Mo–Bi Bimetallic Chalcogenide Nanoparticles Supported on CNTs for the Efficient Electrochemical Reduction of CO2 to Methanol
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Synthesis of Mo–Bi BMC Nanoparticles
2.3. Synthesis of Mo–Bi BMC@CNTs
2.4. Materials Characterization
2.5. Electrochemical Study
2.6. Product Analysis
3. Results and Discussion
3.1. XRD Patterns of the Mo–Bi BMC and Mo–Bi BMC@CNTs
3.2. SEM Images of the Mo–Bi BMC and Mo–Bi BMC@CNTs
3.3. TEM Images of the Mo–Bi BMC and Mo–Bi BMC@CNTs
3.4. Electrochemical Performances of the Mo–Bi BMC and Mo–Bi BMC@CNTs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wentuan, B.; Wu, C.Z.; Xie, Y. Atomically thin two-dimensional solids: An emerging platform for CO2 electroreduction. ACS Energy Lett. 2018, 3, 624–633. [Google Scholar] [CrossRef]
- Zhuang, T.T.; Pang, Y.; Liang, Z.Q.; Wang, Z.Y.; Li, Y.; Tan, C.S.; Li, J.; Luna, P.D. Copper nanocavities confine intermediates for efficient electrosynthesis of C3 alcohol fuels from carbon monoxide. Nat. Catal. 2018, 1, 421. [Google Scholar] [CrossRef]
- Birdja, Y.Y.; Perezgallent, E.; Figueiredo, M.C.; Gottle, A.J.; Calle-Vallejo, F.; Koper, M.T. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 2019, 4, 732–745. [Google Scholar] [CrossRef]
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef]
- Ting, L.R.L.; Yeo, B.S. Recent advances in understanding mechanisms for the electrochemical reduction of carbon dioxide. Curr. Opin. Electrochem. 2018, 8, 126–134. [Google Scholar] [CrossRef]
- Glasing, J.P.; Champagne, M.F. Cunningham, graft modification of chitosan, cellulose and alginate using reversible deactivation radical polymerization (RDRP). Green Sustain. Chem. 2016, 15–21. [Google Scholar] [CrossRef]
- Whipple, D.T.; Kenis, P.J.A. Prospects of CO2 utilization via direct heterog-eneous electrochemical reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458. [Google Scholar] [CrossRef]
- Rosen, B.A.; Salehikhojin, A.; Thorson, M.R.; Zhu, W.; Whipple, D.T.; Kenis, P.J.A.; Masel, R.I. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 2011, 334, 643–644. [Google Scholar] [CrossRef]
- Dimeglio, J.L.; Rosenthal, J. Selective conversion of CO2 to CO with high efficiency using an inexpensive bismuth-based electrocatalyst. J. Am. Chem. Soc. 2013, 135, 8798–8801. [Google Scholar] [CrossRef]
- Shen, J.; Kortlever, R.; Kas, R.; Birdja, Y.Y.; Diazmorales, O.; Kwon, Y.; Ledezmayanez, I.; Schouten, K.J.P.; Mul, G.; Koper, M.T.M. Electrocatalytic reduction of carbon dioxide to carbon monoxide and methane at an immobilized cobalt protoporphyrin. Nat. Commun. 2015, 6, 8177. [Google Scholar] [CrossRef]
- Wang, Y.X.; Niu, C.; Zhu, Y. Copper–silver bimetallic nanowire arrays for electrochemical reduction of carbon dioxide. Nanomaterials 2019, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, N.; Taylor, S.F.R.; Galante, M.T.; Jacquemin, J.; Longo, C.; Holt, K.B.; de Leeuw, N.H.; Hardacre, C. Reduction of carbon dioxide to formate at low overpotential using a superbase ionic liquid. Angew. Chem. 2015, 54, 14164–14168. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.C.; Zhu, Q.G.; Sun, X.F.; Hu, J.Y.; Zhang, J.L.; Liu, Z.M.; Han, B.X. Highly efficient electrochemical reduction of CO2 to CH4 in an ionic liquid using a metal–organic framework cathode. Chem. Sci. 2016, 7, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, K.; Beberwyck, B.J.; Alivisatos, A.P. Enhanced electrochemical methanation of carbon dioxide with a dispersible nanoscale copper catalyst. J. Am. Chem. Soc. 2014, 136, 13319–13325. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.R.; Shaigan, N.; Neburchilov, V.; Zhang, L.; Malek, K.; Eikerling, M.; Luna, P.D. Three-dimensional cathodes for electrochemical reduction of CO2: From macro to nano-engineering. Nanomaterials 2020, 10, 1884. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.L.; Liu, Y.Y.; Zhang, J.J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef]
- Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber, A.E.; Evans, J.; Senanayake, S.D.; Stacchiola, D.J.; Liu, P.; Hrbek, J.; Sanz, J.F.; et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 2014, 345, 546–550. [Google Scholar] [CrossRef]
- Studt, F.; Sharafutdinov, I.; Abildpedersen, F.; Elkjaer, C.F.; Hummelshoj, J.S.; Dahl, S.; Chorkendorff, I.; Norskov, J.K. Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat. Commun. 2014, 6, 320–324. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Opportunities and prospects in the chemical recycling of carbon dioxide to fuels. Catal. Today 2009, 148, 191–205. [Google Scholar] [CrossRef]
- Omae, I. Recent developments in carbon dioxide utilization for the production of organic chemicals. Coord. Chem. Rev. 2012, 256, 1384–1405. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Cadena, C.; Anthony, J.L.; Shah, J.K.; Brennecke, J.F. Why is CO2 so soluble in imidazolium-based ionic liquids? J. Am. Chem. Soc. 2004, 126, 5300–5308. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Kumar, B.; Behranginia, A.; Rosen, B.A.; Baskin, A.; Repnin, N.; Pisasale, D.; Phillips, P.; Zhu, W.; Haasch, R.; et al. Robust carbon dioxide reduction on molybdenum disulphide edges. Nat. Commun. 2014, 5, 4470–4478. [Google Scholar] [CrossRef]
- Summers, D.P.; Leach, S.; Frese, K.W. The electrochemical reduction of aqueous carbon dioxide to methanol at molybdenum electrodes with low overpotentials. J. Electroanal. Chem. Interfacial Electrochem. 1986, 205, 219–232. [Google Scholar] [CrossRef]
- Sun, X.F.; Zhu, Q.G.; Kang, X.C.; Liu, H.Z.; Qian, Q.L.; Zhang, Z.F.; Han, B.X. Molybdenum-Bismuth bimetallic chalcogenide nanosheets for highly efficient electrocatalytic reduction of carbon dioxide to methanol. Angew. Chem. 2016, 55, 6771–6775. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, X.Z.; Chen, S.M.; Yang, D.R.; Zhang, Q.; Song, L.; Xiao, H.; Zhang, Q.H.; Gu, L.; Wang, X. Edge-exposed molybdenum disulfide with N-doped carbon hybridization: A hierarchical hollow electrocatalyst for carbon dioxide Reduction. Adv. Energy Mater. 2019, 9, 1900072. [Google Scholar] [CrossRef]
- Calizzi, M.; Mutschler, R.; Patelli, N.; Migliori, A.; Zhao, K.; Züttel, A. CO2 hydrogenation over unsupported Fe-Co nanoalloy catalysts. Nanomaterials 2020, 10, 1360. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified scherrer equation to estimate more accurately nano-crystallite size using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Hatchard, T.D.; Dahn, J.R. In situ XRD and electrochemical study of the reaction of lithium with amorphous silicon. J. Electrochem. Soc. 2004, 151. [Google Scholar] [CrossRef]
- Celebi, A.T.; Kirca, M.; Baykasoglu, C.; Mugan, A.; To, A.C. Tensile behavior of heat welded CNT network structures. Comput. Mater. Sci. 2014, 88, 14–21. [Google Scholar] [CrossRef]
- Wang, H.W.; Skeldon, P.; Thompson, G.E. Thompson, Xps studies of mos 2 formation from ammonium tetrathiomolybdate solutions. Surf. Coat. Technol. 1997, 91, 200–207. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Gao, P.; Wang, H.; Li, X.; Zhong, L.; Wei, W.; Sun, Y. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons. Catal. Sci. Technol. 2017, 7, 4580–4598. [Google Scholar] [CrossRef]
- Sorribes, I.; Liu, L.C.; Corma, A. Nanolayered Co–Mo–S catalysts for the chemoselective hydrogenation of nitroarenes. ACS Catal. 2017, 7, 2698–2708. [Google Scholar] [CrossRef]
- Wang, S.G.; Li, X.; Chen, Y.; Cai, X.J.; Yao, H.L.; Gao, W.; Zheng, Y.Y.; An, X.; Shi, J.L.; Chen, H.R. A facile one-pot synthesis of a two-dimensional MoS2/Bi2S3 composite theranostic nanosystem for multi-modality tumor imaging and therapy. Adv. Mater. 2015, 27, 2775–2782. [Google Scholar] [CrossRef]
- Liu, Z.L.; Peng, W.X.; Zare, Y.; Hui, D.; Rhee, K.Y. Predicting the electrical conductivity in polymer carbon nanotube nanocomposites based on the volume fractions and resistances of the nanoparticle, interphase, and tunneling regions in conductive. RSC Adv. 2018, 8, 19001–19010. [Google Scholar] [CrossRef]
- Yang, P.; You, X.; Yi, J.H.; Fang, D.; Bao, R.; Shen, T.; Liu, Y.C.; Tao, J.; Li, C. Influence of dispersion state of carbon nanotubes on electrical conductivity of copper matrix composites. J. Alloys Compd. 2018, 752, 376–380. [Google Scholar] [CrossRef]
- Albo, J.; Saez, A.; Sollagullon, J.; Irabien, A. Production of methanol from CO2 electroreduction at Cu2O and Cu2O/ZnO-based electrodes in aqueous solution. Appl. Catal. B Environ. 2015, 176, 709–717. [Google Scholar] [CrossRef]
- Albo, J.; Beobide, G.; Castano, P.; Irabien, A. Methanol electrosynthesis from CO2 at Cu2O/ZnO prompted by pyridine-based aqueous solutions. J. CO2 Util. 2017, 18, 164–172. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Zhao, Z.J.; Yang, P.; Greeley, J.; Mu, R.; Zhang, G.; Gong, Z.; Luo, Z.; Chen, J.; et al. Tuning Cu/Cu2O interfaces for the reduction of carbon dioxide to methanol in aqueous solutions. Angew. Chem. 2018, 130, 15507. [Google Scholar] [CrossRef]
- Jang, Y.J.; Jeong, I.; Lee, J.; Ko, M.J.; Lee, J.S. Unbiased sunlight-driven artificial photosynthesis of carbon monoxide from CO2 using a ZnTe-based photocathode and a perovskite solar cell in tandem. ACS Nano 2016, 10, 6980–6987. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zeng, G.T.; Ha, M.; Ge, M.Y.; Lin, Y.J.; Hettick, M.; Hou, B.Y.; Alexandrova, A.N.; Javey, A.; Cronin, S.B. Artificial photosynthesis on TiO2-passivated InP nanopillars. Nano Lett. 2015, 15, 6177–6181. [Google Scholar] [CrossRef] [PubMed]
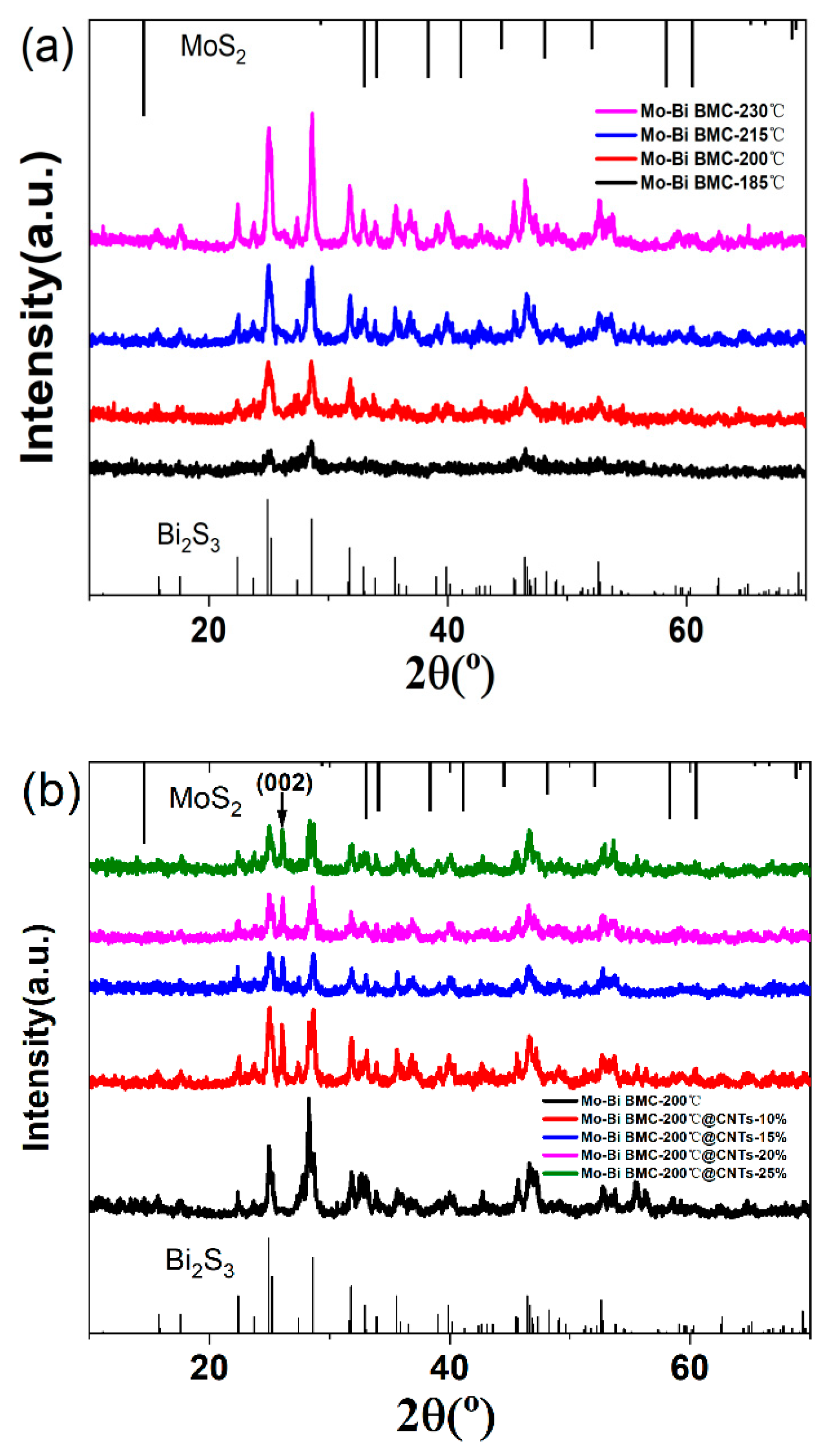

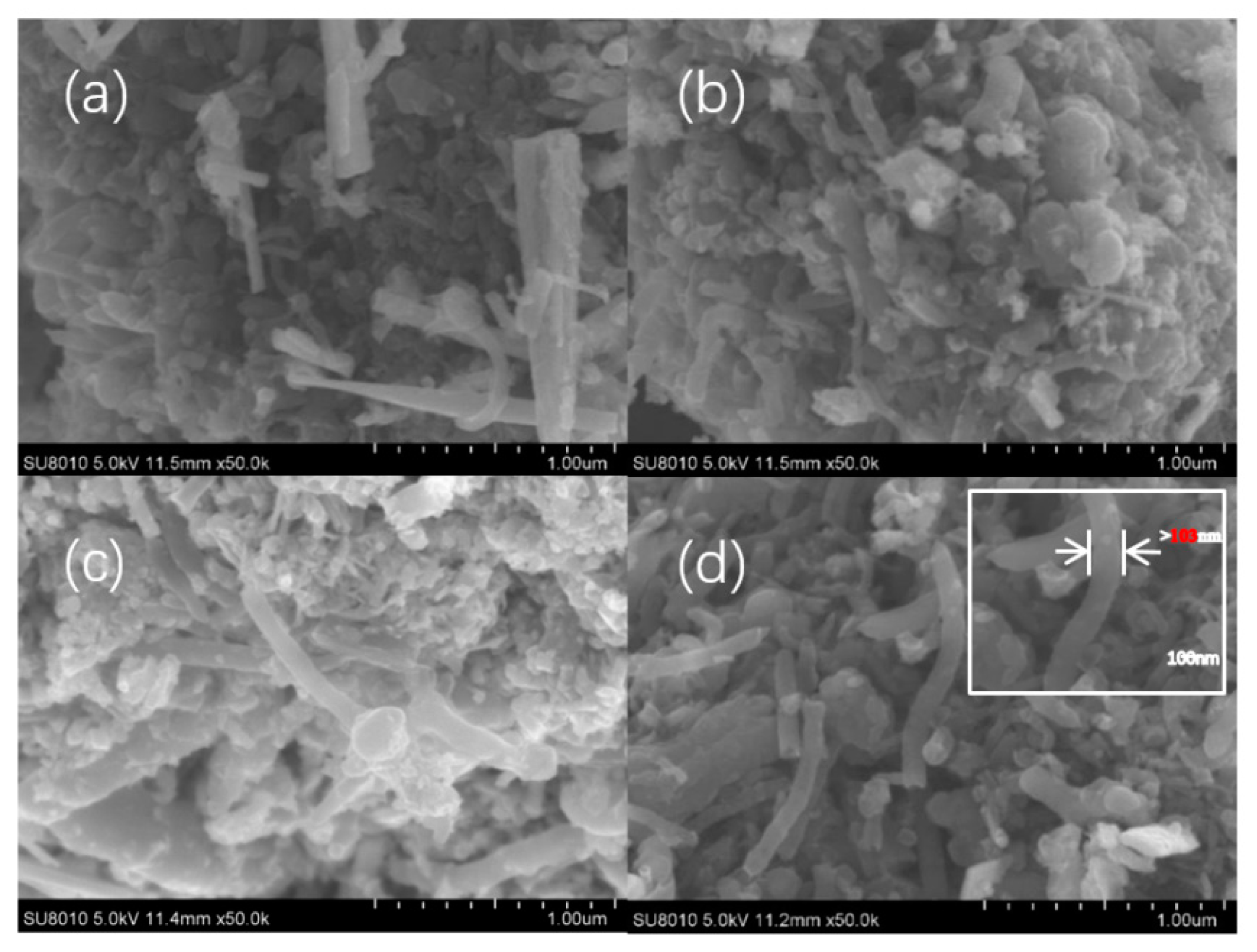






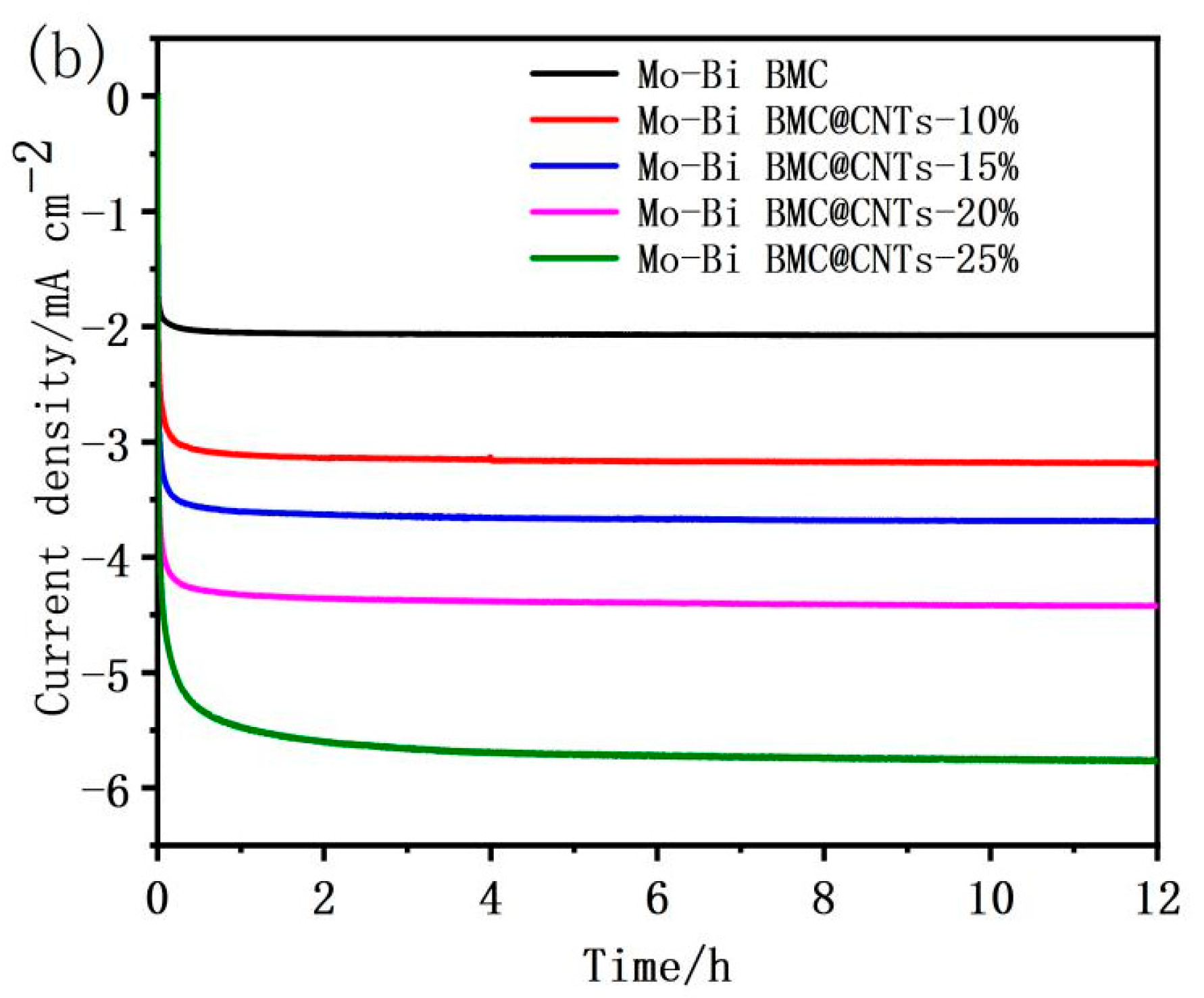
| Sample | 2θ | Peak | Crystallite Size |
|---|---|---|---|
| Mo–Bi BMC-185 °C | 28.06° | (2 1 1) | 13.03 nm |
| Mo–Bi BMC-200 °C | 28.04° | (2 1 1) | 12.16 nm |
| Mo–Bi BMC-215 °C | 28.07° | (2 1 1) | 12.25 nm |
| Mo–Bi BMC-230 °C | 28.06° | (2 1 1) | 12.57 nm |
| Mo–Bi BMC-200 °C@ CNTs-10% | 28.03° | (2 1 1) | 7.81 nm |
| Mo–Bi BMC-200 °C@ CNTs-15% | 28.05° | (2 1 1) | 7.57 nm |
| Mo–Bi BMC-200 °C@ CNTs-20% | 28.06° | (2 1 1) | 7.59 nm |
| Mo–Bi BMC-200 °C@ CNTs-25% | 28.08° | (2 1 1) | 7.63 nm |
| Name | FWHM eV | Area (P) CPS.eV | Area (N) TPP-2M | Atomic % |
|---|---|---|---|---|
| C 1s | 1.81 | 30,611.72 | 0.7 | 75.4 |
| Mo 3d | 2 | 20,229.59 | 0.04 | 4.47 |
| Bi 4f7 Scan A | 1.36 | 29,915.32 | 0.03 | 3.35 |
| Bi 4f7 Scan B | 1.32 | 7831.45 | 0.01 | 0.88 |
| S 2p3 | 1.34 | 4122.18 | 0.07 | 7.95 |
| S 2p1 | 1.34 | 2105.65 | 0.07 | 7.95 |
| Electrode/Electrocatalysts | Electrode Potential/V | Electrolyte | FE/% | CD/mA cm−2 | Reference |
|---|---|---|---|---|---|
| Mo–Bi BMC@CNTs | −0.3 V vs. SCE | 60 wt.% [Emim] BF4 aqueous electrolyte | 81 | 5.6 | This work |
| Mo–Bi BMC | −0.3 V vs. SCE | 60 wt.% [Emim] BF4 aqueous electrolyte | 46 | 2.1 | This work |
| co-protoporphyrin/pyrolytic graphite electrode | −0.5 V vs. RHE | 0.5 M KHCO3 | 38 | Not mentioned | [10] |
| Mo–Bi BMC nanosheet | −0.7 V vs. SHE | 0.5 M [Bmim] BF4 in MeCN | 71.2 | 12.1 | [26] |
| Mo | −0.7 V vs. SCE | 0.2 M Na2SO4 (aq) | 55 | 0.05 | [26] |
| carbon paper/Cu2O | −1.3 V vs. Ag/AgCl | 0.5 M KHCO3 | 45.7 | 6.93 | [38] |
| Cu2O/ZnO and pyridine-based cocatalyst | −0.8 V vs. Ag/AgCl | 10 mM 2-mPy | 25.6 | 10 | [39] |
| Cu2O/TioO2 | −0.7 V vs. RHE | 0.5 M KHCO3 | 53.6 | 1.3 | [40] |
| ZnO@ZnTe@CdTe nanorod | −0.3 V vs. RHE | 0.5 M KHCO3 | 74.9 | 3.88 | [41] |
| TiO2-protected InP nanopillars | −0.6 V vs. NHE | 0.5 M NaHCO3 | 8.7 | Not mentioned | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, C.; Duan, D.; Zhang, Z.; Wei, G.; Li, Y.; Liu, S. Mo–Bi Bimetallic Chalcogenide Nanoparticles Supported on CNTs for the Efficient Electrochemical Reduction of CO2 to Methanol. Coatings 2020, 10, 1142. https://doi.org/10.3390/coatings10121142
Chi C, Duan D, Zhang Z, Wei G, Li Y, Liu S. Mo–Bi Bimetallic Chalcogenide Nanoparticles Supported on CNTs for the Efficient Electrochemical Reduction of CO2 to Methanol. Coatings. 2020; 10(12):1142. https://doi.org/10.3390/coatings10121142
Chicago/Turabian StyleChi, Chen, Donghong Duan, Zhonglin Zhang, Guoqiang Wei, Yu Li, and Shibin Liu. 2020. "Mo–Bi Bimetallic Chalcogenide Nanoparticles Supported on CNTs for the Efficient Electrochemical Reduction of CO2 to Methanol" Coatings 10, no. 12: 1142. https://doi.org/10.3390/coatings10121142
APA StyleChi, C., Duan, D., Zhang, Z., Wei, G., Li, Y., & Liu, S. (2020). Mo–Bi Bimetallic Chalcogenide Nanoparticles Supported on CNTs for the Efficient Electrochemical Reduction of CO2 to Methanol. Coatings, 10(12), 1142. https://doi.org/10.3390/coatings10121142





