Pathogen-Induced Expression of OsDHODH1 Suggests Positive Regulation of Basal Defense Against Xanthomonas oryzae pv. oryzae in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice Materials and Growth Conditions
2.2. Xa R Genes Tagged with DNA Markers
2.3. Genomic DNA Extraction and Genotyping of Rice Plants
2.4. Cloning and Sequencing of Xa21
2.5. Xanthomonas Oryzae pv. Oryzae Growth and Inoculation into Rice Plants
2.6. Lesion Length (LL) Measurement and Disease Scoring
2.7. Arabidopsis Materials, Growth Conditions, and Genotyping
2.8. Pseudomonas Syringae pv. Tomato (Pst) Growth and Inoculum Preparation
2.9. Symptoms Development in Arabidopsis Genotypes Challenged with Pst DC3000 vir
2.10. Total RNA Isolation, cDNA Synthesis and qPCR Analysis
3. Results
3.1. Polymorphic Bands of Amplified DNA SSR and STS Markers Linked to Xa R Genes in Different Rice Cultivars
3.2. Differential Phenotypic Response of Nine Rice Cultivars Towards Xoo K3 Infection
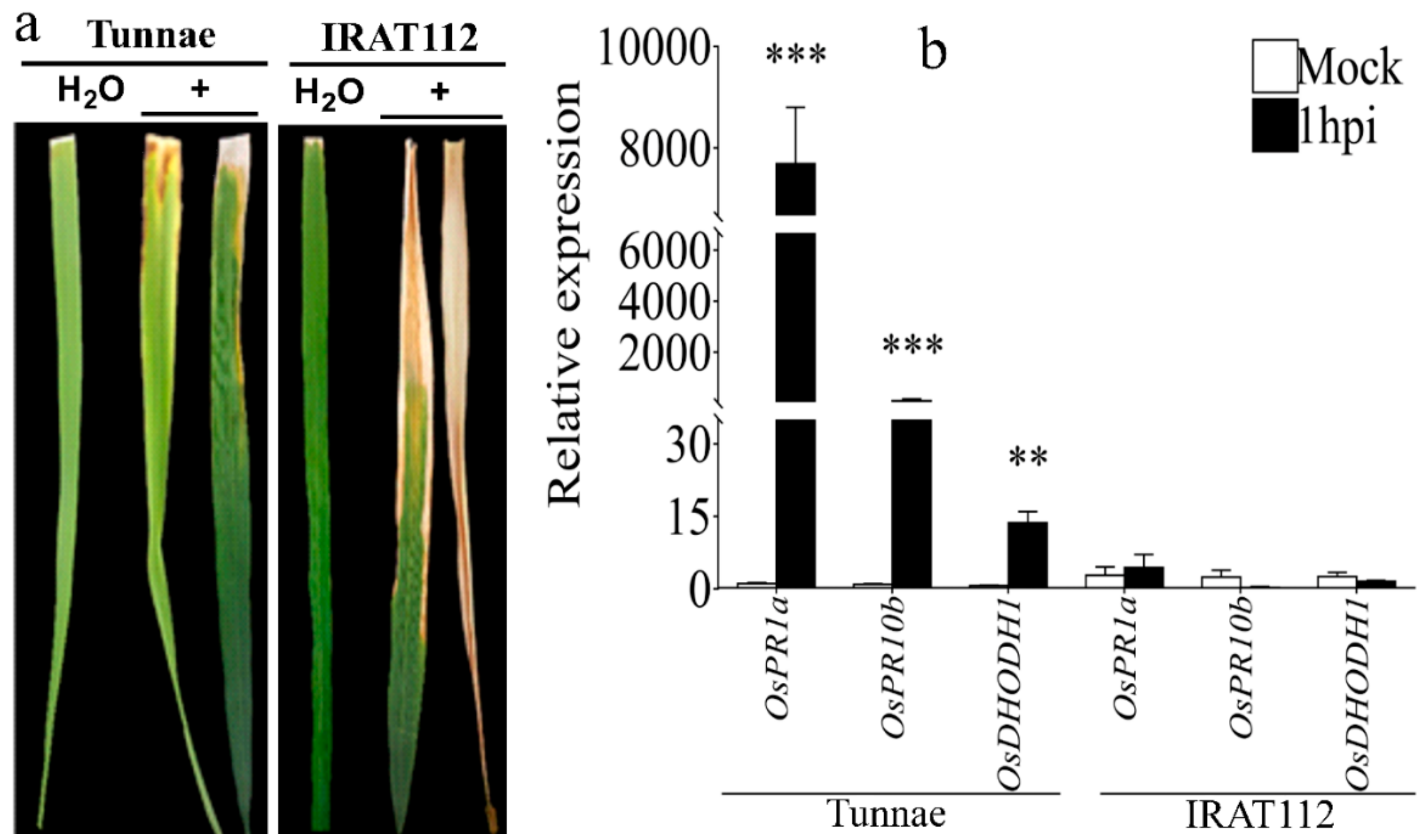
3.3. Xoo K3 Induced OsDHODH1 Expression in Tunnae, the Topmost Resistant, while Being Downregulated in IRAT112, the Highly Susceptible Cultivar Early after Inoculation
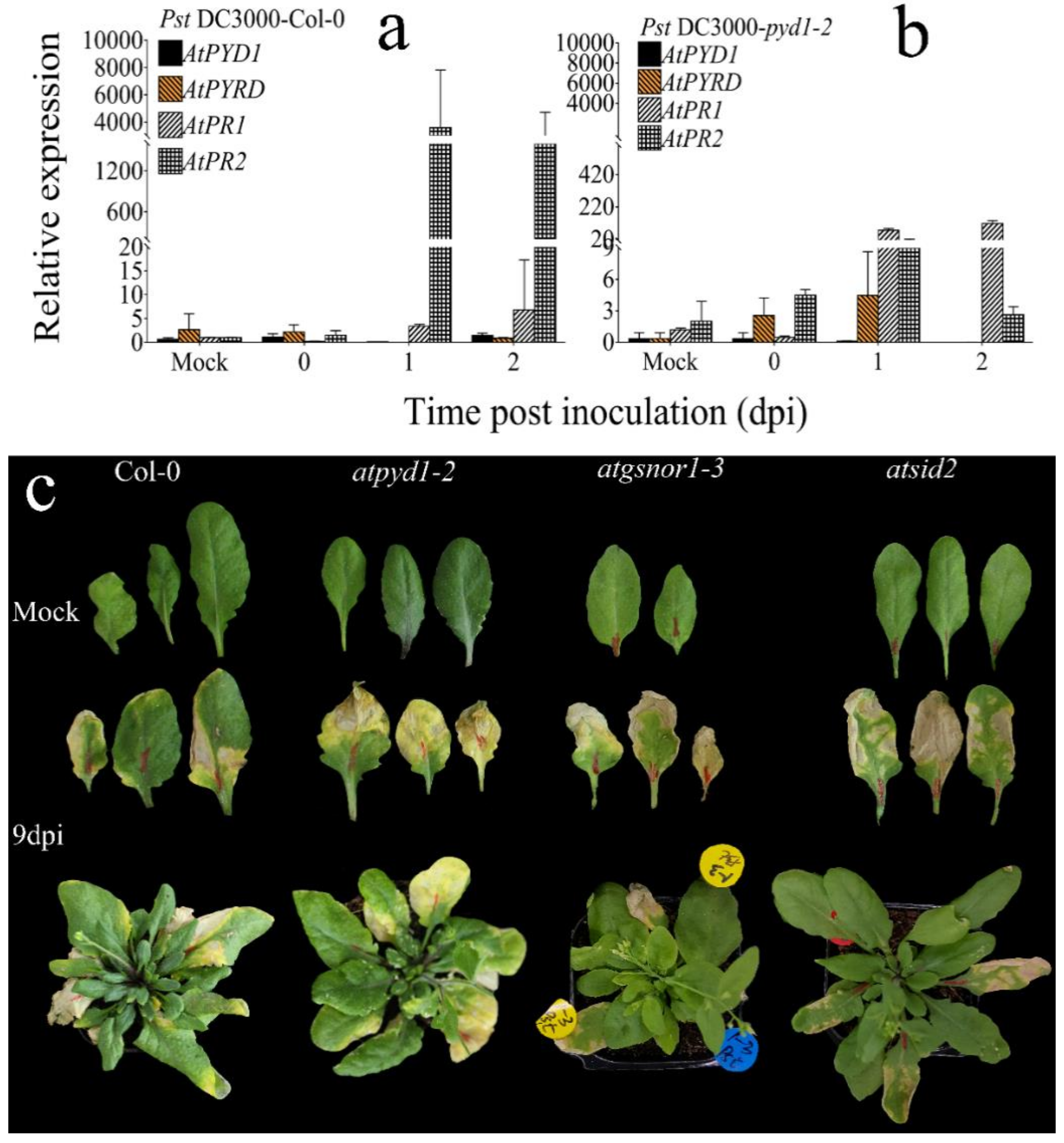
3.4. The Expression of the Arabidopsis PR1 and PR2 was Differentially Regulated in atpyd1-2 Knockout Line
4. Discussion
4.1. Differential Phenotypic Response of Rice Cultivars towards Xoo K3 Inoculation
4.2. The Expression Patterns of OsDHODH1 and PR Genes in Resistant and Susceptible Rice Cultivars Suggest a Positive Regulation of Plant Basal Defense
4.3. AtPYD1 Positively Regulates Plant Basal Defense against Pst DC3000
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khush, G.S. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.K.; Nayak, D.K.; Mohanty, S.; Behera, L.; Barik, S.R.; Pandit, E.; Lenka, S.; Anandan, A. Pyramiding of three bacterial blight resistance genes for broad-spectrum resistance in deepwater rice variety, Jalmagna. Rice 2015, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Lebailly, P.; Michel, B.; M’Vubu, N.; Roger, A. Quel Développement Agricole pour la RDC? Conjonctures Congolaises 2014: Politiques, Territoires et Ressources Naturelles: Changements et Continuités; Éditions L’Harmattan: Paris, France, 2015; pp. 45–64. [Google Scholar]
- Seck, P.A.; Diagne, A.; Mohanty, S.; Wopereis, M.C. Crops that feed the world 7: Rice. Food Secur. 2012, 4, 7–24. [Google Scholar] [CrossRef]
- Serraj, R.; McNally, K.L.; Slamet-Loedin, I.; Kohli, A.; Haefele, S.M.; Atlin, G.; Kumar, A. Drought resistance improvement in rice: An integrated genetic and resource management strategy. Plant Prod. Sci. 2011, 14, 1–14. [Google Scholar] [CrossRef]
- Gregorio, G.; Senadhira, D.; Mendoza, R.; Manigbas, N.; Roxas, J.; Guerta, C. Progress in breeding for salinity tolerance and associated abiotic stresses in rice. Field Crops Res. 2002, 76, 91–101. [Google Scholar] [CrossRef]
- Ou, S.H. Rice Diseases; Commonwealth Mycology Institute: Kew, UK, 1985. [Google Scholar]
- Zhang, H.T.; Wang, S.P. Rice versus Xanthomonas oryzae pv oryzae: A unique pathosystem. Curr. Opin. Plant Biol. 2013, 16, 188–195. [Google Scholar] [CrossRef]
- He, Y.W.; Wu, J.E.; Cha, J.S.; Zhang, L.H. Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiol. 2010, 10. [Google Scholar] [CrossRef]
- Niño-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef]
- Mubassir, M.; Nasiruddin, K.M.; Shahin, N.H.; Begum, S.N.; Sultana, A.; Rashid, A.B. Measurement of Phenotypic Variation for Control and Bacterial Leaf Blight Inoculated Rice Lines and Varieties. Am. J. Biosci. Bioeng. 2016, 4, 59–64. [Google Scholar] [CrossRef][Green Version]
- Djedatin, G.; Ndjiondjop, M.-N.; Sanni, A.; Lorieux, M.; Verdier, V.; Ghesquiere, A. Identification of novel major and minor QTLs associated with Xanthomonas oryzae pv. oryzae (African strains) resistance in rice (Oryza sativa L.). Rice 2016, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Sabar, M.; Bibi, T.; Farooq, H.U.; Haider, Z.; Naseem, I.; Mahmood, A.; Akhter, M. Molecular screening of rice (Oryza sativa L.) germplasm for Xa4, xa5 and Xa21 bacterial leaf blight (BLB) resistant genes using linked marker approach. Afr. J. Biotechnol. 2016, 15, 2317–2324. [Google Scholar]
- Jeung, J.; Heu, S.; Shin, M.; Vera Cruz, C.; Jena, K. Dynamics of Xanthomonas oryzae pv. oryzae populations in Korea and their relationship to known bacterial blight resistance genes. Phytopathology 2006, 96, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Mew, T.; Alvarez, A.; Leach, J.; Swings, J. Focus on bacterial blight of rice. Plant Dis. 1993, 77, 5–12. [Google Scholar] [CrossRef]
- Yu, C.; Chen, H.; Tian, F.; Leach, J.E.; He, C. Differentially-expressed genes in rice infected by Xanthomonas oryzae pv. oryzae relative to a flagellin-deficient mutant reveal potential functions of flagellin in host–pathogen interactions. Rice 2014, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Song, W.-Y.; Wang, G.-L.; Chen, L.-L.; Kim, H.-S. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 1995, 270, 1804. [Google Scholar] [CrossRef]
- Martin, G.B.; Bogdanove, A.J.; Sessa, G. Understanding the functions of plant disease resistance proteins. Annu. Rev. Plant Biol. 2003, 54, 23–61. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Zou, L.; Chen, G. Gene-for-gene relationships between rice and diverse avrBs3/pthA avirulence genes in Xanthomonas oryzae pv. oryzae. Plant Pathol. 2007, 56, 26–34. [Google Scholar] [CrossRef]
- Chen, G.; Zou, L.; Wang, X.; Xiang, Y.; Wang, J.-s. Molecular genetics of pathogenicity determinants of Xanthomonas oryzae pv. oryzae. Sci. Agric. Sin. 2004, 9, 1301–1307. [Google Scholar]
- John, V.; Dobson, R.; Alluri, K.; Zan, K.; Efron, Y.; Wasano, K.; Thottapilly, G.; Gibbons, J.; Rossel, H. Rice: Pathology, virology. Annu. Report Int. Inst. Trop. Agric. 1983 1984, 1984, 19–22. [Google Scholar]
- Xu, J.; Audenaert, K.; Hofte, M.; De Vleesschauwer, D. Abscisic acid promotes susceptibility to the rice leaf blight pathogen Xanthomonas oryzae pv oryzae by suppressing salicylic acid-mediated defenses. PLoS ONE 2013, 8, e67413. [Google Scholar]
- Subramoni, S.; Sonti, R.V. Growth deficiency of a Xanthomonas oryzae pv. oryzae fur mutant in rice leaves is rescued by ascorbic acid supplementation. Mol. Plant-Microbe Interact. 2005, 18, 644–651. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, J.A.; Afroz, S.; Arshad, H.M.I.; Sarwar, N.; Anwar, H.S.; Saleem, K.; Babar, M.M.; Jamil, F.F. Biochemical basis of resistance in rice against Bacterial leaf blight disease caused by Xanthomonas oryzae pv. oryzae. Adv. Life Sci. 2014, 1, 181–190. [Google Scholar]
- Yang, B.; Bogdanove, A. Inoculation and virulence assay for bacterial blight and bacterial leaf streak of rice. Rice Protoc. 2013, 249–255. [Google Scholar]
- Kim, S.-I.; Kwak, J.S.; Song, J.T.; Seo, H.S. Long-term effect of niclosamide on inhibition of bacterial leaf blight in rice. J. Plant Prot. Res. 2016, 56, 323–327. [Google Scholar] [CrossRef]
- Mew, T.; Mew, I.-P.; Huang, J. Scanning electron microscopy of virulent and avirulent strains of Xanthomonas campestris pv. oryzae on rice leaves. Phytopathology 1984, 74, 635–641. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, T.-W.; Wang, J.-Y.; Sun, S.; Chen, G.; Poplawsky, A.; He, Y.-W. The rice bacterial pathogen Xanthomonas oryzae pv. oryzae produces 3-hydroxybenzoic acid and 4-hydroxybenzoic acid via XanB2 for use in xanthomonadin, ubiquinone, and exopolysaccharide biosynthesis. Mol. Plant-Microbe Interact. 2013, 26, 1239–1248. [Google Scholar] [CrossRef]
- Rajagopal, L.; Sundari, C.S.; Balasubramanian, D.; Sonti, R.V. The bacterial pigment xanthomonadin offers protection against photodamage. FEBS Lett. 1997, 415, 125–128. [Google Scholar] [CrossRef]
- Lee, K.; Rasabandith, S.; Angeles, E.; Khush, G. Inheritance of resistance to bacterial blight in 21 cultivars of rice. Phytopathology 2003, 93, 147–152. [Google Scholar] [CrossRef]
- Rao, K.K.; Lakshminarasu, M.; Jena, K. DNA markers and marker-assisted breeding for durable resistance to bacterial blight disease in rice. Biotechnol. Adv. 2002, 20, 33–47. [Google Scholar]
- Yang, Z.; Sun, X.; Wang, S.; Zhang, Q. Genetic and physical mapping of a new gene for bacterial blight resistance in rice. Theor. Appl. Genet. 2003, 106, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, D.; Zhu, Y.; Tan, M.; Zhang, D.; Lin, X. Fine mapping of Xa2, a bacterial blight resistance gene in rice. Mol. Breed. 2006, 17, 1–6. [Google Scholar] [CrossRef]
- Gu, K.; Yang, B.; Tian, D.; Wu, L. R gene expression induced by a type-III effector triggers disease resistance in rice. Nature 2005, 435, 1122. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.S.; McCouch, S.R. The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol. Plant-Microbe Interact. 2004, 17, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.W.; Chittoor, J.; Yano, M.; Sasaki, T.; White, F. Development and mapping of markers linked to the rice bacterial blight resistance gene. Crop Sci. 2003, 43, 1484–1492. [Google Scholar] [CrossRef]
- Sanchez, A.; Ilag, L.; Yang, D.; Brar, D.; Ausubel, F.; Khush, G.; Yano, M.; Sasaki, T.; Li, Z.; Huang, N. Genetic and physical mapping of xa13, a recessive bacterial blight resistance gene in rice. TAG Theor. Appl. Genet. 1999, 98, 1022–1028. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Z.; Wang, S.; Zhang, Q. Identification of a 47-kb DNA fragment containing Xa4, a locus for bacterial blight resistance in rice. Theor. Appl. Genet. 2003, 106, 683–687. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.; Han, S.; Lee, D.; Lee, B. Distribution of Xanthomonas oryzae pv. oryzae strains virulent to Xa21 in Korea. Phytopathology 1999, 89, 928–933. [Google Scholar] [CrossRef]
- Singh, S.; Sidhu, J.; Huang, N.; Vikal, Y.; Li, Z.; Brar, D.; Dhaliwal, H.; Khush, G. Pyramiding three bacterial blight resistance genes (xa5, xa13 and Xa21) using marker-assisted selection into indica rice cultivar PR106. Theor. Appl. Genet. 2001, 102, 1011–1015. [Google Scholar] [CrossRef]
- Dilla-Ermita, C.J.; Tandayu, E.; Juanillas, V.M.; Detras, J.; Lozada, D.N.; Dwiyanti, M.S.; Cruz, C.V.; Mbanjo, E.G.N.; Ardales, E.; Diaz, M.G. Genome-wide Association Analysis Tracks Bacterial Leaf Blight Resistance Loci In Rice Diverse Germplasm. Rice 2017, 10, 8. [Google Scholar] [CrossRef]
- Lee, D.; Seo, J.; Choi, J.; Park, K.; Bae, S. Pathotypes of Xanthomonas campestris pv. oryzae in Honam District, Korea. Korean J. Plant Pathol. 1986, 2, 102–106. [Google Scholar]
- Noh, T.; Lee, D.; Kang, M.; Shin, M.; Na, S. Identification of new race of Xanthomonas oryzae pv. oryzae (Xoo) in Korea. Phytopathology 2003, 93, S66. [Google Scholar]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323. [Google Scholar] [CrossRef] [PubMed]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010, 11, 539. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Xu, J.; Audenaert, K.; Hofte, M.; De Vleesschauwer, D. Correction: Abscisic Acid Promotes Susceptibility to the Rice Leaf Blight Pathogen Xanthomonas oryzae pv oryzae by Suppressing Salicylic Acid-Mediated Defenses. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Van Buyten, E.; Satoh, K.; Balidion, J.; Mauleon, R.; Choi, I.-R.; Vera-Cruz, C.; Kikuchi, S.; Höfte, M. Brassinosteroids antagonize gibberellin-and salicylate-mediated root immunity in rice. Plant Physiol. 2012, 158, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Cao, Y.; Huang, L.; Zhao, J.; Xu, C.; Li, X.; Wang, S. Activation of the indole-3-acetic acid–amido synthetase GH3-8 suppresses expansin expression and promotes salicylate-and jasmonate-independent basal immunity in rice. Plant Cell 2008, 20, 228–240. [Google Scholar] [CrossRef]
- Fang, J.; Uchiumi, T.; Yagi, M.; Matsumoto, S.; Amamoto, R.; Takazaki, S.; Yamaza, H.; Nonaka, K.; Kang, D. Dihydro-orotate dehydrogenase is physically associated with the respiratory complex and its loss leads to mitochondrial dysfunction. Biosci. Rep. 2013, 33, e00021. [Google Scholar] [CrossRef]
- Kafer, C.; Thornburg, R. Pyrimidine metabolism in plants. Paths Pyrimidines 1999, 15, 14–24. [Google Scholar]
- Boldt, R.; Zrenner, R. Purine and pyrimidine biosynthesis in higher plants. Physiol. Plant. 2003, 117, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, R.; Stitt, M.; Sonnewald, U.; Boldt, R. Pyrimidine and purine biosynthesis and degradation in plants. Annu. Rev. Plant Biol. 2006, 57, 805–836. [Google Scholar] [CrossRef] [PubMed]
- DiMauro, S.; Schon, E.A. Mitochondrial disorders in the nervous system. Annu. Rev. Neurosci. 2008, 31, 91–123. [Google Scholar] [CrossRef]
- Frenzel, M.; Rommelspacher, H.; Sugawa, M.D.; Dencher, N.A. Ageing alters the supramolecular architecture of OxPhos complexes in rat brain cortex. Exp. Gerontol. 2010, 45, 563–572. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial DNA mutations in disease and aging. Environ. Mol. Mutagen. 2010, 51, 440–450. [Google Scholar] [CrossRef]
- Khutornenko, A.; Dalina, A.; Chernyak, B.; Chumakov, P.; Evstafieva, A. The role of dihydroorotate dehydrogenase in apoptosis induction in response to inhibition of the mitochondrial respiratory chain complex III. Acta Nat. 2014, 6, 69–75. [Google Scholar] [CrossRef]
- Somado, E.; Guei, R.; Keya, S. NERICA: The New Rice for Africa—A Compendium; Africa Rice Center (WARDA): Cotonou, Benin, 2008; pp. 10–14. [Google Scholar]
- Fred, A.K.; Kiswara, G.; Yi, G.; Kim, K.-M. Screening rice cultivars for resistance to bacterial leaf blight. J. Microbiol. Biotechnol. 2016, 26, 938–945. [Google Scholar] [CrossRef]
- Mateso, B.; Kasongo, K.; Mbuya, K.; Anzolo, N.; Mbuluku, E. Lioto, a short-duration rice variety suitable for Northern Zaire. Int. Rice Res. Notes 1993, 18, 19–20. [Google Scholar]
- Turner, H.; Black, R. Rice leaf scald: Pathogen biology and diversity. In Major Fungal Diseases of Rice; Springer: New York, NY, USA, 2001; pp. 307–319. [Google Scholar]
- Habarurema, I.; Asea, G.; Lamo, J.; Gibson, P.; Edema, R.; Séré, Y.; Onasanya, R. Genetic analysis of resistance to rice bacterial blight in Uganda. Afr. Crop Sci. J. 2012, 20, 105–112. [Google Scholar]
- Lamo, J.; Tongoona, P.; Sie, M.; Semon, M.; Onaga, G.; Okori, P. Upland Rice Breeding in Uganda: Initiatives and Progress. In Advances in International Rice Research; InTech: London, UK, 2017. [Google Scholar]
- Ji, Z.-J.; Yang, S.-D.; Zeng, Y.-X.; Liang, Y.; Yang, C.-D.; Qian, Q. Pyramiding blast, bacterial blight and brown planthopper resistance genes in rice restorer lines. J. Integr. Agric. 2016, 15, 1432–1440. [Google Scholar] [CrossRef]
- Hajira, S.; Sundaram, R.; Laha, G.; Yugander, A.; Balachandran, S.; Viraktamath, B.; Sujatha, K.; Balachiranjeevi, C.; Pranathi, K.; Anila, M. A Single-Tube, Functional Marker-Based Multiplex PCR Assay for Simultaneous Detection of Major Bacterial Blight Resistance Genes Xa21, xa13 and xa5 in Rice. Rice Sci. 2016, 23, 144–151. [Google Scholar] [CrossRef]
- Singh, A.K.; Dharmraj, E.; Nayak, R.; Singh, P.K.; Singh, N.K. Identification of bacterial leaf blight resistance genes in wild rice of eastern India. Turk. J. Bot. 2015, 39, 1060–1066. [Google Scholar] [CrossRef]
- Keb-Llanes, M.; González, G.; Chi-Manzanero, B.; Infante, D. A rapid and simple method for small-scale DNA extraction in Agavaceae and other tropical plants. Plant Mol. Biol. Rep. 2002, 20, 299–300. [Google Scholar] [CrossRef]
- Froger, A.; Hall, J.E. Transformation of plasmid DNA into E. coli using the heat shock method. JoVE J. Vis. Exp. 2007, 6, e253. [Google Scholar] [CrossRef]
- Wang, G.-L.; Song, W.-Y.; Ruan, D.-L.; Sideris, S.; Ronald, P.C. The cloned gene, Xa21, confers resistance to multiple Xanthomonas oryzae pv. oryzae isolates in transgenic plants. Mol. Plant-Microbe Interact. MPMI 1996, 9, 850–855. [Google Scholar] [CrossRef]
- Yin, Z.C.; Gu, K.Y.; Tian, D.S. Molecular Interaction between XA10 and AVRXA10. U.S. Patent 9,650,647, 16 May 2017. [Google Scholar]
- Kauffman, H. An improved technique for evaluation of resistance of rice varieties to Xanthomonas oryzae. Plant Dis. Rep. 1973, 57, 537–541. [Google Scholar]
- Zeng, X.; Tian, D.; Gu, K.; Zhou, Z.; Yang, X.; Luo, Y.; White, F.F.; Yin, Z. Genetic engineering of the Xa10 promoter for broad-spectrum and durable resistance to Xanthomonas oryzae pv. oryzae. Plant Biotechnol. J. 2015, 13, 993–1001. [Google Scholar] [CrossRef]
- Busungu, C.; Taura, S.; Sakagami, J.-I.; Ichitani, K. Identification and linkage analysis of a new rice bacterial blight resistance gene from XM14, a mutant line from IR24. Breed. Sci. 2016, 66, 636–645. [Google Scholar] [CrossRef]
- Chaudhary, R. Internationalization of elite germplasm for farmers: Collaborative mechanisms to enhance evaluation of rice genetic resources. Charact. Eval. 1996, 26, 1–27. [Google Scholar]
- International Rice Research Institute. Standard Evaluation System (SES) for Rice; IRRI: Manila, Philippines, 2013. [Google Scholar]
- Khan, J.A.; Arshad, H.M.I.; Jamil, F.F.; Hasnain, S. Evaluation of rice genotypes against bacterial leaf blight (BLB) disease. Pak. J. Phytopathol. 2009, 21, 26–30. [Google Scholar]
- Feechan, A.; Kwon, E.; Yun, B.-W.; Wang, Y.; Pallas, J.A.; Loake, G.J. A central role for S-nitrosothiols in plant disease resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 8054–8059. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, S.; Vanderauwera, S.; Vuylsteke, M.; Rombauts, S.; Langebartels, C.; Seidlitz, H.K.; Zabeau, M.; Van Montagu, M.; Inze, D.; Van Breusegem, F. Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J. 2004, 39, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Whalen, M.C.; Innes, R.W.; Bent, A.F.; Staskawicz, B.J. Identification of Pseudomonas syringae pathogens of Arabidopsis and a bacterial locus determining avirulence on both Arabidopsis and soybean. Plant Cell 1991, 3, 49–59. [Google Scholar] [CrossRef]
- Hockin, N.L.; Mock, T.; Mulholland, F.; Kopriva, S.; Malin, G. The response of diatom central carbon metabolism to nitrogen starvation is different from that of green algae and higher plants. Plant Physiol. 2012, 158, 299–312. [Google Scholar] [CrossRef]
- Imran, Q.M.; Hussain, A.; Lee, S.-U.; Mun, B.-G.; Falak, N.; Loake, G.J.; Yun, B.-W. Transcriptome profile of NO-induced Arabidopsis transcription factor genes suggests their putative regulatory role in multiple biological processes. Sci. Rep. UK 2018, 8, 771. [Google Scholar] [CrossRef]
- León, J.; Rojo, E.; Sánchez-Serrano, J.J. Wound signalling in plants. J. Exp. Bot. 2001, 52, 1–9. [Google Scholar]
- Wang, W.; Barnaby, J.Y.; Tada, Y.; Li, H.; Tör, M.; Caldelari, D.; Lee, D.-u.; Fu, X.-D.; Dong, X. Timing of plant immune responses by a central circadian regulator. Nature 2011, 470, 110. [Google Scholar] [CrossRef]
- Hua, J. Modulation of plant immunity by light, circadian rhythm, and temperature. Curr. Opin. Plant Biol. 2013, 16, 406–413. [Google Scholar] [CrossRef]
- Mun, B.-G.; Lee, S.-U.; Hussain, A.; Kim, H.-H.; Rolly, N.K.; Jung, K.-H.; Yun, B.-W. S-nitrosocysteine-responsive genes modulate diverse regulatory pathways in Oryza sativa: A transcriptome profiling study. Funct. Plant Biol. 2018, 45, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Revalska, M.; Vassileva, V.; Zechirov, G.; Iantcheva, A. Is the auxin influx carrier LAX3 essential for plant growth and development in the model plants Medicago truncatula, Lotus japonicus and Arabidopsis thaliana? Biotechnol. Biotechnol. Equip. 2015, 29, 786–797. [Google Scholar] [CrossRef]
- Pha, N.T.; Lang, N.T. Marker assisted selection in rice breeding for bacterial leaf blight. Omon Rice 2004, 12, 19–26. [Google Scholar]
- Ramalingam, J.; Basharat, H.; Zhang, G. STS and microsatellite marker-assisted selection for bacterial blight resistance and waxy genes in rice, Oryza sativa L. Euphytica 2002, 127, 255–260. [Google Scholar] [CrossRef]
- Akhtar, M.A.; Abbasi, F.M.; Ahmad, H.; Shahzad, M.; Shah, M.A.; Shah, A.H. Evaluation of rice germplasm against Xanthomonas oryzae causing bacterial leaf blight. Pak. J. Bot. 2011, 43, 3021–3023. [Google Scholar]
- Khoshkdaman, M.; Ebadi, A.A.; Majidi-Shilsar, F.; Dariush, S. Preliminary evaluation of resistance genes in rice against bacterial leaf blight in Guilan Province—Iran. Agric. Sci. 2014, 5, 94. [Google Scholar] [CrossRef]
- Hasan Naqvi, S.A.; Perveen, R.; Chohan, S. Evaluation of Virulence of Xanthomonas oryzae pv. oryzae against Rice Genotypes. Int. J. Agric. Biol. 2015, 17, 1186–1192. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.P.; Singh, H.; Singh, O.; Samantray, S.; Singh, M.; Jaiswal, H. Inheritance of resistance in indica rice cultivar HUR 4-3 against bacterial leaf blight (Xanthomonas oryzae pv. oryzae). Int. J. Agric. Environ. Biotechnol. 2014, 7, 777. [Google Scholar] [CrossRef]
- Sykes, D.B.; Kfoury, Y.S.; Mercier, F.E.; Wawer, M.J.; Law, J.M.; Haynes, M.K.; Lewis, T.A.; Schajnovitz, A.; Jain, E.; Lee, D. Inhibition of dihydroorotate dehydrogenase overcomes differentiation blockade in acute myeloid leukemia. Cell 2016, 167, 171–186.e115. [Google Scholar] [CrossRef]
- Baldwin, J.; Michnoff, C.H.; Malmquist, N.A.; White, J.; Roth, M.G.; Rathod, P.K.; Phillips, M.A. High-throughput screening for potent and selective inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase. J. Biol. Chem. 2005, 280, 21847–21853. [Google Scholar] [CrossRef]
- Liu, W.Y.; Wang, M.M.; Huang, J.; Tang, H.J.; Lan, H.X.; Zhang, H.S. The OsDHODH1 gene is involved in salt and drought tolerance in rice. J. Integr. Plant Biol. 2009, 51, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Rolly, N.K.; Lee, S.-U.; Imran, Q.M.; Hussain, A.; Mun, B.-G.; Kim, K.-M.; Yun, B.-W. Nitrosative stress-mediated inhibition of OsDHODH1 gene expression suggests roots growth reduction in rice (Oryza sativa L.). 3 Biotech 2019, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Rolly, N.K.; Imran, Q.M.; Shahid, M.; Imran, M.; Khan, M.; Lee, S.-U.; Hussain, A.; Lee, I.-J.; Yun, B.-W. Drought-induced AtbZIP62 transcription factor regulates drought stress response in Arabidopsis. Plant Physiol. Biochem. 2020, 156, 384–395. [Google Scholar]
- Rolly, N.K.; Imran, Q.M.; Lee, I.-J.; Yun, B.-W. Salinity Stress-Mediated Suppression of Expression of Salt Overly Sensitive Signaling Pathway Genes Suggests Negative Regulation by AtbZIP62 Transcription Factor in Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 1726. [Google Scholar] [CrossRef] [PubMed]
- Rushton, P.J.; Torres, J.T.; Parniske, M.; Wernert, P.; Hahlbrock, K.; Somssich, I. Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J. 1996, 15, 5690–5700. [Google Scholar] [CrossRef]
- Willmott, R.L.; Rushton, P.J.; Hooley, R.; Lazarus, C.M. DNase1 footprints suggest the involvement of at least three types of transcription factors in the regulation of α-Amy2/A by gibberellin. Plant Mol. Biol. 1998, 38, 817–825. [Google Scholar] [CrossRef]
- Turck, F.; Zhou, A.; Somssich, I.E. Stimulus-dependent, promoter-specific binding of transcription factor WRKY1 to its native promoter and the defense-related gene PcPR1-1 in parsley. Plant Cell 2004, 16, 2573–2585. [Google Scholar] [CrossRef]
- Xing, D.-H.; Lai, Z.-B.; Zheng, Z.-Y.; Vinod, K.; Fan, B.-F.; Chen, Z.-X. Stress-and pathogen-induced Arabidopsis WRKY48 is a transcriptional activator that represses plant basal defense. Mol. Plant 2008, 1, 459–470. [Google Scholar] [CrossRef]
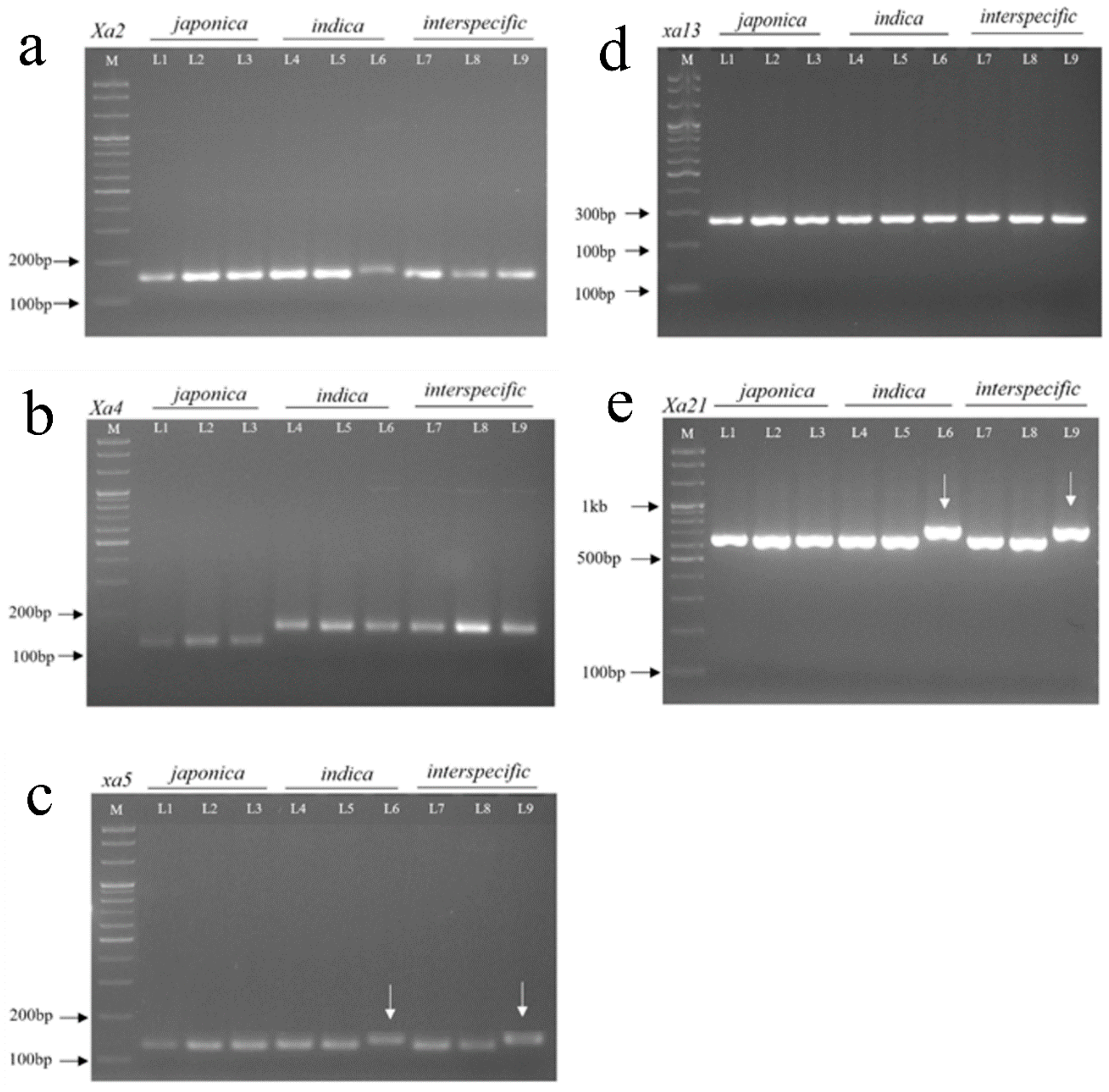
| Markers | Primer Sequences (5′->3′) | Linked Gene | Distance (cM) | Chr | Expected Band Size (bp) | References |
|---|---|---|---|---|---|---|
| Npb197 d, RM-317 | F-CATACTTACCAGTTCACCGCC | Xa2 | 18.5 | 4 | 154 | Singh et al., 2015 |
| R-CTGGAGAGTGTCAGCTAGTTGA | ||||||
| Npb181 a, MP1 | F-ATCGATCGATCTTCACGAGG | Xa4 | 1.7 | 11 | 150 | Ma Bo-Jun et al., 1999 |
| R-TCGTATAAAAGGCATTCGGG | ||||||
| RM122 b, SSR | F-GAGTCGATGTAATGTCATCAGTGC | xa5 | 0.4 | 5 | 227 | Blair et al., 2003 |
| R-GAAGGAGGTATCGCTTTGTTGGAC | ||||||
| RG136 c- SSR | F-GGCCATGGCTCAGTGTTTAT | xa13 | 3.8 | 8 | 450 | Zhang et al., 1996 |
| R-GAGCTCCAGCTCTCCAAATG | ||||||
| pTA248 c, STS | F-AGACGCGGAAGGGTGGTTCCCGGA | Xa21 | 0–1 | 11 | 950 | Ronald et al., 1992 |
| R-AGACGCGGTAATCGAAAGATGAAA |
| Lesion Length (cm) | Disease Leaf Area (%) | Disease Score | Host Response |
|---|---|---|---|
| 0 | No disease observed | 1 | Highly Resistant (HR) |
| >0–5 | Less than 1% | 2 | Resistant (R) |
| 1–3 | 3 | Resistant (R) | |
| 4–10 | 4 | Resistant (R) | |
| >5–10 | 11–15 | 5 | Moderately Resistant (MR) |
| 16–25 | 6 | Moderately Resistant (MR) | |
| >10–15 | 26–50 | 7 | Susceptible (MS) |
| >15 | 51–75 | 8 | Susceptible (S) |
| 76–100 | 9 | Highly Susceptible (HS) |
| Gene Name/Genotype | Locus/SALK | Forward Primer (5′->3′) | Reverse Primer (5′->3′) | Gene Name |
|---|---|---|---|---|
| Genotyping primers of the T-DNA insertion atpyd1-2 (Left border and right border) | ||||
| atpyd1-2 | SALK_083897C | TTGGGTGGCAGAACATAGAAC | ATGAATTCAGCGGCATCATAG | Arabidopsis pyd1-2 loss of function mutant |
| Primers for gene expression in rice | ||||
| OsDHODH1 | LOC_Os02g50350 | GAGGTCTGCGGTTGGATAAA | CTATAGGGTGCACGGCTCTC | Dehydroorotate dihydrogenase encoded gene |
| OsPR1a | LOC_Os07g03710 | AGTTCGTCGAGCAGGTTATC | AGATTGGCCGACGAAGTTG | Rice Pathogenesis related gene 1a |
| OsPR10b | LOC_Os12g36850 | ATGGCTCCGGCCTTCGTCTC | GGTTAAGCTTCATGATGTGGATGG | Rice Pathogenesis related gene 10b |
| OsUBI | LOC_Os03g03920 | GCCATTAATGCTACCACTGC | GTTCTCGGATAGCTGTTGTTGC | Rice ubiquitin encoding gene |
| Primers for gene expression in Arabidopsis | ||||
| AtPYD1 | AT3G17810 | AGTGAGGATCGCTCGCTTTC | TCATCACACCGGTGCATACC | PYRIMIDINE 1 |
| AtPYRD | AT5G23300 | AAGACGAGTGAGGATGCTGC | GCAGTCCTGCAGTATTGGGT | PYRIMIDINE D |
| AtPR1 | AT2G14610 | GTGCAATGGAGTTTGTGGTC | TCACATAATTCCCACGAGGA | Arabidopsis pathogenesis-related gene 1 |
| AtPR2 | AT3G57260 | CAGATTCCGGTACATCAACG | AGTGGTGGTGTCAGTGGCTA | Arabidopsis pathogenesis-related gene 2 |
| AtACT2 | AT3G18780 | AGGTTCTGTTCCAGCCATC | TTAGAAGCATTTCCTGTGAAC | Arabidopsis Actin coding gene 2 |
| Cultivars | Lesion length (cm) 21 dpi | SEM | % DLA 1 | Disease Score (0–9) 2 | Host Response to Xoo K3 Inoculation 3 |
|---|---|---|---|---|---|
| Jinbu | 8.3 | ±4.92 | 29.2 | 7 | Moderately Susceptible (MS) |
| Odae | 14.2 | ±1.49 | 78.8 | 9 | Highly Susceptible (HS) |
| Tunnae | 5.4 | ±0.33 | 21.3 | 6 | Moderately Resistant (MR) |
| Lioto | 15.8 | ±3.44 | 71.8 | 8 | Susceptible (S) |
| IRAT112 | 17.6 | ±1.04 | 90.1 | 9 | Highly Susceptible (HS) |
| Sipi | 4.6 | ±1.25 | 23.3 | 6 | Moderately Resistant (MR) |
| NERICA 4 | 9.2 | ±1.92 | 36.2 | 7 | Moderately Susceptible (MS) |
| NERICA 7 | 17.5 | ±4.05 | 52.7 | 8 | Susceptible (S) |
| NERICA-L14 | 4.7 | ±2.11 | 21.5 | 6 | Moderately Resistant (MR) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolly, N.K.; Imran, Q.M.; Kim, H.-H.; Aye, N.C.; Hussain, A.; Kim, K.-M.; Yun, B.-W. Pathogen-Induced Expression of OsDHODH1 Suggests Positive Regulation of Basal Defense Against Xanthomonas oryzae pv. oryzae in Rice. Agriculture 2020, 10, 573. https://doi.org/10.3390/agriculture10110573
Rolly NK, Imran QM, Kim H-H, Aye NC, Hussain A, Kim K-M, Yun B-W. Pathogen-Induced Expression of OsDHODH1 Suggests Positive Regulation of Basal Defense Against Xanthomonas oryzae pv. oryzae in Rice. Agriculture. 2020; 10(11):573. https://doi.org/10.3390/agriculture10110573
Chicago/Turabian StyleRolly, Nkulu Kabange, Qari Muhammad Imran, Hyun-Ho Kim, Nay Chi Aye, Adil Hussain, Kyung-Min Kim, and Byung-Wook Yun. 2020. "Pathogen-Induced Expression of OsDHODH1 Suggests Positive Regulation of Basal Defense Against Xanthomonas oryzae pv. oryzae in Rice" Agriculture 10, no. 11: 573. https://doi.org/10.3390/agriculture10110573
APA StyleRolly, N. K., Imran, Q. M., Kim, H.-H., Aye, N. C., Hussain, A., Kim, K.-M., & Yun, B.-W. (2020). Pathogen-Induced Expression of OsDHODH1 Suggests Positive Regulation of Basal Defense Against Xanthomonas oryzae pv. oryzae in Rice. Agriculture, 10(11), 573. https://doi.org/10.3390/agriculture10110573







