Genome-Wide Analysis of the β-Amylase Gene Family in Brassica L. Crops and Expression Profiles of BnaBAM Genes in Response to Abiotic Stresses
Abstract
:1. Background
2. Materials and Methods
2.1. Identification of BAM Genes in B. napus, B.oleracea and B. rapa
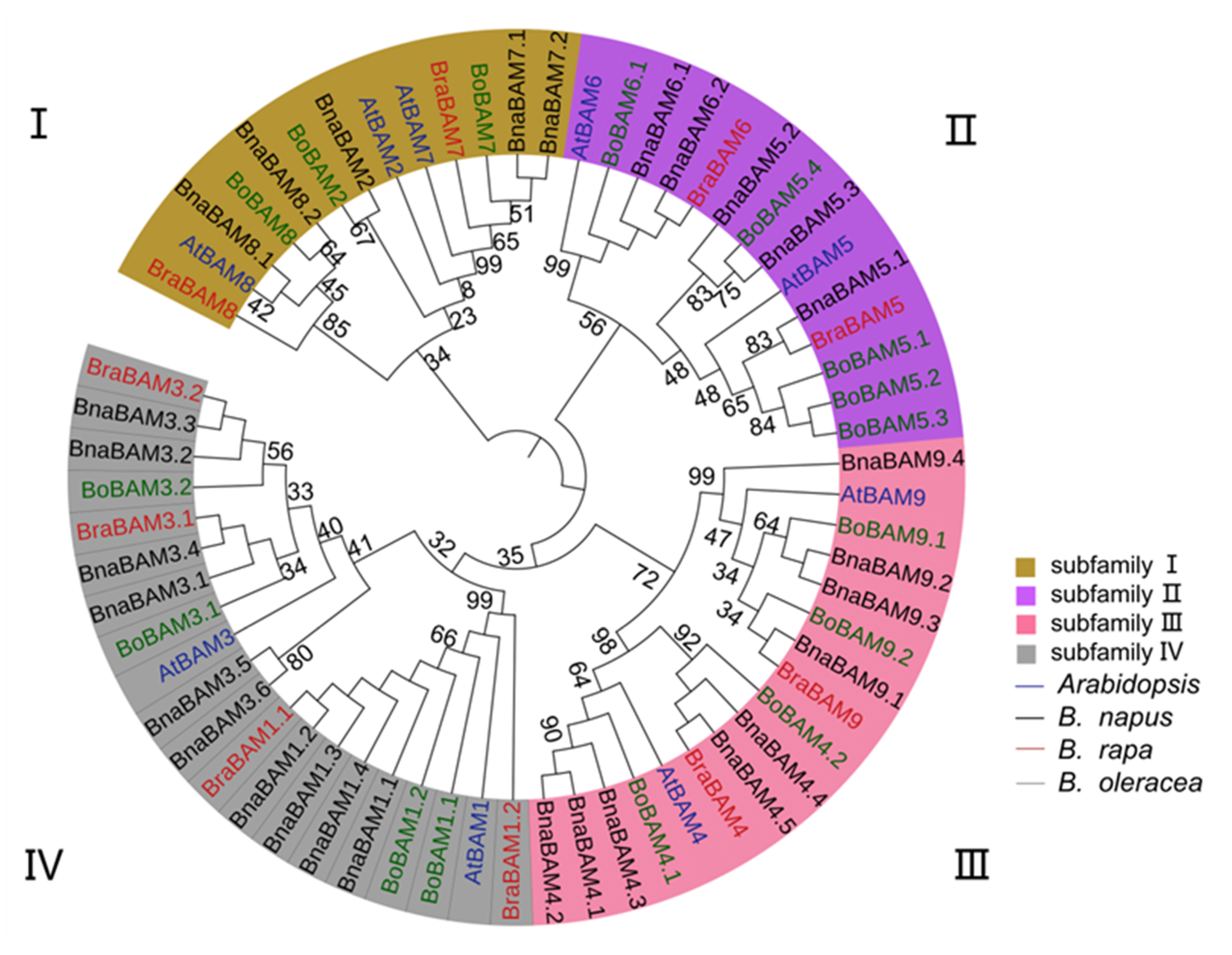
2.2. Phylogenetic Analysis
2.3. Gene Structure, Chromosomal Location and Synteny Analysis
2.4. Materials and Treatments
2.5. Expression Analysis
2.6. Starch Content and β-Amylase Activity Determination
2.7. Statistical Analysis
3. Results
3.1. Characterization of BAM Genes in Brassica Crops
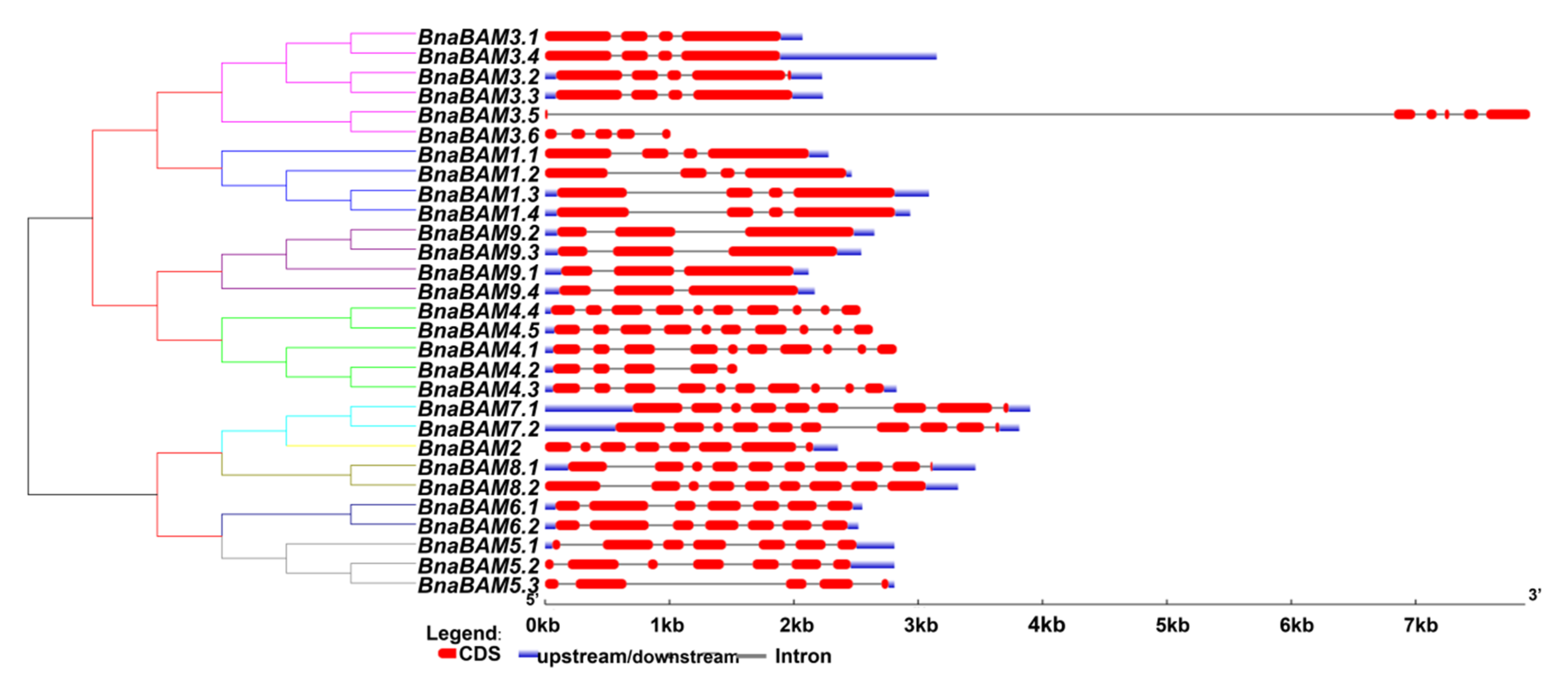
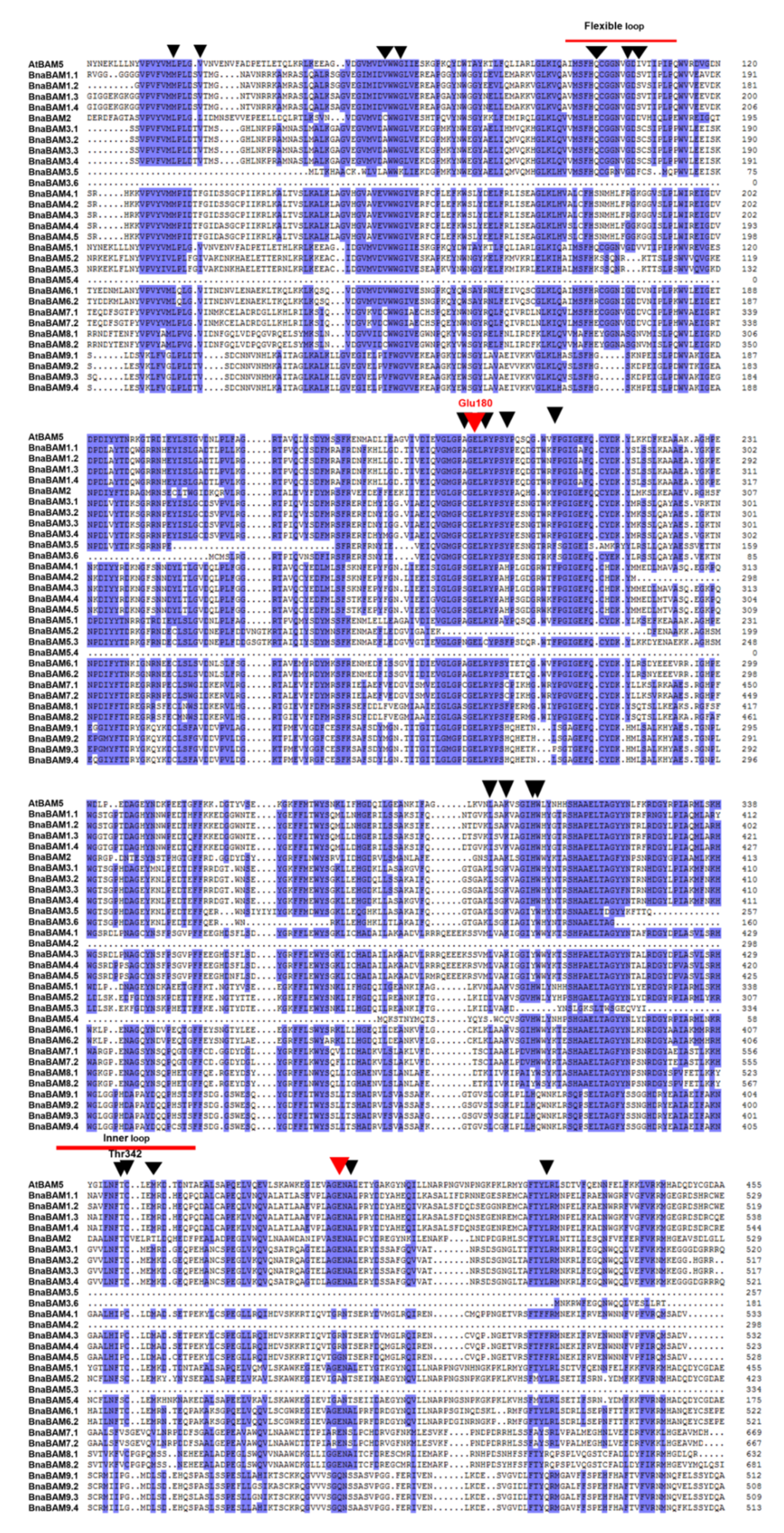
3.2. Identification of the Gene Structure and Conserved Regions of BAM Genes
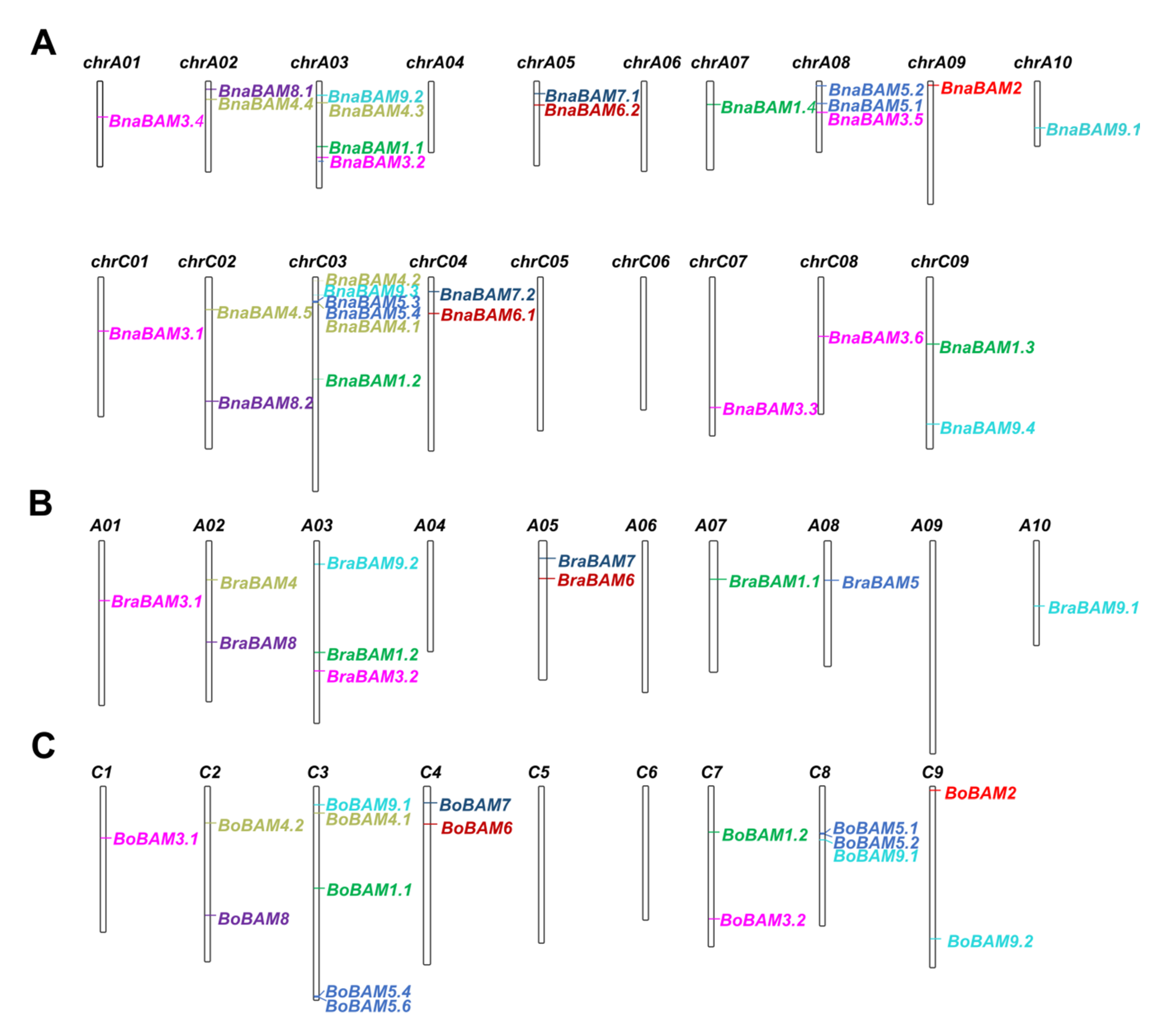
3.3. Chromosomal Localization Analysis of the BAM Gene Family in Brassica Crops
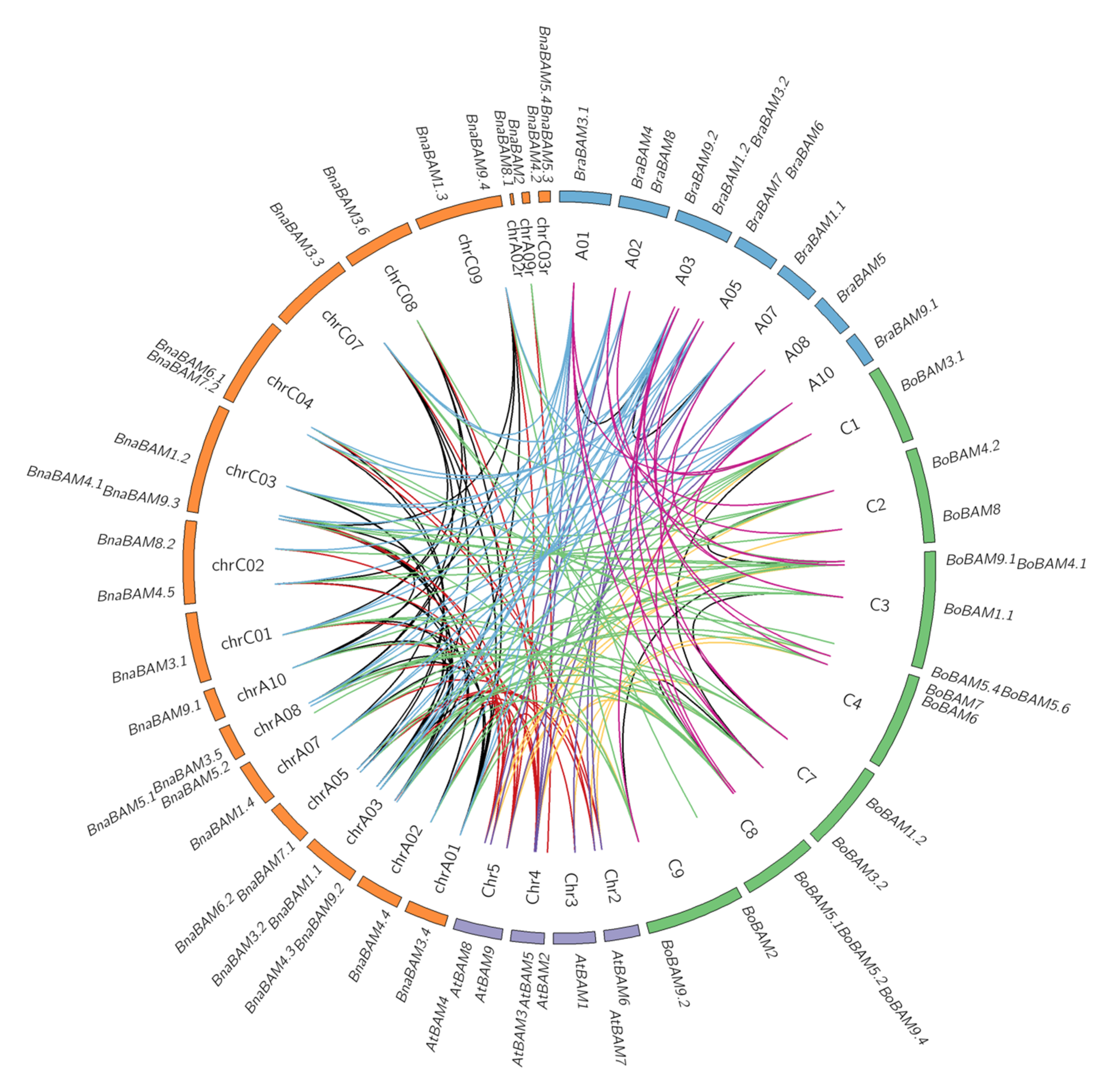
3.4. Collinearity Analysis of Detected BAM Genes
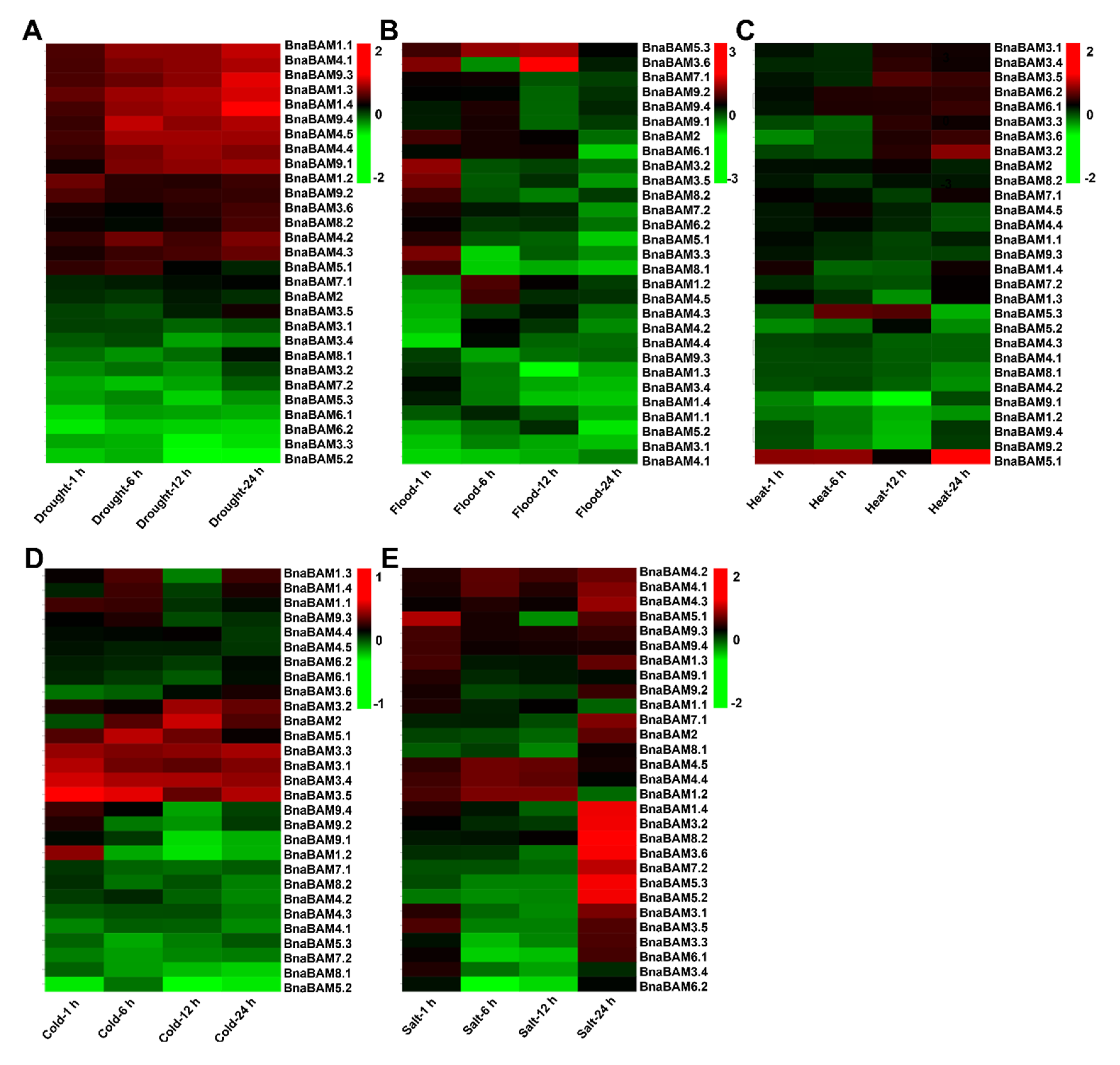
3.5. BnaBAM Genes Response to Various Abiotic Stresses
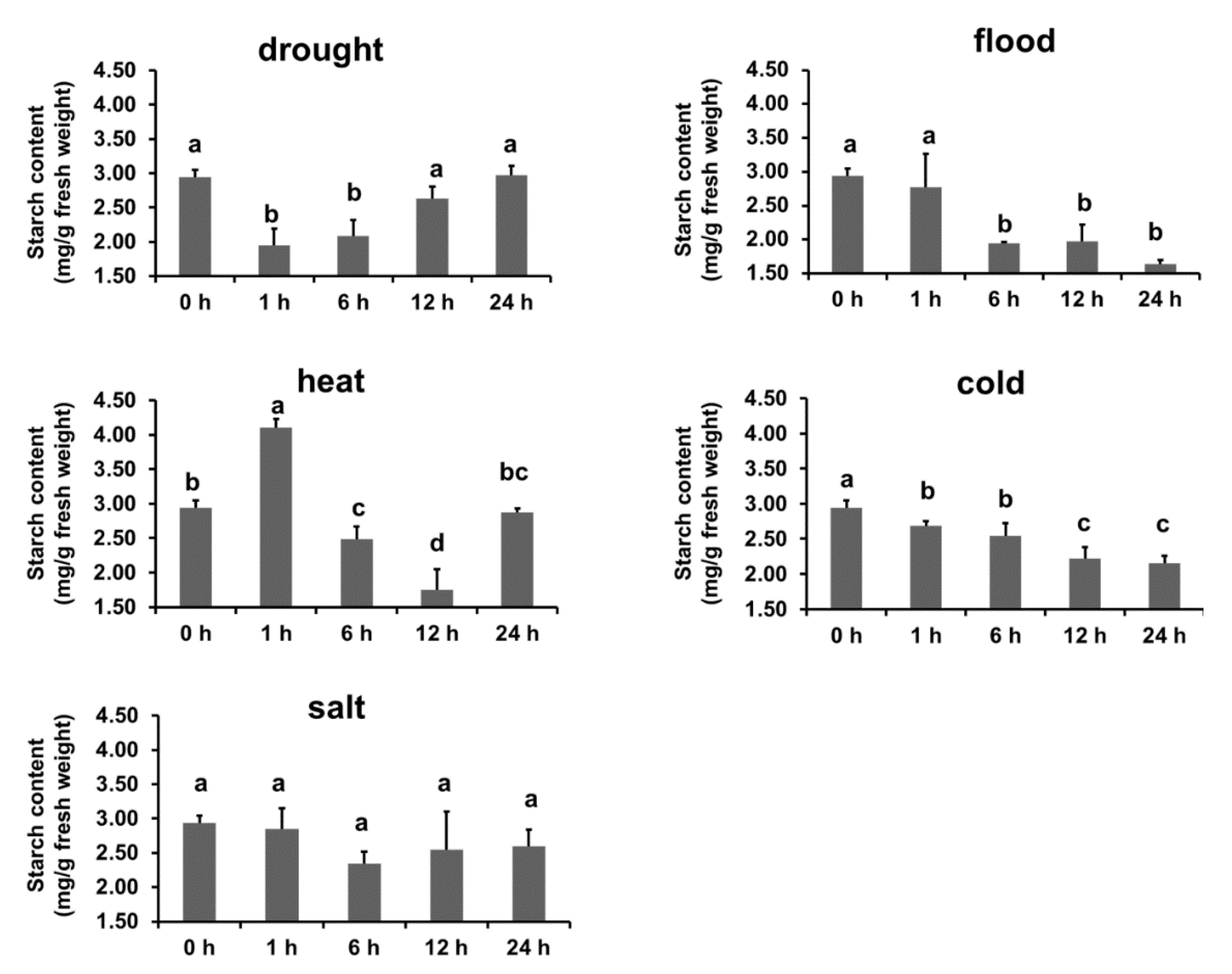
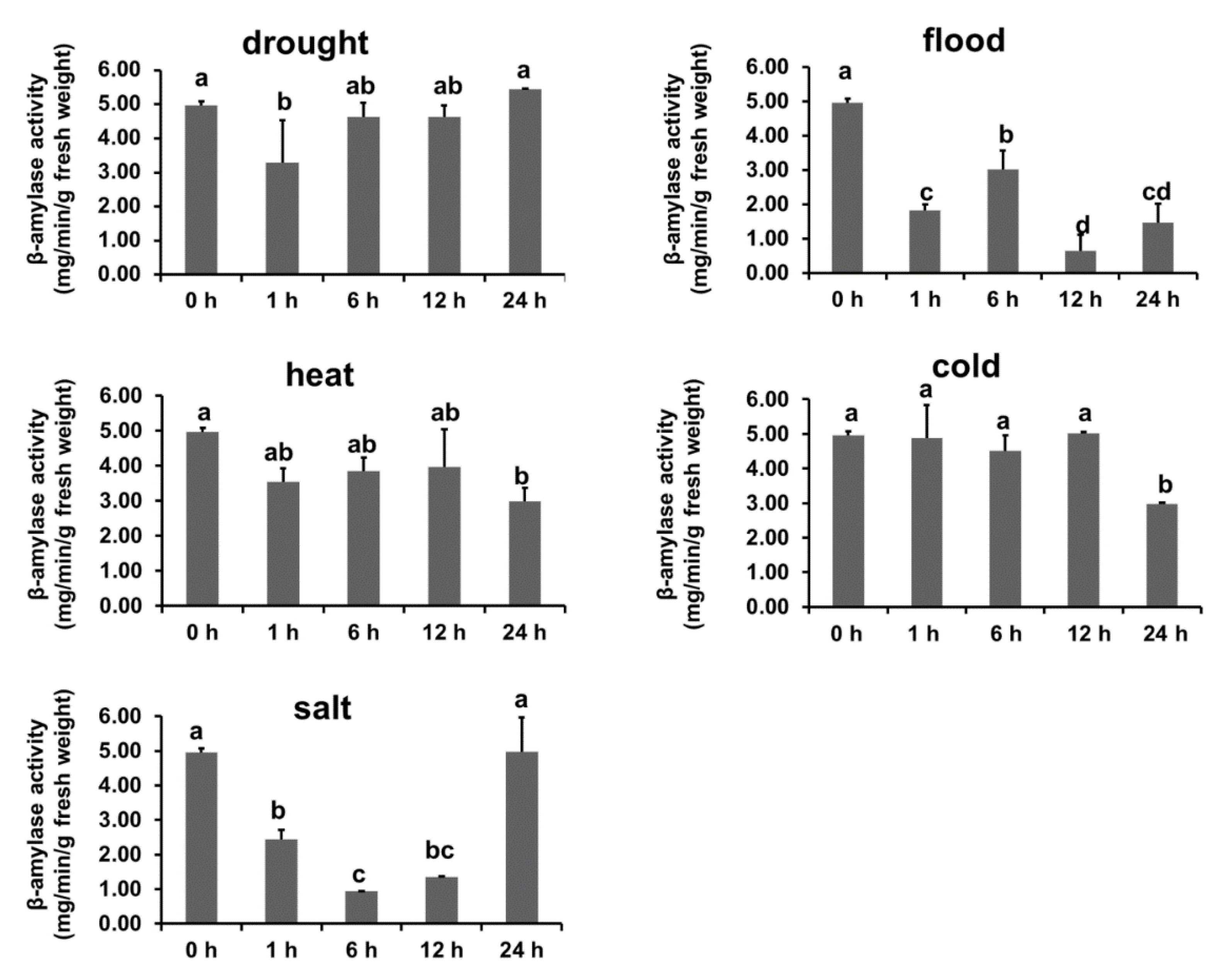
3.6. Abiotic Stresses Affect Starch Content and β-Amylase Activity in B. napus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gong, Z.Z.; Xiong, L.M.; Shi, H.Z.; Yang, S.H.; Herrera-Estrella, L.R.; Xu, G.H.; Chao, D.Y.; Li, J.R.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Monroe, J.D.; Storm, A.R.; Badley, E.M.; Lehman, M.D.; Platt, S.M.; Saunders, L.K.; Schmitz, J.M.; Torres, C.E. β-Amylase1 and β-Amylase3 are Plastidic Starch Hydrolases in Arabidopsis That Seem to Be Adapted for Different Thermal, pH, and Stress Conditions. Plant Physiol. 2014, 166, 1748–1763. [Google Scholar] [CrossRef] [Green Version]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Watkinson, J.I.; Hendricks, L.; Sioson, A.A.; Heath, L.S.; Bohnert, H.J.; Grene, R. Tuber development phenotypes in adapted and acclimated, drought-stressed Solanum tuberosum ssp. andigena have distinct expression profiles of genes associated with carbon metabolism. Plant Physiol. Biochem. 2008, 46, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Hummel, I.; Pantin, F.; Sulpice, R.; Piques, M.; Rolland, G.; Dauzat, M.; Christophe, A.; Pervent, M.; Bouteille, M.; Stitt, M.; et al. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: An integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol. 2010, 154, 357–372. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Zhu, Q.S. Activities of starch hydrolytic enzymes and sucrose-phosphate synthase in the stems of rice subjected to water stress during grain filling. J. Exp. Bot. 2001, 52, 2169–2179. [Google Scholar] [CrossRef]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.; Pereira, A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kolling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valerio, C.; Costa, A.; Marri, L.; Issakidis-Bourguet, E.; Pupillo, P.; Trost, P.; Sparla, F. Thioredoxin-regulated β-amylase (BAM1) triggers diurnal starch degradation guard cells, and in mesophyll cells under osmotic stress. J. Exp. Bot. 2011, 62, 545–555. [Google Scholar] [CrossRef] [Green Version]
- Vasseur, F.; Pantin, F.; Vile, D. Changes in light intensity reveal a major role for carbon balance in Arabidopsis responses to high temperature. Plant Cell Environ. 2011, 34, 1563–1576. [Google Scholar] [CrossRef]
- Kaplan, F.; Guy, C.L. β-amylase induction and the protective role of maltose during temperature shock. Plant Physiol. 2004, 135, 1674–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempa, S.; Krasensky, J.; Dal, S.S.; Kopka, J.; Jonak, C. A central role of abscisic acid in stress-regulated carbohydrate metabolism. PLoS ONE 2008, 3, e3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.J.; Chen, J.Y.; Wang, S.J. Molecular regulation of starch accumulation in rice seedling leaves in response to salt stress. Acta Physiol. Plant. 2008, 30, 135–142. [Google Scholar] [CrossRef]
- Scarpeci, T.E.; Valle, E.M. Rearrangement of carbon metabolism in Arabidopsis thaliana subjected to oxidative stress condition: An emergency survival strategy. Plant Growth Regul. 2008, 54, 133–142. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [Green Version]
- Hoermiller, I.I.; Naegele, T.; Augustin, H.; Stutz, S.; Weckwerth, W.; Heyer, A.G. Subcellular reprogramming of metabolism during cold acclimation in Arabidopsis thaliana. Plant Cell Environ. 2016, 40, 602–610. [Google Scholar] [CrossRef]
- Kaplan, F.; Guy, C.L. RNA interference of Arabidopsis beta-amylase8 prevents maltose accumulation upon cold shock and increases sensitivity of PSII photochemical efficiency to freezing stress. Plant J. 2005, 44, 730–743. [Google Scholar] [CrossRef]
- Zanella, M.; Borghi, G.L.; Pirone, C.; Thalmann, M.; Pazmino, D.; Costa, A.; Santelia, D.; Trost, P.; Sparla, F. β-amylase1 (BAM1) degrades transitory starch to sustain proline biosynthesis during drought stress. J. Exp. Bot. 2016, 67, 1819–1826. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.Y.; Gong, X.; Gao, J.Z.; Dong, H.Z.; Zhang, S.L.; Tao, S.T.; Huang, X.S. Transcriptomic and evolutionary analyses of white pear (Pyrusbretschneideri) β-amylase genes reveals their importance for cold and drought stress responses. Gene 2019, 20, 102–113. [Google Scholar] [CrossRef]
- Stitt, M.; Zeeman, S.C. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292. [Google Scholar] [CrossRef]
- Streb, S.; Zeeman, S.C. Starch metabolism in Arabidopsis. Arab. Book 2012, 10, 73–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulton, D.C.; Stettler, M.; Mettler, T.; Vaughan, C.K.; Li, J.; Francisco, P.; Gil, M.; Reinhold, H.; Eicke, S.; Messerli, G.; et al. β-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active β-amylases in Arabidopsis chloroplasts. Plant Cell 2008, 20, 1040–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroe, J.D.; Breault, J.S.; Pope, L.E.; Torres, C.E.; Gebrejesus, T.B.; Berndsen, C.E.; Storm, A.R. Arabidopsis β-amylase2 is a K+-requiring, catalytic tetramer with sigmoidal kinetics. Plant Physiol. 2017, 175, 1525–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lao, N.T.; Schoneveld, O.; Mould, R.M.; Hibberd, J.M.; Gray, J.C.; Kavanagh, T.A. An Arabidopsis gene encoding a chloroplast-targeted β-amylase. Plant J. 1999, 20, 519–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Francisco, P.; Zhou, W.X.; Edner, C.; Steup, M.; Ritte, G.; Bond, C.S.; Smith, S.M. Catalytically-inactive β-amylase BAM4 required for starch breakdown in Arabidopsis leaves is a starch-binding-protein. Arch. Biochem. Biophys. 2009, 489, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Steidle, E.A. Investigation of the Role of BAM9 in Starch Metabolism in Arabidopsis thaliana. Master’s Thesis, James Madison University, Harrisonburg, VA, USA, 2010. [Google Scholar]
- Soyk, S.; Simkova, K.; Zurcher, E.; Luginbuhl, L.; Brand, L.H.; Vaughan, C.K.; Wanke, D.; Zeeman, S.C. The Enzyme-Like Domain of Arabidopsis Nuclear β-Amylases Is Critical for DNA Sequence Recognition and Transcriptional Activation. Plant Cell 2014, 26, 1746–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhold, H.; Soyk, S.; Simkova, K.; Hostettler, C.; Marafino, J.; Mainiero, S.; Vaughan, C.K.; Monroe, J.D.; Zeeman, S.C. β-Amylase-Like Proteins Function as Transcription Factors in Arabidopsis, Controlling Shoot Growth and Development. Plant Cell 2011, 23, 1391–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Zhang, H.L.; Liu, J.; Reid, S.; Liu, T.F.; Xu, S.J.; Tian, Z.D.; Sonnewald, U.; Song, B.; Xie, C.H. Amylases StAmy23, StBAM1 and StBAM9 regulate cold-induced sweetening of potato tubers in distinct ways. J. Exp. Bot. 2017, 68, 2317–2331. [Google Scholar] [CrossRef] [Green Version]
- Niittyla, T.; Messerli, G.; Trevisan, M.; Chen, J.; Smith, A.M.; Zeeman, S.C. A Previously Unknown Maltose Transporter Essential for Starch Degradation in Leaves. Science 2004, 303, 87–89. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, M.; Hu, D.; Yang, Z.Y.; Ma, S.Q.; Li, X.H.; Xiong, L.Z. The OsMYB30 Transcription Factor Suppresses Cold Tolerance by Interacting with a JAZ Protein and Suppressing β-Amylase Expression. Plant Physiol. 2017, 173, 1475–1491. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Thompson, W.A. OsmiR528 Enhances Cold Stress Tolerance by Repressing Expression of Stress Response-related Transcription Factor Genes in Plant Cells. Curr. Genom. 2019, 20, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zhu, X.F.; Duan, N.; Liu, J.H. PtrBAM1, a β-amylase-coding gene of Poncirus trifoliata, is a CBF regulon member with function in cold tolerance by modulating soluble sugar levels. Plant Cell Environ. 2014, 37, 2754–2767. [Google Scholar] [CrossRef] [PubMed]
- Chalhoub, B.; Denoeud, F.; Liu, S.Y.; Parkin, I.A.P.; Tang, H.B.; Wang, X.Y.; Chiquet, J.; Belcram, H.; Tong, C.B.; Samans, B.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georges, F.; Das, S.; Ray, H.; Bock, C.; Nokhrina, K.; Kolla, V.A.; Keller, W. Over-expression of Brassica napus phosphatidylinositol-phospholipase C2 in canola induces significant changes in gene expression and phytohormone distribution patterns, enhances drought tolerance and promotes early flowering and maturation. Plant Cell Environ. 2009, 32, 1664–1681. [Google Scholar] [CrossRef] [PubMed]
- Diksaityte, A.; Virsile, A.; Zaltauskaite, J.; Januskaitiene, I.; Juozapaitiene, G. Growth and photosynthetic responses in Brassica napus differ during stress and recovery periods when exposed to combined heat, drought and elevated CO2. Plant Physiol. Biochem. 2019, 142, 59–72. [Google Scholar] [CrossRef]
- Wollmer, A.C.; Pitann, B.; Muhling, K.H. Timing of Waterlogging Is Crucial for the Development of Micronutrient Deficiencies or Toxicities in Winter Wheat and Rapeseed. J. Plant Growth Regul. 2019, 38, 824–830. [Google Scholar] [CrossRef]
- Cui, J.Q.; Hua, Y.P.; Zhou, T.; Liu, Y.; Huang, J.Y.; Yue, C.P. Global Landscapes of the Na+/H+ Antiporter (NHX) Family Members Uncover their Potential Roles in Regulating the Rapeseed Resistance to Salt Stress. Int. J. Mol. Sci. 2020, 21, 3429. [Google Scholar] [CrossRef]
- Yan, L.; Shah, T.; Cheng, Y.; Lv, Y.; Zhang, X.K.; Zou, X.L. Physiological and molecular responses to cold stress in rapeseed (Brassica napus L.). J. Integr. Agric. 2019, 18, 2742–2752. [Google Scholar] [CrossRef]
- Huang, R.Z.; Liu, Z.H.; Xing, M.Q.; Yang, Y.; Wu, X.L.; Liu, H.Q.; Liang, W.F. Heat Stress Suppresses Brassica napus Seed Oil Accumulation by Inhibition of Photosynthesis and BnWRI1 Pathway. Plant Cell Physiol. 2019, 60, 1457–1470. [Google Scholar] [CrossRef]
- Smith, S.M.; Fulton, D.C.; Chia, T.; Thorneycroft, D.; Chapple, A.; Dunstan, H.; Hylton, C.; Zeeman, S.C.; Smith, A.M. Diurnal Changes in the Transcriptome Encoding Enzymes of Starch Metabolism Provide Evidence for Both Transcriptional and Posttranscriptional Regulation of Starch metabolism in Arabidopsis leaves. Plant Physiol. 2004, 136, 2687–2699. [Google Scholar] [CrossRef] [Green Version]
- Koide, T.; Ohnishi, Y.; Horinouchi, S. Characterization of recombinant β-amylases from Oryza sativa. Bios. Biot. Bioch. 2011, 75, 793–796. [Google Scholar] [CrossRef] [Green Version]
- Monroe, J.D.; Storm, A.R. Review: The Arabidopsis β-amylase (BAM) gene family: Diversity of form and function. Plant Sci. 2018, 276, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, A.; Nong, H.V.; Kadokawa, H.; Kim, C.S.; Itoh, Y.; Fukazawa, C. Residues essential for catalytic activity of soybean β-amylase. Eur. J. Biochem. 1994, 221, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Liu, M.M.; Ran, F.; Guo, P.C.; Ke, Y.Z.; Wu, Y.W.; Wen, J.; Li, P.F.; Li, J.N.; Du, H. Global Analysis of WOX Transcription Factor Gene Family in Brassica napus Reveals Their Stress- and Hormone-Responsive Patterns. Int. J. Mol. Sci. 2018, 19, 3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clamp, M.; Cuff, J.; Searle, S.M.; Barton, G.J. The Jalview Java alignment editor. Bioinformatics 2004, 20, 426–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, L.M.; Gao, Y.Y.; Zhao, K.; Kong, L.J.; Yu, S.B.; Li, R.R.; Liu, K.W.; Yu, X.L. Comparative analysis of basic helix-loop-helix gene family among Brassica oleracea, Brassica rapa, and Brassica napus. BMC Genom. 2020, 21, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Chen, Y.; Fang, H.D.; Shi, H.F.; Chen, K.P.; Zhang, Z.Y.; Tan, X.L. Selection of reference genes for quantitative reverse-transcription polymerase chain reaction normalization in Brassica napus under various stress conditions. Mol. Genet. Genom. 2014, 289, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.N.; Adachi, M.; Utsumi, S.; Mikami, B. The roles of Glu186 and Glu380 in the catalytic reaction of soybean beta-amylase. J. Mol. Biol. 2004, 339, 1129–1140. [Google Scholar] [CrossRef]
- Kang, Y.N.; Tanabe, A.; Adachi, M.; Utsumi, S.; Mikami, B. Structural Analysis of Threonine 342 Mutants of Soybean β-Amylase: Role of a Conformational Change of the Inner Loop in the Catalytic Mechanism. Biochemistry 2005, 44, 5106–5116. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, M.; Coiro, M.; Meier, T.; Wicker, T.; Zeeman, S.C.; Santelia, D. The evolution of functional complexity within the β-amylase gene family in land plants. BMC Evol. Biol. 2019, 19, 66. [Google Scholar] [CrossRef] [Green Version]
- Cheung, F.; Trick, M.; Drou, N.; Lim, Y.P.; Park, J.Y.; Kwon, S.; Kim, J.A.; Scott, R.; Pires, J.C.; Paterson, A.H.; et al. Comparative Analysis between Homoeologous Genome Segments of Brassica napus and Its Progenitor Species Reveals Extensive Sequence-Level Divergence. Plant Cell 2009, 21, 1912–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.Y.; Tehrim, S.; Zhang, F.Q.; Tong, C.B.; Huang, J.Y.; Cheng, X.H.; Dong, C.H.; Zhou, Y.Q.; Qin, R.; Hua, W.; et al. Genome-wide comparative analysis of NBS-encoding genes between Brassica species and Arabidopsis thaliana. BMC Genom. 2014, 15, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Kang, K.; Gan, L.; Ning, S.B.; Xiong, J.Y.; Song, S.Y.; Xi, L.Z.; Lai, S.Y.; Yin, Y.G.; Gu, J.W.; et al. Drought-responsive genes, late embryogenesis abundant group3 (LEA3) and vicinal oxygen chelate, function in lipid accumulation in Brassica napus and Arabidopsis mainly via enhancing photosynthetic efficiency and reducing ROS. Plant Biotechnol. J. 2019, 17, 2123–2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahal, K.; Gadapati, W.; Savitch, L.V.; Singh, J.; Huner, N.P.; Norman, P.A. Cold acclimation and BnCBF17-over-expression enhance photosynthetic performance and energy conversion efficiency during long-term growth of Brassica napus under elevated CO2 conditions. Planta 2012, 236, 1639–1652. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.L.; Tang, S.H.; Mei, F.L.; Peng, X.J.; Li, J.; Li, X.F.; Yan, X.H.; Zeng, X.H.; Liu, F.; Wu, Y.H.; et al. BnSIP1-1, a Trihelix Family Gene, Mediates Abiotic Stress Tolerance and ABA Signaling in Brassica napus. Front. Plant Sci. 2017, 8, e71136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Chen, H.Y.; Cai, W.M. BnNAC485 is involved in abiotic stress responses and flowering time in Brassica Napus. Plant Physiol. Biochem. 2014, 79, 77–78. [Google Scholar]
- Liu, L.D.; Ding, Q.Y.; Liu, J.; Yang, C.L.; Chen, H.; Zhang, S.F.; Zhu, J.C.; Wang, D.J. Brassica napus COL transcription factor BnCOL2 negatively affects the tolerance of transgenic Arabidopsis to drought stress. Environ. Exp. Bot. 2020, 178, 104171. [Google Scholar] [CrossRef]
- Mita, S.; Hirano, H.; Murano, N.; Nakamura, K. Genetic analysis on sugar-induced expression of a gene for β-amylase in Arabidopsis thaliana. Plant Cell Physiol. 1995, 36, S83. [Google Scholar]
- Bang, C.B.; Huyen, T.T.T. Analysis of β-amylase gene family in soybean (Glycine max). Tap. Chi. Sinh. Hoc. 2015, 37, 165–176. [Google Scholar] [CrossRef]
- Miao, H.X.; Sun, P.G.; Miao, Y.L.; Liu, J.H.; Zhang, J.B.; Jia, C.H.; Wang, J.K.; Wang, Z.; Jin, Z.Q.; Xu, B.Y. Genome-wide identification and expression analysis of the β-amylase genes strongly associated with fruit development, ripening, and abiotic stress response in two banana cultivars. Front. Agric. Sci. Eng. 2016, 3, 346–356. [Google Scholar] [CrossRef] [Green Version]
- Mason-Gamer, R.J. The beta-amylase genes of grasses and a phylogenetic analysis of the Triticeae (Poaceae). Am. J. Bot. 2005, 92, 1045–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowers, J.E.; Chapman, B.A.; Rong, J.; Paterson, A.H. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 2003, 422, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.Y.; Liu, D.F.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Balibrea, M.E.; Amico, J.D.; Bolarin, M.C.; Perez-Alfocea, F. Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol. Plant. 2000, 110, 503–511. [Google Scholar] [CrossRef]
- Kerepesi, I.; Galiba, G. Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci. 2000, 40, 482–487. [Google Scholar] [CrossRef]
- Liu, T.; Staden, J.V. Partitioning of carbohydrates in salt-sensitive and salt-tolerant soybean callus cultures under salinity stress and its subsequent relief. Plant Growth Regul. 2001, 33, 13–17. [Google Scholar] [CrossRef]
- Brumos, J.; Colmenero-Flores, J.M.; Conesa, A.; Izquierdo, P.; Sanchez, G.; Iglesias, D.J.; Lopez-Climent, M.F.; Gomez-Cadenas, A.; Talon, M. Membrane transporters and carbon metabolism implicated in chloride homeostasis differentiate salt stress responses in tolerant and sensitive Citrus rootstocks. Funct. Integr. Genom. 2009, 9, 293–309. [Google Scholar] [CrossRef]
- Gonzalez-Cruz, J.; Pastenes, C. Water-stress-induced thermotolerance of photosynthesis in bean (Phaseolus vulgaris L.) plants: The possible involvement of lipid composition and xanthophyll cycle pigments. Environ. Exp. Bot. 2012, 77, 127–140. [Google Scholar] [CrossRef]
- Goyal, A. Osmoregulation in Dunaliella, Part II: Photosynthesis and starch contribute carbon for glycerol synthesis during a salt stress in Dunaliella tertiolecta. Plant Physiol. Biochem. 2007, 45, 705–710. [Google Scholar] [CrossRef]
- Horrer, D.; Flütsch, S.; Pazmino, D.; Matthews, J.S.A.; Thalmann, M.; Nigro, A.; Leonhardt, N.; Lawson, T.; Santelia, D. Blue Light Induces a Distinct Starch Degradation Pathway in Guard Cells for Stomatal Opening. Curr. Biol. 2016, 26, 362–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Serial No. | Gene Name | Accession Numbers | ORF (bp) | Introns No. | Genomic Location | Protein | PI | |
|---|---|---|---|---|---|---|---|---|
| Length (a.a) | MW (kDa) | |||||||
| 1 | BraBAM1.1 | Bra015025 | 1713 | 3 | A07: 4200669-4203428 | 570 | 63.38 | 5.31 |
| 2 | BraBAM1.2 | Bra001937 | 1695 | 3 | A03: 19461784-19463944 | 564 | 62.99 | 5.68 |
| 3 | BraBAM3.1 | Bra026230 | 1650 | 3 | A01: 10498540-10500425 | 549 | 61.72 | 6.98 |
| 4 | BraBAM3.2 | Bra012676 | 1647 | 3 | A03: 22804184-22806081 | 548 | 61.45 | 6.28 |
| 5 | BraBAM4 | Bra035586 | 1587 | 9 | A02: 7408259-7410757 | 528 | 59.80 | 7.54 |
| 6 | BraBAM5 | Bra038088 | 1497 | 6 | A08: 6619783-6622218 | 498 | 56.18 | 5.07 |
| 7 | BraBAM6 | Bra005581 | 1746 | 6 | A05: 6338859-6341224 | 581 | 66.53 | 5.58 |
| 8 | BraBAM7 | Bra004944 | 2019 | 8 | A05: 2587413-2590431 | 672 | 75.08 | 5.64 |
| 9 | BraBAM8 | Bra021962 | 1306 | 8 | A02: 17928394-17931297 | 653 | 73.63 | 5.60 |
| 10 | BraBAM9.1 | Bra002178 | 1614 | 2 | A10: 11069000-11070867 | 537 | 58.59 | 5.82 |
| 11 | BraBAM9.2 | Bra006473 | 2112 | 0 | A03: 3657746-3659857 | 703 | 79.70 | 6.03 |
| 12 | BoBAM1.1 | Bo3g082110.1 | 1638 | 3 | C3: 30584504-30586921 | 545 | 61.16 | 5.62 |
| 13 | BoBAM1.2 | Bo7g034320.1 | 1713 | 3 | C7: 13225248-13228060 | 570 | 63.41 | 5.31 |
| 14 | BoBAM2 | Bo9g002380.1 | 1623 | 7 | C9: 245084-247243 | 540 | 61.17 | 5.20 |
| 15 | BoBAM3.1 | Bo1g052940.1 | 1647 | 3 | C1: 15029435-15031326 | 548 | 61.86 | 7.18 |
| 16 | BoBAM3.2 | Bo7g104960.1 | 1644 | 3 | C7: 40419711-40421605 | 547 | 61.37 | 6.28 |
| 17 | BoBAM4.1 | Bo3g021390.1 | 1656 | 9 | C3: 7167639-7170389 | 551 | 62.65 | 7.91 |
| 18 | BoBAM4.2 | Bo2g037740.1 | 1917 | 10 | C2: 10399330-10402605 | 638 | 71.78 | 5.76 |
| 19 | BoBAM5.1 | Bo8g040610.1 | 1179 | 4 | C8: 13860892-13862943 | 392 | 44.89 | 5.59 |
| 20 | BoBAM5.2 | Bo8g040630.1 | 915 | 3 | C8: 13904477-13905924 | 304 | 34.18 | 4.77 |
| 21 | BoBAM5.3 | Bo02108s010.1 | 915 | 3 | Unknown chromosome | 304 | 34.18 | 4.77 |
| 22 | BoBAM5.4 | Bo3g185170.1 | 1005 | 4 | C3: 64572299-64575054 | 334 | 38.65 | 5.57 |
| 23 | BoBAM5.5 | Bo03100s010.1 | 498 | 2 | Unknown chromosome | 165 | 18.60 | 5.20 |
| 24 | BoBAM5.6 | Bo3g185180.1 | 645 | 3 | C3:64580047-64581495 | 214 | 24.43 | 6.11 |
| 25 | BoBAM6 | Bo4g045870.1 | 1818 | 7 | C4: 10696860-10699390 | 605 | 69.45 | 5.66 |
| 26 | BoBAM7 | Bo4g025050.1 | 2037 | 9 | C4: 4131171-4134476 | 678 | 75.88 | 5.62 |
| 27 | BoBAM8 | Bo2g127050.1 | 2031 | 9 | C2: 39136275-39139346 | 676 | 76.20 | 5.58 |
| 28 | BoBAM9.1 | Bo3g013270.1 | 1596 | 2 | C3: 4592661-4594899 | 531 | 57.85 | 5.59 |
| 29 | BoBAM9.2 | Bo9g154650.1 | 1749 | 2 | C9: 46305431-46307444 | 582 | 64.25 | 6.34 |
| 30 | BoBAM9.3 | Bo08131s010.1 | 243 | 0 | Unknown chromosome | 81 | 8.98 | 9.01 |
| 31 | BoBAM9.4 | Bo8g046130.1 | 609 | 4 | C8: 15583635-15585005 | 202 | 23.04 | 8.45 |
| 32 | BnaBAM1.1 | BnaA03g37260D | 1668 | 3 | chrA03: 18437399-18439520 | 555 | 61.91 | 6.36 |
| 33 | BnaBAM1.2 | BnaC03g43570D | 1638 | 3 | chrC03: 28618604-28621024 | 545 | 61.16 | 5.88 |
| 34 | BnaBAM1.3 | BnaC09g21440D | 1695 | 3 | chrC09: 18644151-18647237 | 564 | 62.62 | 5.53 |
| 35 | BnaBAM1.4 | BnaA07g05790D | 1713 | 3 | chrA07: 6121654-6124373 | 570 | 63.31 | 5.55 |
| 36 | BnaBAM2 | BnaA09g51890D | 1620 | 7 | chrA09_random: 126993-129149 | 539 | 61.12 | 5.22 |
| 37 | BnaBAM3.1 | BnaC01g21190D | 1647 | 3 | chrC01: 14819094-14820988 | 548 | 61.76 | 7.65 |
| 38 | BnaBAM3.2 | BnaA03g42940D | 1626 | 4 | chrA03: 21561950-21563838 | 541 | 60.72 | 6.46 |
| 39 | BnaBAM3.3 | BnaC07g34180D | 1647 | 3 | chrC07: 37099448-37101348 | 548 | 61.40 | 6.59 |
| 40 | BnaBAM3.4 | BnaA01g17940D | 1650 | 3 | chrA01: 9483862-9485751 | 549 | 61.75 | 7.64 |
| 41 | BnaBAM3.5 | BnaA08g08230D | 774 | 5 | chrA08: 8068950-8076869 | 257 | 29.80 | 8.20 |
| 42 | BnaBAM3.6 | BnaC08g10900D | 546 | 4 | chrC08: 16342627-16343635 | 181 | 20.86 | 8.73 |
| 43 | BnaBAM4.1 | BnaC03g14120D | 1602 | 9 | chrC03: 6768572-6771333 | 533 | 60.49 | 8.74 |
| 44 | BnaBAM4.2 | BnaC03g71580D | 897 | 4 | chrC03_random: 222415-223959 | 298 | 33.68 | 9.32 |
| 45 | BnaBAM4.3 | BnaA03g11260D | 1599 | 9 | chrA03: 5050886-5053547 | 532 | 60.21 | 8.57 |
| 46 | BnaBAM4.4 | BnaA02g08900D | 1572 | 9 | chrA02: 4429617-4432105 | 523 | 59.15 | 8.89 |
| 47 | BnaBAM4.5 | BnaC02g12830D | 1587 | 9 | chrC02: 8167152-8169713 | 528 | 59.65 | 8.51 |
| 48 | BnaBAM5.1 | BnaA08g05660D | 1497 | 6 | chrA08: 5553480-5555924 | 498 | 56.22 | 5.20 |
| 49 | BnaBAM5.2 | BnaA08g00480D | 1389 | 6 | chrA08: 296747-299203 | 462 | 53.3 | 5.84 |
| 50 | BnaBAM5.3 | BnaC03g78310D | 1005 | 4 | chrC03_random: 6484548-6487309 | 334 | 38.65 | 5.57 |
| 51 | BnaBAM5.4 | BnaC03g78320D | 645 | 3 | chrC03_random: 6492299-6493747 | 214 | 24.43 | 6.11 |
| 52 | BnaBAM6.1 | BnaC04g12480D | 1749 | 6 | chrC04: 9717378-9719767 | 582 | 66.78 | 5.77 |
| 53 | BnaBAM6.2 | BnaA05g10780D | 1746 | 6 | chrA05: 5901832-5904179 | 581 | 66.75 | 6.20 |
| 54 | BnaBAM7.1 | BnaA05g05170D | 2031 | 8 | chrA05: 2697113-2700136 | 676 | 75.57 | 5.86 |
| 55 | BnaBAM7.2 | BnaC04g04570D | 2019 | 9 | chrC04: 3393159-3396247 | 672 | 75.19 | 5.71 |
| 56 | BnaBAM8.1 | BnaA02g36650D | 1899 | 9 | chrA02_random: 1168405-1171865 | 632 | 71.06 | 5.62 |
| 57 | BnaBAM8.2 | BnaC02g31510D | 2103 | 8 | chrC02: 33718069-33721130 | 700 | 79.06 | 5.99 |
| 58 | BnaBAM9.1 | BnaA10g16290D | 1611 | 2 | chrA10: 12426993-12428859 | 536 | 58.52 | 6.03 |
| 59 | BnaBAM9.2 | BnaA03g07240D | 1593 | 2 | chrA03: 3214063-3216446 | 530 | 57.83 | 5.73 |
| 60 | BnaBAM9.3 | BnaC03g09200D | 1596 | 2 | chrC03: 4394436-4396680 | 531 | 57.95 | 5.76 |
| 61 | BnaBAM9.4 | BnaC09g39140D | 1614 | 2 | chrC09: 41822923-41824840 | 537 | 58.86 | 6.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Jia, Z.; Cheng, Y.; Zou, X.; Lv, Y. Genome-Wide Analysis of the β-Amylase Gene Family in Brassica L. Crops and Expression Profiles of BnaBAM Genes in Response to Abiotic Stresses. Agronomy 2020, 10, 1855. https://doi.org/10.3390/agronomy10121855
Luo D, Jia Z, Cheng Y, Zou X, Lv Y. Genome-Wide Analysis of the β-Amylase Gene Family in Brassica L. Crops and Expression Profiles of BnaBAM Genes in Response to Abiotic Stresses. Agronomy. 2020; 10(12):1855. https://doi.org/10.3390/agronomy10121855
Chicago/Turabian StyleLuo, Dan, Ziqi Jia, Yong Cheng, Xiling Zou, and Yan Lv. 2020. "Genome-Wide Analysis of the β-Amylase Gene Family in Brassica L. Crops and Expression Profiles of BnaBAM Genes in Response to Abiotic Stresses" Agronomy 10, no. 12: 1855. https://doi.org/10.3390/agronomy10121855
APA StyleLuo, D., Jia, Z., Cheng, Y., Zou, X., & Lv, Y. (2020). Genome-Wide Analysis of the β-Amylase Gene Family in Brassica L. Crops and Expression Profiles of BnaBAM Genes in Response to Abiotic Stresses. Agronomy, 10(12), 1855. https://doi.org/10.3390/agronomy10121855




