AL Amyloidosis: The Effect of Maintenance Therapy on Autologous Stem Cell Transplantation Outcomes
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patient Population
2.2. FISH Abnormalities
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
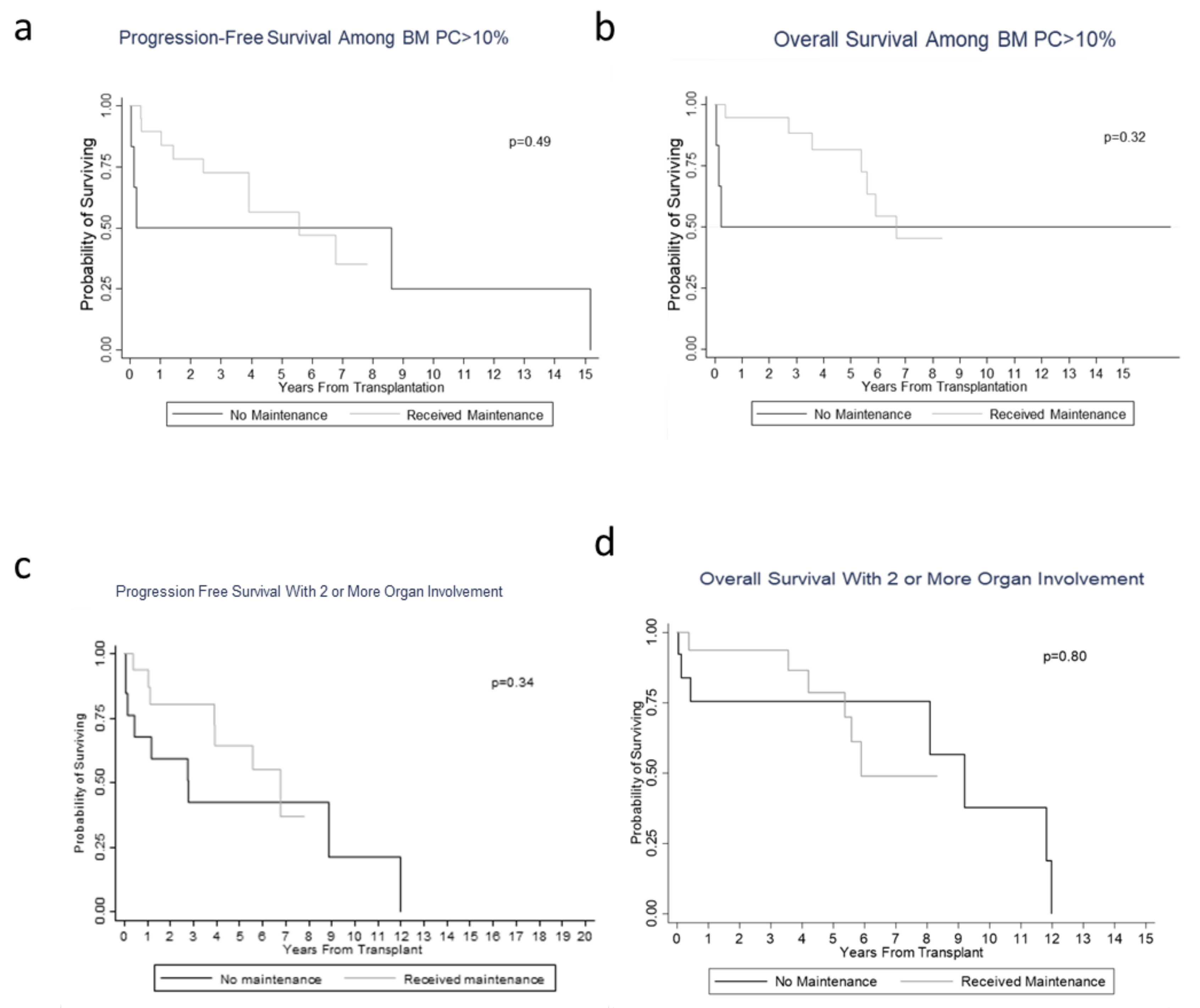
3.2. Hematological/Organ Response and Survival/Prognosis Outcomes
3.3. Toxicity Profiles in the Maintenance Group
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Kyle, R.A.; Linos, A.; Beard, C.M.; Linke, R.P.; Gertz, M.A.; O’Fallon, W.M.; Kurland, L.T. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood 1992, 79, 1817–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gertz, M.A. Immunoglobulin light chain amyloidosis: 2014 update on diagnosis, prognosis, and treatment. Am. J. Hematol. 2014, 89, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Systemic Light Chain Amyloidosis. Version 1. 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/amyloidosis.pdf. (accessed on 1 February 2019).
- Merlini, G.; Seldin, D.C.; Gertz, M.A. Amyloidosis: Pathogenesis and New Therapeutic Options. J. Clin. Oncol. 2011, 29, 1924–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desport, E.; Bridoux, F.; Sirac, C.; Delbes, S.; Bender, S.; Fernandez, B.; Quellard, N.; Lacombe, C.; Goujon, J.-M.; Lavergne, D.; et al. AL Amyloidosis. Orphanet J. Rare Dis. 2012, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comenzo, R.L.; Vosburgh, E.; Falk, R.H.; Sanchorawala, V.; Reisinger, J.; Dubrey, S.; O’Hara, C. Dose-intensive melphalan with blood stem-cell support for the treatment of AL (amyloid light-chain) amyloidosis: Survival and responses in 25 patients. Blood 1998, 91, 3662–3670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandit, A.; Wei, L.; Bustamante, L.; Elder, P.; Falk, W. Improved Treatment Related Mortality in Patients with Primary Systemic Amyloidosis (AL Amyloidosis) Undergoing Autologous Hematopoietic Stem Cell Transplant (aHSCT). Arch. Hematol. Blood Dis. 2019, 2, 12–18. [Google Scholar]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Warsame, R.; Kumar, S.K.; Gertz, M.A.; Lacy, M.Q.; Buadi, F.K.; Hayman, S.R.; Leung, N.; Dingli, D.; Lust, J.A.; Ketterling, R.P.; et al. Abnormal FISH in patients with immunoglobulin light chain amyloidosis is a risk factor for cardiac involvement and for death. Blood Cancer J. 2015, 5, e310. [Google Scholar] [CrossRef] [PubMed]
- Varga, C.; Comenzo, R.L. High-dose melphalan and stem cell transplantation in systemic AL amyloidosis in the era of novel anti-plasma cell therapy: A comprehensive review. Bone Marrow Transplant. 2018, 54, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Gertz, M.A.; Comenzo, R.; Falk, R.H.; Fermand, J.P.; Hazenberg, B.P.; Hawkins, P.N.; Merlini, G.; Moreau, P.; Ronco, P.; Sanchorawala, V.; et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am. J. Hematol. 2005, 79, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; Gertz, M.A.; Kyle, R.A.; Lacy, M.Q.; Burritt, M.F.; Therneau, T.M.; Greipp, P.R.; Witzig, T.E.; Lust, J.A.; Rajkumar, S.V.; et al. Serum Cardiac Troponins and N-Terminal Pro-Brain Natriuretic Peptide: A Staging System for Primary Systemic Amyloidosis. J. Clin. Oncol. 2004, 22, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Colby, C.; Laumann, K.; Zeldenrust, S.R.; Leung, N.; Dingli, D.; et al. Revised Prognostic Staging System for Light Chain Amyloidosis Incorporating Cardiac Biomarkers and Serum Free Light Chain Measurements. J. Clin. Oncol. 2012, 30, 989–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landau, H.J.; Hassoun, H.T.; Rosenzweig, M.A.; Maurer, M.; Liu, J.; Flombaum, C.; Di Bello, C.; Hoover, E.L.; Riedel, E.; Giralt, S.; et al. Bortezomib and dexamethasone consolidation following risk-adapted melphalan and stem cell transplantation for patients with newly diagnosed light-chain amyloidosis. Leukemia 2013, 27, 823–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landau, H.; Lahoud, O.; Devlin, S.; Lendvai, N.; Chung, D.J.; Dogan, A.; Landgren, C.O.; Giralt, S.; Hassoun, H. Pilot Study of Bortezomib and Dexamethasone Pre- and Post-Risk-Adapted Autologous Stem Cell Transplantation in AL Amyloidosis. Biol. Blood Marrow Transplant. 2020, 26, 204–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, G.P.; Schrier, S.L.; Lafayette, R.A.; Arai, S.; Witteles, R.; Liedtke, M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood 2017, 130, 900–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milani, P.; Merlini, G.; Palladini, G. Novel Therapies in Light Chain Amyloidosis. Kidney Int. Rep. 2018, 3, 530–541. [Google Scholar] [CrossRef] [Green Version]
- Palladini, G.; Kastritis, E.; Maurer, M.S.; Zonder, J.A.; Minnema, M.C.; Wechalekar, A.D.; Jaccard, A.; Lee, H.C.; Bumma, N.; Kaufman, J.L.; et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: Safety run-in results of ANDROMEDA. Blood 2020, 136, 71–80. [Google Scholar] [CrossRef]

| Characteristics | All Patients (n = 50) | No Maintenance Therapy (n = 22) | Maintenance Therapy (n = 28) | p-Value |
|---|---|---|---|---|
| Median age at diagnosis (range), years | 58 (33–71) | 58 (33–71) | 58 (39–70) | 0.990 |
| Gender, male, n (%) | 33 (66.0) | 15 (68.2) | 18 (64.3) | 0.773 |
| Specific induction regimen received, n (%) | Lenalidomide–dexamethasone 5 (10%) Melphalan–prednisone 4 (8%) Bortezomib–dexamethasone 15 (30%) Bortezomib–lenalidomide–dexamethasone 2 (4%) Cyclophosphamide–bortezomib–dexamethasone 14 (28%) None 10 (20%) | |||
| Specific maintenance therapy used, n (%) Immunomodulator (lenalidomide, thalidomide), bortezomib | 0 (0) 0 (0) | 24 (86) 4(14) | ||
| Melphalan dose received, 140 mg/m2 200 mg/m2 | 23 (46.0) 27 (54.0) | 11 (50.0) 11 (50.0) | 12 (42.9) 16 (57.1) | 0.620 |
| Light chain restriction (kappa) | 14 (28) | 4 (18.2) | 10 (35.7) | 0.215 |
| Light chain restriction (lambda) | 35 (70) | 18 (81.8) | 17 (60.7) | 0.131 |
| dFLC mg/dla median (range) | 17.9 (1209–7001) | 15.9 (300–607) | 25 (1209–7001) | 0.640 |
| BMPCb <10% ≥10% | 24 (48%) 26 (52%) | 16 (72.7) 6 (27.3) | 8 (28.6) 20 (71.4) | 0.004 |
| Urine total protein, mg/24 h, median (range) | 6849 (0–81,921) | 10,308 (0–81,921) | 3758 (0–22,500) | 0.06 |
| No. of involved organs, median (range) | 2 (0–5) | 1 (0–4) | 2 (1–5) | 0.910 |
| Cardiac involvement, n (%) | 18 (36.0) | 10 (45.5) | 8 (28.6) | 0.217 |
| Renal involvement, n (%) | 40 (80.0) | 19 (86.4) | 21 (75.0) | 0.319 |
| NT-proBNP ≥ 332 ng/L, n (%) | 19 (70.4) | 8 (72.7) | 11 (68.8) | 0.824 |
| NT-proBNP ≥ 1800 ng/L, n (%) | 11 (40.7) | 6 (54.5) | 5 (31.3) | 0.226 |
| Mayo stage (2012), n (%) | 0.460 | |||
| I | 13 (50.0) | 5 (50.0) | 8 (50.0) | |
| II | 7 (26.9) | 4 (40.0) | 3 (18.8) | |
| III | 2 (7.7) | 0 (0.0) | 2 (12.5) | |
| IV | 4 (15.4) | 1 (10.0) | 3 (18.8) | |
| Missing | 24 | 12 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozga, M.; Zhao, Q.; Benson, D.; Elder, P.; Williams, N.; Bumma, N.; Rosko, A.; Chaudhry, M.; Khan, A.; Devarakonda, S.; et al. AL Amyloidosis: The Effect of Maintenance Therapy on Autologous Stem Cell Transplantation Outcomes. J. Clin. Med. 2020, 9, 3778. https://doi.org/10.3390/jcm9113778
Ozga M, Zhao Q, Benson D, Elder P, Williams N, Bumma N, Rosko A, Chaudhry M, Khan A, Devarakonda S, et al. AL Amyloidosis: The Effect of Maintenance Therapy on Autologous Stem Cell Transplantation Outcomes. Journal of Clinical Medicine. 2020; 9(11):3778. https://doi.org/10.3390/jcm9113778
Chicago/Turabian StyleOzga, Michael, Qiuhong Zhao, Don Benson, Patrick Elder, Nita Williams, Naresh Bumma, Ashley Rosko, Maria Chaudhry, Abdullah Khan, Srinivas Devarakonda, and et al. 2020. "AL Amyloidosis: The Effect of Maintenance Therapy on Autologous Stem Cell Transplantation Outcomes" Journal of Clinical Medicine 9, no. 11: 3778. https://doi.org/10.3390/jcm9113778





