Teaghrelin Protects SH-SY5Y Cells against MPP+-Induced Neurotoxicity through Activation of AMPK/SIRT1/PGC-1α and ERK1/2 Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Teaghrelin
2.3. Cell Culture and Differentiation
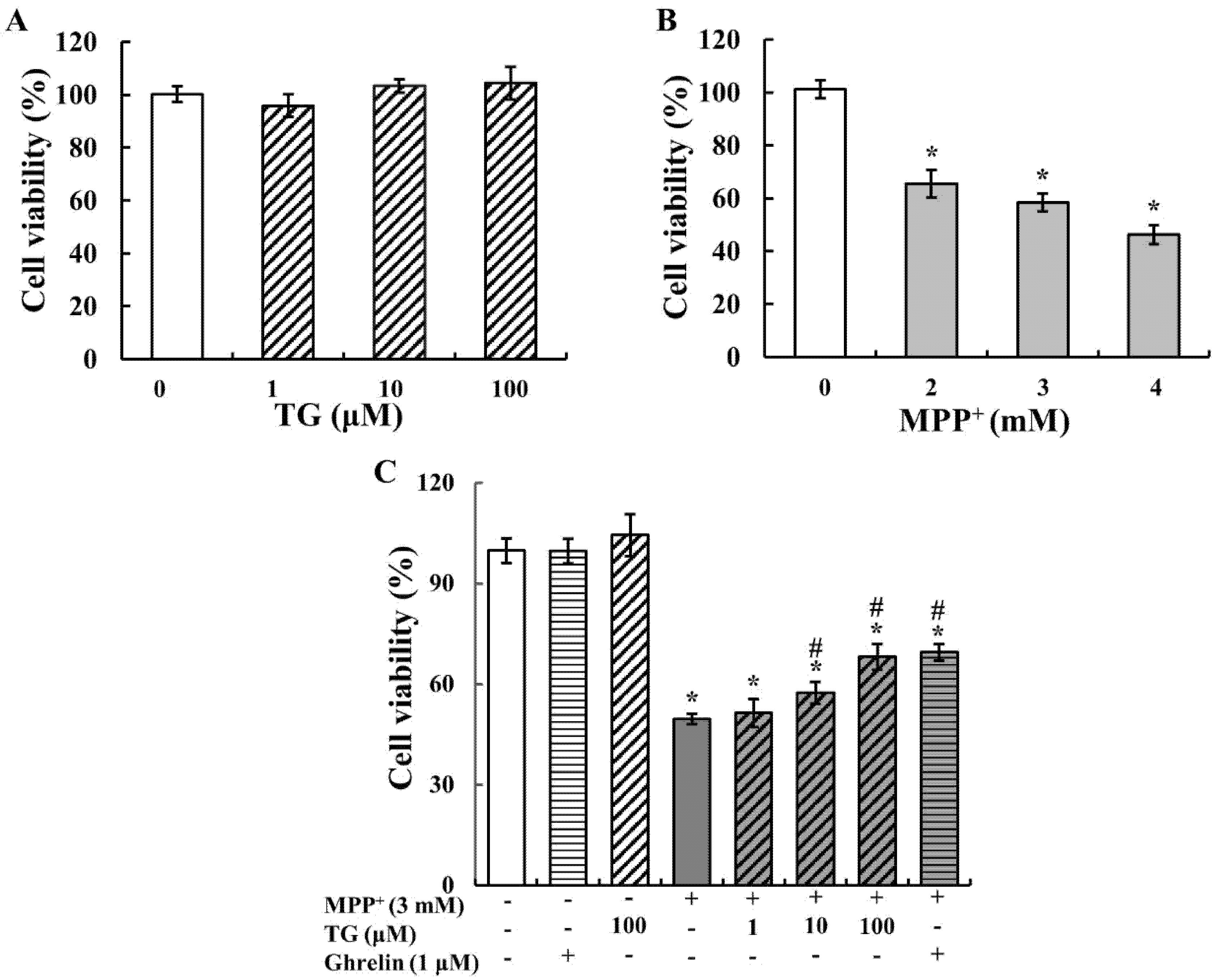
2.4. Cell Viability Assay
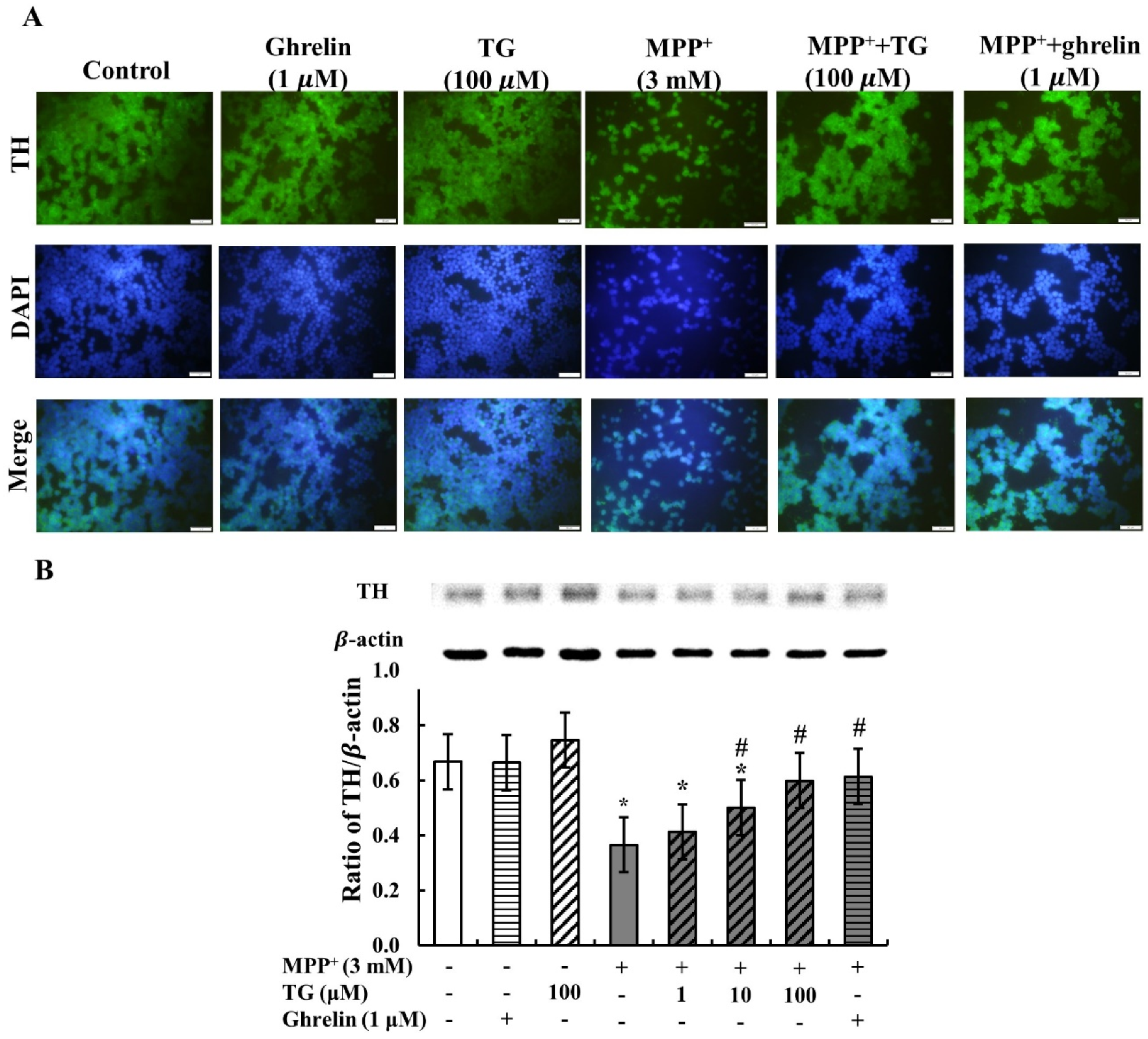
2.5. Immunofluorescence Staining
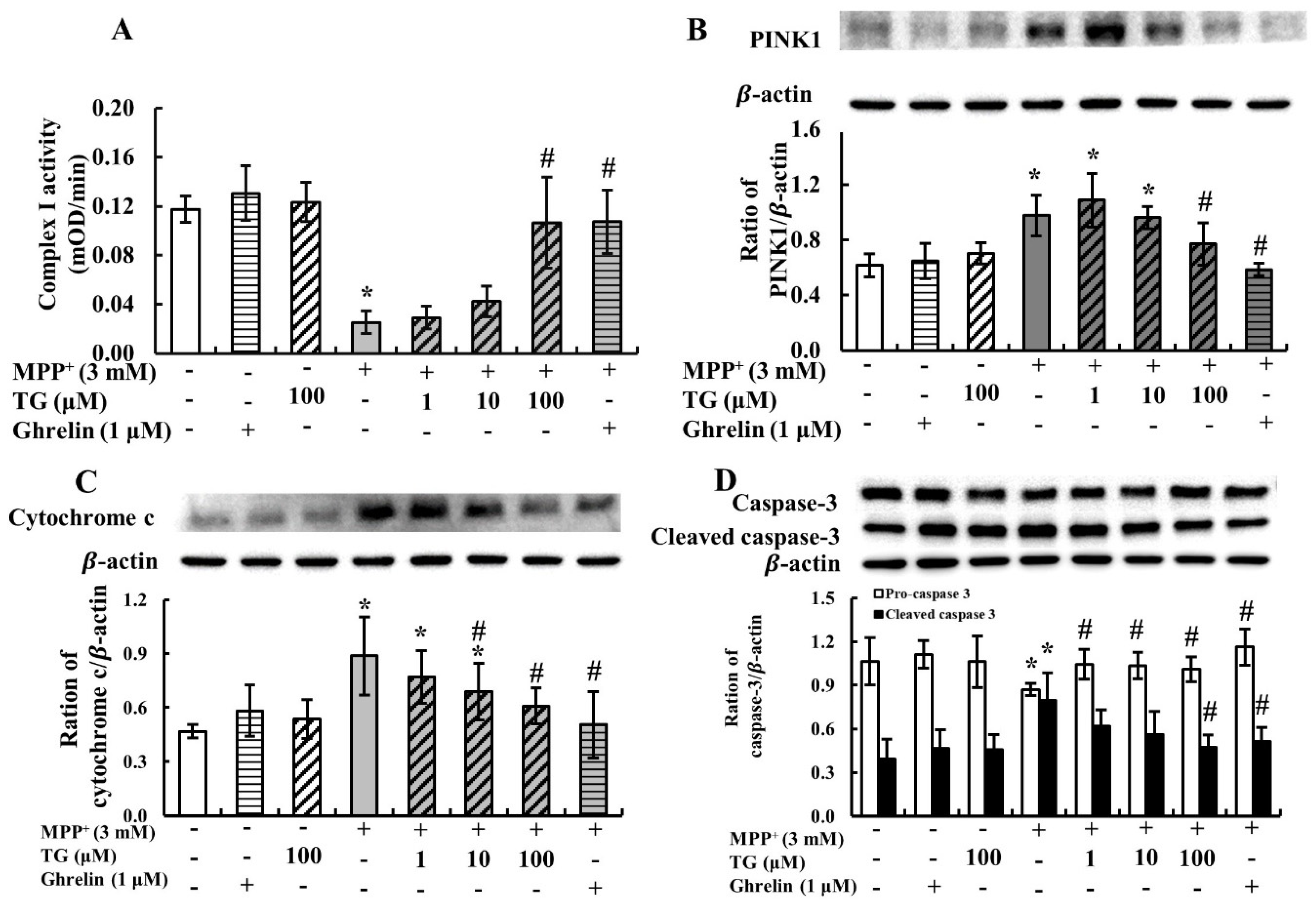
2.6. Measurement of Mitochondrial Complex I Activity
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
3.1. Teaghrelin Attenuated MPP+-Induced Cytotoxicity
3.2. Teaghrelin Alleviated Mitochondrial Dysfunction and Apoptosis in the MPP+-Induced SH-SY5Y Cell Model of PD
3.3. Teaghrelin Attenuated MPP+-Induced Loss of Tyrosine Hydroxylase Expression in SH-SY5Y Cells
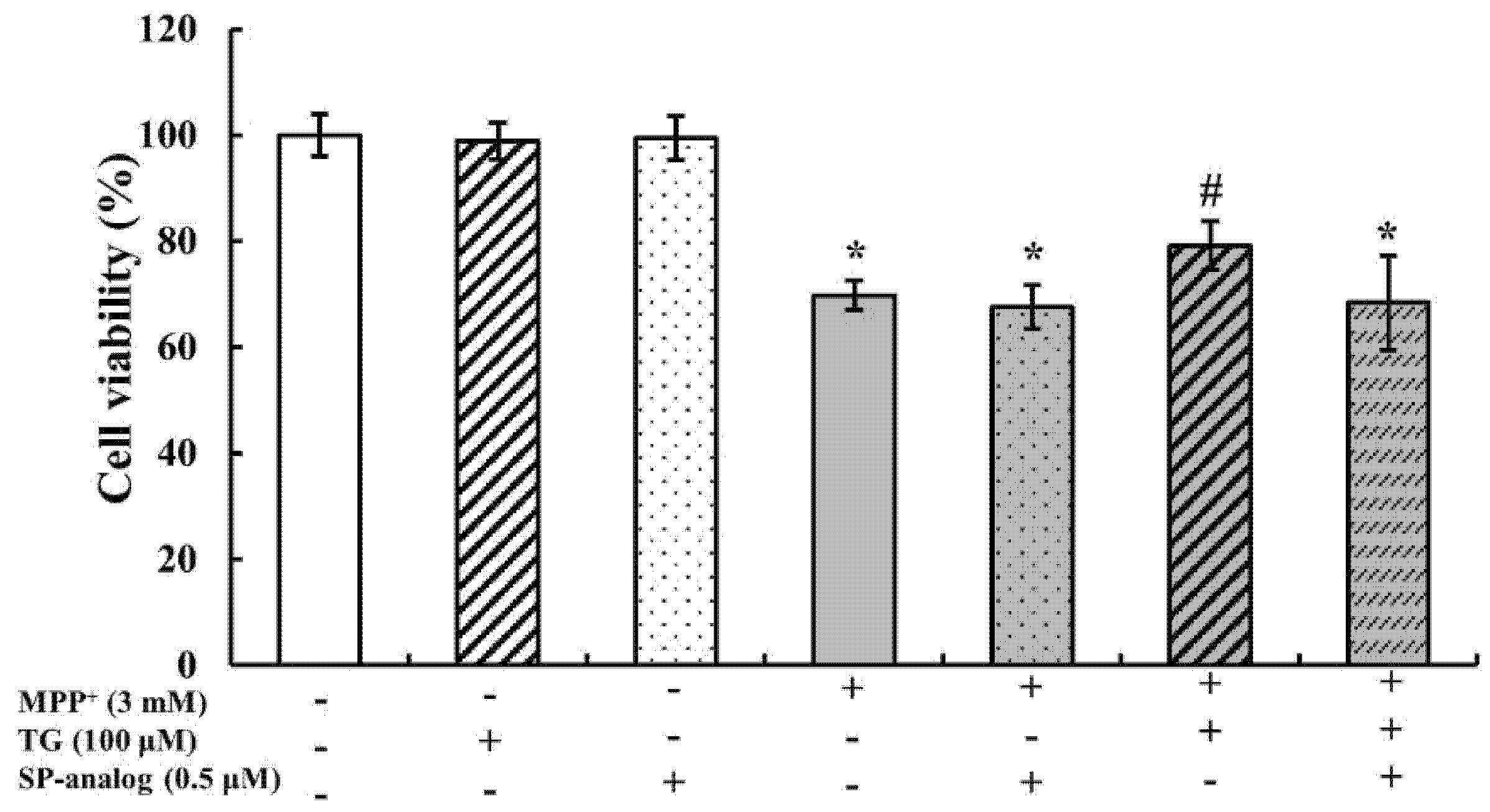
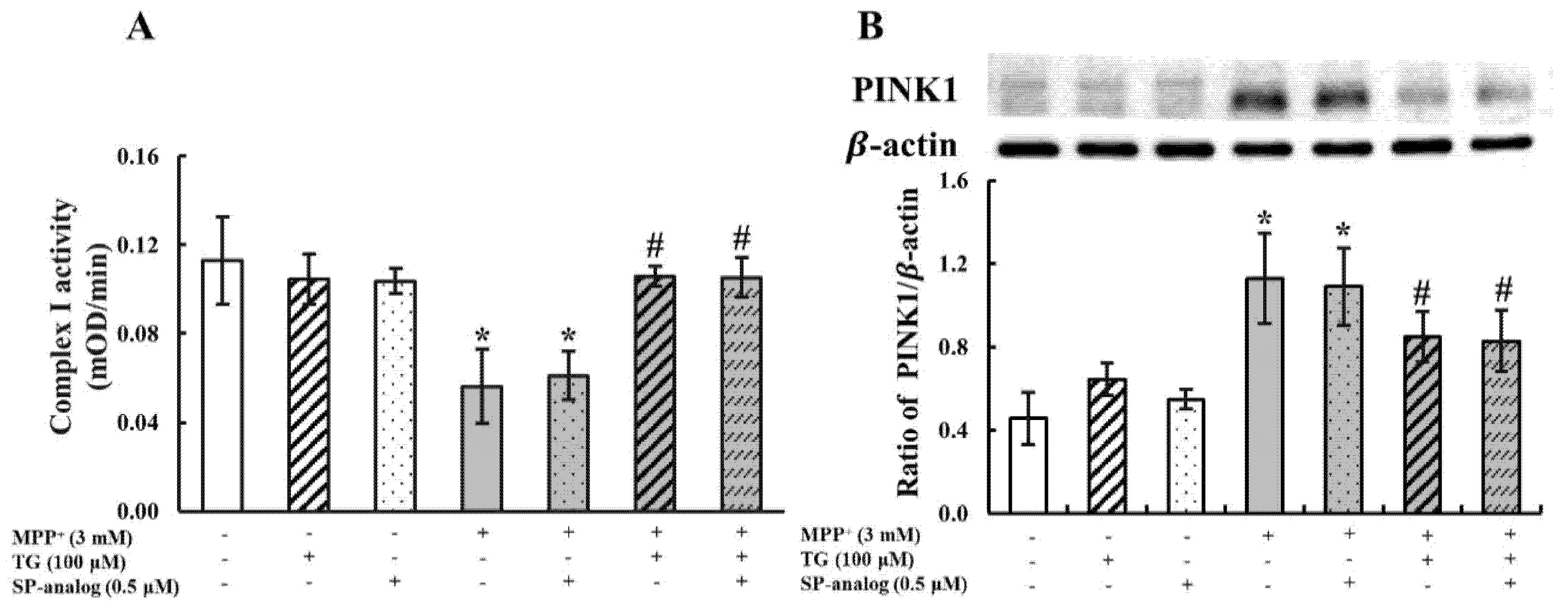
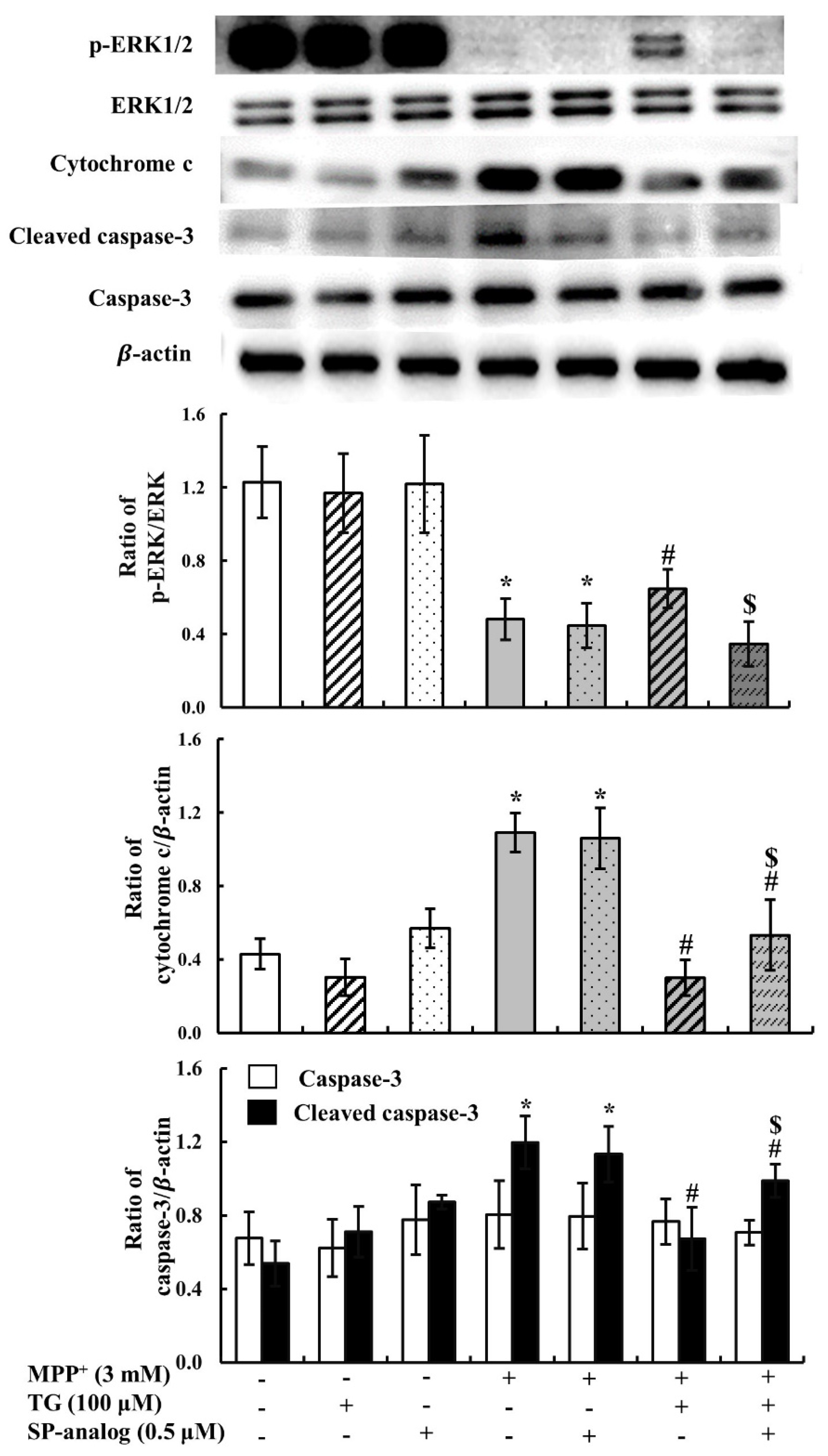
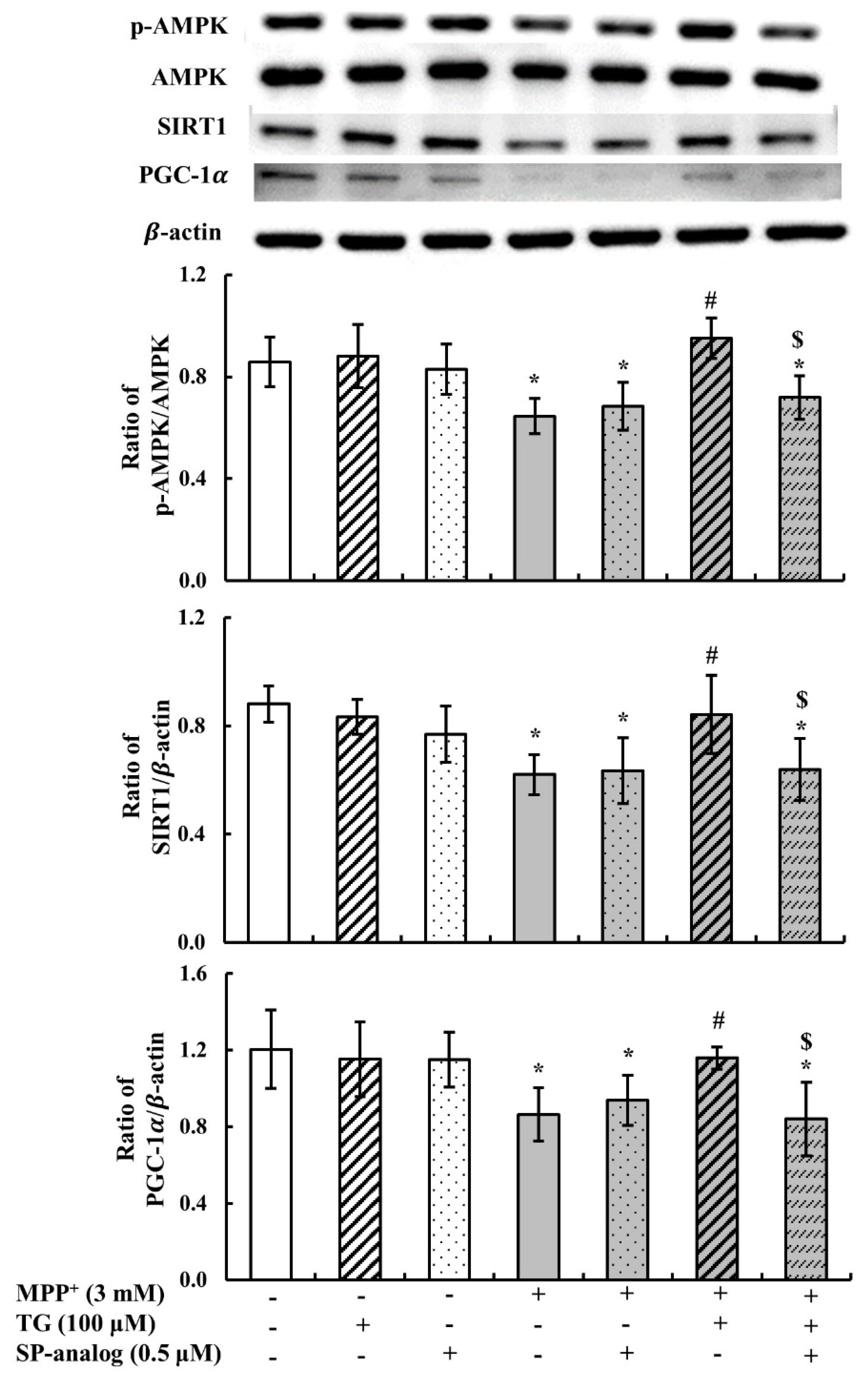
3.4. Substance P Attenuated the Protective Effect of Teaghrelin on MPP+-Induced Neurotoxicity in SH-SY5Y Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef]
- Alves, G.; Forsaa, E.B.; Pedersen, K.F.; Dreetz Gjerstad, M.; Larsen, J.P. Epidemiology of Parkinson’s disease. J. Neurol. 2008, 255, 18–32. [Google Scholar] [CrossRef]
- Thanvi, B.R.; Lo, T.C.N. Long term motor complications of levodopa: Clinical features, mechanisms, and management strategies. Postgrad. Med. J. 2004, 80, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.E.; Hess, C.W.; Fox, S.H. Motor Complications of Dopaminergic Medications in Parkinson’s Disease. Semin. Neurol. 2017, 37, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.M.; Yu, H.; Palyha, O.C.; McKee, K.K.; Feighner, S.D.; Sirinathsinghji, D.J.; Smith, R.G.; Van der Ploeg, L.H.; Howard, A.D. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res. Mol. Brain Res. 1997, 48, 23–29. [Google Scholar] [CrossRef]
- Zigman, J.M.; Jones, J.E.; Lee, C.E.; Saper, C.B.; Elmquist, J.K. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J. Comp. Neurol. 2006, 494, 528–548. [Google Scholar] [CrossRef]
- Shi, L.; Du, X.; Jiang, H.; Xie, J. Ghrelin and Neurodegenerative Disorders-a Review. Mol. Neurobiol. 2017, 54, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Collden, G.; Tschop, M.H.; Muller, T.D. Therapeutic Potential of Targeting the Ghrelin Pathway. Int. J. Mol. Sci. 2017, 18, 798. [Google Scholar] [CrossRef]
- Minalyan, A.; Gabrielyan, L.; Pietra, C.; Taché, Y.; Wang, L. Multiple Beneficial Effects of Ghrelin Agonist, HM01 on Homeostasis Alterations in 6-Hydroxydopamine Model of Parkinson’s Disease in Male Rats. Front. Integr. Neurosci. 2019, 13, 13. [Google Scholar] [CrossRef]
- Rhodes, L.; Zollers, B.; Wofford, J.A.; Heinen, E. Capromorelin: A ghrelin receptor agonist and novel therapy for stimulation of appetite in dogs. Vet. Med. Sci. 2017, 4, 3–16. [Google Scholar] [CrossRef]
- Dou, Q.P. Tea in Health and Disease. Nutrients 2019, 11, 929. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Romero-Garcia, R.; Suckling, J.; Feng, L. Habitual tea drinking modulates brain efficiency: Evidence from brain connectivity evaluation. Aging 2019, 11, 3876–3890. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.H.; Chen, Y.J.; Chang, C.I.; Lin, Y.W.; Chen, C.Y.; Lee, M.R.; Lee, V.S.; Tzen, J.T. Teaghrelins, unique acylated flavonoid tetraglycosides in Chin-shin oolong tea, are putative oral agonists of the ghrelin receptor. J. Agric. Food Chem. 2014, 62, 5085–5091. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.K.; Lin, H.Y.; Chen, C.J.; Jhuo, C.F.; Liao, K.Y.; Chen, W.Y.; Tzen, J.T.C. Promotion of myotube differentiation and attenuation of muscle atrophy in murine C2C12 myoblast cells treated with teaghrelin. Chem. Biol. Interact. 2019, 315, 108893. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.K.; Lo, Y.H.; Wu, C.C.; Chung, T.Y.; Tzen, J.T.C. Identification of biosynthetic intermediates of teaghrelins and teaghrelin-like compounds in oolong teas, and their molecular docking to the ghrelin receptor. J. Food Drug Anal. 2015, 23, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.H.; Rees, D.J.; Andrews, Z.B.; Davies, J.S. Ghrelin mediated neuroprotection—A possible therapy for Parkinson’s disease? Neuropharmacology 2018, 136, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, J.A.; Andrews, Z.B. Ghrelin is neuroprotective in Parkinson’s disease: Molecular mechanisms of metabolic neuroprotection. Ther. Adv. Endocrinol. Metab. 2013, 4, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Wu, C.J.; Lin, Y.C.; Wu, R.H.; Chen, W.Y.; Kuo, P.C.; Tzen, J.T.C. Identification of two teaghrelins in Shy-jih-chuen oolong tea. J. Food Biochem. 2019, 43, e12810. [Google Scholar] [CrossRef]
- Cecarini, V.; Bonfili, L.; Cuccioloni, M.; Keller, J.N.; Bruce-Keller, A.J.; Eleuteri, A.M. Effects of Ghrelin on the Proteolytic Pathways of Alzheimer’s Disease Neuronal Cells. Mol. Neurobiol. 2016, 53, 3168–3178. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Wu, L.; Wang, J.-L.; Yang, J.-D.; Zhang, J.; Zhang, J.; Li, L.-H.; Xia, Y.; Yao, L.-B.; Qin, H.-Z.; et al. Astragaloside IV prevents MPP⁺-induced SH-SY5Y cell death via the inhibition of Bax-mediated pathways and ROS production. Mol. Cell. Biochem. 2012, 364, 209–216. [Google Scholar] [CrossRef]
- Popelová, A.; Kákonová, A.; Hrubá, L.; Kuneš, J.; Maletínská, L.; Železná, B. Potential neuroprotective and anti-apoptotic properties of a long-lasting stable analog of ghrelin: An in vitro study using SH-SY5Y cells. Physiol. Res. 2018, 67, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Korecka, J.A.; van Kesteren, R.E.; Blaas, E.; Spitzer, S.O.; Kamstra, J.H.; Smit, A.B.; Swaab, D.F.; Verhaagen, J.; Bossers, K. Phenotypic characterization of retinoic acid differentiated SH-SY5Y cells by transcriptional profiling. PLoS ONE 2013, 8, e63862. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, T.K.; Mackenzie, C.J.; Plevin, R.; Lutz, E.M. PACAP-38 induces neuronal differentiation of human SH-SY5Y neuroblastoma cells via cAMP-mediated activation of ERK and p38 MAP kinases. J. Neurochem. 2008, 104, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Jarpe, M.B.; Knall, C.; Mitchell, F.M.; Buhl, A.M.; Duzic, E.; Johnson, G.L. [D-Arg1,D-Phe5,D-Trp7,9,Leu11]Substance P acts as a biased agonist toward neuropeptide and chemokine receptors. J. Biol. Chem. 1998, 273, 3097–3104. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Cygankiewicz, A.; Jensen, T.H.; Ankersen, M.; Schwartz, T.W. High constitutive signaling of the ghrelin receptor—Identification of a potent inverse agonist. Mol. Endocrinol. 2003, 17, 2201–2210. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.K.; Chung, T.Y.; Li, Y.C.; Lo, Y.H.; Lin, N.H.; Kuo, P.C.; Chen, W.Y.; Tzen, J.T. Ginkgoghrelins, unique acylated flavonoid diglycosides in Folium Ginkgo, stimulate growth hormone secretion via activation of the ghrelin receptor. J. Ethnopharmacol. 2016, 193, 237–247. [Google Scholar] [CrossRef]
- Frago, L.M.; Baquedano, E.; Argente, J.; Chowen, J.A. Neuroprotective actions of ghrelin and growth hormone secretagogues. Front. Mol. Neurosci. 2011, 4, 23. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Doycheva, D.M.; Gamdzyk, M.; Lu, W.; Tang, J.; Zhang, J.H. Ghrelin attenuates oxidative stress and neuronal apoptosis via GHSR-1α/AMPK/Sirt1/PGC-1α/UCP2 pathway in a rat model of neonatal HIE. Free Radic. Biol. Med. 2019, 141, 322–337. [Google Scholar] [CrossRef]
- Cheng, H.C.; Ulane, C.M.; Burke, R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010, 67, 715–725. [Google Scholar] [CrossRef]
- Suda, Y.; Kuzumaki, N.; Sone, T.; Narita, M.; Tanaka, K.; Hamada, Y.; Iwasawa, C.; Shibasaki, M.; Maekawa, A.; Matsuo, M.; et al. Down-regulation of ghrelin receptors on dopaminergic neurons in the substantia nigra contributes to Parkinson’s disease-like motor dysfunction. Mol. Brain 2018, 11, 6. [Google Scholar] [CrossRef]
- Chen, C.; Turnbull, D.M.; Reeve, A.K. Mitochondrial Dysfunction in Parkinson’s Disease-Cause or Consequence? Biology 2019, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, D.S.; Parks, J.K.; Parker, W.D.; Bennett, J.P. The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim. Biophys. Acta 1999, 1453, 49–62. [Google Scholar] [CrossRef]
- Subramaniam, S.R.; Chesselet, M.-F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Song, N.; Xie, J.; Jiang, H. Ghrelin antagonized 1-methyl-4-phenylpyridinium (MPP(+))-induced apoptosis in MES23.5 cells. J. Mol. Neurosci. 2009, 37, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, L.J.; Wang, J.; Xie, J.X. Ghrelin antagonizes MPTP-induced neurotoxicity to the dopaminergic neurons in mouse substantia nigra. Exp. Neurol. 2008, 212, 532–537. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Ovais, M.; Ullah, I.; Ahmed, J.; Shahid, M. Flavonoids as Prospective Neuroprotectants and Their Therapeutic Propensity in Aging Associated Neurological Disorders. Front. Aging. Neurosci. 2019, 11, 155. [Google Scholar] [CrossRef]
- Tabrez, S.; Jabir, N.R.; Shakil, S.; Greig, N.H.; Alam, Q.; Abuzenadah, A.M.; Damanhouri, G.A.; Kamal, M.A. A synopsis on the role of tyrosine hydroxylase in Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2012, 11, 395–409. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nakashima, A.; Ichinose, H.; Kobayashi, K. Human tyrosine hydroxylase in Parkinson’s disease and in related disorders. J. Neural. Transm. 2019, 126, 397–409. [Google Scholar] [CrossRef]
- Andrews, Z.B.; Erion, D.; Beiler, R.; Liu, Z.W.; Abizaid, A.; Zigman, J.; Elsworth, J.D.; Savitt, J.M.; DiMarchi, R.; Tschoep, M.; et al. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J. Neurosci. 2009, 29, 14057–14065. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhuo, C.-F.; Hsieh, S.-K.; Chen, C.-J.; Chen, W.-Y.; Tzen, J.T.C. Teaghrelin Protects SH-SY5Y Cells against MPP+-Induced Neurotoxicity through Activation of AMPK/SIRT1/PGC-1α and ERK1/2 Pathways. Nutrients 2020, 12, 3665. https://doi.org/10.3390/nu12123665
Jhuo C-F, Hsieh S-K, Chen C-J, Chen W-Y, Tzen JTC. Teaghrelin Protects SH-SY5Y Cells against MPP+-Induced Neurotoxicity through Activation of AMPK/SIRT1/PGC-1α and ERK1/2 Pathways. Nutrients. 2020; 12(12):3665. https://doi.org/10.3390/nu12123665
Chicago/Turabian StyleJhuo, Cian-Fen, Sheng-Kuo Hsieh, Chun-Jung Chen, Wen-Ying Chen, and Jason T.C. Tzen. 2020. "Teaghrelin Protects SH-SY5Y Cells against MPP+-Induced Neurotoxicity through Activation of AMPK/SIRT1/PGC-1α and ERK1/2 Pathways" Nutrients 12, no. 12: 3665. https://doi.org/10.3390/nu12123665
APA StyleJhuo, C.-F., Hsieh, S.-K., Chen, C.-J., Chen, W.-Y., & Tzen, J. T. C. (2020). Teaghrelin Protects SH-SY5Y Cells against MPP+-Induced Neurotoxicity through Activation of AMPK/SIRT1/PGC-1α and ERK1/2 Pathways. Nutrients, 12(12), 3665. https://doi.org/10.3390/nu12123665




