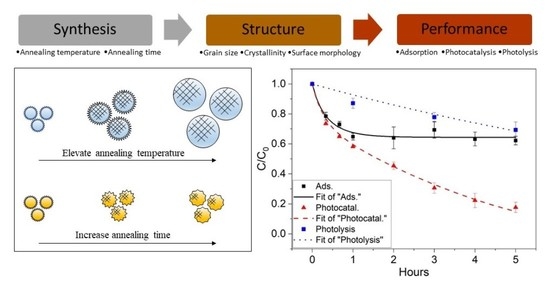
Modeling and Experimental Studies on Adsorption and Photocatalytic Performance of Nitrogen-Doped TiO2 Prepared via the Sol–Gel Method
Abstract
1. Introduction
2. Result and Discussion
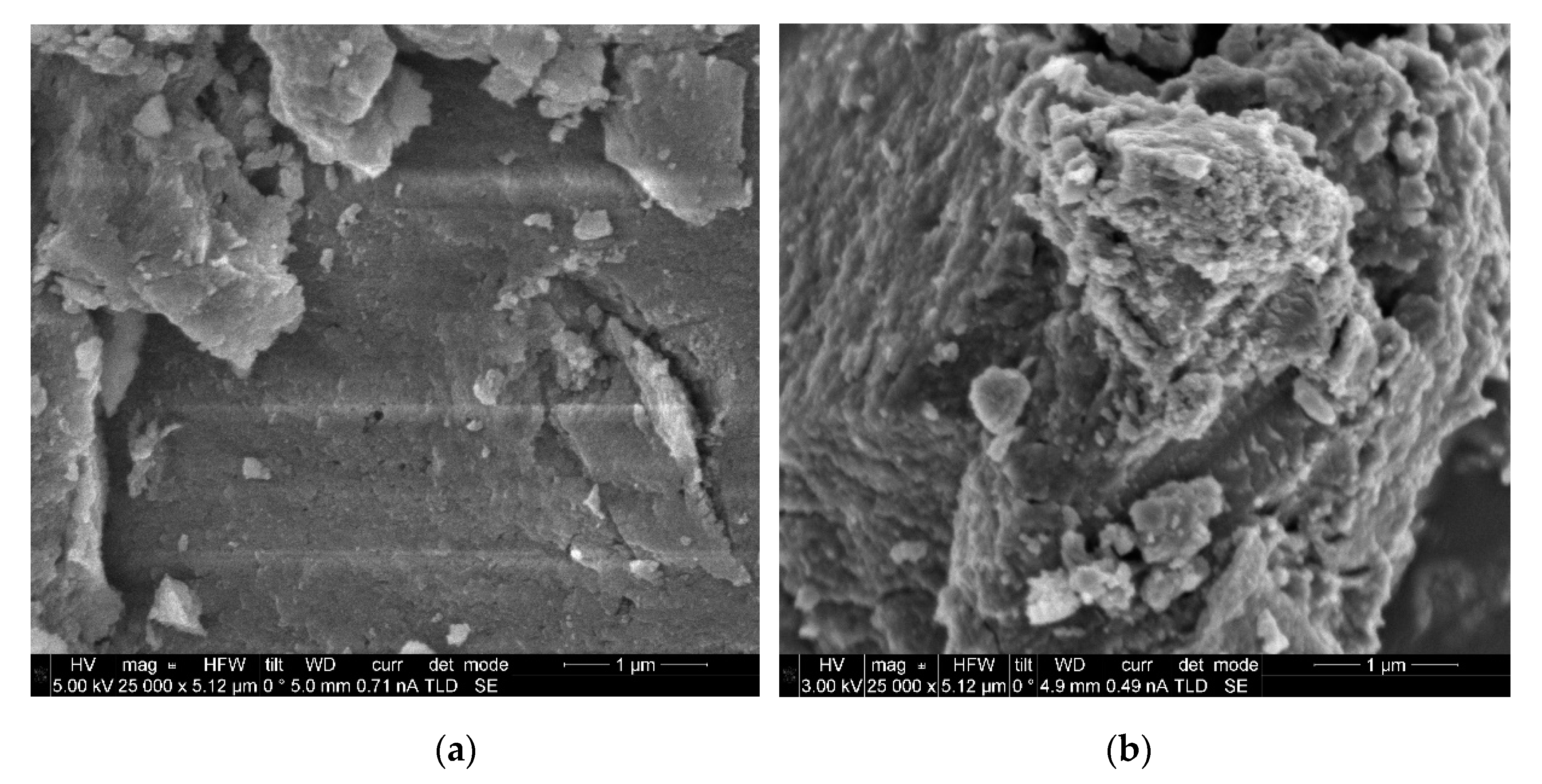
2.1. Characterization of N-TiO2 Photocatalysts
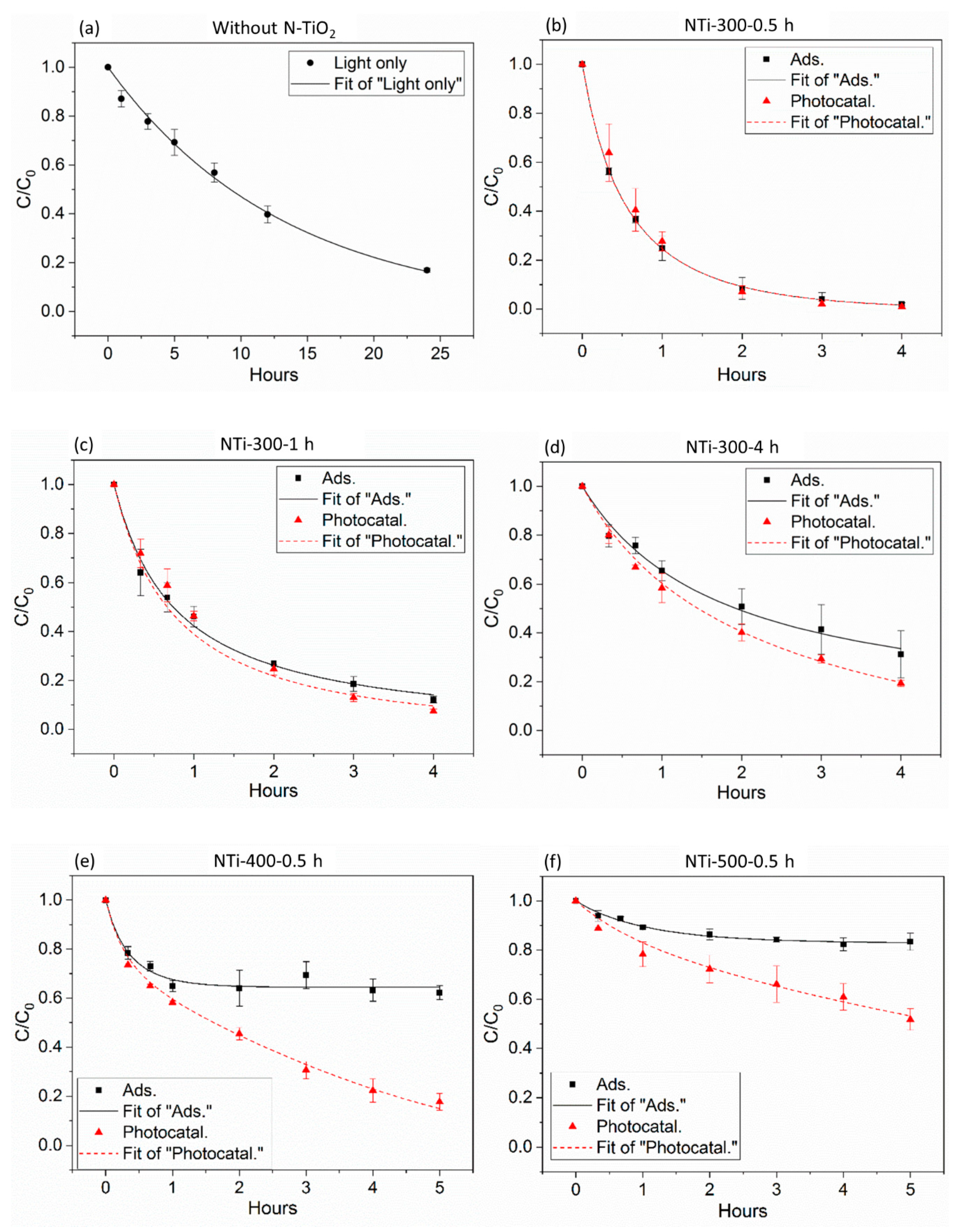
2.2. Measurement of Photocatalytic Activity and Kinetic Modeling Results
3. Experimental Approach
3.1. Photocatalyst Preparation
3.2. Photocatalyst Characterization
3.3. Measurement of Photocatalytic Activities
3.4. Model of Adsorption and Photocatalysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ji, J.; Xu, Y.; Huang, H.; He, M.; Liu, S.; Liu, G.; Xie, R.; Feng, Q.; Shu, Y.; Zhan, Y.; et al. Mesoporous TiO2 under VUV irradiation: Enhanced photocatalytic oxidation for VOCs degradation at room temperature. Chem. Eng. J. 2017, 327, 490–499. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C.; Yu, J.Z.; Xu, J.H. Photodegradation of formaldehyde by photocatalyst TiO2: Effects on the presences of NO, SO2 and VOCs. Appl. Catal. B Environ. 2004, 54, 41–50. [Google Scholar] [CrossRef]
- Jiménez-Tototzintle, M.; Ferreira, I.J.; Duque, S.D.S.; Barrocas, P.R.G.; Saggioro, E.M. Removal of contaminants of emerging concern (CECs) and antibiotic resistant bacteria in urban wastewater using UVA/TiO2/H2O2 photocatalysis. Chemosphere 2018, 210, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Corena, J.R.A.; Bergendahl, J.A.; Hart, F.L. Advanced oxidation of five contaminants in water by UV/TiO2: Reaction kinetics and byproducts identification. J. Environ. Manag. 2016, 181, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Ohama, Y.; Van Gemert, D. Application of Titanium Dioxide Photocatalysis to Construction Materials: State-of-the-Art Report of the RILEM Technical Committee 194-TDP; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Karácsonyi, É.; Baia, L.; Dombi, A.; Danciu, V.; Mogyorósi, K.; Pop, L.C.; Pap, Z. The photocatalytic activity of TiO2/WO3/noble metal (Au or Pt) nanoarchitectures obtained by selective photodeposition. Catal. Today 2013, 208, 19–27. [Google Scholar] [CrossRef]
- Quill, J.; Fedor, G.; Brennan, P.; Everett, E. Quantifying the Indoor Light Environment. Q-Lab Corporation. 2007. Available online: https://www.q-lab.com/documents/public/1e85c327-1aa2-4e87-808e-df04653e4d5e.pdf (accessed on 10 December 2020).
- Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Prospects for LED lighting. Nat. Photon. 2009, 3, 180–182. [Google Scholar] [CrossRef]
- Gascon, J.; SantaClara, J.; Oar-Arteta, L.; Van Koppen, L.; Osadchii, D.; Gascon, J.; Kapteijn, F. Photocatalytic properties of TiO2 and Fe-doped TiO2 prepared by metal organic framework-mediated synthesis. Chem. Eng. J. 2019, 360, 75–88. [Google Scholar] [CrossRef]
- Karafas, E.S.; Romanias, M.N.; Stefanopoulos, V.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Papagiannakopoulos, P. Effect of metal doped and co-doped TiO2 photocatalysts oriented to degrade indoor/outdoor pollutants for air quality improvement. A kinetic and product study using acetaldehyde as probe molecule. J. Photochem. Photobiol. A Chem. 2019, 371, 255–263. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Yang, Y.; Guo, T.; Zhuang, X.; Ji, S.; Zhang, T.; Shang, Y.; Zhou, Z. Enhanced photocatalytic degradation of sulfamethazine by Bi-doped TiO2 nano-composites supported by powdered activated carbon under visible light irradiation. Sep. Purif. Technol. 2019, 211, 673–683. [Google Scholar] [CrossRef]
- Islam, S.Z.; Nagpure, S.; Kim, D.-Y.; Rankin, S.E. Synthesis and Catalytic Applications of Non-Metal Doped Mesoporous Titania. Inorganics 2017, 5, 15. [Google Scholar] [CrossRef]
- Peng, X.; Wang, M.; Hu, F.; Qiu, F.; Dai, H.; Cao, Z. Facile fabrication of hollow biochar carbon-doped TiO2/CuO composites for the photocatalytic degradation of ammonia nitrogen from aqueous solution. J. Alloys Compd. 2019, 770, 1055–1063. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kim, J.-T. Application of visible-light photocatalysis with nitrogen-doped or unmodified titanium dioxide for control of indoor-level volatile organic compounds. J. Hazard. Mater. 2009, 164, 360–366. [Google Scholar] [CrossRef]
- Chen, C.; Bai, H.; Chang, S.-M.; Chang, C.; Den, W. Preparation of N-doped TiO2 photocatalyst by atmospheric pressure plasma process for VOCs decomposition under UV and visible light sources. J. Nanoparticle Res. 2007, 9, 365–375. [Google Scholar] [CrossRef]
- Fisher, M.B.; Keane, D.A.; Fernández-Ibáñez, P.; Colreavy, J.; Hinder, S.J.; McGuigan, K.G.; Pillai, S.C. Nitrogen and copper doped solar light active TiO2 photocatalysts for water decontamination. Appl. Catal. B Environ. 2013, 130, 8–13. [Google Scholar] [CrossRef]
- Choi, H.; Antoniou, M.G.; Pelaez, M.; De La Cruz, A.A.; Shoemaker, J.A.; Dionysiou, D.D. Mesoporous Nitrogen-Doped TiO2 for the Photocatalytic Destruction of the Cyanobacterial Toxin Microcystin-LR under Visible Light Irradiation. Environ. Sci. Technol. 2007, 41, 7530–7535. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.A.K.; Reddy, P.V.L.; Sharma, V.M.; Srinivas, B.; Kumari, V.D.; Subrahmanyam, M. Photocatalytic Degradation of Isoproturon Pesticide on C, N and S Doped TiO2. J. Water Resour. Prot. 2010, 2, 235–244. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Trouts, T.D.; Chin, Y.-P. Direct and indirect photolysis of triclocarban in the presence of dissolved organic matter. Elem. Sci. Anthr. 2015, 3, 000050. [Google Scholar] [CrossRef]
- Liu, Q.-T.; Williams, H.E. Kinetics and Degradation Products for Direct Photolysis of β-Blockers in Water. Environ. Sci. Technol. 2007, 41, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir–Hinshelwood kinetics—A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Coleman, H.M.; Eggins, B.R.; Byrne, J.A.; Palmer, F.L.; King, E. Photocatalytic degradation of 17-β-oestradiol on immobilised TiO2. Appl. Catal. B Environ. 2000, 24, L1–L5. [Google Scholar] [CrossRef]
- Yu, T.; Tan, X.; Zhao, L.; Yin, Y.; Chen, P.; Wei, J. Characterization, activity and kinetics of a visible light driven photocatalyst: Cerium and nitrogen co-doped TiO2 nanoparticles. Chem. Eng. J. 2010, 157, 86–92. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N.W. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Zhou, X.; Peng, F.; Wang, H.; Yu, H.; Yang, J. Preparation of nitrogen doped TiO2 photocatalyst by oxidation of titanium nitride with H2O2. Mater. Res. Bull. 2011, 46, 840–844. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Xing, Z.; Yang, L. Synthesis and characterization of N-doped TiO2 and its enhanced visible-light photocatalytic activity. Arab. J. Chem. 2016, 9, S1706–S1711. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, X.; Burda, C. The Effects of Sintering on the Photocatalytic Activity of N-Doped TiO2 Nanoparticles. Chem. Mater. 2008, 20, 2629–2636. [Google Scholar] [CrossRef]
- Qiu, X.; Burda, C. Chemically synthesized nitrogen-doped metal oxide nanoparticles. Chem. Phys. 2007, 339, 1–10. [Google Scholar] [CrossRef]

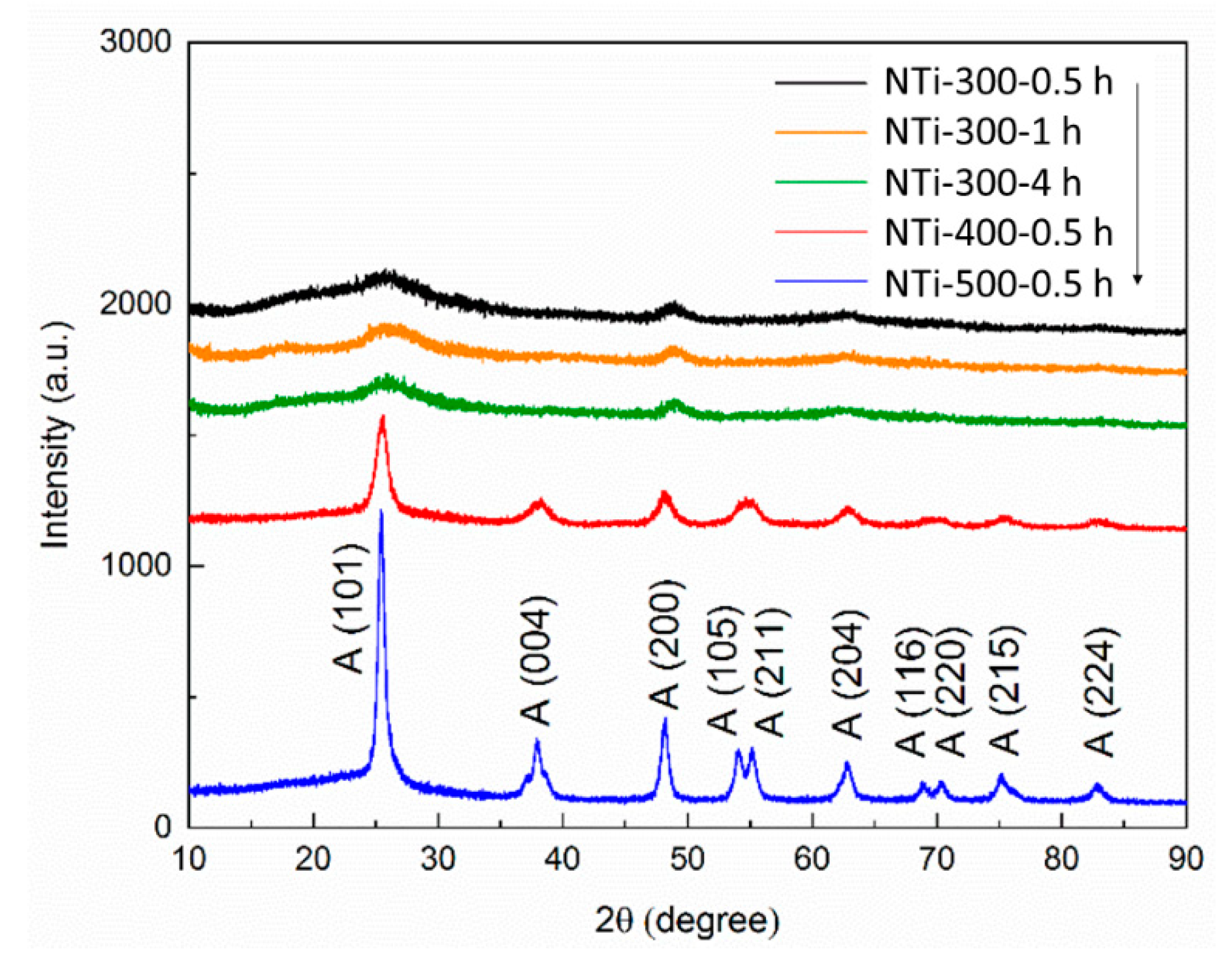

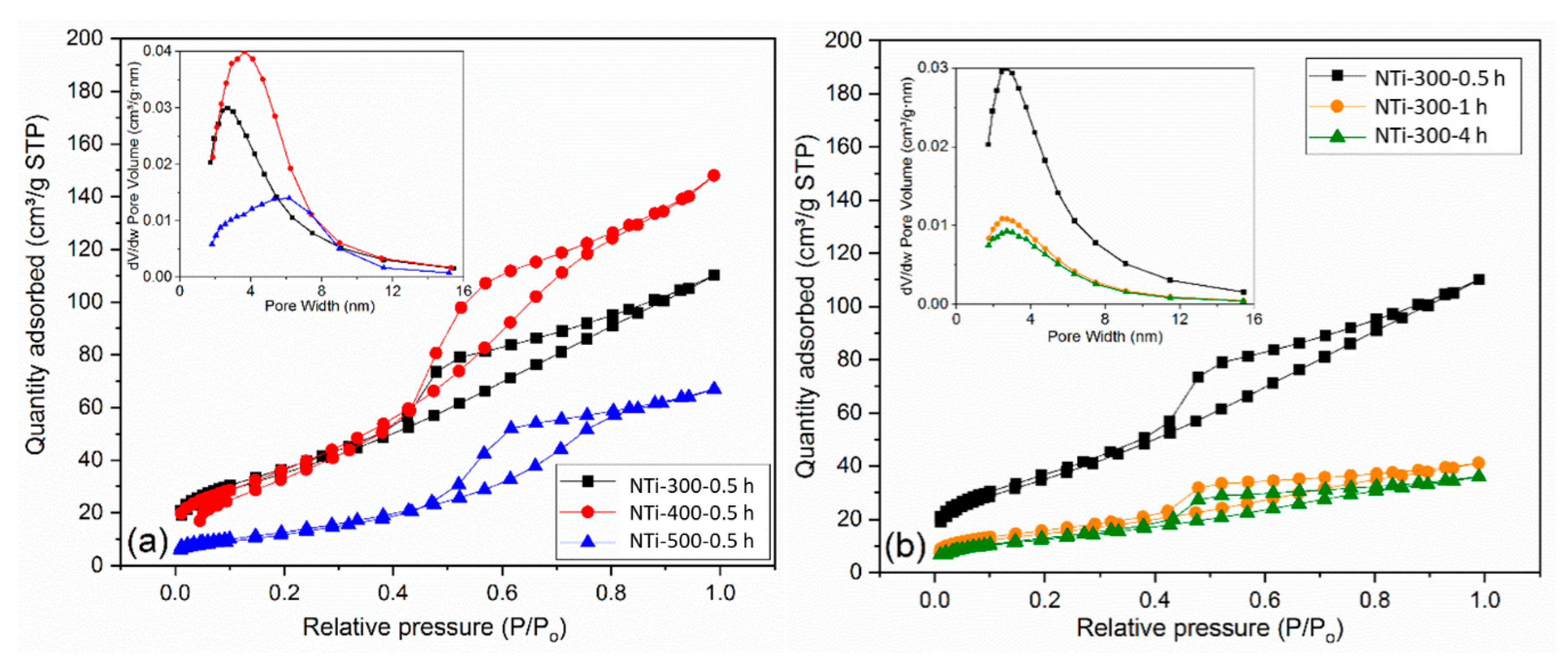
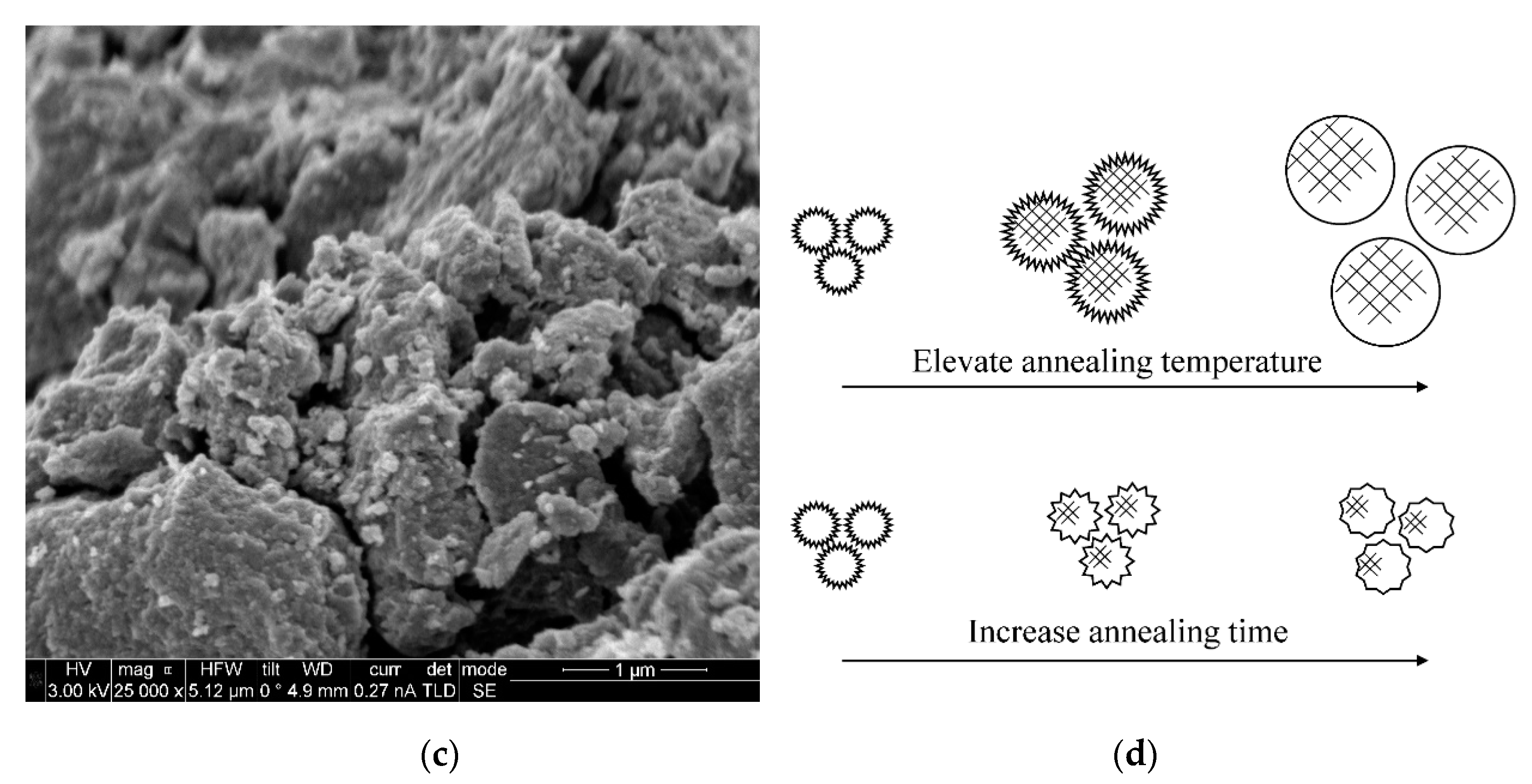
| Photocatalysts | Crystallite Size (nm) | Degree of Crystallinity (%) | BET Surface Area (m2 g−1) | Average Pore Size (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|---|---|
| NTi-300-0.5 h | 1.82 | 37.0 | 132.0 | 2.6 | 0.154 |
| NTi-300-1 h | 2.37 | 46.4 | 53.9 | 2.6 | 0.054 |
| NTi-300-4 h | 1.96 | 50.9 | 46.1 | 2.7 | 0.048 |
| NTi-400-0.5 h | 8.44 | 68.3 | 143.0 | 3.8 | 0.177 |
| NTi-500-0.5 h | 13.74 | 71.8 | 57.4 | 6.0 | 0.095 |
| Photocatalysts | k1 (m3 mol−1 h−1) | CP0 (mol m−3) | k1·CP0 (h−1) | k2 (h−1) | k3 (h−1) |
|---|---|---|---|---|---|
| NTi-300-0.5 h | 30.32 | 0.0660 | 2.0011 | ≈0 | 0.0753 |
| NTi-300-1 h | 30.12 | 0.0447 | 2.3464 | ≈0 | |
| NTi-300-4 h | 14.09 | 0.0383 | 0.5396 | 0.1669 | |
| NTi-400-0.5 h | 79.55 | 0.0141 | 1.1217 | 0.3861 | |
| NTi-500-0.5 h | 25.10 | 0.0068 | 0.1707 | 0.0996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Wickramasinghe, S.; Tsai, Y.H.; Samia, A.C.S.; Gurarie, D.; Yu, X. Modeling and Experimental Studies on Adsorption and Photocatalytic Performance of Nitrogen-Doped TiO2 Prepared via the Sol–Gel Method. Catalysts 2020, 10, 1449. https://doi.org/10.3390/catal10121449
Jiang Z, Wickramasinghe S, Tsai YH, Samia ACS, Gurarie D, Yu X. Modeling and Experimental Studies on Adsorption and Photocatalytic Performance of Nitrogen-Doped TiO2 Prepared via the Sol–Gel Method. Catalysts. 2020; 10(12):1449. https://doi.org/10.3390/catal10121449
Chicago/Turabian StyleJiang, Zhuoying, Sameera Wickramasinghe, Yu Hsin Tsai, Anna Cristina S. Samia, David Gurarie, and Xiong Yu. 2020. "Modeling and Experimental Studies on Adsorption and Photocatalytic Performance of Nitrogen-Doped TiO2 Prepared via the Sol–Gel Method" Catalysts 10, no. 12: 1449. https://doi.org/10.3390/catal10121449
APA StyleJiang, Z., Wickramasinghe, S., Tsai, Y. H., Samia, A. C. S., Gurarie, D., & Yu, X. (2020). Modeling and Experimental Studies on Adsorption and Photocatalytic Performance of Nitrogen-Doped TiO2 Prepared via the Sol–Gel Method. Catalysts, 10(12), 1449. https://doi.org/10.3390/catal10121449