Impact of an Engineered Copper-Titanium Dioxide Nanocomposite and Parent Substrates on the Bacteria Viability, Antioxidant Enzymes and Fatty Acid Profiling
Abstract
1. Introduction
2. Results
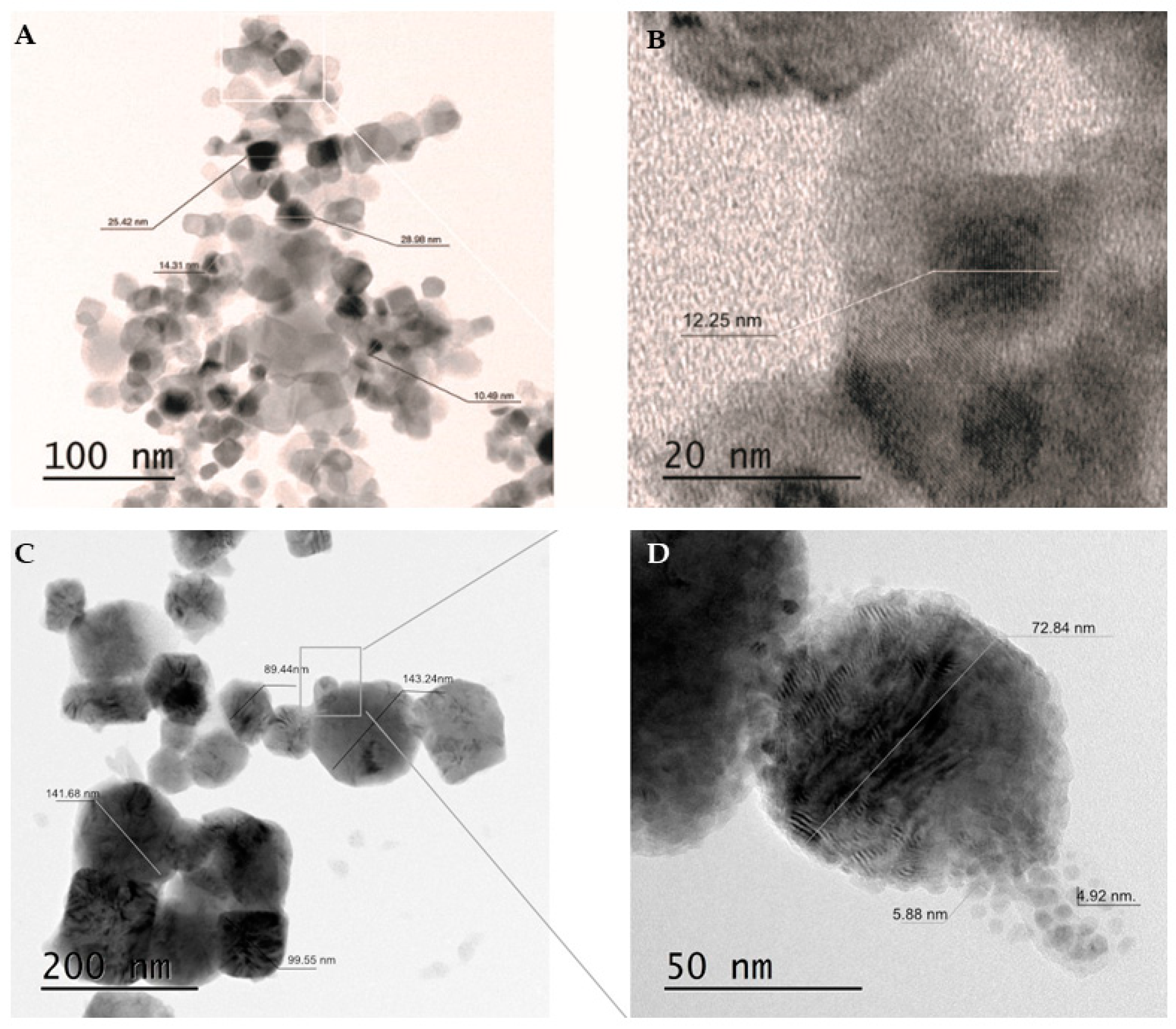
2.1. Size and Shape of the Synthesized Cu/TiO2-NC and Cu-NPs
2.2. Antibacterial Activity of Cu/TiO2-NC, Cu-NPs and TiO2-NPs
2.3. Impact of Cu/TiO2-NC, Cu-NPs and TiO2-NPs on the Activity of Catalase, Superoxide Dismutase and Peroxidase
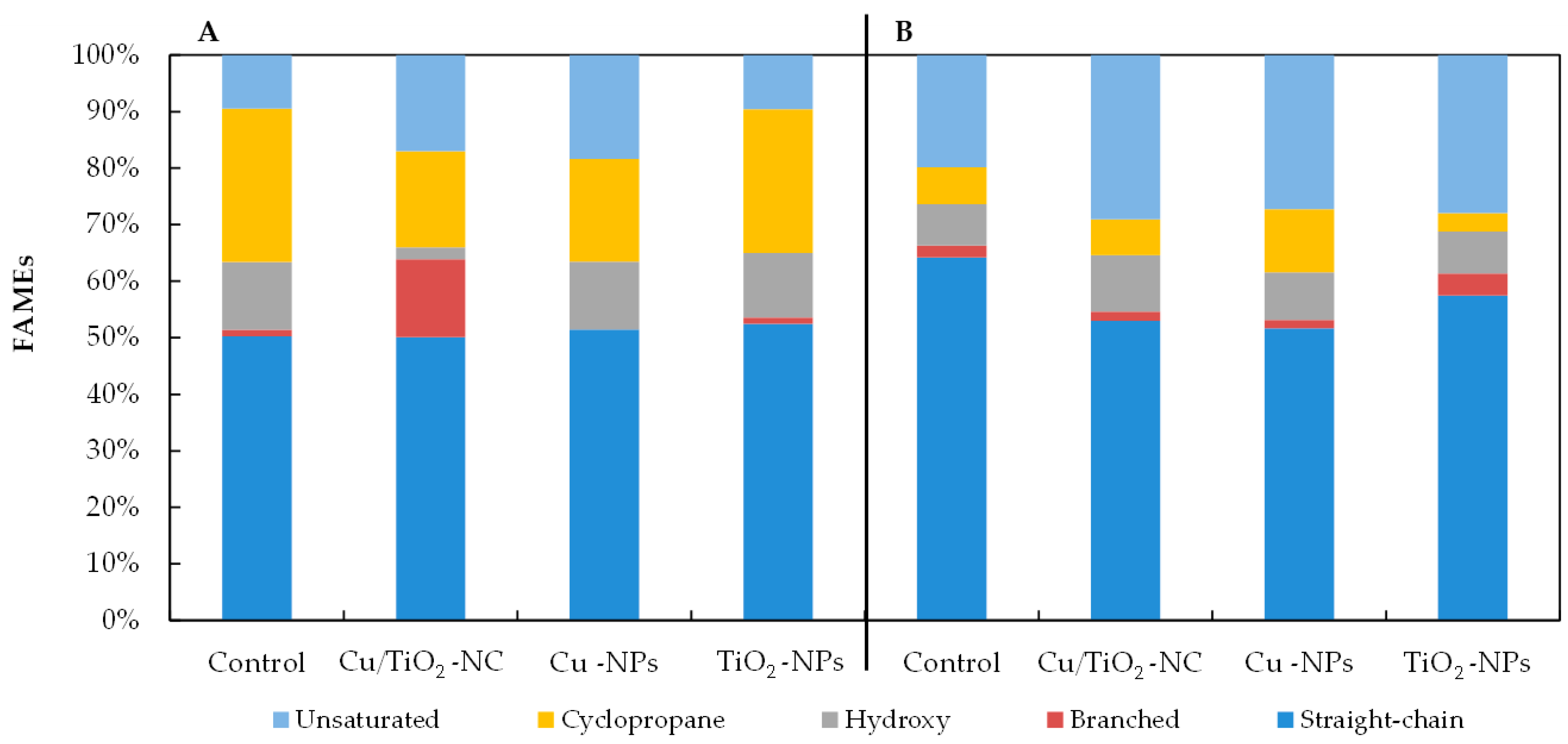
2.4. Effects of Cu/TiO2-NC, Cu-NPs and TiO2-NPs on the Bacterial Fatty acid Composition
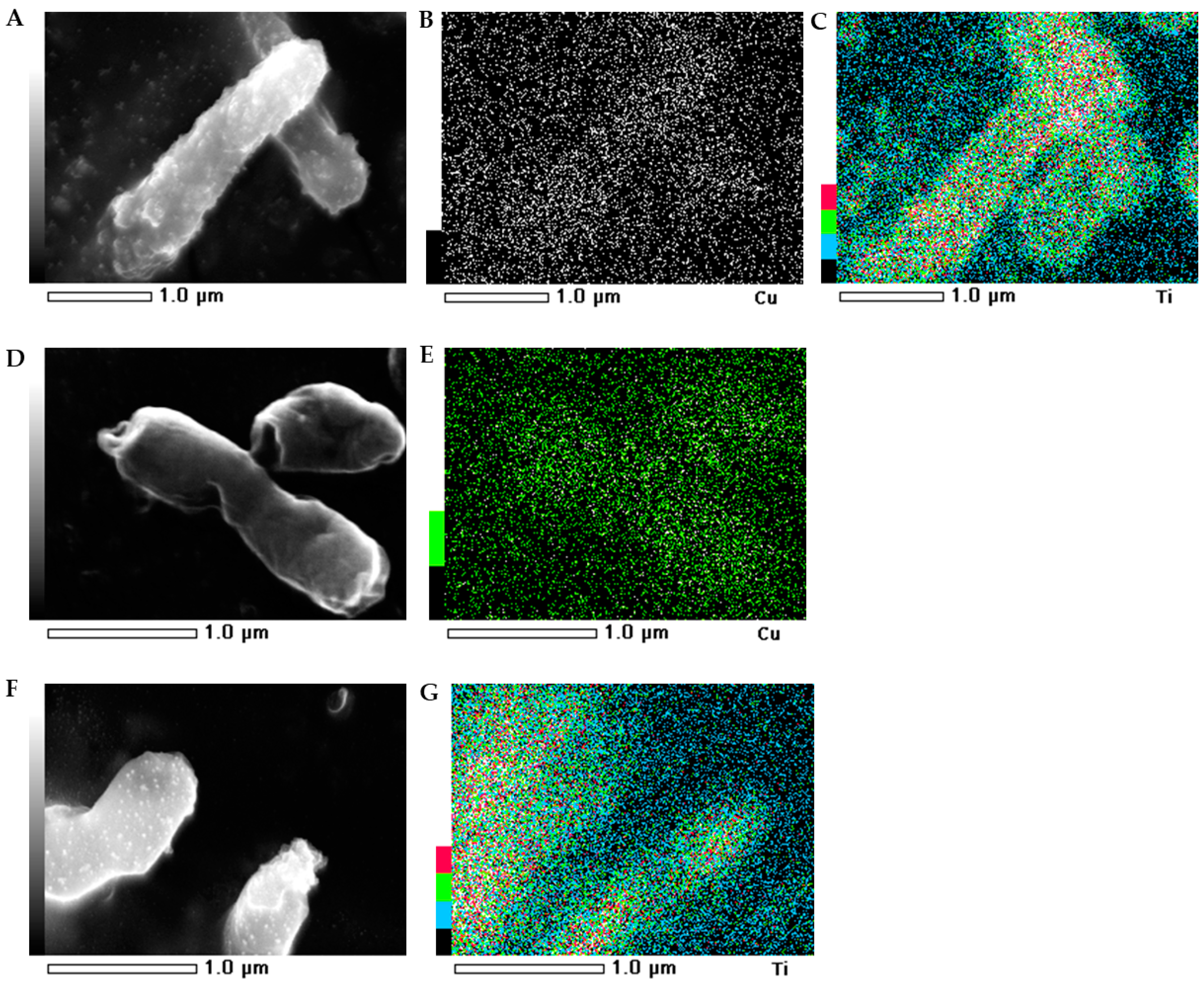
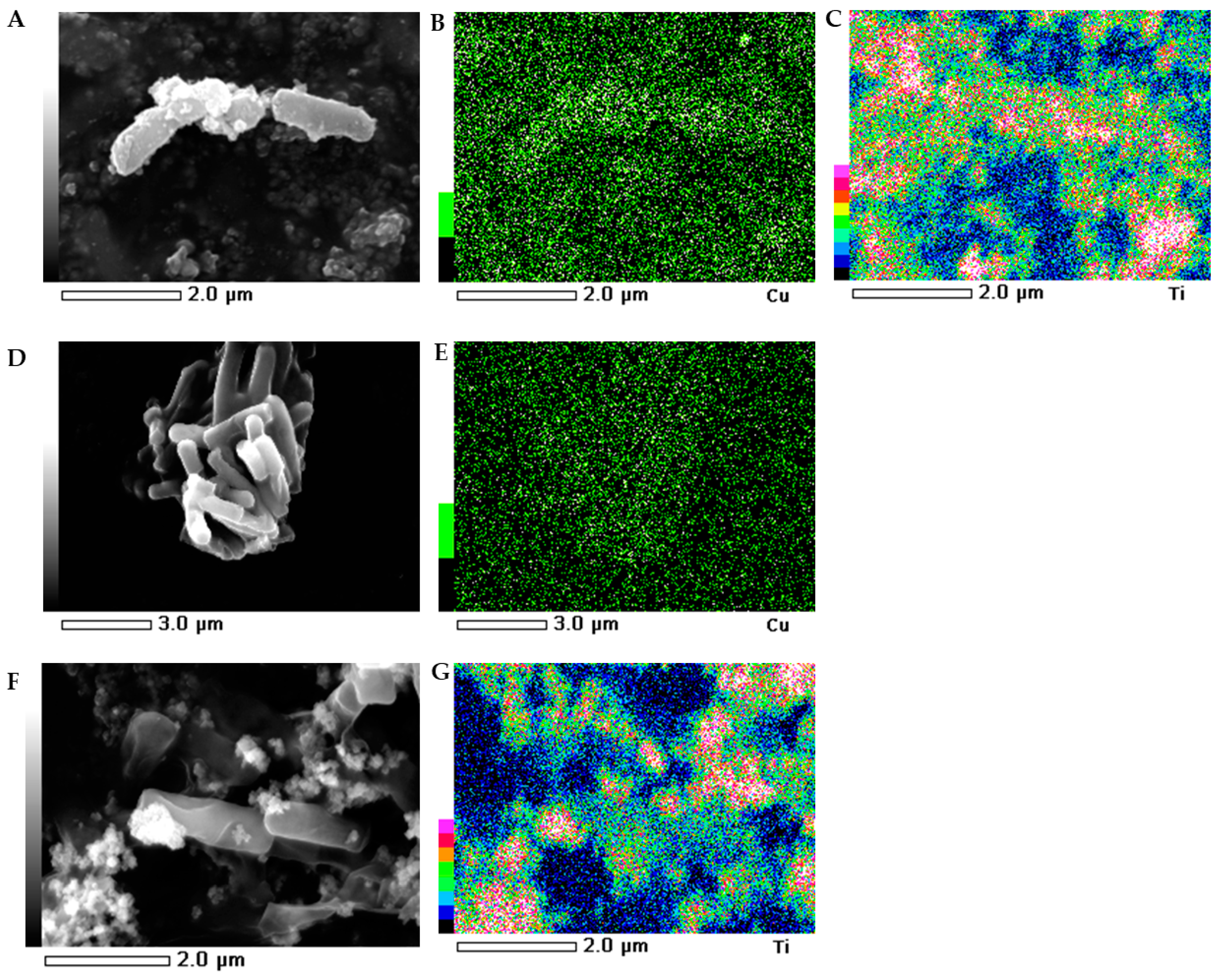
2.5. SEM-EDS Images
2.6. Microbial Assay for Risk Assessment (MARA)
3. Discussion
4. Materials and Methods
4.1. Synthesis of Cu-NPs and Cu/TiO2-NC
4.2. Determining Antibacterial Activity of NMs
4.3. Determining the Activity of Catalase, Peroxidase, Superoxide Dismutase and Dehydrogenases
4.4. Determining the Effect of NMs on the Bacterial Fatty acid Composition
4.5. Imaging of the NMs and Bacterial Cells Using Scanning Electron Microscopy
4.6. Microbial Assay for Risk Assessment (MARA)
4.7. Statistical Analysis
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NMs | Nanomaterials |
| Cu-NPs | Copper nanoparticles |
| TiO2-NPs | Titanium dioxide nanoparticles |
| Cu/TiO2-NC | Copper-titanium dioxide nanocomposite |
| MBC | Minimum bactericidal concentration |
| MTC | Microbial toxic concentration |
| MIC | Minimum inhibitory concentration |
| IC50 | Half maximal inhibitory concentration |
| CAT | Catalase |
| PER | Peroxidase |
| SOD | Superoxide dismutase |
| DEH | Dehydrogenases |
| FAME | Fatty acid methyl esters |
| ROS | Reactive oxygen species |
References
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotech. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, L.; Wang, C.; Zhang, S.; Liu, H.; Li, S.; Wang, X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019, 75, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Report Consultant. Available online: https://www.reportconsultant.com/reports/Global-Copper-Nanoparticle-Industry-Market-Analysis--Forecast-2018-2023-7587 (accessed on 22 June 2020).
- Transparency Market Research. Available online: https://www.transparencymarketresearch.com/copper-nanoparticles-market.html (accessed on 22 June 2020).
- Allied Market Research. Available online: https://www.alliedmarketresearch.com/nano-titanium-dioxide-market (accessed on 22 June 2020).
- Tanaka, A.; Nishino, Y.; Sakaguchi, S.; Yoshikawa, T.; Imamura, K.; Hashimoto, K.; Kominami, H. Functionalization of a plasmonic Au/TiO2 photocatalyst with an Ag co-catalyst for quantitative reduction of nitrobenzene to aniline in 2-propanol suspensions under irradiation of visible light. Chem. Commun. 2013, 49, 2551–2553. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.; Park, J.C.; Park, S.; Park, K.H. Rose-like Pd–Fe3O4 hybrid nanocomposite-supported Au nanocatalysts for tandem synthesis of 2-phenylindoles. Nanoscale 2015, 7, 8356–8360. [Google Scholar] [CrossRef]
- Etape, E.P.; Ngolui, L.J.; Foba-Tendo, J.; Yufanyi, D.M.; Namondo, B.V. Synthesis and characterizisation of CuO, TiO2 and CuO-TiO2 mixed oxide by a modified oxalate route. J. Appl. Chem. 2017, 2017, 4518654. [Google Scholar] [CrossRef]
- Peszke, J.; Dulski, M.; Nowak, A.; Balin, K.; Zubko, M.; Sułowicz, S.; Nowak, B.; Piotrowska-Seget, Z.; Talik, E.; Wojtyniak, M.; et al. Unique properties of silver and copper silica-based nanocomposites as antimicrobial agents. RSC Adv. 2017, 7, 28092–28104. [Google Scholar] [CrossRef]
- Markets and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/nanocomposites-market-141476334.html (accessed on 22 June 2020).
- Chen, S.; Guo, Y.; Zhong, H.; Chen, S.; Li, J.; Ge, Z.; Tang, J. Synergistic antibacterial mechanism and coating application of copper/titanium dioxide nanoparticles. Chem. Eng. J. 2014, 256, 238–246. [Google Scholar] [CrossRef]
- DeSario, P.A.; Pietron, J.J.; Brintlinger, T.H.; McEntee, M.; Parker, J.F.; Baturina, O.; Stroud, R.M.; Rolison, D.R. Oxidation-stable plasmonic copper nanoparticles in photocatalytic TiO2 nanoarchitectures. Nanoscale 2017, 9, 11720–11729. [Google Scholar] [CrossRef]
- Dong, J.; Ye, J.; Ariyanti, D.; Wang, Y.; Wei, S.; Gao, W. Enhancing photocatalytic activities of titanium dioxide via well-dispersed copper nanoparticles. Chemosphere 2018, 204, 193–201. [Google Scholar] [CrossRef]
- Thongpool, V.; Phunpueok, A.; Jaiyen, S.; Sornkwan, T. Synthesis and photocatalytic activity of copper and nitrogen co-doped titanium dioxide nanoparticles. Results Phys. 2020, 16, 102948. [Google Scholar] [CrossRef]
- Boyes, W.K.; Thornton, B.L.M.; Al-Abed, S.R.; Andersen, C.P.; Bouchard, D.C.; Burgess, R.M.; Hubal, E.A.C.; Ho, K.T.; Hughes, M.F.; Kitchin, K.; et al. A comprehensive framework for evaluating the environmental health and safety implications of engineered nanomaterials. Crit. Rev. Toxicol. 2017, 47, 771–814. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies and future prospects. Environ. Sci. Pollut. Res. Int. 2015, 22, 4122–4143. [Google Scholar] [CrossRef]
- Chowdhuri, A.R.; Tripathy, S.; Chandra, S.; Roy, S.; Sahu, S.K. A ZnO decorated chitosan–graphene oxide nanocomposite shows significantly enhanced antimicrobial activity with ROS generation. RSC Adv. 2015, 5, 49420–49428. [Google Scholar] [CrossRef]
- Jain, N.; Bhargava, A.; Rathi, M.; Dilip, R.V.; Panwar, J. Removal of protein capping enhances the antibacterial efficiency of biosynthesized silver nanoparticles. PLoS ONE 2015, 10, e0134337. [Google Scholar] [CrossRef]
- Borkowski, A.; Cłapa, T.; Szala, M.; Gąsiński, A.; Selwet, M. Synthesis of SiC/Ag/cellulose nanocomposite and its antibacterial activity by reactive oxygen species generation. Nanomaterials 2016, 6, 171. [Google Scholar] [CrossRef]
- Karimi, F.; Dabbagh, S.; Alizadeh, S.; Rostamnia, S. Evaluation of AgClNPs@SBA-15/IL nanoparticle-induced oxidative stress and DNA mutation in Escherichia coli. Appl. Microbiol. Biotechnol. 2016, 100, 7161–7170. [Google Scholar] [CrossRef]
- Zhang, X.F.; Shen, W.; Gurunathan, S. Biologically synthesized gold nanoparticles ameliorate cold and heat stress-induced oxidative stress in Escherichia coli. Molecules 2016, 21, 731. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Pan, X.; Zhu, F.; Jiang, C.; Liu, Q.; Cheng, Z.; Dai, G.; Wu, G.; Wang, L.; et al. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int. J. Nanomed. 2019, 14, 1469–1487. [Google Scholar] [CrossRef]
- Borkowski, A.; Szala, M.; Kowalczyk, P.; Cłapa, T.; Narożna, D.; Selwet, M. Oxidative stress in bacteria (Pseudomonas putida) exposed to nanostructures of silicon carbide. Chemosphere 2015, 135, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, B.; Kar, S.; Dey, S.K.; Bhandary, S.; Roy, D.; Mukhopadhyay, T.K.; Das, S.; Nandy, P. In situ synthesis and antibacterial activity of copper nanoparticle loaded natural montmorillonite clay based on contact inhibition and ion release. Colloids Surf. B Biointerfaces 2013, 108, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Kaweeteerawat, C.; Chang, C.H.; Roy, K.R.; Liu, R.; Li, R.; Toso, D.; Fischer, H.; Ivask, A.; Ji, Z.; Zink, J.I.; et al. Cu nanoparticles have different impacts in Escherichia coli and Lactobacillus brevis than their microsized and ionic analogues. ASC Nano 2015, 9, 7215–7225. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Binh, C.T.T.; Kelly, J.J.; Gaillard, J.F.; Gray, K.A. Cytotoxicity of commercial nano-TiO2 to Escherichia coli assessed by high-throughput screening: Effects of environmental factors. Water Res. 2013, 47, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Suppi, S.; Kasemets, K.; Ivask, A.; Künnis-Beres, K.; Sihtmäe, M.; Kurvet, I.; Aruoja, V.; Kahru, A. A novel method for comparison of biocidal properties of nanomaterials to bacteria, yeasts and algae. J. Hazard. Mater. 2015, 286, 75–84. [Google Scholar] [CrossRef]
- Bonnet, M.; Massard, C.; Veisseire, P.; Camares, O.; Awitor, K.O. Environmental toxicity and antimicrobial efficiency of titanium dioxide nanoparticles in suspension. J. Biomater. Nanobiotechnol. 2015, 6, 213–224. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria—“A battle of the titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microb. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Sheline, C.T.; Choi, D.W. Cu2+ toxicity inhibition of mitochondrial dehydrogenases in vitro and in vivo. Ann. Neurol. 2004, 55, 645–653. [Google Scholar] [CrossRef]
- Yuan, Y.G.; Peng, Q.L.; Gurunathan, S. Effects of silver nanoparticles on multiple drug-resistant strains of Staphylococcus aureus and Pseudomonas aeruginosa from mastitis-infected goats: An alternative approach for antimicrobial therapy. Int. J. Mol. Sci. 2017, 18, 569. [Google Scholar] [CrossRef] [PubMed]
- Borkowski, A.; Syczewski, M.; Czarnecka-Skwarek, A. Ionic liquids strongly affect the interaction of bacteria with magnesium oxide and silica nanoparticles. RSC Adv. 2019, 9, 28724–28734. [Google Scholar] [CrossRef]
- De Dicastillo, C.L.; Correa, M.G.; Martínez, F.B.; Streitt, C.; Galotto, M.J. Antimicrobial effect of titanium dioxide nanoparticles. In Antimicrobial Resistance; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, X.; Xia, N.; Zhang, S.; Guo, X. Modification of fatty acids in membranes of bacteria: Implication for an adaptive mechanism to the toxicity of carbon nanotubes. Environ. Sci. Technol. 2014, 48, 4086–4095. [Google Scholar] [CrossRef] [PubMed]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Shah, V.; Walker, V.K. Perturbation of an arctic soil microbial community by metal nanoparticles. J. Hazard. Mater. 2011, 190, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Belozerova, I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009, 197, 143–148. [Google Scholar] [CrossRef]
- Bao, H.; Yu, X.; Xu, C.; Li, X.; Li, Z.; Wei, D.; Liu, Y. New toxicity mechanism of silver nanoparticles: Promoting apoptosis and inhibiting proliferation. PLoS ONE 2015, 10, e0122535. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO2 nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic. Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef]
- Leung, Y.H.; Xu, X.; Ma, A.P.Y.; Liu, F.; Ng, A.M.C.; Shen, Z.; Gethings, L.A.; Guo, M.Y.; Djurišić, A.B.; Lee, P.K.H.; et al. Toxicity of ZnO and TiO2 to Escherichia coli cells. Sci. Rep. 2016, 6, 35243. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Zhao, J.; Urmila, K. Enhancing the antimicrobial activity of natural extraction using the synthetic ultrasmall metal nanoparticles. Sci. Rep. 2015, 5, 11033. [Google Scholar] [CrossRef]
- Blaise, C.; Gagné, F.; Férard, J.F.; Eullaffroy, P. Ecotoxicity of selected nano-materials to aquatic organisms. Environ. Toxicol. 2008, 23, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Monteiro, R.T.R.; Blaise, C.; Gagné, F.; Bull, K.; Férard, J.F. Influence of sediment grain size on elutriate toxicity of inorganic nanomaterials. Water Qual. Res. J. Can. 2009, 44, 201–210. [Google Scholar] [CrossRef]
- Nowak, A.; Szade, J.; Talik, E.; Ratuszna, A.; Ostafin, M.; Peszke, J. Structural, spectroscopic and biological investigation of copper oxides nanoparticles with various capping agents. Mater. Chem. Phys. 2014, 145, 465–470. [Google Scholar] [CrossRef]
- Dulski, M.; Dudek, K.; Podwórny, J.; Sułowicz, S.; Piotrowska-Seget, Z.; Malarz, K.; Mrozek-Wilczkiewicz, A.; Wolnica, K.; Matus, K.; Peszke, J.; et al. Impact of temperature on the physicochemical, structural and biological features of copper-silica nanocomposites. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110274. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Szade, J.; Talik, E.; Zubko, M.; Wasilkowski, D.; Dulski, M.; Balin, K.; Mrozik, A.; Peszke, J. Physicochemical and antibacterial characterization of ionocity Ag/Cu powder nanoparticles. Matter. Charact. 2016, 117, 9–16. [Google Scholar] [CrossRef]
- Hegeman, G.D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida I. Synthesis of enzymes by the wild type. J. Bacteriol. 1966, 91, 1140–1154. [Google Scholar] [CrossRef]
- Banerjee, G.; Pandey, S.; Ray, A.K.; Kumar, R. Bioremediation of heavy metals by a novel bacterial strain Enterobacter cloacae and its antioxidant enzyme activity, flocculant production and protein expression in presence of lead, cadmium and nickel. Water Air Soil Pollut. 2015, 226, 91. [Google Scholar] [CrossRef]
- Muniswamy, D.; Krishna, P.M.; Sangeetha, J. Elucidation of impact of heavy metal pollution on soil bacterial growth and extracellular polymeric substances flexibility. 3 Biotech. 2016, 6, 172. [Google Scholar] [CrossRef]
- Zhang, C.; Bruins, M.E.; Yang, Z.Q.; Liu, S.T.; Rao, P.F. A new formula to calculate activity of superoxide dismutase in indirect assays. Anal. Biochem. 2016, 503, 65–67. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nweke, C.O.; Alisi, C.S.; Okolo, J.C.; Nwanyanwu, C.E. Toxicity of zinc to heterotrophic bacteria from a tropical river sediment. Appl. Ecol. Env. Res. 2007, 5, 123–132. [Google Scholar] [CrossRef]
- Sasser, M. Bacterial Identification by Gas Chromatographic Analysis of Fatty Acid Methyl Esters (GC-FAME); Technical Note #101; MIDI: Newark, DE, USA, 1990; revised 2006. [Google Scholar]
- Karcz, J.; Woźnica, A.; Binkowski, M.; Klonowska-Olejnik, M.; Bernas, T.; Karczewski, J.; Migula, P. SEM-EDS and X-ray micro computed tomography studies of skeletal surface pattern and body structure in the freshwater sponge Spongilla lacustris collected from Goczalkowice reservoir habit (Southern Poland). Folia Histochem. Cytobiol. 2015, 53, 88–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hazrin-Chong, N.H.; Manefield, M. An alternative SEM drying method using hexamethyldisilazane (HMDS) for microbial cell attachment studies on sub-bituminous coal. J. Microbiol. Methods 2012, 90, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Bronowska, M.; Stęborowski, R.; Bystrzejewska-Piotrowska, G. Estimation of the acute cesium toxicity by the microbial assay for risk assessment (MARA) test. Nukleonika 2013, 58, 481–485. [Google Scholar]
- Fai, P.B.; Grant, A. An assessment of the potential of the microbial assay for risk assessment (MARA) for ecotoxicological testing. Ecotoxicology 2010, 19, 1626–1633. [Google Scholar] [CrossRef] [PubMed]


| Type of Nanomaterials | Escherichia coli ATCC 25922 | Bacillus subtilis ATCC 6633 | ||||
|---|---|---|---|---|---|---|
| MIC | MBC | IC50 | MIC | MBC | IC50 | |
| Cu/TiO2-NC | 500 | 500 | 100.61 | 525 | 575 | 464.22 |
| Cu-NPs | 575 | 600 | 506.35 | 40 | 50 | 4.84 |
| TiO2-NPs | 500 | 500 | 102.16 | 575 | 1000 | 95.83 |
| Type of Nanomaterial | Strain | MTCav. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (G+) | 2 (G-) | 3 (G-) | 4 (G-) | 5 (G+) | 6 (G-) | 7 (G+) | 8 (G+) | 9 (G-) | 10 (G-) | 11 | ||
| Cu/TiO2-NC | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | 973 | >1000 | >1000 | >1000 | >1000 | >1000 |
| Cu-NPs | 555 | >1000 | 986 | 683 | 747 | 423 | 463 | 376 | 790 | 97 | >1000 | 559 |
| TiO2-NPs | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metryka, O.; Wasilkowski, D.; Nowak, A.; Adamczyk-Habrajska, M.; Mrozik, A. Impact of an Engineered Copper-Titanium Dioxide Nanocomposite and Parent Substrates on the Bacteria Viability, Antioxidant Enzymes and Fatty Acid Profiling. Int. J. Mol. Sci. 2020, 21, 9089. https://doi.org/10.3390/ijms21239089
Metryka O, Wasilkowski D, Nowak A, Adamczyk-Habrajska M, Mrozik A. Impact of an Engineered Copper-Titanium Dioxide Nanocomposite and Parent Substrates on the Bacteria Viability, Antioxidant Enzymes and Fatty Acid Profiling. International Journal of Molecular Sciences. 2020; 21(23):9089. https://doi.org/10.3390/ijms21239089
Chicago/Turabian StyleMetryka, Oliwia, Daniel Wasilkowski, Anna Nowak, Małgorzata Adamczyk-Habrajska, and Agnieszka Mrozik. 2020. "Impact of an Engineered Copper-Titanium Dioxide Nanocomposite and Parent Substrates on the Bacteria Viability, Antioxidant Enzymes and Fatty Acid Profiling" International Journal of Molecular Sciences 21, no. 23: 9089. https://doi.org/10.3390/ijms21239089
APA StyleMetryka, O., Wasilkowski, D., Nowak, A., Adamczyk-Habrajska, M., & Mrozik, A. (2020). Impact of an Engineered Copper-Titanium Dioxide Nanocomposite and Parent Substrates on the Bacteria Viability, Antioxidant Enzymes and Fatty Acid Profiling. International Journal of Molecular Sciences, 21(23), 9089. https://doi.org/10.3390/ijms21239089






