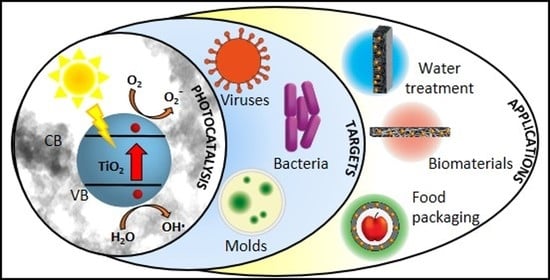
Photocatalytic TiO2-Based Nanostructured Materials for Microbial Inactivation
Abstract
1. Introduction
2. Preparation of TiO2-Based Nanostructured Materials for Photocatalytic Inactivation of Microorganisms
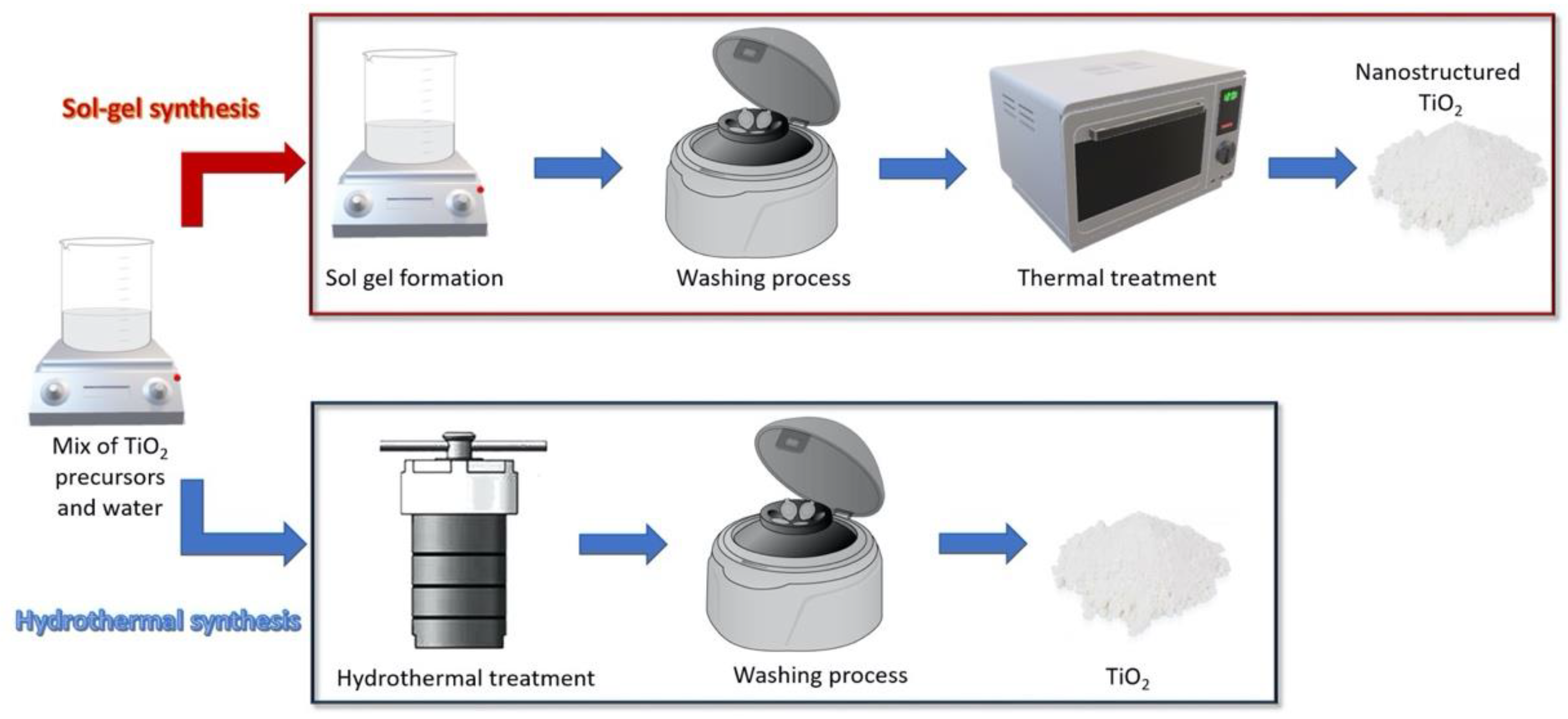
2.1. Synthesis of Photocatalytic TiO2 Nanoparticles with Antimicrobial Function
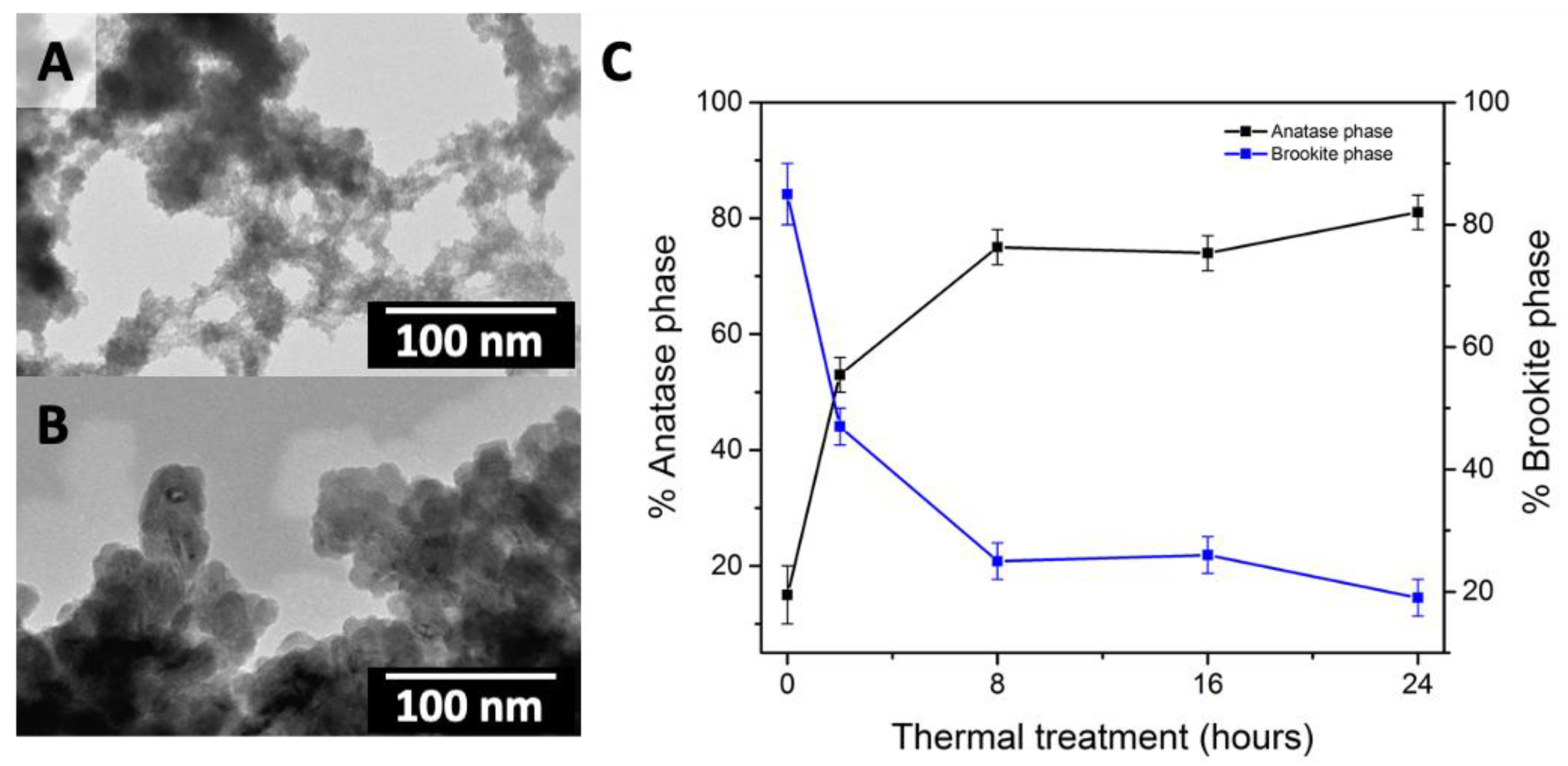
2.1.1. Sol-Gel Methods
2.1.2. Hydrothermal Methods
2.2. Preparation of TiO2/Metal Nanocomposites
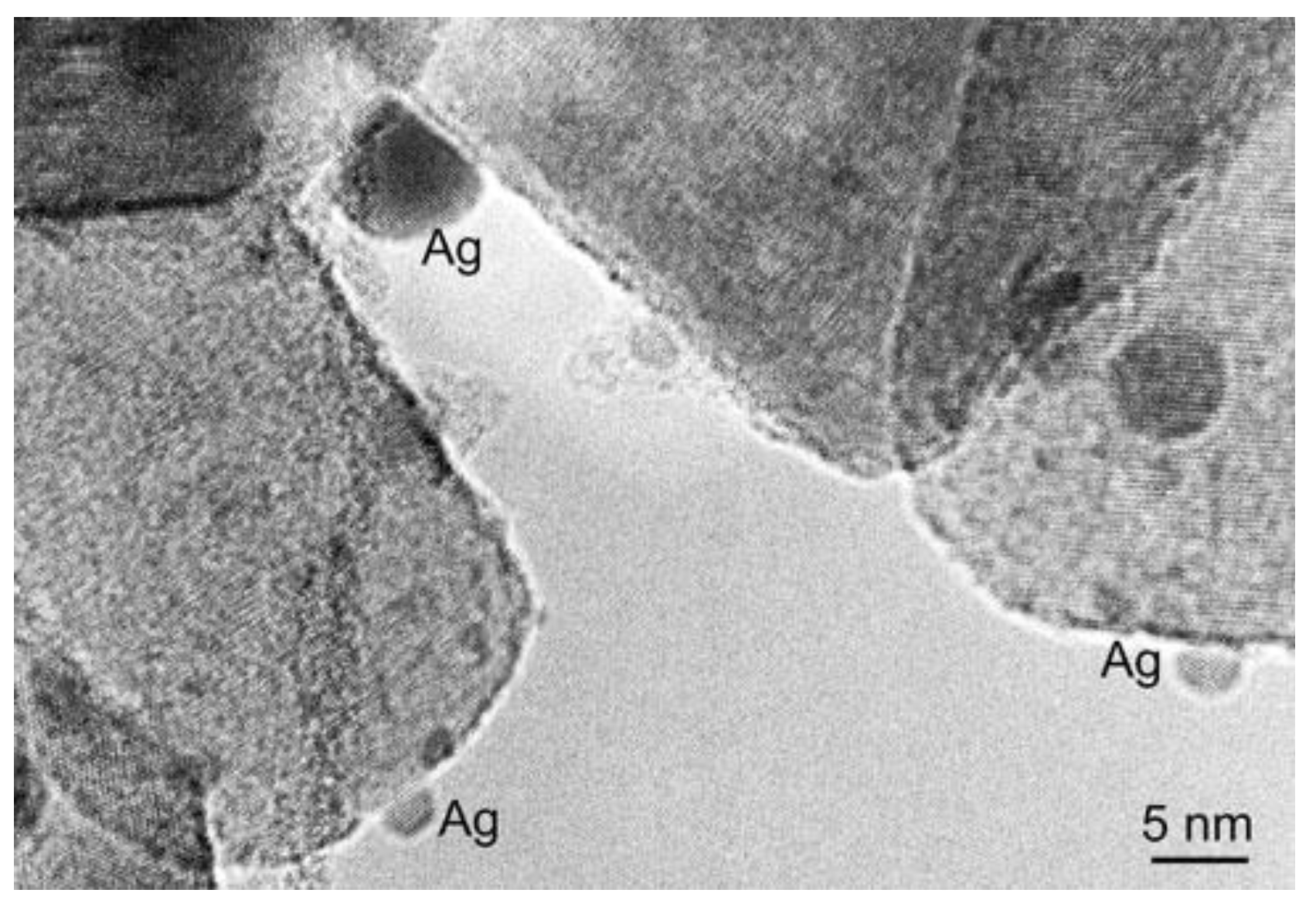
2.2.1. TiO2/Ag-Based Nanocomposites
2.2.2. Coupling TiO2 with Other Metals and Metal Oxides
3. Activity of TiO2-Based Nanostructured Materials against Bacteria, Fungi, and Virus
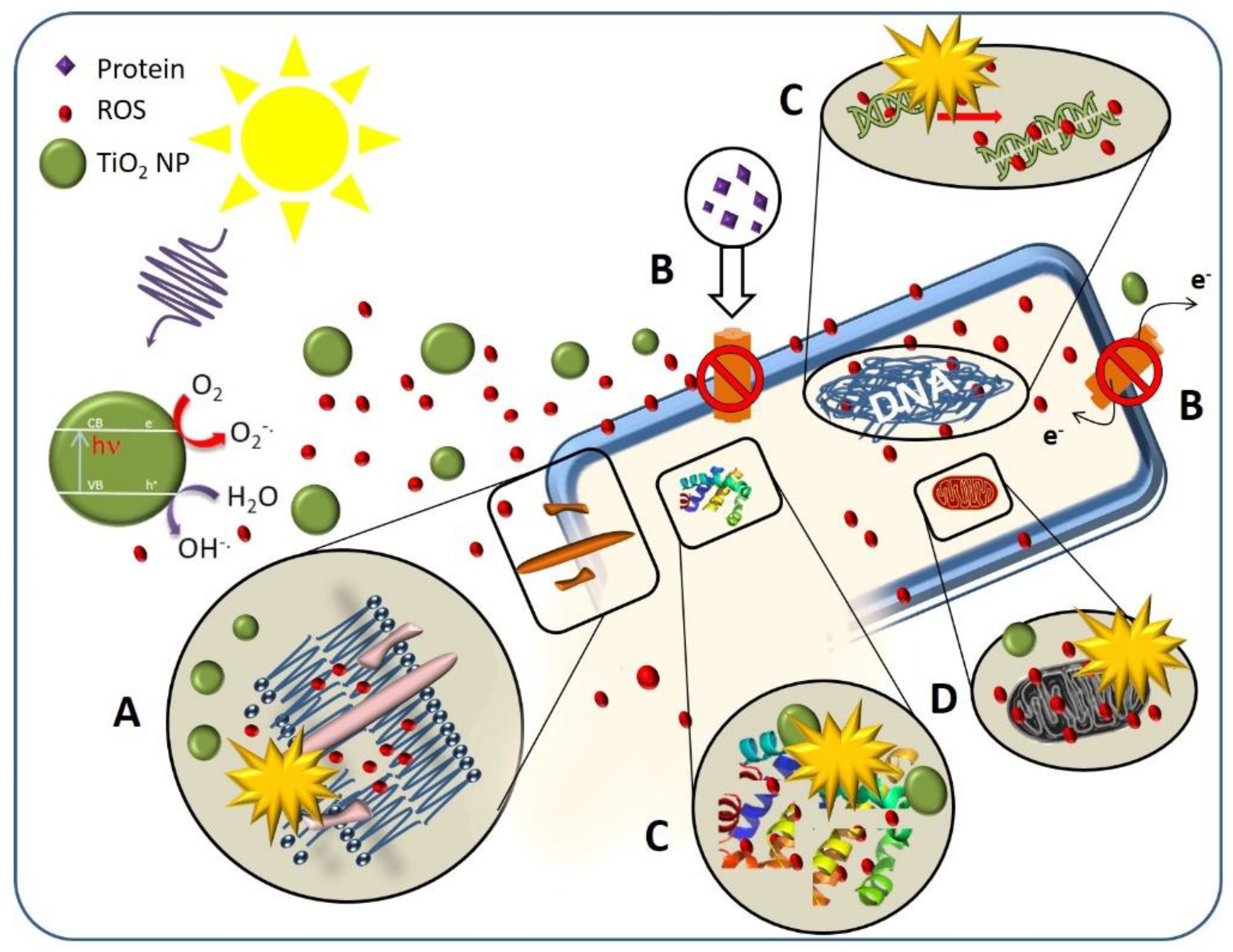
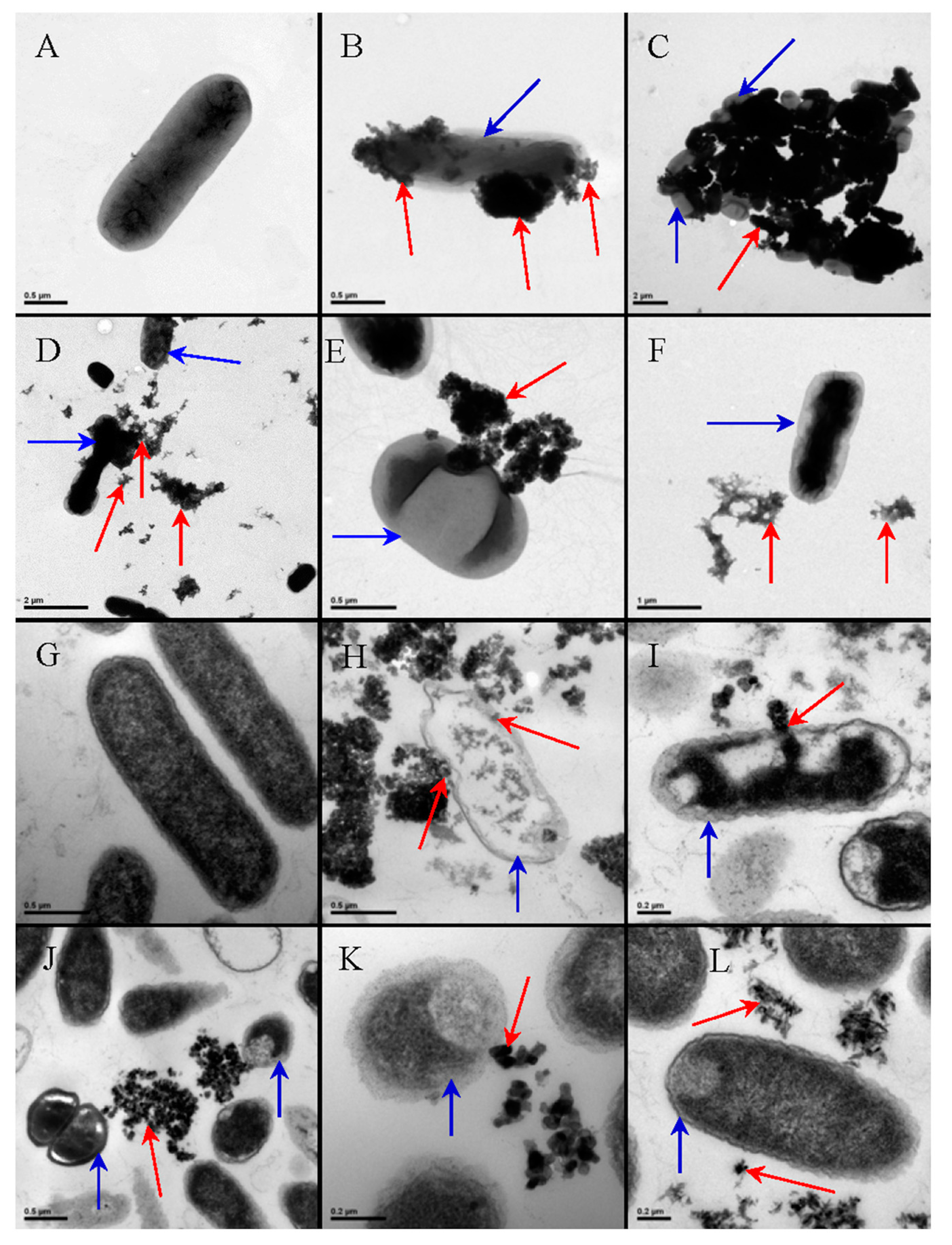
3.1. Antibacterial Activity of TiO2 Nanostructured Materials
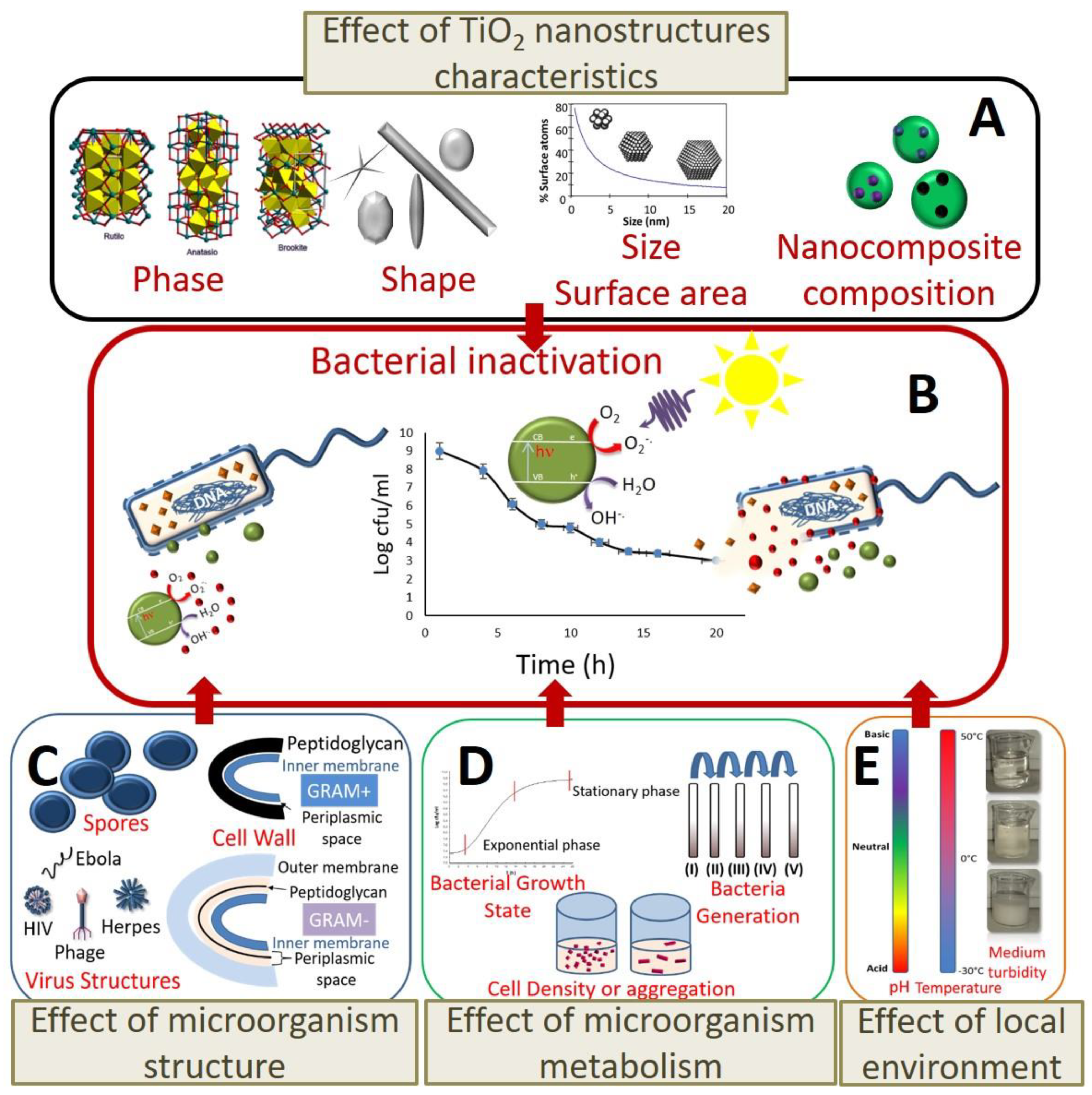
3.1.1. Effect of TiO2 Nanostructure Characteristics
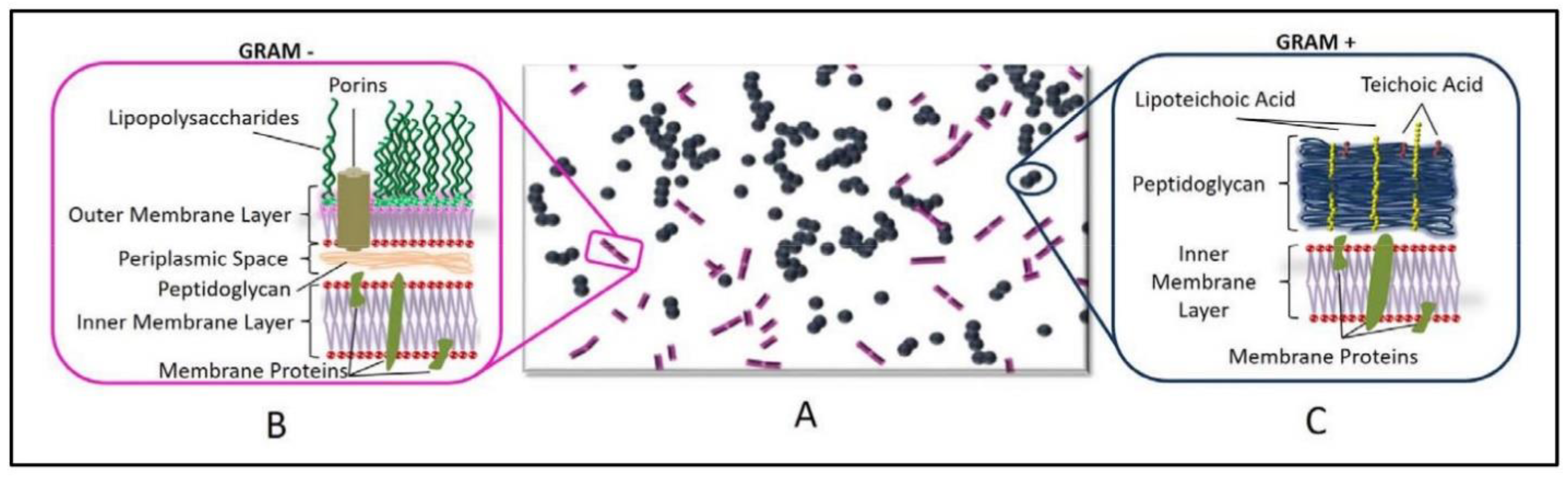
3.1.2. Effect of the Cell Membrane Structure
3.1.3. Effect of Bacterial Metabolism
3.1.4. Effect of Physiological State of Bacteria Cell and Environmental Stress
3.1.5. Intrinsic Antibacterial Activity of TiO2
3.2. Virus Inactivation
3.3. Fungi Inactivation
3.4. General Considerations
4. Technological Applications of Antimicrobial TiO2-Based Nanostructured Materials
4.1. Environmental Applications
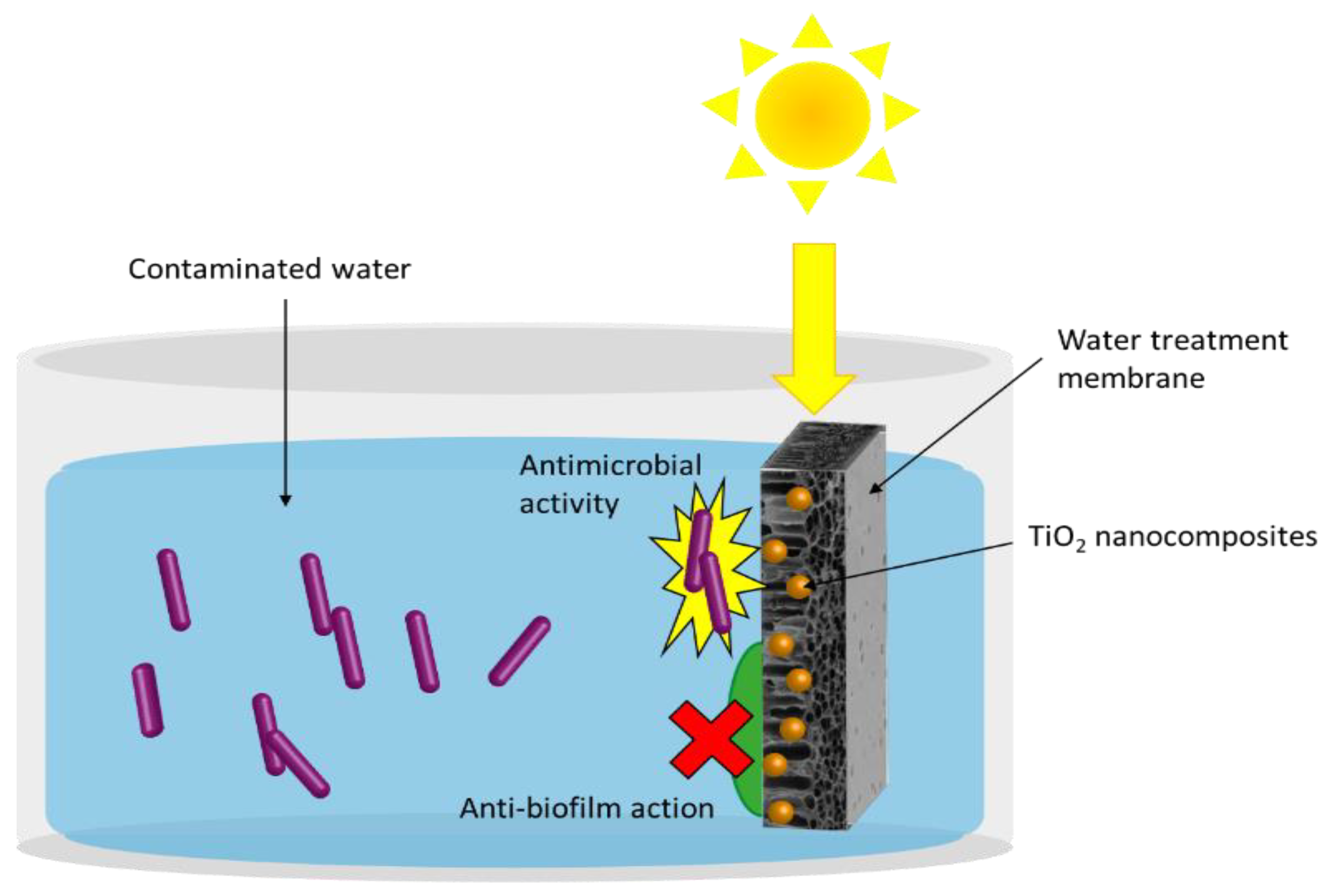
4.1.1. TiO2-Based Nanostructured Materials for Water Disinfection
4.1.2. Immobilization of Nanocomposites on Membranes or Recoverable Supports
4.1.3. Anti-Biofouling Membranes for Water Treatment
4.2. TiO2 NPs-Based Nanocomposite against Biofouling on Building Materials
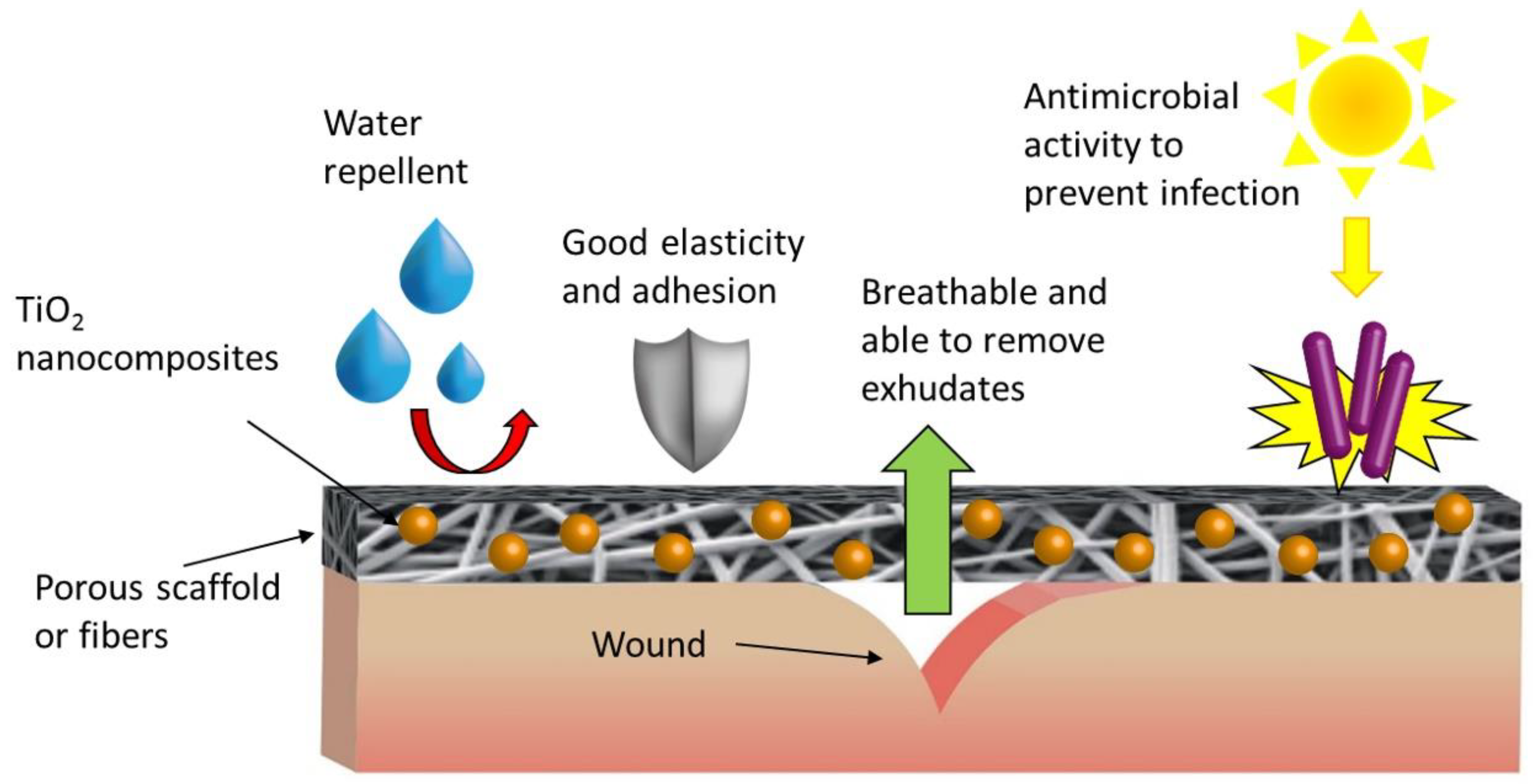
4.3. Photocatalytic TiO2 NPs-Based Nanocomposites for Biomaterials Disinfection
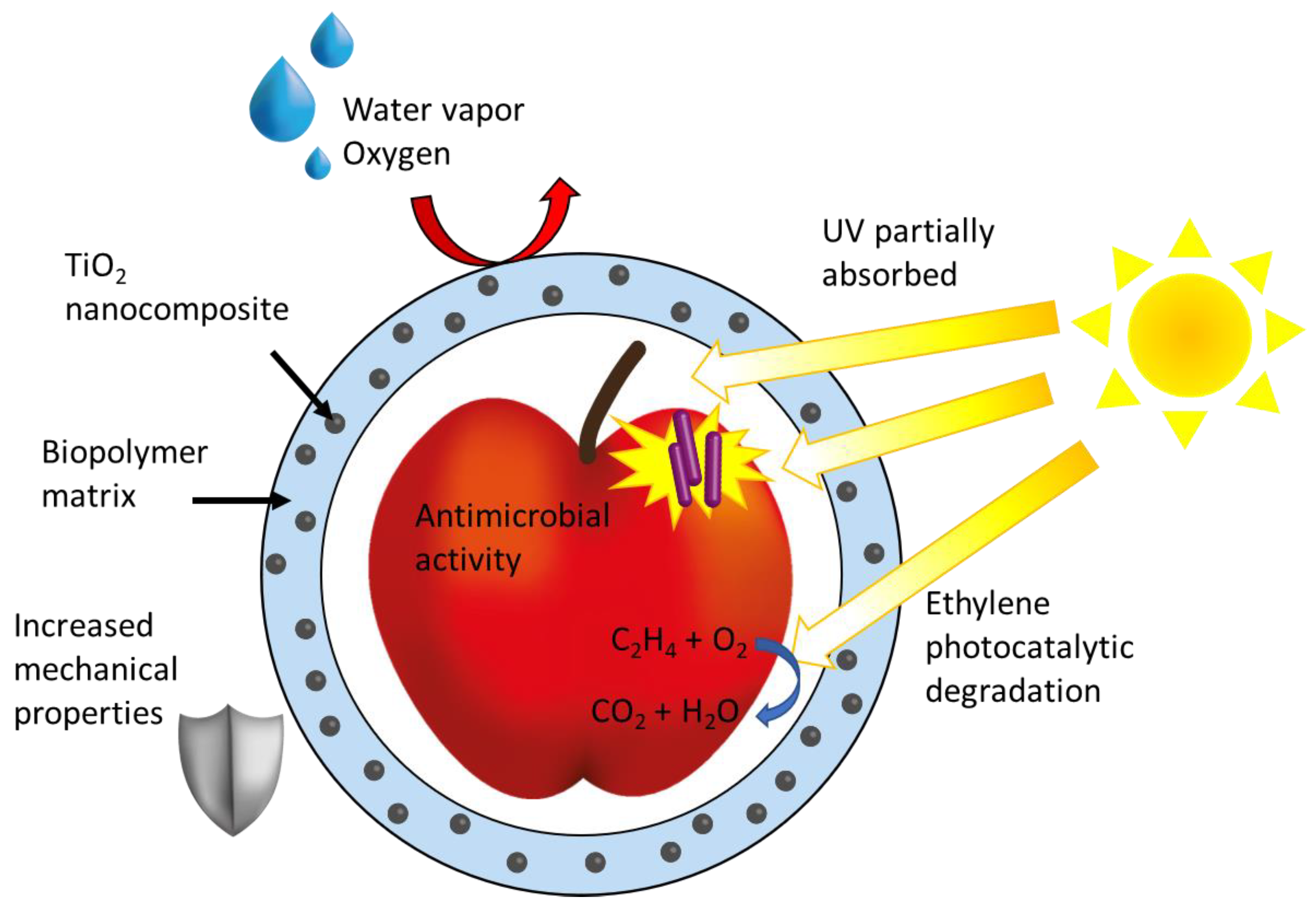
4.4. TiO2 NPs-Based Nanocomposites Designed for Disinfection of Food Packaging and Processing Materials
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Laxma Reddy, P.V.; Kavitha, B.; Kumar Reddy, P.A.; Kim, K.-H. TiO2-based photocatalytic disinfection of microbes in aqueous media: A review. Environ. Res. 2017, 154, 296–303. [Google Scholar] [CrossRef]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Sibillano, T.; Giannini, C.; Striccoli, M.; Comparelli, R.; Curri, M.L. Multifunctional TiO2/FexOy/Ag based nanocrystalline heterostructures for photocatalytic degradation of a recalcitrant pollutant. Catal. Today 2017, 284, 100–106. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Ingrosso, C.; Placido, T.; Striccoli, M.; Curri, M.L.; Agostiano, A.; Comparelli, R. Nanocomposite materials for photocatalytic degradation of pollutants. Catal. Today 2017, 281, 85–100. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Lusvardi, G.; Barani, C.; Giubertoni, F.; Paganelli, G. Synthesis and Characterization of TiO2 Nanoparticles for the Reduction of Water Pollutants. Materials 2017, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Qarni, F.; Alomair, N.; Mohamed, H. Environment-Friendly Nanoporous Titanium Dioxide with Enhanced Photocatalytic Activity. Catalysts 2019, 9, 799. [Google Scholar] [CrossRef]
- Petronella, F.; Truppi, A.; Dell’Edera, M.; Agostiano, A.; Curri, M.L.; Comparelli, R. Scalable Synthesis of Mesoporous TiO2 for Environmental Photocatalytic Applications. Materials 2019, 12, 1853. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.H.; Jun, Y.; Li, H.G.; Du, S.G. Using a Sol-Gel Method to Prepare the TiO2/CNTs Nanocomposite. Appl. Mech. Mater. 2014, 529, 108–111. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- Kang, X.; Liu, S.; Dai, Z.; He, Y.; Song, X.; Tan, Z. Titanium Dioxide: From Engineering to Applications. Catalysts 2019, 9, 191. [Google Scholar] [CrossRef]
- Cassaignon, S.; Koelsch, M.; Jolivet, J.-P. From TiCl3 to TiO2 nanoparticles (anatase, brookite and rutile): Thermohydrolysis and oxidation in aqueous medium. J. Phys. Chem. Solids 2007, 68, 695–700. [Google Scholar] [CrossRef]
- Lee, D.S.; Liu, T.K. Preparation of TiO2 Sol Using TiCl4 as a Precursor. J. Sol-Gel Sci. Technol. 2002, 25, 121–136. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, C.; Cao, L. The synthesis of nanosized TiO2 powder using a sol-gel method with TiCl4 as a precursor. J. Mater. Sci. 2000, 35, 4049–4054. [Google Scholar]
- Ullattil, S.; Periyat, P. Sol-Gel Synthesis of Titanium Dioxide. In Sol-Gel Materials for Energy, Environment and Electronic Applications. Advances in Sol-Gel Derived Materials and Technologies; Springer: Cham, Switzerland, 2017; pp. 271–283. [Google Scholar]
- Dell’Edera, M.; Petronella, F.; Truppi, A.; Liotta, L.F.; Gallì, N.; Sibillano, T.; Giannini, C.; Brescia, R.; Milano, F.; Striccoli, M.; et al. Low Temperature Synthesis of Photocatalytic Mesoporous TiO2 Nanomaterials. Catalysts 2020, 10, 893. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rodríguez-Páez, J.E. Facile Synthesis of TiO2 Nanoparticles of Different Crystalline Phases and Evaluation of Their Antibacterial Effect Under Dark Conditions Against E. coli. J. Clust. Sci. 2019, 30, 379–391. [Google Scholar] [CrossRef]
- Daels, N.; Radoicic, M.; Radetic, M.; De Clerck, K.; Van Hulle, S.W.H. Electrospun nanofibre membranes functionalised with TiO2 nanoparticles: Evaluation of humic acid and bacterial removal from polluted water. Sep. Purif. Technol. 2015, 149, 488–494. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwak, S.Y.; Sohn, B.H.; Park, T.H. Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem. J. Membr. Sci. 2003, 211, 157–165. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Sreekantan, S. Effect of pH on TiO2 Nanoparticles via Sol-Gel Method. Adv. Mater. Res. 2011, 173, 184–189. [Google Scholar] [CrossRef]
- Galkina, O.L.; Sycheva, A.; Blagodatskiy, A.; Kaptay, G.; Katanaev, V.L.; Seisenbaeva, G.A.; Kessler, V.G.; Agafonov, A.V. The sol–gel synthesis of cotton/TiO2 composites and their antibacterial properties. Surf. Coat. Technol. 2014, 253, 171–179. [Google Scholar] [CrossRef]
- Bahar, M.; Mozaffari, M.; Esmaeili, S. Effect of different alcohols, gelatinizing times, calcination and microwave on characteristics of TiO2 nanoparticles synthesized by sol–gel method. J. Theoretic. Appl. Phys. 2017, 11, 79–86. [Google Scholar] [CrossRef]
- Duymaz, B.; Yigit, Z.V.; Şeker, M.G.; Dündar, F. Antibacterial Properties of Sol-Gel Derived TiO2 Nanoparticles. Acta Phys. Pol. A 2016, 129, 872–874. [Google Scholar] [CrossRef]
- Rasheed, R.; Algawi, S.; Rhoomi, Z. Synthesis and Antibacterial Activity of Rutile-TiO2 Nano Powder Prepared by Hydrothermal Process. J. Univ. Babylon Pure Appl. Sci. 2017, 25, 1744–1754. [Google Scholar]
- Kőrösi, L.; Prato, M.; Scarpellini, A.; Kovács, J.; Dömötör, D.; Kovács, T.; Papp, S. H2O2-assisted photocatalysis on flower-like rutile TiO2 nanostructures: Rapid dye degradation and inactivation of bacteria. Appl. Surf. Sci. 2016, 365, 171–179. [Google Scholar] [CrossRef]
- Zárate, R.A.; Fuentes, S.; Wiff, J.P.; Fuenzalida, V.M.; Cabrera, A.L. Chemical composition and phase identification of sodium titanate nanostructures grown from titania by hydrothermal processing. J. Phys. Chem. Solids 2007, 68, 628–637. [Google Scholar] [CrossRef]
- León-Ríos, S.; Espinoza González, R.; Fuentes, S.; Chávez Ángel, E.; Echeverría, A.; Serrano, A.E.; Demergasso, C.S.; Zárate, R.A. One-Dimensional TiO2-B Crystals Synthesised by Hydrothermal Process and Their Antibacterial Behaviour on Escherichia coli. J. Nanomater. 2016, 2016, 7213672. [Google Scholar] [CrossRef]
- Ben-Shahar, Y.; Banin, U. Hybrid Semiconductor–Metal Nanorods as Photocatalysts. Top. Curr. Chem. 2016, 374, 54. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Banin, U.; Ben-Shahar, Y.; Vinokurov, K. Hybrid Semiconductor–Metal Nanoparticles: From Architecture to Function. Chem. Mater. 2014, 26, 97–110. [Google Scholar] [CrossRef]
- Kedziora, A.; Strek, W.; Kepinski, L.; Bugla-Ploskonska, G.; Doroszkiewicz, W. Synthesis and antibacterial activity of novel titanium dioxide doped with silver. J. Sol-Gel Sci. Technol. 2012, 62, 79–86. [Google Scholar] [CrossRef]
- Carvalho, I.; Ferdov, S.; Mansilla, C.; Marques, S.M.; Cerqueira, M.A.; Pastrana, L.M.; Henriques, M.; Gaidau, C.; Ferreira, P.; Carvalho, S. Development of antimicrobial leather modified with Ag–TiO2 nanoparticles for footwear industry. Sci. Technol. Mater. 2018, 30, 60–68. [Google Scholar] [CrossRef]
- Ahmed, A.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A.; Pervaiz, E.; Janjua, H.A.; Hussain, Z. In-vitro and in-vivo study of superabsorbent PVA/Starch/g-C3N4/Ag@TiO2 NPs hydrogel membranes for wound dressing. Eur. Polym. J. 2020, 130, 109650. [Google Scholar] [CrossRef]
- Page, K.; Palgrave, R.G.; Parkin, I.P.; Wilson, M.; Savin, S.L.P.; Chadwick, A.V. Titania and silver–titania composite films on glass—potent antimicrobial coatings. J. Mater. Chem. 2007, 17, 95–104. [Google Scholar] [CrossRef]
- Amarjargal, A.; Tijing, L.D.; Ruelo, M.T.G.; Lee, D.H.; Kim, C.S. Facile synthesis and immobilization of Ag-TiO2 nanoparticles on electrospun PU nanofibers by polyol technique and simple immersion. Mater. Chem. Phys. 2012, 135, 277–281. [Google Scholar] [CrossRef]
- Skorb, E.V.; Antonouskaya, L.I.; Belyasova, N.A.; Shchukin, D.G.; Möhwald, H.; Sviridov, D.V. Antibacterial activity of thin-film photocatalysts based on metal-modified TiO2 and TiO2:In2O3 nanocomposite. Appl. Catal. B 2008, 84, 94–99. [Google Scholar] [CrossRef]
- Kaushik, R.; Samal, P.K.; Halder, A. Degradation of Fluoroquinolone-Based Pollutants and Bacterial Inactivation by Visible-Light-Active Aluminum-Doped TiO2 Nanoflakes. ACS Appl. Nano Mater. 2019, 2, 7898–7909. [Google Scholar] [CrossRef]
- Venieri, D.; Gounaki, I.; Bikouvaraki, M.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Solar photocatalysis as disinfection technique: Inactivation of Klebsiella pneumoniae in sewage and investigation of changes in antibiotic resistance profile. J. Environ. Manag. 2017, 195, 140–147. [Google Scholar] [CrossRef]
- Binas, V.D.; Sambani, K.; Maggos, T.; Katsanaki, A.; Kiriakidis, G. Synthesis and photocatalytic activity of Mn-doped TiO2 nanostructured powders under UV and visible light. Appl. Catal. B 2012, 113–114, 79–86. [Google Scholar] [CrossRef]
- Venieri, D.; Fraggedaki, A.; Kostadima, M.; Chatzisymeon, E.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Solar light and metal-doped TiO2 to eliminate water-transmitted bacterial pathogens: Photocatalyst characterization and disinfection performance. Appl. Catal. B 2014, 154–155, 93–101. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Yang, O.B.; Kim, H.-C.; Khil, M.-S. TiO2 nanofibers doped with rare earth elements and their photocatalytic activity. Ceram. Int. 2012, 38, 5925–5930. [Google Scholar] [CrossRef]
- Nithya, N.; Bhoopathi, G.; Magesh, G.; Kumar, C.D.N. Neodymium doped TiO2 nanoparticles by sol-gel method for antibacterial and photocatalytic activity. Mater. Sci. Semicond. Process 2018, 83, 70–82. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Dobrowolska, A.; Czaczyk, K.; Motylenko, M.; Rafaja, D.; Ehrlich, H.; Jesionowski, T. Hydrothermal synthesis of multifunctional TiO2-ZnO oxide systems with desired antibacterial and photocatalytic properties. Appl. Surf. Sci. 2019, 463, 791–801. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.K.; Hu, X.Y.; Hu, J.; Wang, Z.Y.; Yin, Y.C.; Sun, C.H.; Zhang, X.W. Two-dimensional g-C3N4/TiO2 nanocomposites as vertical Z-scheme heterojunction for improved photocatalytic water disinfection. Catal. Today 2019, 335, 243–251. [Google Scholar] [CrossRef]
- Sikora, P.; Cendrowski, K.; Markowska-Szczupak, A.; Horszczaruk, E.; Mijowska, E. The effects of silica/titania nanocomposite on the mechanical and bactericidal properties of cement mortars. Constr. Build. Mater. 2017, 150, 738–746. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Z.H. The Fabrication of Magnetically Recyclable La-Doped TiO2/Calcium Ferrite/Diatomite Composite for Visible-Light-Driven Degradation of Antibiotic and Disinfection of Bacteria. Environ. Eng. Sci. 2020, 37, 109–119. [Google Scholar] [CrossRef]
- Xu, W.R.; Xie, W.J.; Huang, X.Q.; Chen, X.; Huang, N.; Wang, X.; Liu, J. The graphene oxide and chitosan biopolymer loads TiO2 for antibacterial and preservative research. Food Chem. 2017, 221, 267–277. [Google Scholar] [CrossRef]
- Ghosh, M.; Mondal, M.; Mandal, S.; Roy, A.; Chakrabarty, S.; Chakrabarti, G.; Pradhan, S.K. Enhanced photocatalytic and antibacterial activities of mechanosynthesized TiO2-Ag nanocomposite in wastewater treatment. J. Mol. Struct. 2020, 1211, 11. [Google Scholar] [CrossRef]
- Liga, M.V.; Bryant, E.L.; Colvin, V.L.; Li, Q.L. Virus inactivation by silver doped titanium dioxide nanoparticles for drinking water treatment. Water Res. 2011, 45, 535–544. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, X.; Zhang, W.Y.; Zhang, S.; Su, H.J.; Tan, T.W. Visible-light-mediated synergistic photocatalytic antimicrobial effects and mechanism of Ag-nanoparticles@chitosan-TiO2 organic-inorganic composites for water disinfection. Appl. Catal. B-Environ. 2015, 170, 255–262. [Google Scholar] [CrossRef]
- Haghighat, N.; Vatanpour, V.; Sheydaei, M.; Nikjavan, Z. Preparation of a novel polyvinyl chloride (PVC) ultrafiltration membrane modified with Ag/TiO2 nanoparticle with enhanced hydrophilicity and antibacterial activities. Sep. Purif. Technol. 2020, 237, 116374. [Google Scholar] [CrossRef]
- Kim, B.C.; Jeong, E.; Kim, E.; Hong, S.W. Bio-organic–inorganic hybrid photocatalyst, TiO2 and glucose oxidase composite for enhancing antibacterial performance in aqueous environments. Appl. Catal., B 2019, 242, 194–201. [Google Scholar] [CrossRef]
- Monmaturapoj, N.; Sri-On, A.; Klinsukhon, W.; Boonnak, K.; Prahsarn, C. Antiviral activity of multifunctional composite based on TiO2-modified hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xiao, Y.R.; Guo, D.X.; Shen, L.G.; Li, R.J.; Jiao, Y.; Xu, Y.C.; Lin, H.J. In-situ coating TiO2 surface by plant-inspired tannic acid for fabrication of thin film nanocomposite nanofiltration membranes toward enhanced separation and antibacterial performance. J. Colloid Interface Sci. 2020, 572, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, J.; Ma, S.; Liu, G.; Yang, K.; Tong, M.; Lin, D. Toxicity of TiO2 Nanoparticles to Escherichia coli: Effects of Particle Size, Crystal Phase and Water Chemistry. PLoS ONE 2014, 9, e110247. [Google Scholar] [CrossRef]
- Shirai, R.; Miura, T.; Yoshida, A.; Yoshino, F.; Ito, T.; Yoshinari, M.; Yajima, Y. Antimicrobial effect of titanium dioxide after ultraviolet irradiation against periodontal pathogen. Dent. Mater. J. 2016, 35, 511–516. [Google Scholar] [CrossRef]
- Rtimi, S.; Giannakis, S.; Bensimon, M.; Pulgarin, C.; Sanjines, R.; Kiwi, J. Supported TiO2 films deposited at different energies: Implications of the surface compactness on the catalytic kinetics. Appl. Catal. B 2016, 191, 42–52. [Google Scholar] [CrossRef]
- Sunada, K.; Kikuchi, Y.; Hashimoto, K.; Fujishima, A. Bactericidal and Detoxification Effects of TiO2 Thin Film Photocatalysts. Environ. Sci. Technol. 1998, 32, 726–728. [Google Scholar] [CrossRef]
- Wang, W.; Li, G.; Xia, D.; An, T.; Zhao, H.; Wong, P.K. Photocatalytic nanomaterials for solar-driven bacterial inactivation: Recent progress and challenges. Environ. Sci. Nano 2017, 4, 782–799. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Q.; Yang, H.; Shi, D.; Qian, J. Photocatalytic antibacterial properties of copper doped TiO2 prepared by high-energy ball milling. Ceram. Int. 2020, 46, 16716–16724. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Y.; Li, Z.; Liu, X.; Zhu, S.; Liang, Y.; Yeung, K.W.K.; Wu, S. Ce and Er Co-doped TiO2 for rapid bacteria- killing using visible light. Bioact. Mater. 2020, 5, 201–209. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, Q.; Zhang, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Negishi, N.; Yang, Y. Superior disinfection effect of Escherichia coli by hydrothermal synthesized TiO2-based composite photocatalyst under LED irradiation: Influence of environmental factors and disinfection mechanism. Environ. Pollut. 2019, 247, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Vary, P.S.; Lin, C.-T. Anatase TiO2 Nanocomposites for Antimicrobial Coatings. J. Phys. Chem. B 2005, 109, 8889–8898. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food. Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguro, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad Spectrum Microbicidal Activity of Photocatalysis by TiO2. Catalysts 2013, 3, 310–323. [Google Scholar] [CrossRef]
- Backhaus, K.; Marugan, J.; van Grieken, R.; Sordo, C. Photocatalytic inactivation of E. faecalis in secondary wastewater plant effluents. Water. Sci. Technol. 2010, 61, 2355–2361. [Google Scholar] [CrossRef]
- Pal, A.; Pehkonen, S.O.; Yu, L.E.; Ray, M.B. Photocatalytic inactivation of Gram-positive and Gram-negative bacteria using fluorescent light. J. Photochem. Photobiol. A 2007, 186, 335–341. [Google Scholar] [CrossRef]
- Ripolles-Avila, C.; Martinez-Garcia, M.; Hascoët, A.-S.; Rodríguez-Jerez, J.J. Bactericidal efficacy of UV activated TiO2 nanoparticles against Gram-positive and Gram-negative bacteria on suspension. J. Food 2019, 17, 408–418. [Google Scholar] [CrossRef]
- Tsuang, Y.H.; Sun, J.S.; Huang, Y.C.; Lu, C.H.; Chang, W.H.; Wang, C.C. Studies of photokilling of bacteria using titanium dioxide nanoparticles. Artif. Organs. 2008, 32, 167–174. [Google Scholar] [CrossRef] [PubMed]
- van Grieken, R.; Marugán, J.; Pablos, C.; Furones, L.; López, A. Comparison between the photocatalytic inactivation of Gram-positive E. faecalis and Gram-negative E. coli faecal contamination indicator microorganisms. Appl. Catal. B 2010, 100, 212–220. [Google Scholar] [CrossRef]
- Haider, A.J.; AL-Anbar, R.H.; Kadhim, G.R.; Salame, C.T. Exploring potential Environmental applications of TiO2 Nanoparticles. Energy Proc. 2017, 119, 332–345. [Google Scholar] [CrossRef]
- Rincón, A.-G.; Pulgarin, C. Bactericidal action of illuminated TiO2 on pure Escherichia coli and natural bacterial consortia: Post-irradiation events in the dark and assessment of the effective disinfection time. Appl. Catal. B 2004, 49, 99–112. [Google Scholar] [CrossRef]
- Child, M.; Strike, P.; Pickup, R.; Edwards, C. Salmonella typhimurium displays cyclical patterns of sensitivity to UV-C killing during prolonged incubation in the stationary phase of growth. FEMS Microbiol. Lett. 2002, 213, 81–85. [Google Scholar] [CrossRef]
- Munro, P.M.; Flatau, G.N.; Clément, R.L.; Gauthier, M.J. Influence of the RpoS (KatF) sigma factor on maintenance of viability and culturability of Escherichia coli and Salmonella typhimurium in seawater. Appl. Environ. Microbiol. 1995, 61, 1853. [Google Scholar] [CrossRef]
- Eisenstark, A.; Calcutt, M.J.; Becker-Hapak, M.; Ivanova, A. Role of escherichia coli rpos and associated genes in defense against oxidative damage. Free Radic. Biol. Med. 1996, 21, 975–993. [Google Scholar] [CrossRef]
- Prieto-Calvo, M.A.; López, M.; Prieto, M.; Alvarez-Ordóñez, A. Variability in resistance to Cold Atmospheric Plasma (CAP) and Ultraviolet light (UV) and multiple stress resistance analysis of pathogenic verocytotoxigenic Escherichia coli (VTEC). Food Res. Int. 2016, 79, 88–94. [Google Scholar] [CrossRef]
- Janulczyk, R.; Ricci, S.; Bjorck, L. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect. Immun. 2003, 71, 2656–2664. [Google Scholar] [CrossRef]
- Mandel, G.L. Catalase, superoxide dismutase, and virulence of Staphylococcus aureus. In vitro and in vivo studies with emphasis on staphylococcal--leukocyte interaction. J. Clin. Investig. 1975, 55, 561–566. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, V.; Obregon, S.; Patron-Soberano, O.A.; Terashima, C.; Fujishima, A. An approach to the photocatalytic mechanism in the TiO2-nanomaterials microorganism interface for the control of infectious processes. Appl. Catal. B 2020, 270, 118853. [Google Scholar] [CrossRef]
- Bonnet, M.; Massard, C.; Veisseire, P.; Camares, O.; Awitor, K.O. Environmental Toxicity and Antimicrobial Efficiency of Titanium Dioxide Nanoparticles in Suspension. J. Biomater. Nanobiotechnol. 2015, 06, 213–224. [Google Scholar] [CrossRef]
- Pagnout, C.; Jomini, S.; Dadhwal, M.; Caillet, C.; Thomas, F.; Bauda, P. Role of electrostatic interactions in the toxicity of titanium dioxide nanoparticles toward Escherichia coli. Colloids Surf. B Biointerfaces 2012, 92, 315–321. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Nesic, J.; Rtimi, S.; Laub, D.; Roglic, G.M.; Pulgarin, C.; Kiwi, J. New evidence for TiO2 uniform surfaces leading to complete bacterial reduction in the dark: Critical issues. Colloids Surf. B Biointerfaces 2014, 123, 593–599. [Google Scholar] [CrossRef]
- Kiwi, J.; Rtimi, S.; Sanjines, R.; Pulgarin, C. TiO2 and TiO2-Doped Films Able to Kill Bacteria by Contact: New Evidence for the Dynamics of Bacterial Inactivation in the Dark and under Light Irradiation. Int. J. Photoenergy 2014, 2014, 785037. [Google Scholar] [CrossRef]
- Rtimi, S.; Nesic, J.; Pulgarin, C.; Sanjines, R.; Bensimon, M.; Kiwi, J. Effect of surface pretreatment of TiO2 films on interfacial processes leading to bacterial inactivation in the dark and under light irradiation. Interface Focus 2015, 5, 20140046. [Google Scholar] [CrossRef]
- Erdem, A.; Metzler, D.; Cha, D.; Huang, C.P. Inhibition of bacteria by photocatalytic nano-TiO2 particles in the absence of light. Int. J. Environ. Sci. Technol. 2014, 12, 2987–2996. [Google Scholar] [CrossRef][Green Version]
- Carré, G.; Hamon, E.; Ennahar, S.; Estner, M.; Lett, M.C.; Horvatovich, P.; Gies, J.P.; Keller, V.; Keller, N.; Andre, P. TiO2 photocatalysis damages lipids and proteins in Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2573–2581. [Google Scholar] [CrossRef]
- Sułek, A.; Pucelik, B.; Kuncewicz, J.; Dubin, G.; Dąbrowski, J.M. Sensitization of TiO2 by halogenated porphyrin derivatives for visible light biomedical and environmental photocatalysis. Catal. Today 2019, 335, 538–549. [Google Scholar] [CrossRef]
- Gibson, K.E. Viral pathogens in water: Occurrence, public health impact, and available control strategies. Curr. Opin. Virol. 2014, 4, 50–57. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezli, S.; Salkeld, J.A.; French, G.L. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am. J. Infect. Control 2013, 41, S6–S11. [Google Scholar] [CrossRef]
- Barker, J.; Vipond, I.B.; Bloomfield, S.F. Effects of cleaning and disinfection in reducing the spread of Norovirus contamination via environmental surfaces. J. Hosp. Infect. 2004, 58, 42–49. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Li, Q.; Page, M.; Marinas, B.J.; Ahang, F.K. Treatment of Coliphage MS2 with Palladium-Modified Nitrogen-Doped Titanium Oxide Photocatalyst Illuminated by Visible Light. Environ. Sci. Technol. 2008, 42, 6148–6153. [Google Scholar] [CrossRef]
- Syngouna, V.I.; Chrysikopoulos, C.V. Inactivation of MS2 bacteriophage by titanium dioxide nanoparticles in the presence of quartz sand with and without ambient light. J. Colloid Interface Sci. 2017, 497, 117–125. [Google Scholar] [CrossRef]
- Koizumi, Y.; Taya, M. Kinetic evaluation of biocidal activity of titanium dioxide against phage MS2 considering interaction between the phage and photocatalyst particles. Biochem. Eng. J. 2002, 12, 107–116. [Google Scholar] [CrossRef]
- Ditta, I.B.; Steele, A.; Liptrot, C.; Tobin, J.; Tyler, H.; Yates, H.M.; Sheel, D.W.; Foster, H.A. Photocatalytic antimicrobial activity of thin surface films of TiO2, CuO and TiO2/CuO dual layers on Escherichia coli and bacteriophage T4. Appl. Microbiol. Biotechnol. 2008, 79, 127–133. [Google Scholar] [CrossRef]
- Ishiguro, H.; Nakano, R.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, M.; Hashimoto, K.; Kubota, Y. Photocatalytic inactivation of bacteriophages by TiO2-coated glass plates under low-intensity, long-wavelength UV irradiation. Photochem. Photobiol. Sci. 2011, 10, 1825–1829. [Google Scholar] [CrossRef]
- Ishiguro, H.; Yao, Y.; Nakano, R.; Hara, M.; Sunada, K.; Hashimoto, K.; Kajioka, J.; Fujishima, A.; Kubota, Y. Photocatalytic activity of Cu2+/TiO2-coated cordierite foam inactivates bacteriophages and Legionella pneumophila. Appl. Catal. B 2013, 129, 56–61. [Google Scholar] [CrossRef]
- Soylemez, E.; de Boer, M.P.; Sae-Ueng, U.; Evilevitch, A.; Stewart, T.A.; Nyman, M. Photocatalytic Degradation of Bacteriophages Evidenced by Atomic Force Microscopy. PLoS ONE 2013, 8, e53601. [Google Scholar] [CrossRef]
- Gerrity, D.; Ryu, H.; Crittenden, J.; Abbaszadegan, M. Photocatalytic inactivation of viruses using titanium dioxide nanoparticles and low-pressure UV light. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2008, 43, 1261–1270. [Google Scholar] [CrossRef]
- Hajkova, P.; Spatenka, P.; Horsky, J.; Horska, I.; Kolouch, A. Photocatalytic Effect of TiO2 Films on Viruses and Bacteria. Plasma Processes Polym. 2007, 4, S397–S401. [Google Scholar] [CrossRef]
- Nakano, R.; Ishiguro, H.; Yao, Y.; Kajioka, J.; Fujishima, A.; Sunada, K.; Minoshima, M.; Hashimoto, K.; Kubota, Y. Photocatalytic inactivation of influenza virus by titanium dioxide thin film. Photochem. Photobiol. Sci. 2012, 11, 1293–1298. [Google Scholar] [CrossRef]
- Guillard, C.; Bui, T.H.; Felix, C.; Moules, V.; Lina, B.; Lejeune, P. Microbiological disinfection of water and air by photocatalysis. C. R. Chim. 2008, 11, 107–113. [Google Scholar] [CrossRef]
- Mazurkova, N.A.; Spitsyna, Y.E.; Shikina, N.V.; Ismagilov, Z.R.; Zagrebel’nyi, S.N.; Ryabchikova, E.I. Interaction of titanium dioxide nanoparticles with influenza virus. Nanotechnol. Russia 2010, 5, 417–420. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-p.; Cheng, C.; Shi, L.; Cheng, R.; Yuan, D.-H. Photocatalytic disinfection performance in virus and virus/bacteria system by Cu-TiO2 nanofibers under visible light. Environ. Pollut. 2018, 237, 452–459. [Google Scholar] [CrossRef]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and potential applications of ultraviolet light in the food industry–a critical review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Xu, R.; Liu, X.; Zhang, P.; Ma, H.; Liu, G.; Xia, Z. The photodestruction of virus in Nano-TiO2 suspension. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2007, 22, 422–425. [Google Scholar] [CrossRef]
- Liga, M.V.; Maguire-Boyle, S.J.; Jafry, H.R.; Barron, A.R.; Li, Q. Silica decorated TiO2 for virus inactivation in drinking water--simple synthesis method and mechanisms of enhanced inactivation kinetics. Environ. Sci. Technol. 2013, 47, 6463–6470. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Matarese, M.; D’Amico, C.; Surace, G.; Paduano, V.; Fiorillo, M.T.; Moschella, A.; Bruna, A.; Romano, G.L.; et al. COVID-19 Surface Persistence: A Recent Data Summary and Its Importance for Medical and Dental Settings. Int. J. Environ. Res. Public Health 2020, 17, 3132. [Google Scholar] [CrossRef] [PubMed]
- Khaiboullina, S.; Uppal, T.; Dhabarde, N.; Subramanian, V.R.; Verma, S.C. In Vitro Inactivation of Human Coronavirus by Titania Nanoparticle Coatings and UVC Radiation: Throwing Light on SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Seven, O.; Dindar, B.; Aydemir, S.; Metin, D.; Ozinel, M.A.; Icli, S. Solar photocatalytic disinfection of a group of bacteria and fungi aqueous suspensions with TiO2, ZnO and Sahara desert dust. J. Photochem. Photobiol. A 2004, 165, 103–107. [Google Scholar] [CrossRef]
- Maneerat, C.; Hayata, Y. Antifungal activity of TiO2 photocatalysis against Penicillium expansum in vitro and in fruit tests. Int. J. Food. Microbiol. 2006, 107, 99–103. [Google Scholar] [CrossRef]
- Polo-López, M.I.; Fernández-Ibáñez, P.; García-Fernández, I.; Oller, I.; Salgado-Tránsito, I.; Sichel, C. Resistance of Fusarium sp spores to solar TiO2 photocatalysis: Influence of spore type and water (scaling-up results). J. Chem. Technol. Biotechnol. 2010, 85, 1038–1048. [Google Scholar] [CrossRef]
- Sichel, C.; Tello, J.; de Cara, M.; Fernández-Ibáñez, P. Effect of UV solar intensity and dose on the photocatalytic disinfection of bacteria and fungi. Catal. Today 2007, 129, 152–160. [Google Scholar] [CrossRef]
- Sichel, C.; de Cara, M.; Tello, J.; Blanco, J.; Fernández-Ibáñez, P. Solar photocatalytic disinfection of agricultural pathogenic fungi: Fusarium species. Appl. Catal. B 2007, 74, 152–160. [Google Scholar] [CrossRef]
- Hochmannova, L.; Vytrasova, J. Photocatalytic and antimicrobial effects of interior paints. Prog. Org. Coat. 2010, 67, 1–5. [Google Scholar] [CrossRef]
- Muranyi, P.; Schraml, C.; Wunderlich, J. Antimicrobial efficiency of titanium dioxide-coated surfaces. J. Appl. Microbiol 2010, 108, 1966–1973. [Google Scholar] [CrossRef]
- Vucetic, S.B.; Rudic, O.; Markov, S.L.; Bera, O.J.; Vidakovic, A.M.; Skapin, A.S.; Ranogajec, J.G. Antifungal efficiency assessment of the TiO2 coating on facade paints. Environ. Sci. Pollut. Res. Int. 2014, 21, 11228–11237. [Google Scholar] [CrossRef]
- Lonnen, J.; Kilvington, S.; Kehoe, S.C.; Al-Touati, F.; McGuigan, K.G. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res. 2005, 39, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Kühn, K.P.; Chaberny, I.F.; Massholder, K.; Stickler, M.; Benz, V.W.; Sonntag, H.-G.; Erdinger, L. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere 2003, 53, 71–77. [Google Scholar] [CrossRef]
- Ohara, T.; Tsuge, T. FoSTUA, encoding a basic helix-loop-helix protein, differentially regulates development of three kinds of asexual spores, macroconidia, microconidia, and chlamydospores, in the fungal plant pathogen Fusarium oxysporum. Eukaryot. Cell 2004, 3, 1412–1422. [Google Scholar] [CrossRef]
- Negishi, N.; Miyazaki, Y.; Kato, S.; Yang, Y. Effect of HCO3− concentration in groundwater on TiO2 photocatalytic water purification. Appl. Catal. B 2019, 242, 449–459. [Google Scholar] [CrossRef]
- Guillard, C.; Puzenat, E.; Lachheb, H.; Houas, A.; Herrmann, J.-M. Why inorganic salts decrease the TiO2 photocatalytic efficiency. Int. J. Photoenergy 2005, 7, 641208. [Google Scholar] [CrossRef]
- Pratap Reddy, M.; Venugopal, A.; Subrahmanyam, M. Hydroxyapatite-supported Ag-TiO2 as Escherichia coli disinfection photocatalyst. Water Res. 2007, 41, 379–386. [Google Scholar] [CrossRef] [PubMed]
- ISO27447:2019. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)-Test Method for Antibacterial Activity of Semiconducting Photocatalytic Materials; International Organization for Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- JISR1702:2012. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method For Antibacterial Activity of Photocatalytic Products under Photoirradiation and Efficacy; Japanese Standards Association, 2012. [Google Scholar]
- ISO18061:2014. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Antiviral Activity of Semiconducting Photocatalytic Materials—Test Method Using Bacteriophage Q-Beta; International Organization for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- JISR1706:2013. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Determination of Antiviral Activity of Photocatalytic Materials—Test Method Using Bacteriophage Q-Beta; Japanese Standard Association, 2013. [Google Scholar]
- ISO13125:2013. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Antifungal Activity of Semiconducting Photocatalytic Materials; International Organization for Standardization, 2013. [Google Scholar]
- JISR1705:2016. Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Antifungal Activity of Photocatalytic Products under Photoirradiation; Japanese Standard Association, 2016. [Google Scholar]
- Karagoz, S.; Kiremitler, N.B.; Sakir, M.; Salem, S.; Onses, M.S.; Sahmetlioglu, E.; Ceylan, A.; Yilmaz, E. Synthesis of Ag and TiO2 modified polycaprolactone electrospun nanofibers (PCL/TiO2-Ag NFs) as a multifunctional material for SERS, photocatalysis and antibacterial applications. Ecotoxicol. Environ. Saf. 2020, 188, 109856. [Google Scholar] [CrossRef]
- Lei, S.; Guo, G.; Xiong, B.; Gong, W.; Mei, G. Disruption of bacterial cells by photocatalysis of montmorillonite supported titanium dioxide. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 557–561. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; Shalan, A.E. An overview of nanomaterials for industrial wastewater treatment. Korean J. Chem. Eng. 2019, 36, 1209–1225. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Reddy, P.V.; Kim, K.H. A review of photochemical approaches for the treatment of a wide range of pesticides. J. Hazard. Mater. 2015, 285, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Truppi, A.; Petronella, F.; Placido, T.; Margiotta, V.; Lasorella, G.; Giotta, L.; Giannini, C.; Sibillano, T.; Murgolo, S.; Mascolo, G.; et al. Gram-scale synthesis of UV–vis light active plasmonic photocatalytic nanocomposite based on TiO2/Au nanorods for degradation of pollutants in water. Appl. Catal. B 2019, 243, 604–613. [Google Scholar] [CrossRef]
- Tahir, M.B.; Ahmad, A.; Iqbal, T.; Ijaz, M.; Muhammad, S.; Siddeeg, S.M. Advances in photo-catalysis approach for the removal of toxic personal care product in aqueous environment. Environ. Dev. Sustain. 2020, 22, 6029–6052. [Google Scholar] [CrossRef]
- Zhang, G.; Li, W.; Chen, S.; Zhou, W.; Chen, J. Problems of conventional disinfection and new sterilization methods for antibiotic resistance control. Chemosphere 2020, 254, 126831. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, S.; Li, Q.L.; Lyon, D.Y.; Brunet, L.; Alvarez, P.J.J. Nanotechnology-Enabled Water Disinfection and Microbial Control: Merits and Limitations; William Andrew Inc.: Norwich, UK, 2009; pp. 157–166. [Google Scholar]
- Mascolo, G.; Comparelli, R.; Curri, M.L.; Lovecchio, G.; Lopez, A.; Agostiano, A. Photocatalytic degradation of methyl red by TiO2: Comparison of the efficiency of immobilized nanoparticles versus conventional suspended catalyst. J. Hazard. Mater. 2007, 142, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Biancullo, F.; Moreira, N.F.F.; Ribeiro, A.R.; Manaia, C.M.; Faria, J.L.; Nunes, O.C.; Castro-Silva, S.M.; Silva, A.M.T. Heterogeneous photocatalysis using UVA-LEDs for the removal of antibiotics and antibiotic resistant bacteria from urban wastewater treatment plant effluents. Chem. Eng. J. 2019, 367, 304–313. [Google Scholar] [CrossRef]
- Rizzo, L.; Sannino, D.; Vaiano, V.; Sacco, O.; Scarpa, A.; Pietrogiacomi, D. Effect of solar simulated N-doped TiO2 photocatalysis on the inactivation and antibiotic resistance of an E. coli strain in biologically treated urban wastewater. Appl. Catal. B 2014, 144, 369–378. [Google Scholar] [CrossRef]
- Rizzo, L.; Della Sala, A.; Fiorentino, A.; Puma, G.L. Disinfection of urban wastewater by solar driven and UV lamp-TiO2 photocatalysis: Effect on a multi drug resistant Escherichia coli strain. Water Res. 2014, 53, 145–152. [Google Scholar] [CrossRef]
- Li, H.Z.; Shen, L.Y.; Zhang, K.F.; Sun, B.J.; Ren, L.P.; Qiao, P.Z.; Pan, K.; Wang, L.; Zhou, W. Surface plasmon resonance-enhanced solar-driven photocatalytic performance from Ag nanoparticle-decorated self-floating porous black TiO2 foams. Appl. Catal. B 2018, 220, 111–117. [Google Scholar] [CrossRef]
- Negishi, N.; Chawengkijwanich, C.; Pimpha, N.; Larpkiattaworn, S.; Charinpanitkul, T. Performance verification of the photocatalytic solar water purification system for sterilization using actual drinking water in Thailand. J. Water Process Eng. 2019, 31, 100835. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Kuna, E. Photoactive Hybrid Catalysts Based on Natural and Synthetic Polymers: A Comparative Overview. Molecules 2017, 22, 790. [Google Scholar] [CrossRef] [PubMed]
- Castro-Munoz, R. The Role of New Inorganic Materials in Composite Membranes for Water Disinfection. Membranes 2020, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Shen, Z.P.; Shi, L.; Cheng, R.; Yuan, D.H. Photocatalytic Membrane Reactors (PMRs) in Water Treatment: Configurations and Influencing Factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.-J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020, 84, 23–41. [Google Scholar] [CrossRef]
- Cheng, R.; Shen, L.J.; Wang, Q.; Xiang, S.Y.; Shi, L.; Zheng, X.; Lv, W.Z. Photocatalytic Membrane Reactor (PMR) for Virus Removal in Drinking Water: Effect of Humic Acid. Catalysts 2018, 8, 284. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, X.; Choo, K.-H. Submerged microfiltration-catalysis hybrid reactor treatment: Photocatalytic inactivation of bacteria in secondary wastewater effluent. Sep. Purif. Technol. 2018, 198, 87–92. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.Y.; Bai, H.W.; Sun, D.D. Concurrent filtration and solar photocatalytic disinfection/degradation using high-performance Ag/TiO2 nanofiber membrane. Water Res. 2012, 46, 1101–1112. [Google Scholar] [CrossRef]
- Ishikawa, T.; Yamaoka, H.; Harada, Y.; Fujii, T.; Nagasawa, T. A general process for in situ formation of functional surface layers on ceramics. Nature 2002, 416, 64–67. [Google Scholar] [CrossRef]
- Nazerah, A.; Ismail, A.F.; Jaafar, J. Incorporation of bactericidal nanomaterials in development of antibacterial membrane for biofouling mitigation: A mini review. J. Teknol. 2016, 78, 53–61. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Zhang, R.X.; Luis, P.; Arsuaga, J.M.; Kim, J.; Van der Bruggen, B. Effect of nanoparticle aggregation at low concentrations of TiO2 on the hydrophilicity, morphology, and fouling resistance of PES-TiO2 membranes. J. Colloid Interface Sci. 2011, 363, 540–550. [Google Scholar] [CrossRef]
- Becerra, J.; Zaderenko, A.P.; Gomez-Moron, M.A.; Ortiz, P. Nanoparticles Applied to Stone Buildings. Int. J. Archit. Herit. 2019, 1–16. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Dyshlyuk, L.; Babich, O.; Ivanova, S.; Vasilchenco, N.; Atuchin, V.; Korolkov, I.; Russakov, D.; Prosekov, A. Antimicrobial potential of ZnO, TiO2 and SiO2 nanoparticles in protecting building materials from biodegradation. Int. Biodeterior. Biodegrad. 2020, 146, 8. [Google Scholar] [CrossRef]
- Chen, L.; Pan, H.; Zhuang, C.F.; Peng, M.Y.; Zhang, L. Joint wound healing using polymeric dressing of chitosan/strontium-doped titanium dioxide with high antibacterial activity. Mater. Lett. 2020, 268, 3. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, S467–S479. [Google Scholar] [CrossRef] [PubMed]
- Malmir, S.; Karbalaei, A.; Pourmadadi, M.; Hamedi, J.; Yazdian, F.; Navaee, M. Antibacterial properties of a bacterial cellulose CQD-TiO2 nanocomposite. Carbohydr. Polym. 2020, 234, 10. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.L.; Chen, K.K.; He, X.C.; Li, N.; Huang, J.B.; Tang, K.Y.; Li, Y.J.; Wang, F. Nano-TiO2/collagen-chitosan porous scaffold for wound repairing. Int. J. Biol. Macromol. 2016, 91, 15–22. [Google Scholar] [CrossRef]
- Marulasiddeshwara, R.; Jyothi, M.S.; Soontarapa, K.; Keri, R.S.; Velmurugan, R. Nonwoven fabric supported, chitosan membrane anchored with curcumin/TiO2 complex: Scaffolds for MRSA infected wound skin reconstruction. Int. J. Biol. Macromol. 2020, 144, 85–93. [Google Scholar] [CrossRef]
- Ansarizadeh, M.; Haddadi, S.A.; Amini, M.; Hasany, M.; SaadatAbadi, A.R. Sustained release of CIP from TiO2-PVDF/starch nanocomposite mats with potential application in wound dressing. J. Appl. Polym. Sci. 2020, 137, 11. [Google Scholar] [CrossRef]
- Ashraf, R.; Sofi, H.S.; Akram, T.; Rather, H.A.; Abdal-Hay, A.; Shabir, N.; Vasita, R.; Alrokayan, S.H.; Khan, H.A.; Sheikh, F.A. Fabrication of multifunctional cellulose/TiO2/Ag composite nanofibers scaffold with antibacterial and bioactivity properties for future tissue engineering applications. J. Biomed. Mater. Res. A 2020, 108, 947–962. [Google Scholar] [CrossRef] [PubMed]
- Monmaturapoj, N.; Thepsuwan, W.; Mai-Ngam, K.; Ngernpimai, S.; Klinsukhon, W.; Prahsarn, C. Preparation and properties of hydroxyapatite/titania composite for microbial filtration application. Adv. Appl. Ceram. 2014, 113, 267–274. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-Based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Azad, Z.R.A.A. Food Nanotechnology: An Emerging Technology in Food Processing and Preservation. In Health and Safety Aspects of Food Processing Technologies; Springer: Cham, Switzerland, 2019; pp. 567–576. [Google Scholar]
- Bajpai, V.K.; Kamle, M.; Shukla, S.; Mahato, D.K.; Chandra, P.; Hwang, S.K.; Kumar, P.; Huh, Y.S.; Han, Y.-K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug Anal. 2018, 26, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 11. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Ruvalcaba-Gomez, J.M.; Maytorena-Verdugo, C.I.; Gonzalez-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Perez-Larios, A.; Montalvo-Gonzalez, E. Chitosan-TiO2: A Versatile Hybrid Composite. Materials 2020, 13, 811. [Google Scholar] [CrossRef]
- Goudarzi, V.; Shahabi-Ghahfarrokhi, I.; Babaei-Ghazvini, A. Preparation of ecofriendly UV-protective food packaging material by starch/TiO2 bio-nanocomposite: Characterization. Int. J. Biol. Macromol. 2017, 95, 306–313. [Google Scholar] [CrossRef]
- Bohmer-Maas, B.W.; Fonseca, L.M.; Otero, D.M.; Zavareze, E.D.; Zambiazi, R.C. Photocatalytic zein-TiO2 nanofibers as ethylene absorbers for storage of cherry tomatoes. Food Packag. Shelf Life 2020, 24, 7. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef]
- Zhang, X.D.; Xiao, G.; Wang, Y.Q.; Zhao, Y.; Su, H.J.; Tan, T.W. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.R.; Yang, Y.M.; Wang, J.; Yan, W.J.; Wu, Z.J.; Chen, H.; Zhang, Q.; Wu, D.T.; Qin, W.; Tu, Z.C. Preparation and characterization of TiO2-Ag loaded fish gelatin-chitosan antibacterial composite film for food packaging. Int. J. Biol. Macromol. 2020, 154, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Hung, Y.C. UV-A activated TiO2 embedded biodegradable polymer film for antimicrobial food packaging application. LWT-Food Sci. Technol. 2018, 96, 307–314. [Google Scholar] [CrossRef]
- Hong, L.; Luo, S.H.; Yu, C.H.; Xie, Y.; Xia, M.Y.; Chen, G.Y.; Peng, Q. Functional Nanomaterials and Their Potential Applications in Antibacterial Therapy. Pharm. Nanotechnol. 2019, 7, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Teymourpour, S.; Mohammadi Nafchi, A.; Nahidi, F. Functional, thermal, and antimicrobial properties of soluble soybean polysaccharide biocomposites reinforced by nano TiO2. Carbohydr. Polym. 2015, 134, 726–731. [Google Scholar]
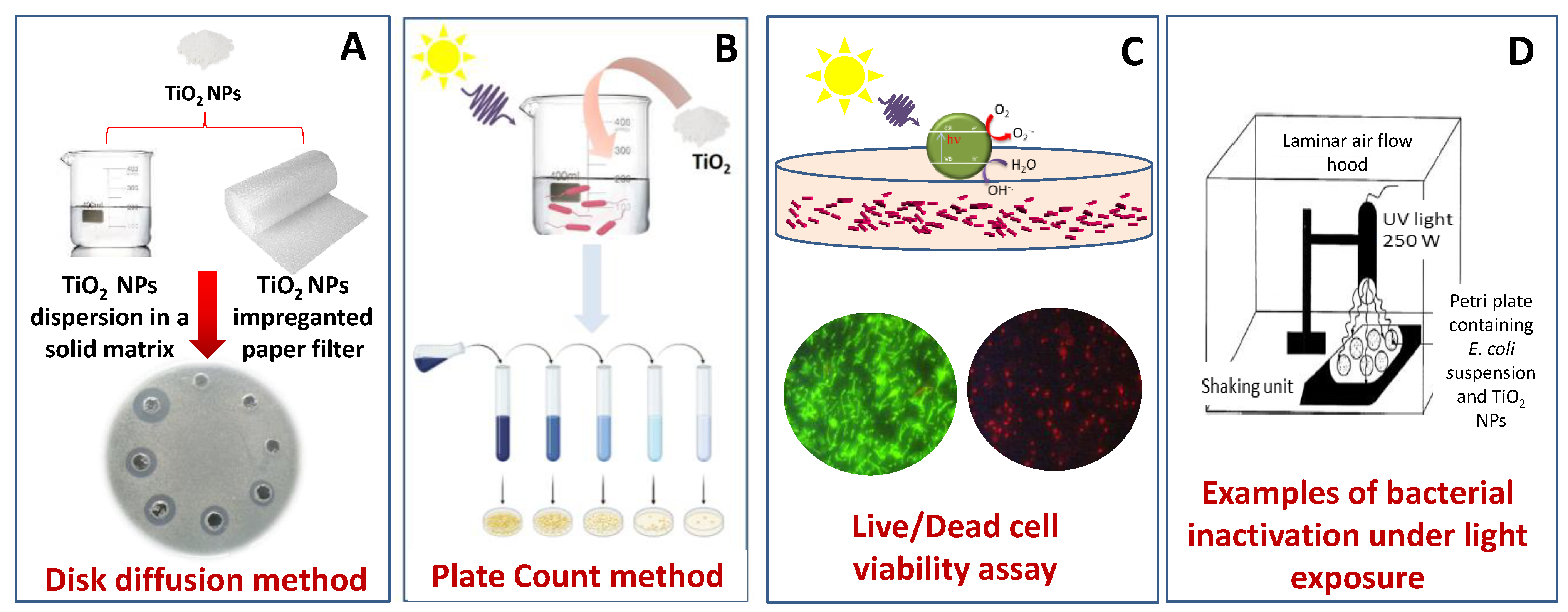
| Photocatalyst | Phase/Size | Experimental Parameters | Bacteria | Disinfection Efficiency | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Catalyst Loading | Light Source/Light Flux | Exposure Time | Strain | Cell Density | ||||
| TiO2 | 66 and 950, nm, 44 µm | 10–5000 ppm | Sunlight | 360 min | E. coli, B. subtilis | OD600 = 0.002 | 45–75% | [85] |
| TiO2 NPs | anatase/10–50 nm; rutile/25 nm; anatase-rutile/25 nm | 10–500 mg/L | Natural light | 180 min | E. coli | OD600 = 1 | 0–100% | [55] |
| TiO2 | anatase/21 nm, 5 µm | 15 mg | BLB°/27W | 60, 180, 360 min | Porphyromonas gingivalis | OD660 = 0.2 | 0–80% | [56] |
| TiO2 NPs | n.a. | 0.01–5 mM | Room light | n.a. | B. megaterium, E. coli | OD600 = 0.8–1 | Size of inhibition zone (disk agar diffusion method) | [66] |
| TiO2 P25 | 20 nm | 0.05 g/L | UV-A bulb lamp/125W | 120–280 min | Enterococcus faecalis, E. coli | 6 log CFU/mL | tmax° = 15.4–204 min | [69] |
| TiO2 NPs | anatase/7 nm; anatase-rutile (80:20 wt/wt) 21 nm | 0.78–100 μg/mL | UV light (315–400 nm)/n.a. | 24 h | Salmonella entericavar. Enteridis, E. coli, St aureus, B. cereus, Lb casei, Lb delbrueckii subsp. bulgaricus, Lb lactis subsp. lactis, Lb acidophilus | 7 log CFU/mL | OD650 = 0–0.8 | [71] |
| TiO2 NPs | anatase-rutile/8–17 nm | 1 mg/cm2 | Sunlight irradiaton | 120 min | P. aeruginosa, St. aureus | 7 log CFU/mL | 100% | [74] |
| TiO2 P25 | n.a. | 0.25–1 g/L | Solar irradiation | 180 min | E. coli, coliforms, Enterococcus spp. | 8 log CFU/mL | Eliminated in 0.5–2.5 h | [75] |
| TiO2 NPs | 8 nm | 50–1200 mg/L | UV lamp/48W | 30 min | St. aureus, Lb casei rhamnosus, E. coli | 6 log CFU/mL | Mortality rate = 80–100% | [83] |
| TiO2 P25 | 20 nm | 0.1–0.8 g/L | BLB/40W | 30–60 min | E. coli | 6 log CFU/mL | Log10(C/C0)° = −0.3–−3 | [90] |
| TiO2 film | 100 nm | n.a. | BLB/15W | 4 h | E. coli | 2 × 105 CFU/mL | Survival ratio = 50% | [58] |
| TiO2-coated glass | 200 nm | n.a. | BLB/0.1 mW/cm2 | 0–16 h | E. coli, Serratia marcescens, K. pneumoniaei, Acin. baumaii, P. aeruginosa, St. aureus, Enterococcus spp., Str. pneumoniae | 107 CFU/mL | 101–105 CFU/mL | [68] |
| PE°-TiO2 film | n.a. | 0.031–0.051 TiO2 wt%/wt PE | Solar simulator/50W | 300 min | E. coli | 6 log CFU/mL | Eliminated in 55–260 min | [57] |
| TiO2; Ag- TiO2 film | n.a. | n.a. | UV lamp (254 nm)/n.a. | 30 min | St. aureus, E. coli, B. cereus | 6 log CFU/mL | 4.5 log CFU/ml | [34] |
| TiO2 NP TiO2:In2O3 TiO2/AgTiO2/Ag/Ni | anatase | n.a. | Hg lamp (filter 300–400 nm)/125W | 10 min | P. fluorescens, Lb lactis spp. lactis | n.a. | 1–3 log CFU/ml | [36] |
| P/Ag/Ag2O/Ag3PO4/TiO2 | n.a. | 0.5 g/L | LED lamp/<0.3W | 20 min | E. coli | 107 CFU/mL | 0–107 CFU/mL | [62] |
| Photocatalyst | Phase/Size | Experimental Parameters | Virus | Disinfection Efficiency | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Catalyst Loading (mg/L) | Light Source/Light Flux | Exposure Time | Strain | Cell Density | ||||
| TiO2 P25 | anatase/25 nm | n.a. | Ambient light/n.a. | 0–30 days | Phage MS2 | 3–8 log PFU°/ml | Log10(C/C0) = 0.02–0.05 | [97] |
| TiO2 P25 | anatase/rutile | n.a. | Low-pressure UV light/n.a. | n.a. | Bacteriophage PRD1, MS2, phi-X174, fr | n.a. | 4 log CFU/ml | [103] |
| TiO2 | n.a. | n.a. | BLB°/1 mW | 8 h | Human influenza A | 4.0 × 108 PFU/ml | Complete in 5 min | [105] |
| TiO2-coated glass | 200 nm | n.a. | BLB/0.1 mW/cm2 | 0–16 h | Influenza virus, feline calicivirus | 107 PFU/mL | 102–106 PFU/mL | [68] |
| Cu-TiO2 nanofibers | n.a. | 25–150 | Xe lamp/0–130 mW/cm2 | 240 min | Bacteriophage f2 | 4 log PFU/ml | Q = 1–5.5 | [108] |
| Cu2+/TiO2-coated cordierite | anatase | n.a. | FL20 BLB (λ = 351 nm)/0.001–0.1 mW/cm2 | 24 h | Qβ and T4 bacteriophage | n.a. | Complete in 4–8 h | [101] |
| nAg/TiO2 | anatase | 100 | UV-A lamp/8W | 2 min | Phage MS2 | 3.0 × 107 PFU/mL | Inactivation rate = 1.6–6 log | [49] |
| TiON/PdO | n.a. | 100 | Xe arc lam/1000W | 120 min | Phage MS2 | 3.0 × 108 PFU/mL | 1.5 log in 60 min | [96] |
| HA°/TiO2 | n.a. | 0.125–0.5 | UV light/n.a. | 180 min | Influenza virus H1N1 | 107 TCID50°/mL | 2 log TCID50/mL | [53] |
| SiO2-TiO2 | 25 nm | 1–102.6 | UV-A/8 W | 2 min | Phage MS2 | 104–1010 PFU/mL | 5 log in 1.8 min | [111] |
| Photocatalyst | Phase/Size | Experimental Parameters | Fungi | Disinfection Efficiency | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Catalyst Loading | Light Source | Exposure Time | Strain | Cell Density | ||||
| TiO2 P25 | anatase-rutile | 0.01 mg/mL | Sodium lamp/400W | 4 h | S. cerevisiae, C. albicans, A.niger | 1 × 105 CFU/mL | Completed in 120 min | [114] |
| TiO2 NPs | 7 nm | 0–10–100 mg | BLB (UV-A)/20W | 72 h–14 gg | P. expansum | 2.5 × 105 conidia/mL | Score = 1.9 vs. 3.2 | [115] |
| TiO2 P25 | anatase-rutile | n.a. | white light (356 nm)/2 × 15 W | 60 min | C. albicans | 106 CFU/mL | 2 log CFU/mL | [123] |
| TiO2 P25 | anatase rutile phase | 100 mg/L | Natural sunlight | 5–6 h | Fusarium sp. spores | 102–103 CFU/mL | Completed in 4–5 h | [116] |
| TiO2/Zn-Al | n.a. | n.a. | UV-A/n.a. | 5 days | A. niger | n.a. | Surface coverage (%) = 0–92.6 | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pasquale, I.; Lo Porto, C.; Dell’Edera, M.; Petronella, F.; Agostiano, A.; Curri, M.L.; Comparelli, R. Photocatalytic TiO2-Based Nanostructured Materials for Microbial Inactivation. Catalysts 2020, 10, 1382. https://doi.org/10.3390/catal10121382
De Pasquale I, Lo Porto C, Dell’Edera M, Petronella F, Agostiano A, Curri ML, Comparelli R. Photocatalytic TiO2-Based Nanostructured Materials for Microbial Inactivation. Catalysts. 2020; 10(12):1382. https://doi.org/10.3390/catal10121382
Chicago/Turabian StyleDe Pasquale, Ilaria, Chiara Lo Porto, Massimo Dell’Edera, Francesca Petronella, Angela Agostiano, Maria Lucia Curri, and Roberto Comparelli. 2020. "Photocatalytic TiO2-Based Nanostructured Materials for Microbial Inactivation" Catalysts 10, no. 12: 1382. https://doi.org/10.3390/catal10121382
APA StyleDe Pasquale, I., Lo Porto, C., Dell’Edera, M., Petronella, F., Agostiano, A., Curri, M. L., & Comparelli, R. (2020). Photocatalytic TiO2-Based Nanostructured Materials for Microbial Inactivation. Catalysts, 10(12), 1382. https://doi.org/10.3390/catal10121382