Critical Leaf Magnesium Concentrations for Adequate Photosynthate Production of Soilless Cultured Cherry Tomato—Interaction with Potassium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Leaf and Plant Measurements
2.3. Statistical Analysis
3. Results
3.1. Fruit Yield, Biomass, and HI Were Affected by Mg Treatment Concentration
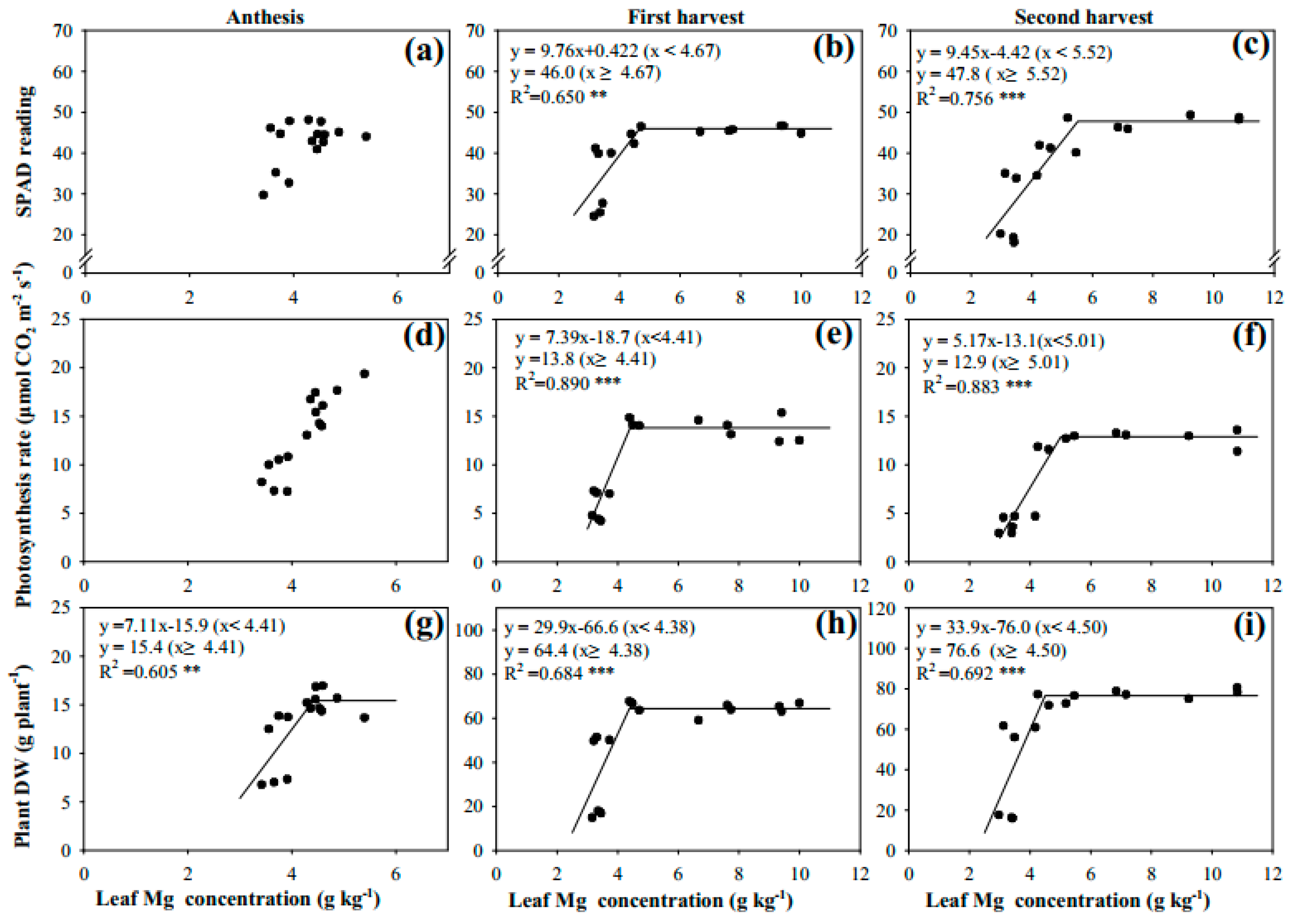
3.2. Leaf Mg Concentration Regulated Leaf Chlorophyll, Photosynthetic Rate, and Plant DW
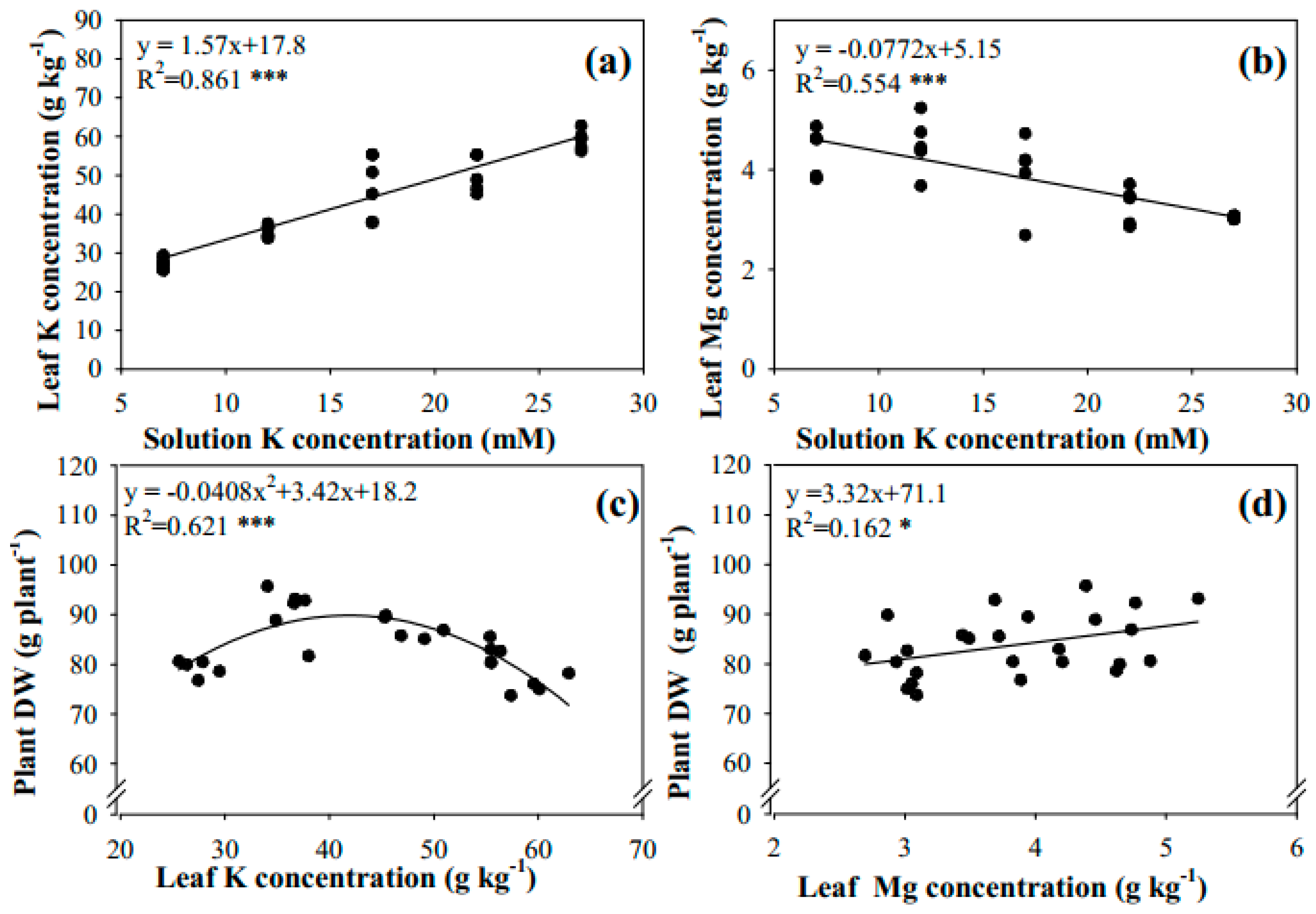
3.3. Oversupply of Mg Reduced Leaf K and Ca Levels, and Limited Plant K and Ca Uptake
3.4. Potassium Supply Affected Leaf K and Mg Levels, in Turn Affecting Plant DW, Mg Uptake, and Fruit Yield
4. Discussion
4.1. Mg Application Affected Photosynthate Production and Distribution
4.2. Relationships among Leaf SPAD Reading, Photosynthetic Rate, Plant DW, and Leaf Mg Concentration
4.3. Two Side Effects of Mg Application on the Plant K and Ca Content
4.4. K Application Influenced Cherry Tomato Growth by Regulating Plant Mg and K
4.5. Mg and K Management in Soilless Vegetable Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sonneveld, C.; Voogt, W. Plant nutrition in future greenhouse production. In Plant Nutrition of Greenhouse Crops; Springer: Dordrecht, The Netherlands, 2009; pp. 393–403. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Good Agricultural Practices for Greenhouse Vegetable Crops. 2013. Available online: http://www.fao.org/publications (accessed on 11 November 2020).
- Putra, P.A.; Yuliando, H. Soilless culture system to support water use efficiency and product quality: A review. Agric. Agric. Sci. Procedia 2015, 3, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.M.; Papadopoulos, A.P. Effects of calcium and magnesium on growth, fruit yield and quality in a fall greenhouse tomato crop grown on rockwool. Can. J. Plant Sci. 2003, 83, 903–912. [Google Scholar] [CrossRef]
- Dorais, M.; Papadoulos, A.P.; Gosselin, A. Greenhouse tomato fruit quality. In Horticulture Review; Timber Press: Portland, OR, USA, 2001; Volume 26, pp. 239–319. [Google Scholar] [CrossRef]
- Gruda, N. Do soilless culture systems have an influence on product quality of vegetables. Invasive Plant Sci. Manag. 2009, 82, 141–147. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.M. Mg: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Savvas, D.; Ntatsi, G.; Passam, H.C. Plant nutrition and physiological disorders in greenhouse grown tomato, pepper and eggplant. Eur. J. Plant Sci. Biotechnol. 2008, 2, 45–61. [Google Scholar]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.Q.; Zhang, F.S.; Li, X.X. Magnesium fertilization improves crop yield in most production systems: A Meta-Analysis. Front. Plant Sci. 2020, 10, 1727. [Google Scholar] [CrossRef] [Green Version]
- Koch, M.; Busse, M.; Naumann, M.; Jákli, B.; Smit, I.; Cakmak, I.; Hermansd, C.; Pawelzik, E. Differential effects of varied potassium and magnesium nutrition on production and partitioning of photoassimilates in potato plants. Physiol. Plant. 2019, 166, 921–935. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef]
- Farhat, N.; Rabhi, M.; Krol, M.; Barhoumi, Z.; Ivanov, A.G.; McCarthy, A.; Abdelly, C.; Smaoui, A.; Huner, N. Starch and sugar accumulation in Sulla carnosa leaves upon Mg2+ starvation. Acta Physiol. Plant. 2014, 36, 2157–2165. [Google Scholar] [CrossRef]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil 2013, 368, 101–128. [Google Scholar] [CrossRef] [Green Version]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems I and II. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermans, C.; Vuylsteke, M.; Coppens, F.; Cristescu, S.M.; Harren, F.J.M.; Inzé, D. Systems analysis of the responses to long-term magnesium deficiency and restoration in Arabidopsis thaliana. New Phytol. 2010, 187, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Hauer-Jákli, M.; Tränkner, M. Critical leaf magnesium thresholds and the impact of magnesium on plant growth and photo-oxidative defense: A systematic review and meta-analysis on 70 years of research. Front. Plant Sci. 2019, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.M.; Papadopoulos, A.P. Effects of calcium and magnesium on plant growth, biomass partitioning, and fruit yield of winter greenhouse tomato. Hortscience 2004, 39, 512–515. [Google Scholar] [CrossRef] [Green Version]
- Chapagain, B.P.; Wiesman, Z. Effect of potassium magnesium chloride in the fertigation solution as partial source of potassium on growth, yield and quality of greenhouse tomato. Sci. Hortic. 2004, 99, 279–288. [Google Scholar] [CrossRef]
- Guo, W.; Chen, S.; Hussain, N.; Cong, Y.; Liang, Z.; Chen, K. Magnesium stress signaling in plant: Just a beginning. Plant Signal. Behav. 2015, 10, 3. [Google Scholar] [CrossRef]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 309–323. [Google Scholar] [CrossRef]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 1–10. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Mg mobility in soils as a challenge for soil and plant analysis, Mg fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef] [Green Version]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J. Eats roots and leaves. Can edible horticultural crops address dietary calcium, magnesium and potassium deficiencies? Proc. Nutr. Soc. 2010, 69, 601–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marschner, H. Mineral nutrition of Higher Plants. In Nutritional Physiology; Academic Elsevier Ltd.: London, UK, 2012; Available online: www.sciencedirect.com/book/9780124735422/mineral-nutrition-of-higher-plants (accessed on 11 November 2020).
- Liu, H.; Meng, F.; Miao, H.; Chen, S.; Yin, T.; Hu, S.; Shao, Z.; Liu, Y.; Gao, L.; Zhu, C.; et al. Effects of postharvest methyl jasmonate treatment on main health-promoting components and volatile organic compounds in cherry tomato fruits. Food Chem. 2018, 263, 194–200. [Google Scholar] [CrossRef]
- Nzanza, B. Yield and Quality of Tomato as Influenced by Differential Ca, Mg and K Nutrition. Ph.D. Thesis, Faculty of Natural and Agricultural Sciences University of Pretoria, Pretoria, South Africa, 2006; pp. 22–39. Available online: http://hdl.handle.net/2263/24683 (accessed on 11 November 2020).
- Rodriguez-Ortega, W.M.; Martinez, V.; Nieves, M.; Simon, I.; Lidon, V.; Fernandez-Zapata, J.C.; Martinez-Nicolas, J.J.; Camara-Zapata, J.M.; Garcia-Sanchez, F. Agricultural and physiological responses of tomato plants grown in different soilless culture systems with saline water under greenhouse conditions. Sci. Rep. 2019, 9, 6733. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Hengeler, C.; Marschner, H. Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Fischer, E.S.; Lohaus, G.; Heineke, D.; Heldt, H.W. Magnesium deficiency results in accumulation of carbohydrates and amino acids in source and sink leaves of spinach. Physiol. Plant. 1998, 102, 16–20. [Google Scholar] [CrossRef]
- Andersson, I. Catalysis and regulation in Rubisco. J. Exp. Bot. 2008, 59, 1555–1568. [Google Scholar] [CrossRef] [Green Version]
- Hannick, A.F.; Waterkeyn, L.; Weissen, F.; van Prag, H.J. Vascular tissue anatomy of Norway spruce needles and twigs in relation to magnesium deficiency. Tree Physiol. 1993, 13, 337–349. [Google Scholar] [CrossRef]
- Hermans, C.; Bourgis, F.; Faucher, M.; Strasser, R.J.; Delrot, S.; Verbruggen, N. Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 2005, 220, 541–549. [Google Scholar] [CrossRef]
- Reuter, D.J.; Robinson, J.B.; Smith, F.W.; Robinson, J.B.; Piggott, T.J.; Price, G.H. Plant Analysis; National Library of Australia Cataloguing-In-Publication Data: Canberra, Australia, 1986; pp. 181–183. [Google Scholar]
- Laing, W.M.; Greer, D.H.; Sun, O.J.; Beets, P.N.; Lowe, A.; Payn, T.W. Physiological Impacts of Mg Deficiency in Pinus radiata growth and photosynthesis. New Phytol. 2000, 146, 47–57. [Google Scholar] [CrossRef]
- Kasinath, B.L.; Ganeshmurthy, A.N.; Nagegowda, N.S. Critical limit of soil and plant magnesium in tomato-growing soils of South Karnataka. J. Hortic. Sci. 2014, 9, 209–212. [Google Scholar]
- Yang, G.; Yang, L.; Jiang, H.; Li, Y.; Wang, P.; Chen, L. Physiological impacts of magnesium-deficiency in Citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Shaul, O.; Hilgemann, D.W.; Almeida-Engler, J.; Van Montagu, M.; Inzé, D.; Galili, G. Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J. 1999, 18, 3973–3980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, I.M.; Sharp, R.E.; Boyer, J.S. Leaf magnesium alters photosynthetic response to low water potentials in sunflower. Plant Physiol. 1987, 84, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Mun, H.I.; Kim, Y.X.; Suh, D.H.; Lee, S.; Singh, D.; Jung, E.S.; Lee, C.H.; Sung, J. Metabolomic response of Perilla frutescens leaves, an edible-medicinal herb, to acclimatize magnesium oversupply. PLoS ONE 2020, 15, e236813. [Google Scholar] [CrossRef] [PubMed]
- Besford, R.T. Uptake and distribution of phosphorus in tomato palnts. Plant Soil 1979, 51, 331–340. [Google Scholar] [CrossRef]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef]
- Wang, X.; Geng, S.; Ma, Y.; Shi, D.; Yang, C.; Wang, H. Growth, photosynthesis, solute accumulation, and ion balance of tomato plant under sodium- or potassium-salt stress and alkali stress. Agron. J. 2015, 107, 651–661. [Google Scholar] [CrossRef]
- Tůma, J.; Skalický, M.; Tůmová, L.; Bláhová, P.; Rosůlková, M. Potassium, magnesium and calcium content in individual parts of Phaseolus vulgaris L. plant as related to potassium and magnesium nutrition. Plant Soil Environ. 2004, 50, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Zengin, M.; Gökmen, F.; Gezgin, S.; Çakmak, İ. Effects of different fertilizers with potassium and magnesium on the yield and quality of potato. Asian J. Chem. 2008, 20, 663–676. [Google Scholar]
- Ding, Y.; Xu, G. Low magnesium with high potassium supply changes sugar partitioning and root growth pattern prior to visible magnesium deficiency in leaves of rice (Oryza sativa L.). Am. J. Plant Sci. 2011, 2, 601–608. [Google Scholar] [CrossRef] [Green Version]
- Toumi, M.; Nedjimi, B.; Halitim, A.; Garcia, M. Effects of K-Mg ratio on growth and cation nutrition of Vitis vinifera L. cv. “Dattier de Beiruth” grafted on SO4 rootstock. J. Plant Nutr. 2016, 39, 907–911. [Google Scholar] [CrossRef]
- Sadowski, A.; Scibisz, K.; Tomala, K.; Kozanecka, T.; Kepka, M. Negative effects of excessive nitrogen and potassium fertilization in a replanted apple orchard. Acta Hortic. 1988, 233, 85–94. [Google Scholar] [CrossRef]
- Yurtseven, E.; Kesmez, G.D.; Ünlükara, A. The effects of water salinity and potassium levels on yield, fruit quality and water consumption of a native central anatolian tomato species (Lycopersicon esculantum). Agric. Water Manag. 2005, 78, 128–135. [Google Scholar] [CrossRef]
- Sonntag, F.; Naumann, M.; Pawelzik, E.; Smit, I. Improvement of cocktail tomato yield and consumer-oriented quality traits by potassium fertilization is driven by the cultivar. J. Sci. Food Agric. 2019, 99, 3350–3358. [Google Scholar] [CrossRef]
- Ali, A.A.; Ikeda, M.; Yamada, Y. Effects of the supply of K, Ca, and Mg on the absorption and assimilation of ammonium- and nitrate-nitrogen in tomato plants. Soil Sci. Plant Nutr. 1991, 37, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Luo, W.; Xu, G. Characterisation of magnesium nutrition and interaction of magnesium and potassium in rice. Ann. Appl. Biol. 2006, 149, 111–123. [Google Scholar] [CrossRef]
- Tränkner, M.; Jákli, B.; Tavakol, E.; Geilfus, C.M.; Cakmak, I.; Dittert, K. Magnesium deficiency decreases biomass water-use efficiency and increases leaf water-use efficiency and oxidative stress in barley plants. Plant Soil 2016, 406, 409–423. [Google Scholar] [CrossRef] [Green Version]
- Farhat, N.; Rabhi, M.; Falleh, H.; Lengliz, K.; Smaoui, A.; Abdelly, C. Interactive effects of excessive potassium and Mg deficiency on safflower. Acta Physiol. Plant. 2013, 35, 2737–2745. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R.; Huang, S.; Jin, J. Effects of potassium application on flavor compounds of cherry tomato fruits. J. Plant Nutr. 2009, 32, 1451–1468. [Google Scholar] [CrossRef]
- Constán-Aguilar, C.; Leyva, R.; Blasco, B.; Sánchez-Rodríguez, E.; Soriano, T.; Ruiz, J.M. Biofortification with potassium: Antioxidant responses during postharvest of cherry tomato fruits in cold storage. Acta Physiol. Plant. 2014, 36, 283–293. [Google Scholar] [CrossRef]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef] [Green Version]
| Mg Concentration in Solution (mM) | Yield | Plant Dry Weight | Harvest Index | |||
|---|---|---|---|---|---|---|
| (g·Plant−1 FW) | (g·Plant−1) | (%) | ||||
| First Harvest | Second Harvest | First Harvest | Second Harvest | First Harvest | Second Harvest | |
| 0 | 29c † | 67d | 16.8e | 16.8d | 21.9b | 31.2c |
| 0.5 | 172b | 187c | 50.6d | 59.7c | 26.3a | 24.4d |
| 1 | 290a | 398a | 66.2ab | 75.5ab | 27.9a | 41.1a |
| 2 | 281a | 425a | 63.2b | 76.5ab | 26.1a | 42.7a |
| 4 | 293a | 397a | 65.3ab | 78.2a | 26.9a | 39.6ab |
| 8 | 284a | 335b | 67.2a | 73.8b | 27.3a | 35.4bc |
| 16 | 152b | 162c | 57.4c | 59.4c | 17.7c | 21.3d |
| Mg Concentration in Solution (mM) | Leaf Mg Concentration (g·kg−1) | SPAD Reading | Photosynthetic Rate (μmol CO2·m−2·s−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Anthesis | First Harvest | Second Harvest | Anthesis | First Harvest | Second Harvest | Anthesis | First Harvest | Second Harvest | |
| 0 | 3.66d † | 3.31f | 3.25e | 32.6b | 26.0d | 19.3e | 7.2d | 4.5d | 3.4c |
| 0.5 | 3.73d | 3.40f | 3.58e | 46.3a | 40.4c | 34.5d | 10.6c | 7.2c | 4.7b |
| 1 | 4.46cd | 4.52e | 4.76de | 46.3a | 44.6b | 41.2c | 14.1b | 14.4a | 12.2a |
| 2 | 4.46cd | 7.33d | 6.39d | 44.1a | 45.6ab | 47.0b | 15.5b | 14.0a | 13.1a |
| 4 | 4.90c | 9.57c | 10.3c | 43.4a | 46.1ab | 48.8a | 17.8a | 13.5a | 12.7a |
| 8 | 6.89b | 13.2b | 14.5b | 44.3a | 47.0a | 49.7a | 18.0a | 12.9a | 12.5a |
| 16 | 7.95a | 16.6a | 17.0a | 46.1a | 47.4a | 48.6ab | 19.1a | 8.9b | 11.9a |
| Mg Concentration in Solution (mM) | Leaf Nutrient Concentration (g·kg−1) | Fruit Nutrient Concentration (g·kg−1) | Plant Nutrient Uptake (mg·Plant−1) | |||||
|---|---|---|---|---|---|---|---|---|
| K | Ca | Mg | K | Ca | Mg | K | Ca | |
| 0 | 35.2b † | 8.68d | 2.39a | 48.2b | 2.33a | 31f | 570e | 95e |
| 0.5 | 42.2a | 14.4b | 2.38a | 59.5a | 1.80b | 135e | 2437c | 536b |
| 1 | 43.4a | 17.8a | 2.39a | 55.9ab | 1.90b | 196d | 3229a | 591a |
| 2 | 42.5a | 17.0a | 2.33a | 50.4ab | 1.26c | 232c | 3102ab | 515b |
| 4 | 37.3b | 15.3b | 2.49a | 52.4ab | 1.17c | 350b | 3138ab | 521b |
| 8 | 37.7b | 14.2b | 2.71a | 53.6ab | 1.28c | 454a | 2869b | 444c |
| 16 | 30.0c | 11.5c | 3.00a | 49.5ab | 1.03c | 456a | 1961d | 334d |
| K Concentrations in Solution (mM) | |||||
|---|---|---|---|---|---|
| 7 | 12 | 17 | 22 | 27 | |
| Cherry tomato fruit † |  | ||||
| Yield (g plant−1 FW) | 552ab | 579a | 499bc | 493c | 463c |
| MFR (%) | 86.1b | 92.4a | 87.0b | 85.6b | 84.9b |
| Plant DW (g plant−1) | 79.3b | 92.6a | 84.3ab | 85.4ab | 77.2b |
| HI (%) | 54.1a | 48.6b | 46.0b | 44.9b | 46.6b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, X.; Liu, D.; Liu, B.; Wu, C.; Liu, C.; Wang, X.; Zou, C.; Chen, X. Critical Leaf Magnesium Concentrations for Adequate Photosynthate Production of Soilless Cultured Cherry Tomato—Interaction with Potassium. Agronomy 2020, 10, 1863. https://doi.org/10.3390/agronomy10121863
Guan X, Liu D, Liu B, Wu C, Liu C, Wang X, Zou C, Chen X. Critical Leaf Magnesium Concentrations for Adequate Photosynthate Production of Soilless Cultured Cherry Tomato—Interaction with Potassium. Agronomy. 2020; 10(12):1863. https://doi.org/10.3390/agronomy10121863
Chicago/Turabian StyleGuan, Xilin, Dunyi Liu, Bin Liu, Changchun Wu, Chuanyun Liu, Xiaozhong Wang, Chunqin Zou, and Xinping Chen. 2020. "Critical Leaf Magnesium Concentrations for Adequate Photosynthate Production of Soilless Cultured Cherry Tomato—Interaction with Potassium" Agronomy 10, no. 12: 1863. https://doi.org/10.3390/agronomy10121863





