An Investigation of the Influence of PEG 400 and PEG-6-Caprylic/Capric Glycerides on Dermal Delivery of Niacinamide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Solubility, Solubility Parameters and HPLC Analytical Method
2.3. Dynamic Vapor Sorption (DVS) Studies
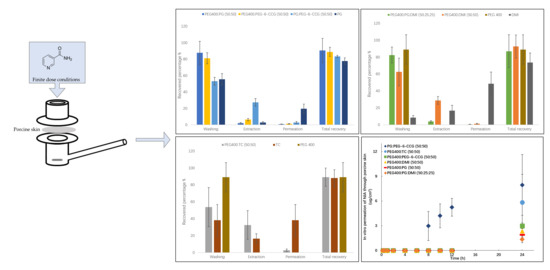
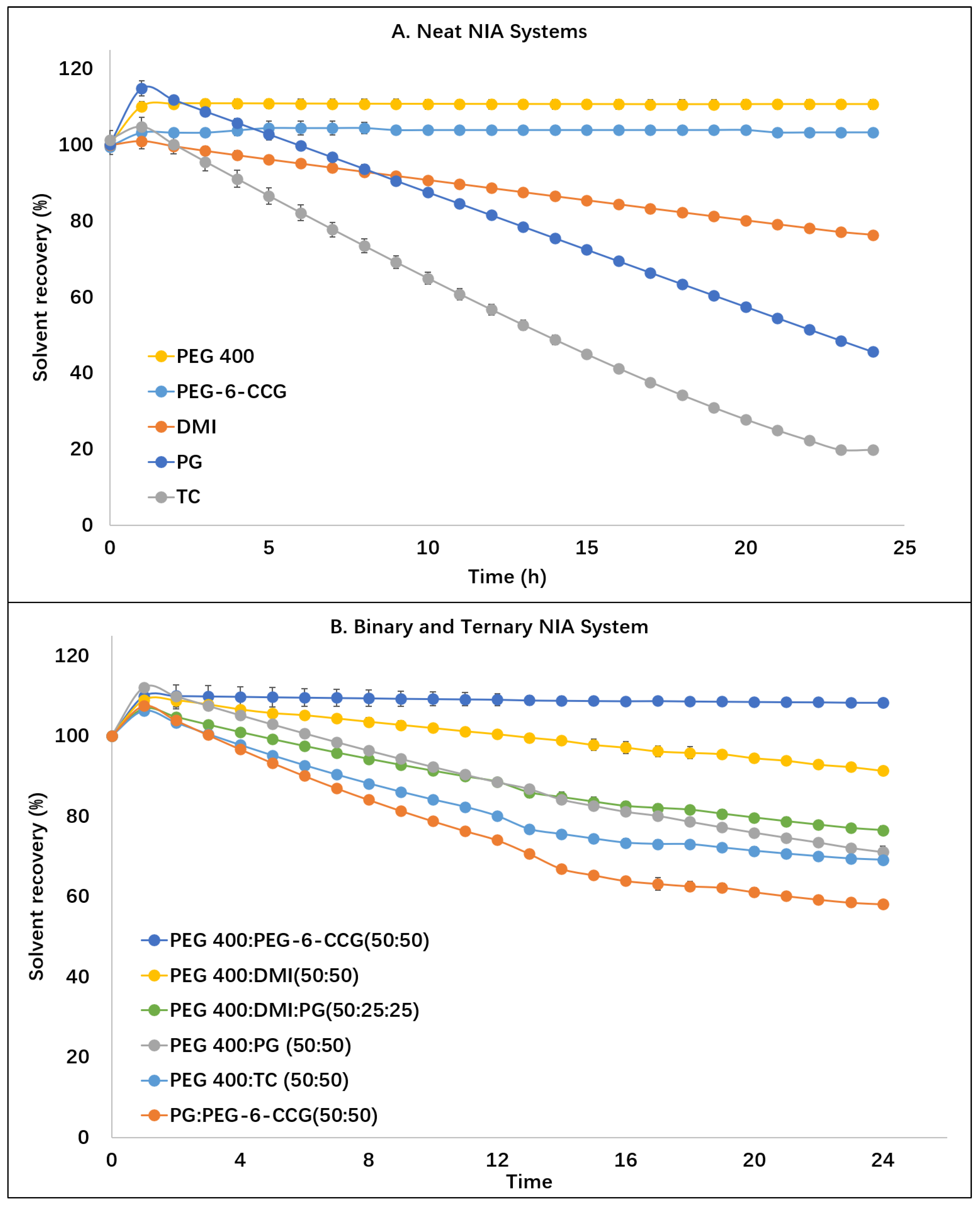
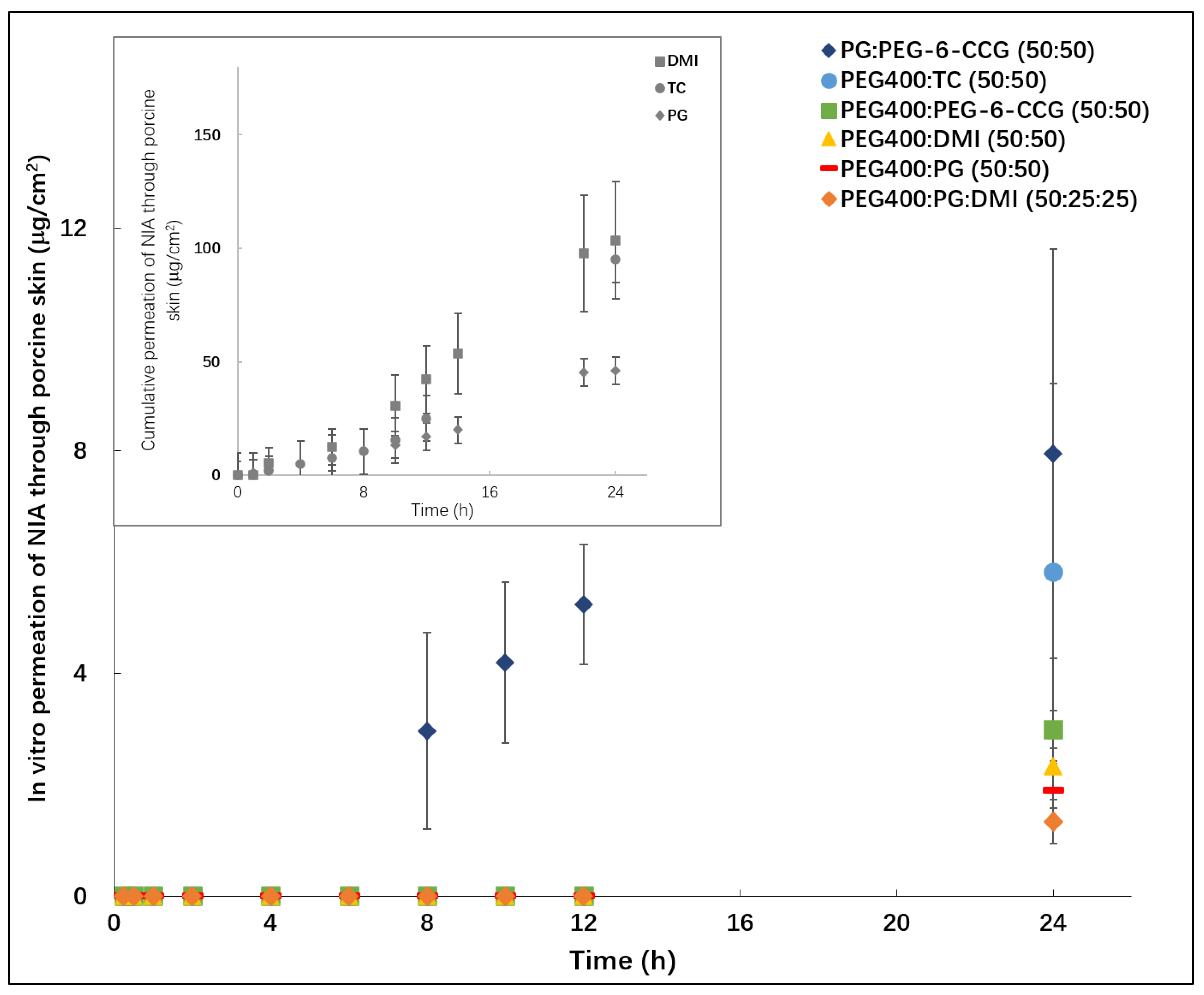
2.4. Permeation Studies and Mass Balance Studies
2.5. Statistical Analysis
3. Results and Discussion
3.1. Solubility Studies
3.2. DVS Studies
3.3. In Vitro Permeation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hadgraft, J. Skin, the final frontier. Int. J. Pharm. 2001, 224, 1–18. [Google Scholar] [CrossRef]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Grice, J.E.; Mohammed, Y.; Namjoshi, S.; Roberts, M.S. Topical and transdermal drug delivery: From simple potions to smart technologies. Curr. Drug Deliv. 2019, 16, 444–460. [Google Scholar] [CrossRef]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The structure and function of the stratum corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef]
- Jang, H.-J.; Shin, C.Y.; Kim, K.-B. Safety evaluation of polyethylene glycol (PEG) compounds for cosmetic use. Toxicol. Res. 2015, 31, 105–136. [Google Scholar] [CrossRef]
- Casiraghi, A.; Selmin, F.; Minghetti, P.; Cilurzo, F.; Montanari, L. Nonionic surfactants: Polyethylene glycol (peg) ethers and fatty acid esters as penetration enhancers. Percutaneous Penetration Enhanc. Chem. Methods Penetration Enhanc. 2015, 10, 251–271. [Google Scholar]
- Fruijtier-Pölloth, C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005, 214, 1–38. [Google Scholar] [CrossRef]
- Sarpotdar, P.P.; Gaskill, J.L.; Giannini, R.P. Effect of polyethylene glycol 400 on the penetration of drugs through human cadaver skin in vitro. J. Pharm. Sci. 1986, 75, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, D.; Crowther, J.M.; Matts, P.J.; Hadgraft, J.; Lane, M.E. Influence of niacinamide containing formulations on the molecular and biophysical properties of the stratum corneum. Int. J. Pharm. 2013, 441, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Comaish, J.S.; Felix, R.H.; McGrath, H. Topically applied niacinamide in isoniazid-induced pellagra. Arch. Dermatol. 1976, 112, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, T.; Minwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillebrand, G.; Bissett, D.; Boissy, R. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002, 147, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Shalita, A.R.; Smith, J.G.; Parish, L.C.; Sofman, M.S.; Chalker, D.K. Topical nicotinamide compared with clindamycin gel in the treatment of inflammatory acne vulgaris. Int. J. Dermatol. 1995, 34, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Damian, D.L.; Halliday, G.M.; Taylor, C.A.; Barnetson, R.S. Ultraviolet radiation induced suppression of Mantoux reactions in humans. J. Investig. Dermatol. 1998, 110, 824–827. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of propylene glycol, tripropylene glycol, and PPGs as used in cosmetics. Int. J. Toxicol. 2012, 31, 245S–260S. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.W.; Gad, S.C.; Julien, M. A review of the nonclinical safety of Transcutol®, a highly purified form of diethylene glycol monoethyl ether (DEGEE) used as a pharmaceutical excipient. Food Chem. Toxicol. 2014, 72, 40–50. [Google Scholar] [CrossRef]
- Osborne, D.W.; Musakhanian, J. Skin penetration and permeation properties of Transcutol®—Neat or diluted mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The importance of poly (ethylene glycol) alternatives for overcoming peg immunogenicity in drug delivery and bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef]
- Fiume, M.M.B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Safety assessment of pegylated alkyl glycerides as used in cosmetics. Int. J. Toxicol. 2020, 39, 26S–58S. [Google Scholar] [CrossRef]
- Zhang, Y.; Lane, M.E.; Hadgraft, J.; Heinrich, M.; Chen, T.; Lian, G.; Sinko, B. A comparison of the in vitro permeation of niacinamide in mammalian skin and in the parallel artificial membrane permeation assay (PAMPA) model. Int. J. Pharm. 2019, 556, 142–149. [Google Scholar] [CrossRef]
- Braon, T.L. Determination of solubility parmeter values for pure solvents and binary mixtures. Drug Dev. Ind. Pharm. 1980, 6, 87–98. [Google Scholar] [CrossRef]
- ICH. Validation of Analytical Procedures: Text and Methodology; ICH Harmonised Tripartite Guideline: Geneva, Switzerland, 2005; pp. 1–13. [Google Scholar]
- Iliopoulos, F.; Sil, B.C.; Monjur Al Hossain, A.S.M.; Moore, D.J.; Lucas, R.A.; Lane, M.E. Topical delivery of niacinamide: Influence of neat solvents. Int. J. Pharm. 2020, 579, 119137. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.C.; Callender, V.D. Postinflammatory hyperpigmentation: A review of the epidemiology, clinical features, and treatment options in skin of color. J. Clin. Aesthet. Dermatol. 2010, 3, 20–31. [Google Scholar] [PubMed]
- Haque, T.; ME, L.; BS, S.; Crowther, J.M.; Moore, D.J. In vitro permeation and disposition of niacinamide in silicone and porcine skin of skin barrier-mimetic formulations. Int. J. Pharm. 2017, 520, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kung, C.P.; Sil, B.C. Topical delivery of niacinamide: Influence of binary and ternary solvent systems. Pharmaceutics 2019, 11, 668. [Google Scholar] [CrossRef] [PubMed]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In vitro skin models as a tool in optimization of drug formulation. Eur. J. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef]
- OECD. Test No. 428: Skin Absorption: In Vitro Method; OECD Publishing: Paris, France, 2004. [Google Scholar]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Oxybutynin permeation in skin: The influence of drug and solvent activity. Int. J. Pharm. 2010, 384, 67–72. [Google Scholar] [CrossRef]
- Davies, D.J.; Ward, R.J.; Heylings, J.R. Multi-species assessment of electrical resistance as a skin integrity marker for in vitro percutaneous absorption studies. Toxicol. Vitr. 2004, 18, 351–358. [Google Scholar] [CrossRef]
- Zhang, G.; Bao, C.; Fu, K.; Lin, Y.; Li, T.; Yang, H. Synthesis, characterization, self-assembly, and irritation studies of polyglyceryl-10 caprylates. Polymers 2020, 12, 294. [Google Scholar] [CrossRef]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical delivery of anthramycin I. Influence of neat solvents. Eur. J. Pharm. Sci. 2017, 104, 188–195. [Google Scholar] [CrossRef]
- SCCS (The Scientific Committee on Concumer Safety). Opinion on Basic Criteria for the In Vitro Assessment of Dermal Absorption of Cosmetic Ingredients; SCCS: Brussels, Belgium, 2010. [Google Scholar]
- Hoelgaard, A.; Møllgaard, B.; Baker, E. Vehicle effect on topical drug delivery. IV. Effect of N-methylpyrrolidone and polar lipids on percutaneous drug transport. Int. J. Pharm. 1988, 43, 233–240. [Google Scholar] [CrossRef]
- Kung, C.-P.; Zhang, Y.; Sil, B.C.; Hadgraft, J.; Lane, M.E.; Patel, B.; McCulloch, R. Investigation of binary and ternary solvent systems for dermal delivery of methadone. Int. J. Pharm. 2020, 586, 119538. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.E.; Watkinson, A.C.; Green, D.M.; Hadgraft, J.; Brain, K. The relative effect of Azone® and Transcutol® on permeant diffusivity and solubility in human stratum corneum. Pharm. Res. 1996, 13, 542–546. [Google Scholar] [CrossRef] [PubMed]

| Solvent Systems (v/v) | Solubility Parameter (cal/cm3 )1/2 | Solubility of NIA ((%, g/100 mL) |
|---|---|---|
| PEG-6-CCG | - | 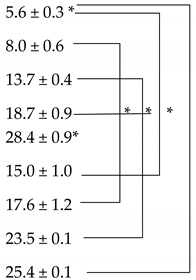 |
| DMI | 10.0 | |
| TC | 10.6 | |
| PEG 400 | 11.7 | |
| PG | 14.1 | |
| PEG 400:PEG-6-CCG (50:50) | - | |
| PEG 400:DMI (50:50) | 10.9 | |
| PEG 400:TC (50:50) | 11.2 | |
| PG:PEG-6-CCG (50:50) | - | |
| PEG 400:PG (50:50) | 12.8 | 25.6 ± 1.1 |
| PEG400:PG:DMI (50:25:25) | 11.9 | 21.9 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lane, M.E.; Moore, D.J. An Investigation of the Influence of PEG 400 and PEG-6-Caprylic/Capric Glycerides on Dermal Delivery of Niacinamide. Polymers 2020, 12, 2907. https://doi.org/10.3390/polym12122907
Zhang Y, Lane ME, Moore DJ. An Investigation of the Influence of PEG 400 and PEG-6-Caprylic/Capric Glycerides on Dermal Delivery of Niacinamide. Polymers. 2020; 12(12):2907. https://doi.org/10.3390/polym12122907
Chicago/Turabian StyleZhang, Yanling, Majella E. Lane, and David J. Moore. 2020. "An Investigation of the Influence of PEG 400 and PEG-6-Caprylic/Capric Glycerides on Dermal Delivery of Niacinamide" Polymers 12, no. 12: 2907. https://doi.org/10.3390/polym12122907
APA StyleZhang, Y., Lane, M. E., & Moore, D. J. (2020). An Investigation of the Influence of PEG 400 and PEG-6-Caprylic/Capric Glycerides on Dermal Delivery of Niacinamide. Polymers, 12(12), 2907. https://doi.org/10.3390/polym12122907