral library of the gas chromatography-mass spectrometry (GC-MS).
4.3. Animal Experiment
This study was carried out according to the animal experiment handling provision by Toho University Animal Experiment Committee. Animal experiment design plan has been approved by the committee. Jcl:ICR male mice at 5 weeks of age purchased from Clea Japan (Tokyo, Japan) were housed in an acrylic cage, three animals per cage, and kept under a 12 h light/dark cycle (light period 08:00–20:00) at 24 ± 2 °C. Food and water were allowed ad libitum. After adapting to the rearing environment over a week, mice were randomly divided into 4 groups of 6 mice: control/saline (normal rearing and saline inhalation) group, stress/saline (stress load and saline inhalation) group, control/sesame (normal breeding and sesame oil aroma inhalation) group, and stress/sesame (stress load and sesame oil aroma inhalation) group. The Stress/saline and stress/sesame groups received water immersion stress. These animals were bred for 24 h in a cage with water in the limbs of mice were immersed to varying degrees (water depth of about 1 cm). This stresses the mice due to discomfort and insomnia induction. Meanwhile, control/saline and control/sesame groups were bred under normal conditions with wood chips in a cage. Since the manure odor of the mouse during the water immersion stress is strong, it may influence the aroma inhalation later. Therefore, cages were put inside the draft (fume hood) to minimize manure odor during the stress loading. For control groups, breeding was carried out in the same draft for 24 h to unify the breeding environment. Inhalation was started from 10:30 just after 24 h of stress loading or normal rearing. The control/saline and stress/saline groups were exposed to saline resulting in them inhaling it, and the control/sesame and stress/sesame groups instead inhaled the sesame oil aroma for 90 min. The odor was placed in a glass box in which a piece of filter paper impregnated with 50 μL of physiological saline or sesame oil was adhered to the inside. This study was carried out according to the animal experiment handling provision by Toho University Animal Experiment Committee. Ethic Committee approval numbers were Dosho 16-52-292, 17-53-292, 18-54-292, 19-55-292, and 20-21-450.
4.4. Weight Measurement
In order to check whether water immersion for 24 h was stressful to the mouse, body weight was measured before and after stress application, and the rate of change was calculated. Measurements before stress were carried out when the mice were randomly divided into 4 groups. Measurements after stress were performed after each aroma inhalation, since moisture was contained in the body hair of mice due to water immersion.
4.5. Behavioral Analysis
Evaluation of anxiety-like behavior was performed by the elevated plus maze test for 10 min just after completion of inhalation of the aroma. The maze consisted of an arm length of 30 cm, arm width of 5 cm, height from the floor of 60 cm, and closed arm wall height of 20 cm. The test was carried out in a dim room with an illuminance of 140 ± 10 lux. Immediately after putting the mouse in the center of the maze, the test room was unattended. The behavior of animals was captured with a video camera on the ceiling. The total moving distance and average moving speed were automatically recorded using behavioral tracking software ANY-maze (Muromachi Kikai, Tokyo, Japan). Regarding the number entering into the open arm and the staying time, the number entering into the closed arm and the staying time, the video image was observed and the average value was calculated by measuring twice for each individual.
4.6. Dissection
After 24 h under the conditions described above, the mice were decapitated from 12:00, and the blood and whole brain were collected. The blood was centrifuged (2000× g/4 °C, 10 min), and the serum was dispensed into another 2 mL tube and stored at −80 °C. The whole brain was dissected on ice, and the striatum and hippocampus were sampled. The brain tissue was put in a 2 mL tube, immediately frozen in liquid nitrogen, and stored at −80 °C. Thereafter, the frozen tissue was pulverized in liquid nitrogen to make it into powder form. Powdery tissue was divided into 2 mL tubes and stored at −80 °C.
4.7. Corticosterone Concentration
Serum corticosterone concentration was measured using the Corticosterone Enzyme Immunoassay Kit (Arbor Assays, Ann Arbor, MI, USA). In each tube, 5 μL of dissociation reagent and 5 μL of serum were mixed and incubated at room temperature for 5 min or more. The serum was diluted 100 times by adding 490 μL of assay buffer. The procedure was completed according to the manufacturer’s protocol. Each reaction was carried out on a 96-well plate, and the absorbance at 450 nm was measured with a plate reader. Corticosterone concentration was calculated using My Assay (
https://www.myassays.com/home.aspx).
4.8. Total RNA Extraction
One mL of Qiazol (Qiagen, Hilden, Germany) was added to each brain tissue sample and stirred until the sample was completely dissolved. Total RNA was extracted with RNeasy Mini Kit (Qiagen) and RNase-Free DNase set (Qiagen) with the manufacturer’s protocols. RNA was dissolved in RNase-free water, and the concentration and the purity of the obtained total RNA was measured using NanoDrop. Samples with low purity were ethanol precipitated with Ethachinmate (Nippon Jean, Tokyo, Japan). Total RNA was stored at −80 °C.
4.9. cDNA Synthesis
Reverse transcription was performed from the obtained RNA to synthesize cDNA using ReverTra Ace® qPCR RT Master Mix (Toyobo, Osaka, Japan). First, the amount of sample was calculated so that the RNA concentration of each sample was 1 pg/μL to 1 μg/μL in 10 μL. To 2 μL of each sample, 6 μL of nuclease-free water was added, and 2 μL of 2 × RT Master Mix (ReverTra Ace® qPCR RT Master Mix, Toyobo) was added. cDNA was synthesized by reverse transcription using GeneAmp PCR System 9700 (Applied Biosystems, Thermo Fisher Scientific). Conditions for cDNA synthesis were 15 min at 37 °C, 5 min at 50 °C and 5 min at 98 °C. The synthesized cDNA was stored at −20 °C.
4.10. Real Time Reverse Transcription-Polymerase Chain Reaction (Real Time RT-PCR)
For real time RT-PCR, we used TB Green Premix Ex Taq II (Takara Bio, Shiga, Japan). To 2 μL of each cDNA, 1.6 μL of primer (Forward and Reverse, 0.8 each) (
Figure 5) (Takara Bio) was added, and 8.5 μL of RNase free water, 12.5 μL of TB Green
Premix Ex Taq II and 0.4 μL of Rox Reference Dye was added to make a total of 25 μL. The expression level of cDNA was measured using an Applied Biosystems
® 7500 real-time PCR system (Thermo Fisher Scientific). Holding Stage was 50 °C for 2 min and 95 °C for 10 min. Cycling Stage for 40 cycles was 95 °C for 15 s and 60 °C for 1 min. Melt Curve Stage was 95 °C for 15 s, 60 °C for 1 min, 95 °C for 30 s, and 60 °C for 15 s. Analysis of the gene expression level of each sample was carried out in comparison with the expression level of glyceraldehyde 3-phosphate dehydrogenase (
GAPDH) which is a housekeeping gene. Expression levels of
Klf-4 and
Dusp-1 were calculated as correction values divided by the expression level of
GAPDH.
4.11. Statistical Analysis
Results were expressed as mean ± SEM. For the significance among 4 groups, ANOVA analysis was performed and further analysis was conducted using the Tukey’s test method. In some cases, the results were analyzed by Student’s t-test to determine significant difference between two groups. p < 0.05 was considered to be statistically significant.