Physiological and Phytochemical Responses of Spinach Baby Leaves Grown in a PFAL System with LEDs and Saline Nutrient Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Light Conditions
2.3. Yield, Color, and Chlorophyll Fluorescence
2.4. Phytochemical Composition
2.5. Statistical Analysis
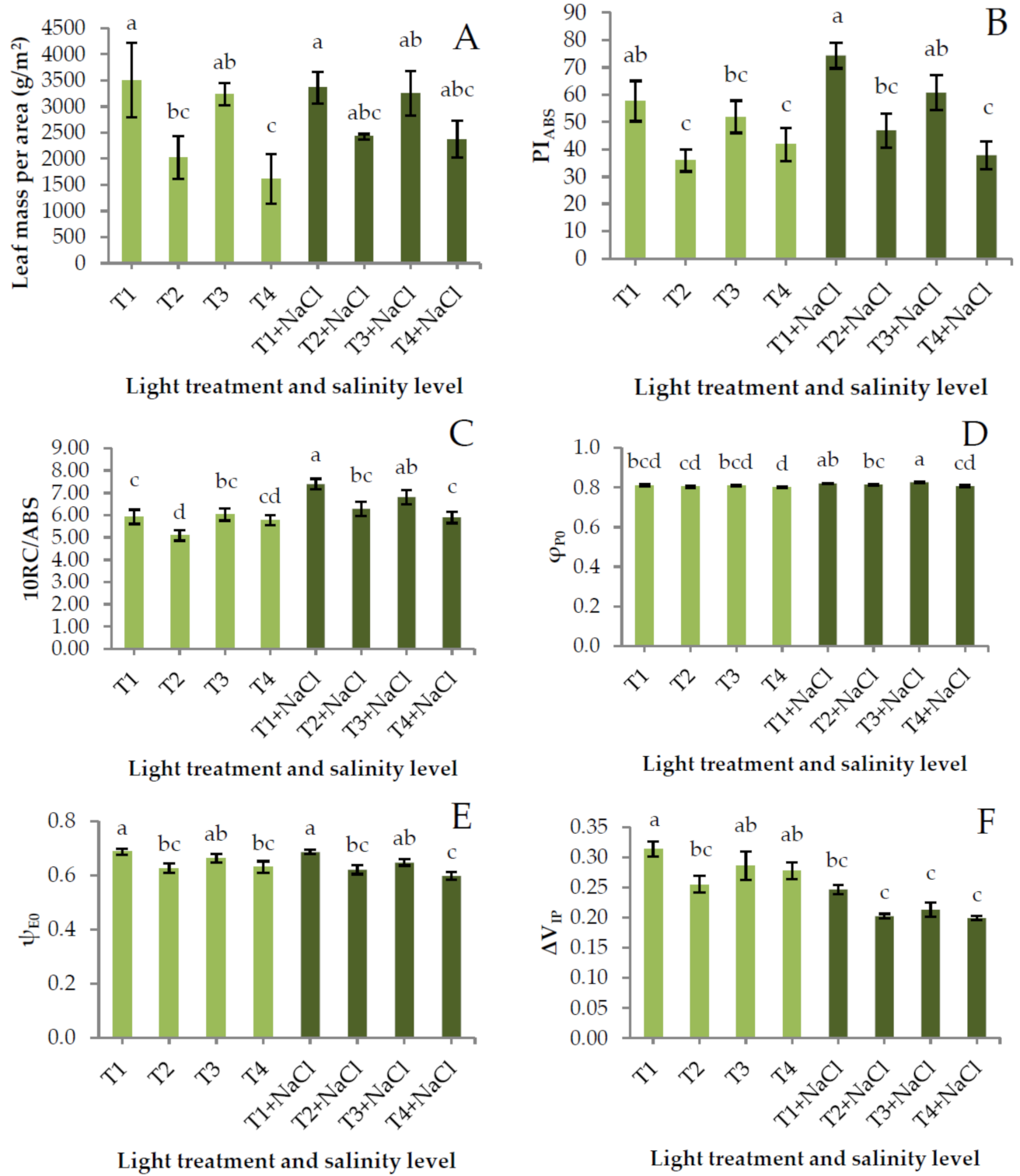
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mani, J.S.; Johnson, J.B.; Steel, J.C.; Broszczak, D.A.; Neilsen, P.M.; Walsh, K.B.; Naiker, M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020, 284, 197989. [Google Scholar] [CrossRef] [PubMed]
- Ostan, R.; Lanzarini, C.; Pini, E.; Scurti, M.; Vianello, D.; Bertarelli, C.; Fabbri, C.; Izzi, M.; Palmas, M.G.; Biondi, F.; et al. Inflammaging and Cancer: A Challenge for the Mediterranean Diet. Nutrients 2015, 7, 2589–2621. [Google Scholar] [CrossRef] [PubMed]
- Koukounaras, A.; Siomos, A.S.; Sfakiotakis, E. Postharvest CO2 and ethylene production and quality of rocket (Eruca sativa Mill.) leaves as affected by leaf age and storage temperature. Postharvest Biol. Technol. 2007, 46, 167–173. [Google Scholar] [CrossRef]
- Alvarado-Camarillo, D.; Valdez-Aguilar, L.A.; González-Fuentes, J.A.; Rascón-Alvarado, E.; Peña-Ramos, F.M. Response of hydroponic lettuce to aeration, nitrate and potassium in the nutrient solution. Acta Agric. Scand. Sect. B Plant Soil Sci. 2020, 70, 341–348. [Google Scholar] [CrossRef]
- Roberts, J.L.; Moreau, R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016, 7, 3337–3353. [Google Scholar] [CrossRef]
- Koukounaras, A.; Bantis, F.; Karatolos, N.; Melissas, C.; Vezyroglou, A. Influence of Pre-Harvest Factors on Postharvest Quality of Fresh-Cut and Baby Leafy Vegetables. Agronomy 2020, 10, 172. [Google Scholar] [CrossRef]
- Gholami, V.; Yousefi, Z.; Rostami, H.Z. Modeling of Ground Water Salinity on the Caspian Southern Coasts. Water Resour. Manag. 2010, 24, 1415–1424. [Google Scholar] [CrossRef]
- Carillo, P.; Giordano, M.; Raimondi, G.; Napolitano, F.; Di Stasio, E.; Kyriacou, M.C.; Sifola, M.I.; Rouphael, Y. Physiological and Nutraceutical Quality of Green and Red Pigmented Lettuce in Response to NaCl Concentration in Two Successive Harvests. Agronomy 2020, 10, 1358. [Google Scholar] [CrossRef]
- Hamilton, J.M.; Fonseca, J.M. Effect of Saline Irrigation Water on Antioxidants in Three Hydroponically Grown Leafy Vegetables: Diplotaxis tenuifolia, Eruca sativa, and Lepidium sativum. HortScience 2010, 45, 546–552. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Role of the plant factory with artificial lighting (PFAL) in urban areas. In Plant Factory; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: London, UK, 2020; pp. 7–34. [Google Scholar]
- Bourget, C.M. An Introduction to Light-emitting Diodes. HortScience 2008, 43, 1944–1946. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G. Plant responses to light. In Plant Factory; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: London, UK, 2020; pp. 153–166. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
- Stutte, G.W. Light-emitting Diodes for Manipulating the Phytochrome Apparatus. HortScience 2009, 44, 231–234. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Far-red radiation interacts with relative and absolute blue and red photon flux densities to regulate growth, morphology, and pigmentation of lettuce and basil seedlings. Sci. Hortic. 2019, 255, 269–280. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Miao, Y.-X.; Wang, X.-Z.; Gao, L.-H.; Chen, Q.-Y.; Qu, M. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Hosseini, A.; Mehrjerdi, M.Z.; Aliniaeifard, S.; Seif, M. Photosynthetic and growth responses of green and purple basil plants under different spectral compositions. Physiol. Mol. Biol. Plants 2019, 25, 741–752. [Google Scholar] [CrossRef]
- Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]
- Baraldi, R.; Canaccini, F.; Cortes, S.; Magnani, F.; Rapparini, F.; Zamboni, A.; Raddi, S. Role of xanthophyll cycle-mediated photoprotection in Arbutus unedo plants exposed to water stress during the Mediterranean summer. Photosynthetica 2008, 46, 378–386. [Google Scholar] [CrossRef]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of Photosystem I in the chlorophyll a fluorescence rise OJIP. BBA Bioenerg. 2005, 1706, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Ahlman, L.; Bånkestad, D.; Wik, T. Relation between Changes in Photosynthetic Rate and Changes in Canopy Level Chlorophyll Fluorescence Generated by Light Excitation of Different Led Colours in Various Background Light. Remote. Sens. 2019, 11, 434. [Google Scholar] [CrossRef]
- Yoshida, H.; Mizuta, D.; Fukuda, N.; Hikosaka, S.; Goto, E. Effects of varying light quality from single-peak blue and red light-emitting diodes during nursery period on flowering, photosynthesis, growth, and fruit yield of everbearing strawberry. Plant Biotechnol. 2016, 33, 267–276. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Trivandrum, KL, India, 2006; pp. 23–67. [Google Scholar]
- Neocleous, D.; Koukounaras, A.; Siomos, A.S.; Vasilakakis, M. Assessing the Salinity Effects on Mineral Composition and Nutritional Quality of Green and Red “Baby” Lettuce. J. Food Qual. 2014, 37, 1–8. [Google Scholar] [CrossRef]
- Długosz-Grochowska, O.; Kołton, A.; Wojciechowska, R. Modifying folate and polyphenol concentrations in Lamb’s lettuce by the use of LED supplemental lighting during cultivation in greenhouses. J. Funct. Foods 2016, 26, 228–237. [Google Scholar] [CrossRef]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED lighting enhances growth characteristics and total phenolic content of Ocimum basilicum, but variably affects transplant success. Sci. Hortic. 2016, 198, 277–283. [Google Scholar] [CrossRef]
- Bantis, F.; Radoglou, K. Testing the potential of LEDs to enhance growth and quality characteristics of Salvia fruticosa. Hortic. Sci. 2019, 46, 98–106. [Google Scholar] [CrossRef]
- Sager, J.C.; McFarlane, J.C. Plant growth chamber handbook, Radiation. In Iowa Agriculture and Home Economics Experimental Station Special Report No. 99; Langhans, R.W., Tibbits, T.W., Eds.; Iowa State University Press: Ames, IA, USA, 1997; pp. 1–29. [Google Scholar]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, O.K. Blue Light added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef]
- Briggs, W.R.; Christie, J.M. Phototropins 1 and 2: Versatile plant blue-light receptors. Trends Plant Sci. 2002, 7, 204–210. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Levizou, E.; Ntatsi, G.; Fernandes, Â.; Petrotos, K.; Akoumianakis, K.; Barros, L.; Ferreira, I. Salinity effect on nutritional value, chemical composition and bioactive compounds content of Cichorium spinosum L. Food Chem. 2017, 214, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Mou, B. Responses of Spinach to Salinity and Nutrient Deficiency in Growth, Physiology, and Nutritional Value. J. Am. Soc. Hortic. Sci. 2016, 141, 12–21. [Google Scholar] [CrossRef]
- Wink, M. Functions and Biotechnology of Plant Secondary Metabolites. Annual Plant Reviews 3; Wiley-Blackwell: Oxford, UK, 2010. [Google Scholar]
- Deleris, A.; Gallego-Bartolome, J.; Bao, J.; Kasschau, K.D.; Carrington, J.C.; Voinnet, O. Hierarchical Action and Inhibition of Plant Dicer-Like Proteins in Antiviral Defense. Science 2006, 313, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Amoozgar, A.; Mohammadi, A.; Sabzalian, M.R. Impact of light-emitting diode irradiation on photosynthesis, phytochemical composition and mineral element content of lettuce cv. Grizzly. Photosynthetica 2017, 55, 85–95. [Google Scholar] [CrossRef]
- Ouzounis, T.; Parjikolaei, B.R.; Rosenqvist, E.; Ottosen, C.-O.; Fretté, X. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal red:blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Długosz-Grochowska, O.; Kołton, A.; Żupnik, M. Effects of LED supplemental lighting on yield and some quality parameters of lamb’s lettuce grown in two winter cycles. Sci. Hortic. 2015, 187, 80–86. [Google Scholar] [CrossRef]
- Chen, X.-L.; Xue, X.-Z.; Guo, W.-Z.; Wang, L.-C.; Qiao, X.-J. Growth and nutritional properties of lettuce affected by mixed irradiation of white and supplemental light provided by light-emitting diode. Sci. Hortic. 2016, 200, 111–118. [Google Scholar] [CrossRef]
- Addiscott, T. Is it nitrate that threatens life or the scare about nitrate? J. Sci. Food Agric. 2006, 86, 2005–2009. [Google Scholar] [CrossRef]
- Chung, J.; Jin, S.; Cho, H. Low Water Potential in Saline Soils Enhances Nitrate Accumulation of Lettuce. Commun. Soil Sci. Plant Anal. 2005, 36, 1773–1785. [Google Scholar] [CrossRef]
- Hill, M. Nitrates and nitrites from food and water in relation to human disease. In Nitrates and Nitrites in Food and Water; Woodhead Publishing: Hampshire, UK, 1996; pp. 163–193. [Google Scholar]
- Lillo, C. Signalling cascades integrating light-enhanced nitrate metabolism. Biochem. J. 2008, 415, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Viršilė, A.; Brazaitytė, A.; Vaštakaitė, V.; Jankauskienė, J.; Miliauskienė, J.; Samuolienė, G.; Novičkovas, A.; Duchovskis, P. Nitrate, nitrite, protein, amino acid contents, and photosynthetic and growth characteristics of tatsoi cultivated under various photon flux densities and spectral light compositions. Sci. Hortic. 2019, 258, 108781. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Umar, S.; Iqbal, M. Nitrate accumulation in plants, factors affecting the process, and human health implications. A review. Agron. Sustain. Dev. 2007, 27, 45–57. [Google Scholar] [CrossRef]
- Madeira, A.C.; Ferreira, A.; de Varennes, A.; Vieira, M.I. SPAD Meter Versus Tristimulus Colorimeter to Estimate Chlorophyll Content and Leaf Color in Sweet Pepper. Commun. Soil Sci. Plant Anal. 2003, 34, 2461–2470. [Google Scholar] [CrossRef]

| Parameters | Light Treatment | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| UV%; 380–399 nm | 0.02 | 0.02 | 0.02 | 0.36 |
| Blue%; 400–499 nm | 7.62 | 10.90 | 11.38 | 20.59 |
| Green%; 500–599 nm | 2.34 | 18.54 | 13.85 | 36.46 |
| Red%; 600–699 nm | 67.25 | 62.20 | 56.48 | 36.92 |
| Far-red%; 700–780 nm | 22.77 | 8.34 | 18.28 | 5.68 |
| Red peak (nm) | 660 | 631 | 660 | 660 |
| Blue peak (nm) | 448 | 448 | 448 | 461 |
| R:B | 8.82 | 5.71 | 4.97 | 1.79 |
| R:FR | 2.95 | 7.46 | 3.09 | 6.50 |
| CCT (K) | - | 1624 | 2143 | 5034 |
| CRI | - | 66.1 | 71.0 | 87.7 |
| Light Treatment | Colorimetric Parameter | |||
|---|---|---|---|---|
| Lightness | Chroma | Hue Angle | a*/b* | |
| T1 | 44.62 ± 0.58 a | 29.23 ± 0.91 c | 125.05 ± 0.37 ab | −0.70 ± 0.01 ab |
| T2 | 44.60 ± 0.42 a | 29.75 ± 0.67 bc | 125.79 ± 0.33 a | −0.72 ± 0.01 b |
| T3 | 46.21 ± 0.52 a | 30.73 ± 0.71 abc | 125.42 ± 0.36 a | −0.71 ± 0.01 b |
| T4 | 45.30 ± 0.57 a | 30.69 ± 0.96 abc | 125.32 ± 0.40 a | −0.71 ± 0.01 b |
| T1+NaCl | 45.75 ± 0.79 a | 34.21 ± 1.02 ab | 123.82 ± 0.41 ab | −0.67 ± 0.01 ab |
| T2+NaCl | 43.76 ± 0.76 a | 32.01 ± 1.24 abc | 125.20 ± 0.47 ab | −0.71 ± 0.01 ab |
| T3+NaCl | 45.74 ± 0.98 a | 34.72 ± 1.56 a | 123.25 ± 0.64 b | −0.66 ± 0.02 a |
| T4+NaCl | 43.62 ± 1.01 a | 32.88 ± 1.35 abc | 124.95 ± 0.59 ab | −0.70 ± 0.02 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bantis, F.; Fotelli, M.; Ilić, Z.S.; Koukounaras, A. Physiological and Phytochemical Responses of Spinach Baby Leaves Grown in a PFAL System with LEDs and Saline Nutrient Solution. Agriculture 2020, 10, 574. https://doi.org/10.3390/agriculture10110574
Bantis F, Fotelli M, Ilić ZS, Koukounaras A. Physiological and Phytochemical Responses of Spinach Baby Leaves Grown in a PFAL System with LEDs and Saline Nutrient Solution. Agriculture. 2020; 10(11):574. https://doi.org/10.3390/agriculture10110574
Chicago/Turabian StyleBantis, Filippos, Mariangela Fotelli, Zoran S. Ilić, and Athanasios Koukounaras. 2020. "Physiological and Phytochemical Responses of Spinach Baby Leaves Grown in a PFAL System with LEDs and Saline Nutrient Solution" Agriculture 10, no. 11: 574. https://doi.org/10.3390/agriculture10110574
APA StyleBantis, F., Fotelli, M., Ilić, Z. S., & Koukounaras, A. (2020). Physiological and Phytochemical Responses of Spinach Baby Leaves Grown in a PFAL System with LEDs and Saline Nutrient Solution. Agriculture, 10(11), 574. https://doi.org/10.3390/agriculture10110574








