The Removal of Platinum Group Metals, Cs, Se, and Te from Nuclear Waste Glass Using Liquid Sb Extraction and Phase Separation Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. The Preparation of the Simulated HLVW
2.2. Reductive Heat Treatment
2.3. The Elution Test
i = K, Cs, Se, Pd, Ru, Rh, Te
2.4. The Extraction of PGMs from the Metal Phase
M = Pd, Ru, Rh, Cs, Te and Se
2.5. Characterization
3. Results and Discussion
3.1. XRD Measurement of the Reduced Metal Phase
3.2. PGMs Extraction in Reduced Metallic Buttons
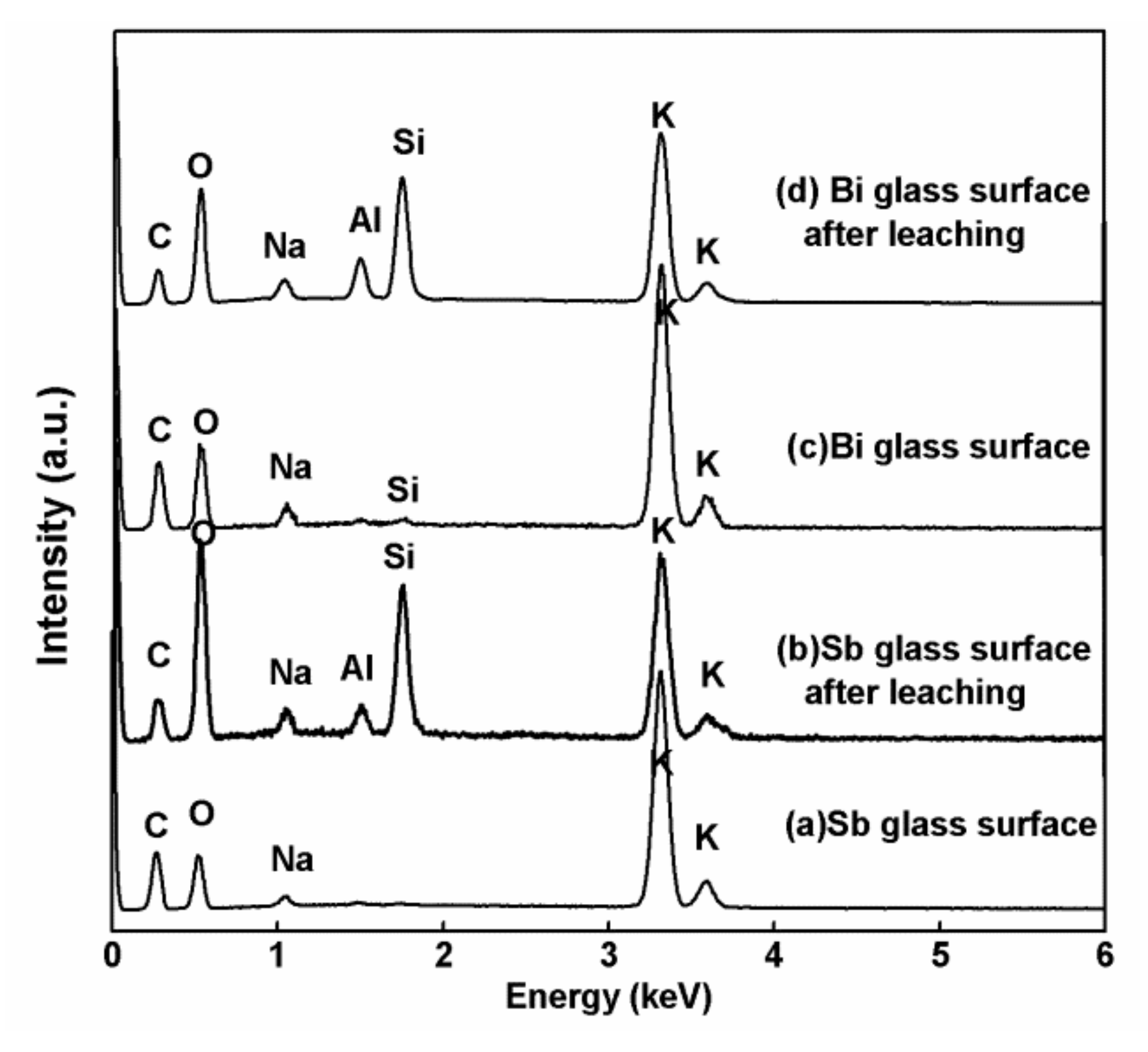
3.3. The Extraction of Other Fission Products from the Heat-Treated Glass Phase
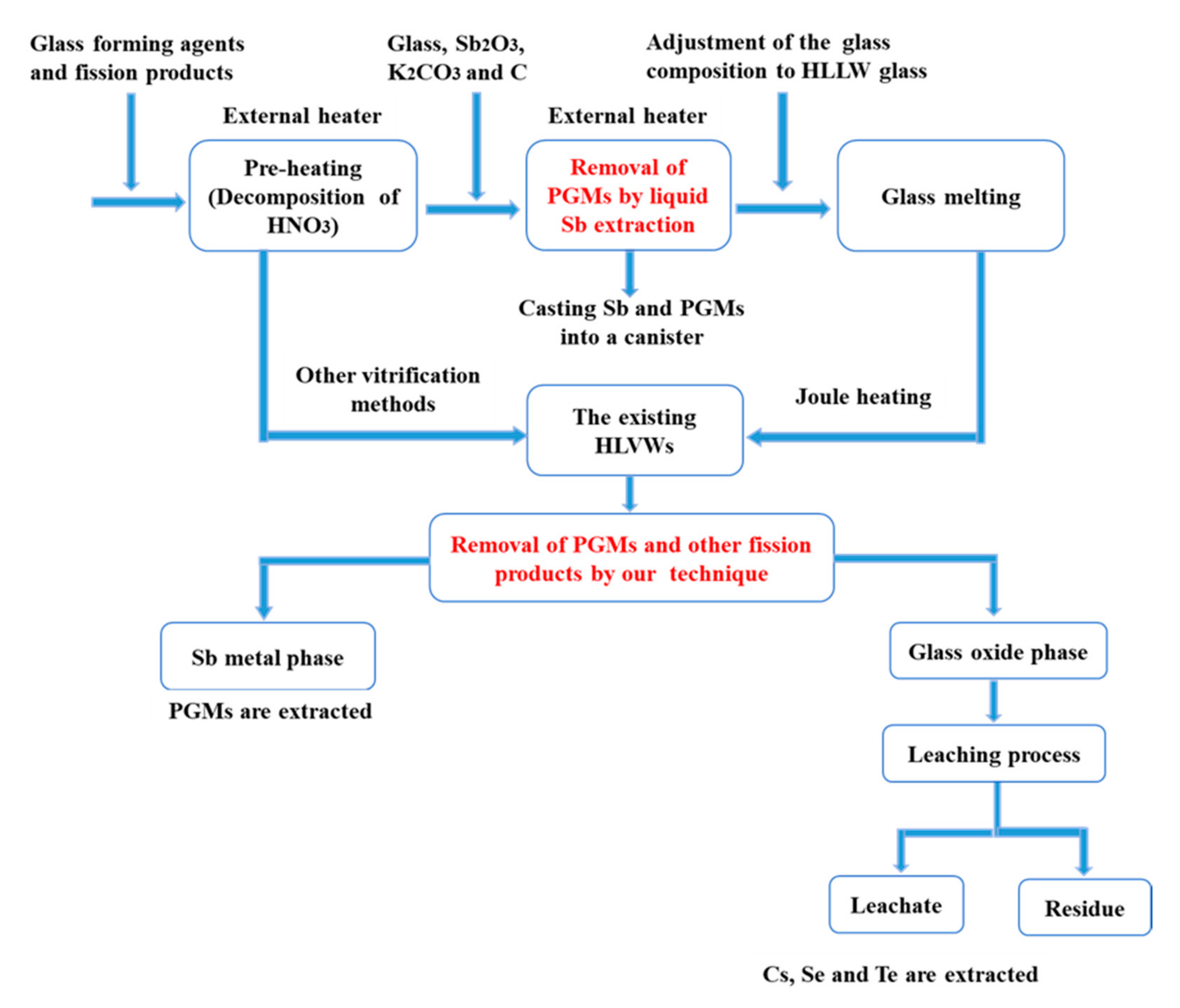
3.4. The Proposed LLFPs Separation Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chapman, C.C.; Pope, J.M.; Barnes, S.M. Electric melting of nuclear waste glasses State of the art. J. Non-Cryst. Solids 1986, 84, 226–240. [Google Scholar] [CrossRef]
- Luckscheiter, B.; Nešović, M. Development of glasses for the vitrification of high level liquid waste (HLLW) in a joule heated ceramic melter. Waste Manag. 1996, 16, 571–578. [Google Scholar] [CrossRef]
- Akai, T.; Nishii, J.; Yamashita, M.; Yamanaka, H. Chemical behavior of platinum-group metals in oxide glasses. J. Non-Cryst. Solids 1997, 222, 304–309. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. An Introduction to Nuclear Waste Immobilisation; Elesevier: Amsterdam, The Netherland, 2005. [Google Scholar]
- Jena, H.; Kutty, K.V.G.; Rao, P.R.V. Effect of temperature on the extraction of Pd by liquid tin from molten borosilicate glass containing simulated radwaste. J. Non-Cryst. Solids 2011, 357, 2911–2919. [Google Scholar] [CrossRef]
- Igarashi, H.; Takahashi, T. The draining of noble metals in vitrified nuclear waste by a melter with a sloping floor. Glass Technol. 1991, 32, 46–50. [Google Scholar]
- Burnham, R.F.; Harry, J.E.; Gibbon, A. Plasma Arc Furnace. European Patent Application 0096 493 A2, 21 December 1987. [Google Scholar]
- Kumar, S.; Pavloudis, T.; Singh, V.; Nguyen, H.; Steinhauer, S.; Pursell, C.; Clemens, B.; Kioseoglou, J.; Grammatikopoulos, P.; Sowwan, M. Hydrogen Flux through Size Selected Pd Nanoparticles into Underlying Mg Nanofilms. Adv. Energy Mater. 2018, 8, 1701326–1701341. [Google Scholar] [CrossRef]
- Vardon, D.R.; Settle, A.E.; Vorotnikov, V.; Menart, M.J.; Eaton, T.R.; Unocic, K.A.; Steirer, K.X.; Wood, K.N.; Cleveland, N.S.; Moyer, K.E.; et al. Ru-Sn/AC for the Aqueous-Phase Reduction of Succinic Acid to 1, 4-Butanediol under Continuous Process Conditions. ACS Catal. 2017, 7, 6207–6219. [Google Scholar] [CrossRef]
- Garrett, C.E.; Prasad, K. The Art of Meeting Palladium Specifications in Active Pharmaceutical Ingredients Produced by Pd-Catalyzed Reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, G.; Tana, L.; Aia, P.; Yang, R.; Tsubakia, N. Direct synthesis of liquefied petroleum gas from syngas over H-ZSM-5 enwrapped Pd-based zeolite capsule catalyst. Catal. Today 2018, 303, 77–85. [Google Scholar] [CrossRef]
- Benson, M.; Bennett, C.R.; Harry, J.E.; Patel, M.K.; Cross, M. The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Resour. Conserv. Recycl. 2000, 31, 1–7. [Google Scholar] [CrossRef]
- Taninouchi, Y.; Watanabe, T.; Okabe, T.H. Recovery of platinum group metals from spent catalysts using electroless nickel plating and magnetic separation. Mater. Trans. 2017, 58, 410–419. [Google Scholar] [CrossRef]
- OECD-NEA. Actinide and Fission Product Partitioning and Transmutation Status and Assessment Report; OECD Publications: Paris, France, 2007. [Google Scholar]
- OECD-NEA. Accelerator-Driven Systems (ADS) and Fast Reactors (FR) in Advanced Nuclear Fuel Cycles—A Comparative Study; OECD Publications: Paris, France, 2003. [Google Scholar]
- Naito, K.; Matsui, T.; Tanaka, Y. Recovery of noble metals from insoluble residue of spent fuel. J. Nucl. Sci. Technol. 1986, 23, 540–549. [Google Scholar] [CrossRef]
- Naito, K.; Matsui, T.; Nakahira, H.; Kitagawa, M.; Okada, H. Recovery and mutural separation of noble metals form the simulated insoluble residue of spent fuel. J. Nucl. Mater. 1991, 184, 30–38. [Google Scholar] [CrossRef]
- Uruga, K.; Arita, Y.; Enokida, Y.; Yamamoto, I. Removal of platinum group metals contained in molten glass using copper. J. Nucl. Sci. Technol. 2007, 44, 1024–1031. [Google Scholar] [CrossRef]
- Xu, Z.; Okada, T.; Nishimura, F.; Yonezawa, S. Phase separation of cesium from lead borosilicate glass by heat treatment under a reducing atmosphere. J. Hazard. Mater. 2016, 317, 622–631. [Google Scholar] [CrossRef]
- Uruga, K.; Sawada, K.; Enokida, Y.; Yamamoto, Y. Liquid metal extraction for removal of molybdenum from molten glass containing simulated nuclear waste elements. J. Nucl. Sci. Technol. 2008, 45, 1063–1071. [Google Scholar] [CrossRef]
- Altin, E.; Oz, E.; Erdem, M.; Demirel, S.; Aydogdu, Y.; Altin, S. Thermoelectric and mechanical properties of Mg-Al-Sb alloys. J. Mater. Sci. Mater. Electron. 2015, 26, 1023–1032. [Google Scholar] [CrossRef]
- Liu, J.; Bai, P.; Zhao, X.S. Ruthenium nanoparticles embedded in mesoporous carbon microfibers: Preparation, characterization and catalytic properties in the hydrogenation of D-glucose. Phys. Chem. Chem. Phys. 2011, 13, 3758–3763. [Google Scholar] [CrossRef]
- Petla, R.K.; Vivekanandhan, S.; Misra, M.; Mohanty, A.K.; Satyanarayana, N. Soybean (Glycine max) leaf extract based green synthesis of palladium nanoparticle. J. Biomater. Nanobiotechnol. 2012, 3, 14–19. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, J.; Pandey, A.C. Rational low temperature synthesis and structural investigations of ultrathin bismuth nanosheets. RSC Adv. 2013, 3, 2313–2317. [Google Scholar] [CrossRef]
- Takao, K.; Mori, T.; Kubo, M.; Uehara, A.; Ikeda, Y. Wet chemical processing for nuclear waste glass to retrieve radionuclides. J. Hazard. Mater. 2019, 362, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Kamizono, H.; Kikkawa, S.; Togashi, Y.; Tashiro , S. Volatilization of 137Cs and 106Ru from borosilicate glass containing actual high-level waste. J. Am. Ceram. Soc. 1989, 72, 1438–1440. [Google Scholar] [CrossRef]
- Kleykamp, H. The chemical-state of fission-products in oxide fuels at different stages of the nuclear fuel cycle. Nucl. Technol. 1988, 80, 412–422. [Google Scholar] [CrossRef]
- Jensen, G.; Platt, A.M.; Mellinger, G.B.; Bjorklund, W.J. Recovery of noble metals from fission products. Nucl. Technol. 1984, 65, 305–332. [Google Scholar] [CrossRef]

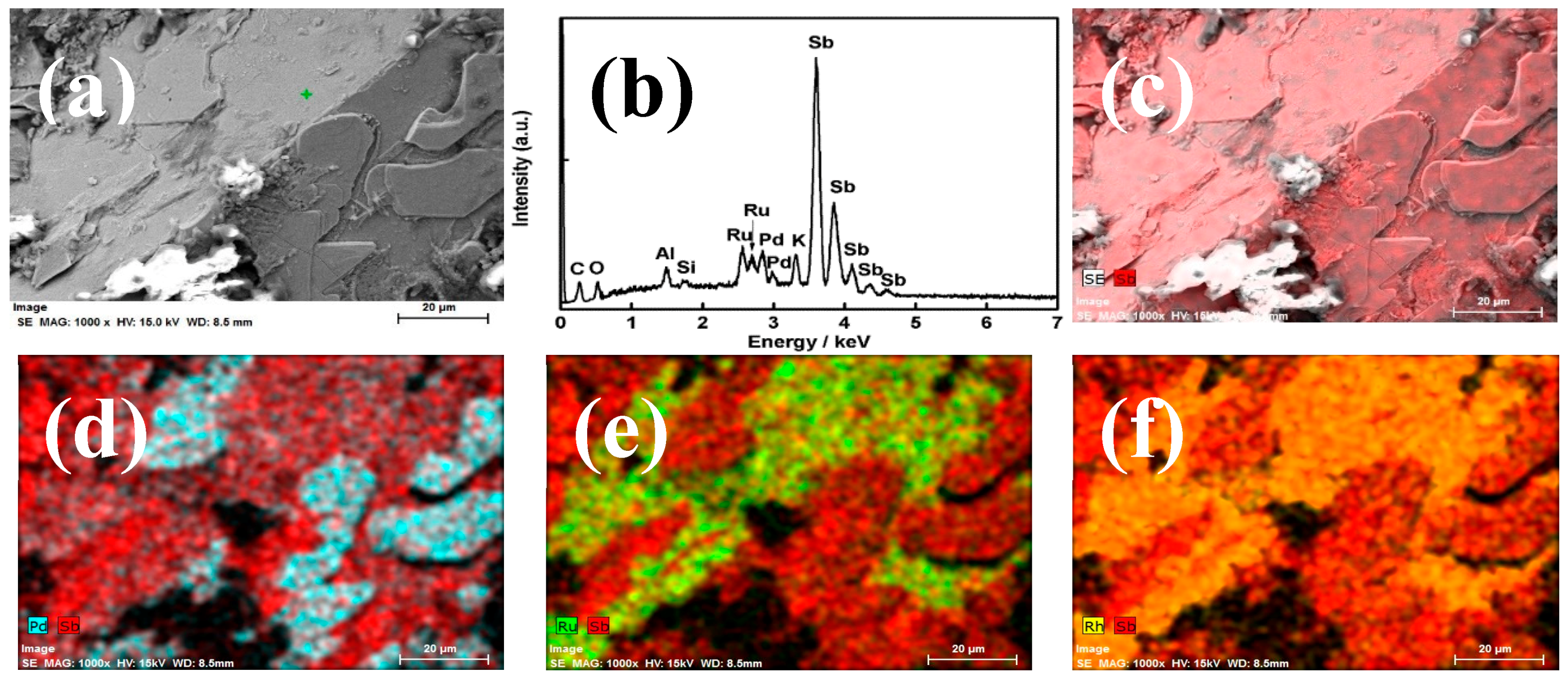

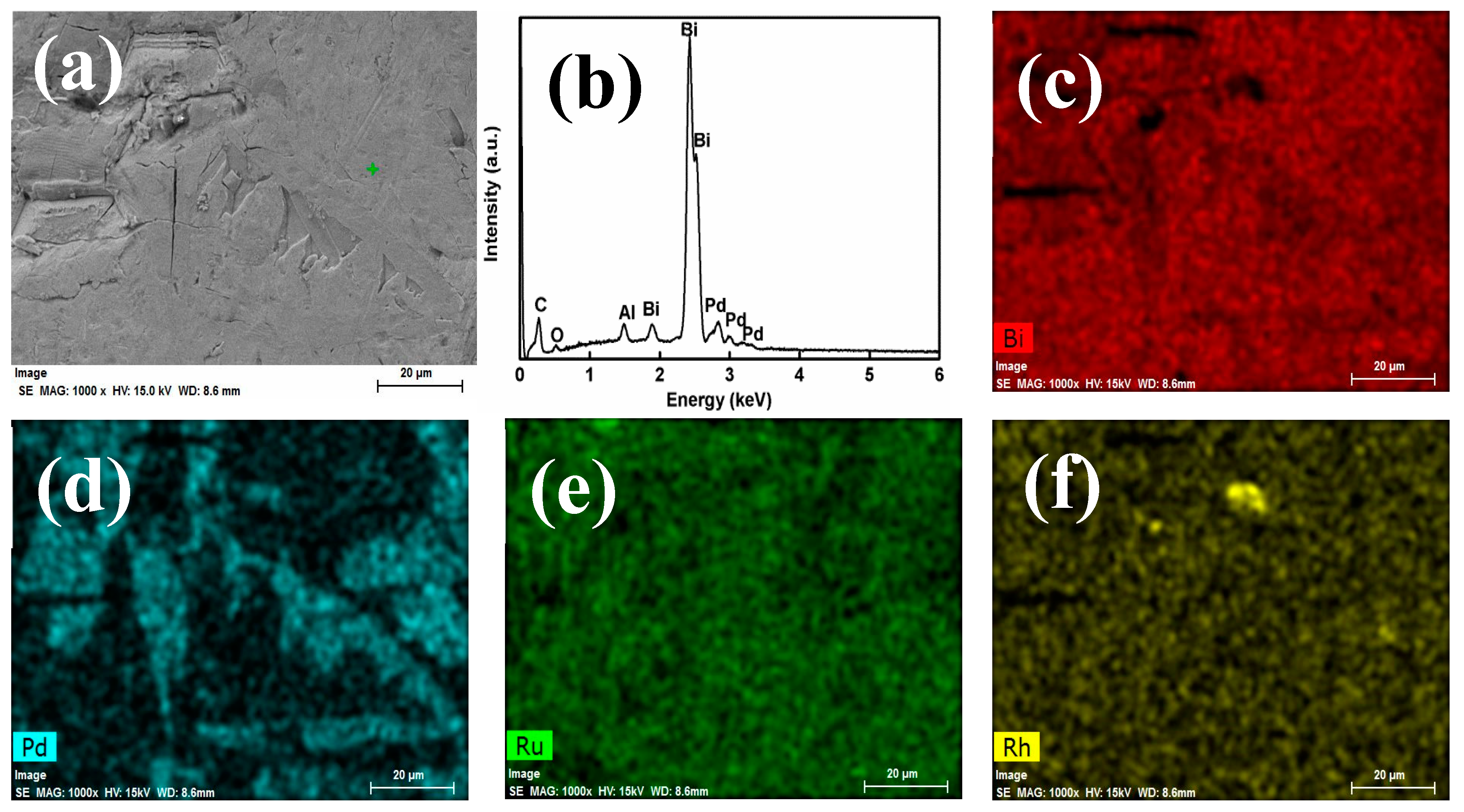
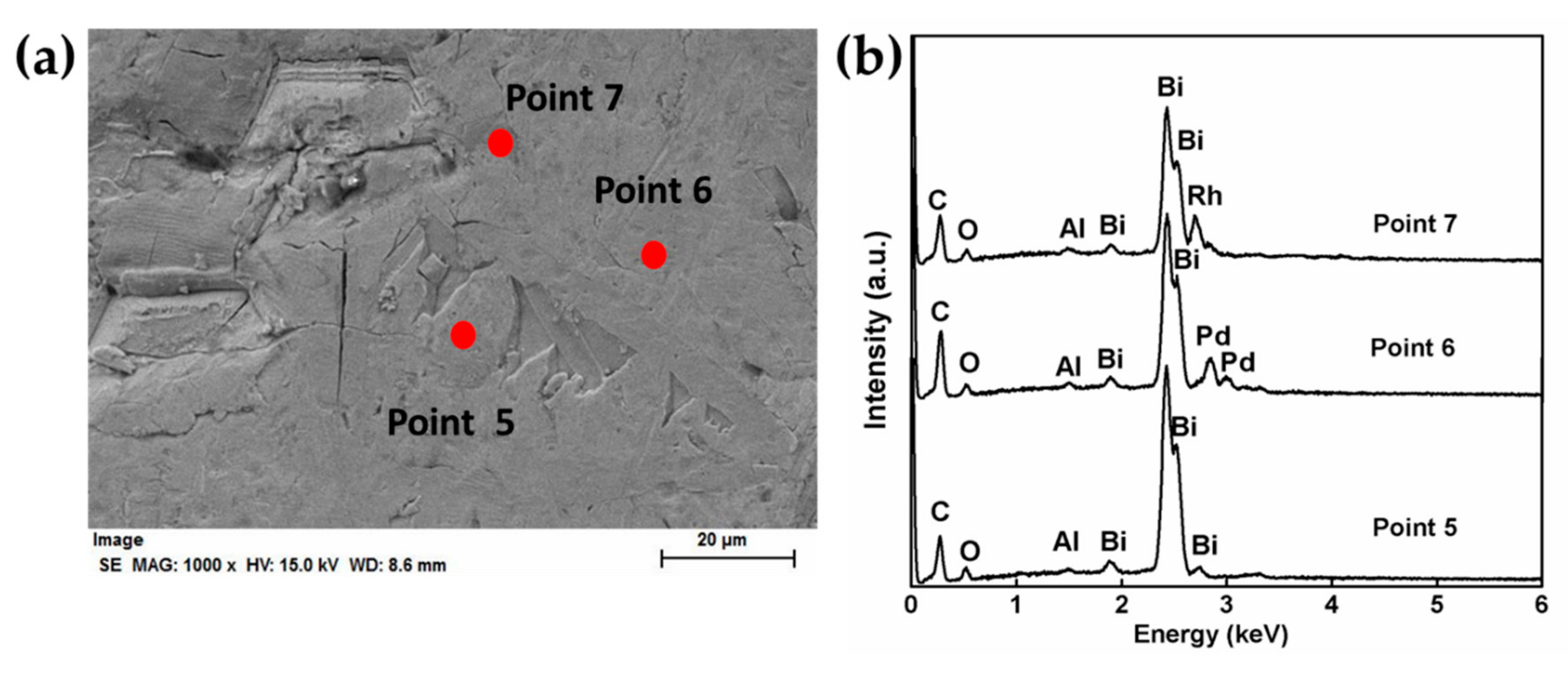
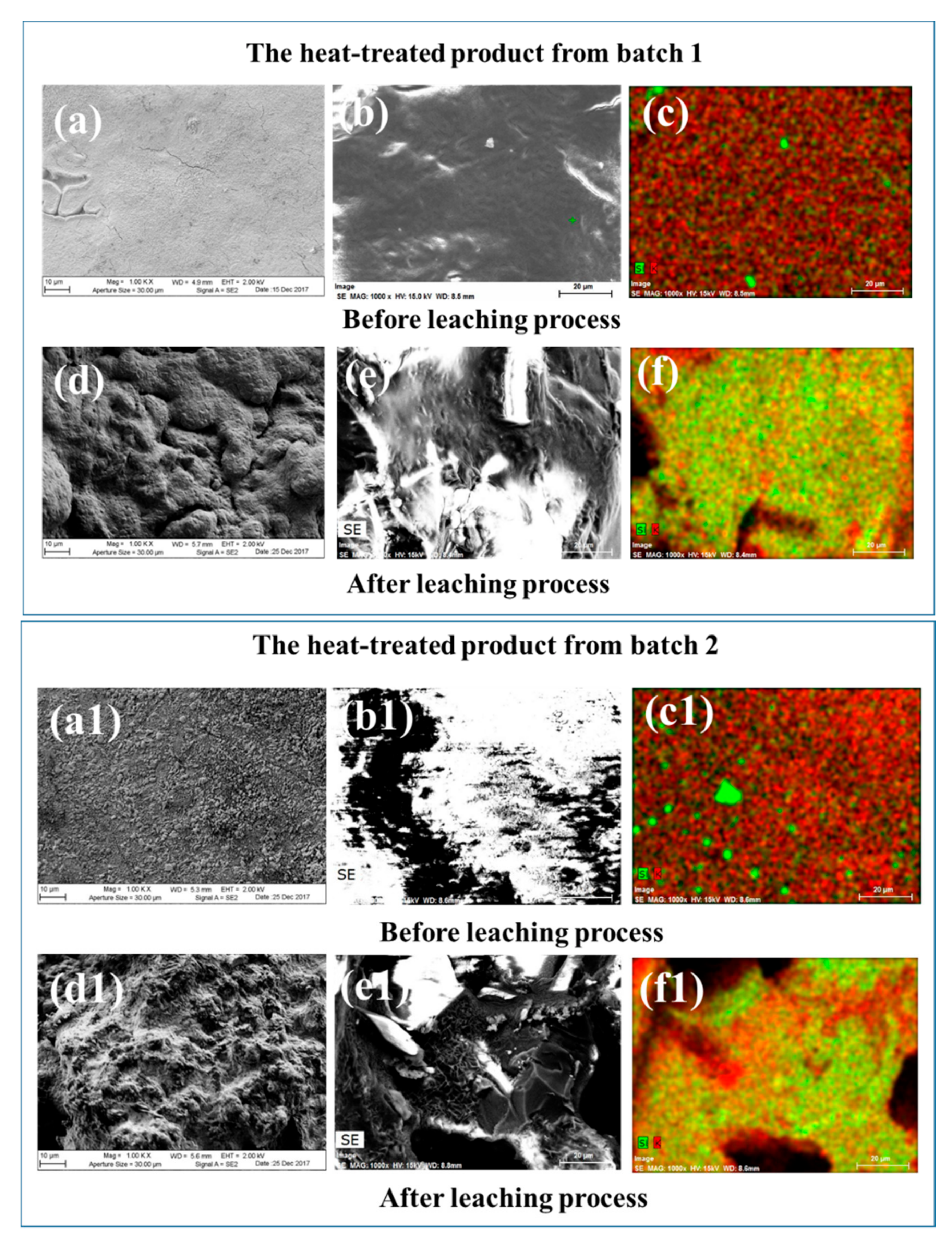
| Concentration (wt %) | Error (%) | |
|---|---|---|
| SiO2 | 50.5 | 1.3 |
| B2O3 | 14.9 | 0.3 |
| Na2O | 10.24 | 0.5 |
| CaO | 3.08 | 0.9 |
| Al2O3 | 5.47 | 1.1 |
| ZnO | 3.21 | 0.8 |
| Li2O | 3.06 | 0.7 |
| RuO2 | 0.65 | 0.09 |
| Rh2O3 | 0.18 | 0.02 |
| PdO | 0.36 | 0.04 |
| SeO2 | 0.12 | 0.02 |
| Cs2O | 0.75 | 0.05 |
| TeO2 | 0.13 | 0.01 |
| ZrO2 | 1.31 | 0.1 |
| MnO | 0.34 | 0.04 |
| BaO | 0.54 | 0.03 |
| La2O3 | 0.33 | 0.1 |
| CeO2 | 1.94 | 0.4 |
| Nd2O3 | 1.39 | 0.6 |
| Gd2O3 | 1.29 | 0.8 |
| Fe2O3 | 0.21 | 0.02 |
| Simulated Glass | K2CO3 | Sb2O3 | Bi2O3 | Carbon | |
| Batch 1 | 2.5 | 5 | 0.5 | 0 | 0.5 |
| Batch 2 | 2.5 | 5 | 0 | 0.5 | 0.5 |
| Elements | Mean Extraction Efficiency (wt %) | |
|---|---|---|
| Sb Metal Phase | Bi Metal Phase | |
| Pd | 78.6 | 88.1 |
| Rh | 107 | 42.1 |
| Ru | 62.1 | 0.53 |
| Se | N.D. | N.D. |
| Te | 0.13 | 0.73 |
| Cs | 1.8 | 2.2 |
| Elements | Concentration of Elements (wt %) | |||
|---|---|---|---|---|
| Heat-Treated Contact Surface from Batch 1 | Heat-Treated Contact Surface from Batch 1 after Leaching | Heat-Treated Contact Surface from Batch 2 | Heat-Treated Contact Surface from Batch 2 after Leaching | |
| K | 30.74 | 13.91 | 26.13 | 17.37 |
| Si | N.D. | 7.61 | 0.25 | 8.68 |
| Na | 1.59 | 2.1 | 2.27 | 1.29 |
| Elements | Mean Extraction Efficiency (wt %) | |
|---|---|---|
| Heat-Treated Contact Surface from Batch 1 | Heat-Treated Contact Surface from Batch 2 | |
| Cs | 93.76 | 73.56 |
| Se | 60.40 | 2.59 |
| Te | 23.65 | 4.93 |
| Pd | N.D. | N.D. |
| Rh | 0.22 | 0.14 |
| Ru | <0.01 | 1.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Lv, Y.; Xu, Z.; Wang, S.; Wang, J. The Removal of Platinum Group Metals, Cs, Se, and Te from Nuclear Waste Glass Using Liquid Sb Extraction and Phase Separation Methods. Materials 2020, 13, 5305. https://doi.org/10.3390/ma13225305
Zhang M, Lv Y, Xu Z, Wang S, Wang J. The Removal of Platinum Group Metals, Cs, Se, and Te from Nuclear Waste Glass Using Liquid Sb Extraction and Phase Separation Methods. Materials. 2020; 13(22):5305. https://doi.org/10.3390/ma13225305
Chicago/Turabian StyleZhang, Meng, Ying Lv, Zhanglian Xu, Sheng Wang, and Jie Wang. 2020. "The Removal of Platinum Group Metals, Cs, Se, and Te from Nuclear Waste Glass Using Liquid Sb Extraction and Phase Separation Methods" Materials 13, no. 22: 5305. https://doi.org/10.3390/ma13225305
APA StyleZhang, M., Lv, Y., Xu, Z., Wang, S., & Wang, J. (2020). The Removal of Platinum Group Metals, Cs, Se, and Te from Nuclear Waste Glass Using Liquid Sb Extraction and Phase Separation Methods. Materials, 13(22), 5305. https://doi.org/10.3390/ma13225305





