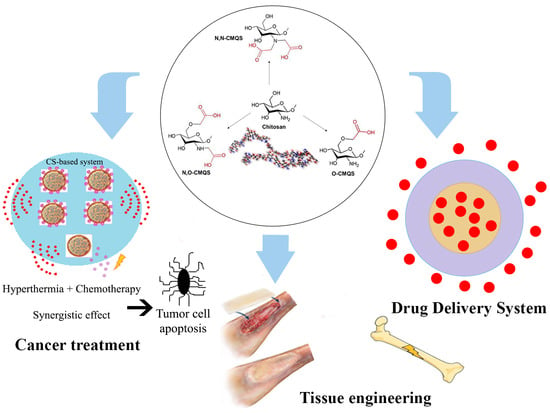
A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment
Abstract
1. Introduction
2. Chitin and Chitosan
Chitosan’s Chemical Modifications
3. Chitosan in Tissue Engineering
3.1. Bone Tissue Engineering
3.2. Cartilage Tissue Engineering
3.3. Neural Tissue Engineering
3.4. Chitosan in Wound Healing
- (1)
- Immediately after the injury, coagulation and hemostasis occur in the wound, which can avoid exsanguination and arrange a matrix for the invasion of cells necessary in the subsequent stages of healing [185].
- (2)
- Soon after, the inflammatory phase of wound healing begins [186], controlled by inflammatory reactions moderated by cytokines, chemokines, growth factors, and their actions on cell receptors. The activation of intracellular signaling cascades contributes to cell proliferation, migration, and differentiation. Additionally, distinct types of cells (such as granulocytes and macrophages) are recruited by chemoattractant factors to initiate repair at the wound site [187]. In this process, an appropriate inflammatory microenvironment conducive to healing is formed by regulating cell activity and factors released by chitosan (CS)-based hydrogels.
- (3)
- In 2 to 10 days after the injury, proliferation begins, featuring proliferation and migration in distinct cell types. The proliferative phase involves neoangiogenesis, development of granulation tissue and ECM, and re-epithelialization [188]. CS provides a non-protein matrix for 3D tissue growth and the activation of macrophages for tumoricidal activity. CS-based hydrogels can promote fibroblast proliferation, angiogenesis, and regular collagen deposition; enhance the natural hyaluronic acid (HA) level at the wound site; accelerate wound healing; and act in scar avoidance [6,96,189].
- (4)
- In this phase, remodeling occurs, in which, by many enzymes and stress actions, the content and disposition of collagen fibers in the scar tissue are adapted to adjust to the physiological work, and this ends in the development of normal epithelium and maturation of scar tissue. A vital component of the dermal tissue present in chitosan is N-acetyl glucosamine (NAG), which is essential for repairing scar tissue [190].
4. The Use of Chitosan as a Drug Delivery System
4.1. Chitosan in Cancer Treatment
- (1)
- Supporting in the solubilization of hydrophobic drugs in aqueous media by polymeric nanocarriers [286];
- (2)
- Longer blood circulation times for nanocomposites with the appropriate size (70–200 nm), which increases permeability and the retention effect (EPR) to passively target tumor tissue;
- (3)
- Microenvironment-responsive properties of DDSs can be obtained by grafting a nanocarrier with stimuli-responsive materials, used to guarantee the drug release solely at the target site;
- (4)
4.1.1. Therapeutic Metal Ions (TMIs)
4.1.2. Photodynamic Therapy (PDT)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120 Pt A, 1181–1189. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Ajun, W.; Yan, S.; Li, G.; Huili, L. Preparation of aspirin and probucol in combination loaded chitosan nanoparticles and in vitro release study. Carbohydr. Polym. 2009, 75, 566–574. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell. Mol. Life Sci. 1997, 53, 131–140. [Google Scholar] [CrossRef]
- Tan, H.; Wu, J.; Lao, L.; Gao, C. Gelatin/chitosan/hyaluronan scaffold integrated with PLGA microspheres for cartilage tissue engineering. Acta Biomater. 2009, 5, 328–337. [Google Scholar] [CrossRef]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K.; Wong, T.W. Chitosan and Its Application as Tissue Engineering Scaffolds. In Nanotechnology Applications for Tissue Engineering; William Andrew: Norwich, NY, USA, 2015; pp. 133–147. [Google Scholar]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-based nanomaterials for drug delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef]
- Yadu Nath, V.; Raghvendra Kumar, M.; Aswathy, V.; Parvathy, P.; Sunija, S.; Neelakandan, M.; Nitheesha, S.; Vishnu, K. Chitosan as promising materials for biomedical application: Review. Res. Dev. Mater. Sci. 2017, 2, 2576–8840. [Google Scholar]
- Colucci, F.; Caligiuri, M.A.; Di Santo, J.P. What does it take to make a natural killer? Nat. Rev. Immunol. 2003, 3, 413–425. [Google Scholar] [CrossRef]
- Li, X.; Dong, W.; Nalin, A.P.; Wang, Y.; Pan, P.; Xu, B.; Zhang, Y.; Tun, S.; Zhang, J.; Wang, L.S.; et al. The natural product chitosan enhances the anti-tumor activity of natural killer cells by activating dendritic cells. Oncoimmunology 2018, 7, e1431085. [Google Scholar] [CrossRef]
- Ehrlich, H.; Maldonado, M.; Spindler, K.D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (demospongia: Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 347–356. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Mathur, N.K.; Narang, C.K. Chitin and chitosan, versatile polysaccharides from marine animals. J. Chem. Educ. 1990, 67, 938–942. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Li, Q.; Dunn, E.T.; Grandmaison, E.W.; Goosen, M.F.A. Applications and Properties of Chitosan. J. Bioact. Compat. Polym. 1992, 7, 370–397. [Google Scholar] [CrossRef]
- Laranjeira, M.C.M.; Fávere, V.T.d. Quitosana: Biopolímero funcional com potencial industrial biomédico. Quim. Nova 2009, 32, 672–678. [Google Scholar] [CrossRef]
- Allan, C.R.; Hadwiger, L.A. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 1979, 3, 285–287. [Google Scholar] [CrossRef]
- Ehrlich, H.; Krautter, M.; Hanke, T.; Simon, P.; Knieb, C.; Heinemann, S.; Worch, H. First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (Hexactinellida: Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 473–483. [Google Scholar] [CrossRef]
- Birolli, W.G.; Delezuk, J.A.d.M.; Campana-Filho, S.P. Ultrasound-assisted conversion of alpha-chitin into chitosan. Appl. Acoust. 2016, 103, 239–242. [Google Scholar] [CrossRef]
- Lamarque, G.; Chaussard, G.; Domard, A. Thermodynamic aspects of the heterogeneous deacetylation of β-chitin: Reaction mechanisms. Biomacromolecules 2007, 8, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczynski, Z.; Bychowska, A.; Brzozowski, K.; Thoming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed]
- Bruck, W.M.; Slater, J.W.; Carney, B.F. Chitin, Chitosan, Oligosaccharides and Their Derivatives; Kim, S., Ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- No, H.K.; Meyers, S.P. Utilization of crawfish processing wastes as carotenoids, chitin and chitosan souces. J. Kor. Soc. Food Nutr. 1992, 21, 319–326. [Google Scholar]
- Tolaimate, A.; Desbrieres, J.; Rhazi, M.; Alagui, A.; Vincendon, M.; Vottero, P. On the influence of deacetylation process on the physicochemical characteristics of chitosan from squid chitin. Polymer 2000, 41, 2463–2469. [Google Scholar] [CrossRef]
- No, H.K.; Meyers, S.P. Preparation and Characterization of Chitin and Chitosan—A Review. J. Aquat. Food Prod. Technol. 1995, 4, 27–52. [Google Scholar] [CrossRef]
- No, H.K.; Cho, Y.I.; Kim, H.R.; Meyers, S.P. Effective deacetylation of chitin under conditions of 15 psi/121 °C. J. Agric. Food Chem. 2000, 48, 2625–2627. [Google Scholar] [CrossRef]
- Knaul, J.Z.; Hudson, S.M.; Creber, K.A.M. Crosslinking of chitosan fibers with dialdehydes: Proposal of a new reaction mechanism. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1079–1094. [Google Scholar] [CrossRef]
- Sonia, T.A.; Sharma, C.P. Chitosan and Its derivatives for drug delivery perspective. In Chitosan for Biomaterials I; Springer: Berlin, Germany, 2011; pp. 23–53. [Google Scholar]
- Silva, E.M.; Coutinho, M.G.F.; Costa, R.B.; de Carvalho, L.H.; Canedo, E.L. Influência da concentração e purificação da argila na estrutura e permeação ao vapor de água de nanocompósitos PEBDL/bentonita. Polímeros 2013, 23, 108–114. [Google Scholar] [CrossRef]
- Damian, C.; Beirão, L.H.; de Francisco, A.; Espírito Santo, M.L.P.; Teixeira, E. Quitosana: Um amino polissacarídeo com características funcionais. Alim. Nutr. 2005, 16, 195–205. [Google Scholar]
- Chung, Y.C.; Chen, C.Y. Antibacterial characteristics and activity of acid-soluble chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; De Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Papineau, A.M.; Hoover, D.G.; Knorr, D.; Farkas, D.F. Antimicrobial effect of water-soluble chitosans with high hydrostatic pressure. Food Biotechnol. 1991, 5, 45–57. [Google Scholar] [CrossRef]
- Thomas, J.; Thomas, S.; Jose, J.; Kalarikkal, N. Recent Trends in Nanomedicine and Tissue Engineering; River Publishers: Gistrup, Denmark, 2017. [Google Scholar]
- Park, P.-J.; Je, J.-Y.; Jung, W.-K.; Ahn, C.-B.; Kim, S.-K. Anticoagulant activity of heterochitosans and their oligosaccharide sulfates. Eur. Food Res. Technol. 2004, 219, 529–533. [Google Scholar] [CrossRef]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Klokkevold, P.R.; Fukayama, H.; Sung, E.C.; Bertolami, C.N. The effect of chitosan (poly-N-Acetyl Glucosamine) on lingual hemostasis in heparinized rabbits. J. Oral Maxillofac. Surg. 1999, 57, 49–52. [Google Scholar] [CrossRef]
- Smith, J.; Wood, E.; Dornish, M. Effect of Chitosan on epithelial cell tight junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kawakami, K.; Miyatake, K.; Morimoto, M.; Shigemasa, Y.; Minami, S. Analgesic effects of chitin and chitosan. Carbohydr. Polym. 2002, 49, 249–252. [Google Scholar] [CrossRef]
- Hein, S.; Wang, K.; Stevens, W.F.; Kjems, J. Chitosan composites for biomedical applications: Status, challenges and perspectives. Mater. Sci. Technol. 2008, 24, 1053–1061. [Google Scholar] [CrossRef]
- Artursson, P.; Lindmark, T.; Davis, S.S.; Illum, L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm. Res. 1994, 11, 1358–1361. [Google Scholar] [CrossRef] [PubMed]
- Domard, A. pH and c.d. measurements on a fully deacetylated, chitosan: Application to Cu"-polymer interactions. Int. J. Biol. Macromol. 1987, 9, 98–104. [Google Scholar] [CrossRef]
- He, P.; Davis, S.S.; Illum, L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int. J. Pharm. 1998, 166, 75–88. [Google Scholar] [CrossRef]
- Hussain, M.R.; Iman, M.; Maji, T.K. Determination of degree of deacetylation of chitosan and their effect on the release behavior of essential oil from chitosan and chitosan-gelatin complex microcapsules. Int. J. Adv. Eng. Appl. 2013, 6, 4–12. [Google Scholar]
- D’Ayala, G.G.; Malinconico, M.; Laurienzo, P. Marine derived polysaccharides for biomedical applications: Chemical modification approaches. Molecules 2008, 13, 2069–2106. [Google Scholar] [CrossRef]
- Vongchan, P.; Sajomsang, W.; Subyen, D.; Kongtawelert, P. Anticoagulant activity of a sulfated chitosan. Carbohydr. Res. 2002, 337, 1239–1242. [Google Scholar] [CrossRef]
- Vongchan, P.; Sajomsang, W.; Kasinrerk, W.; Subyen, D.; Kongtawelert, P. Anticoagulant activities of the chitosan polysulfate synthesized from marine crab shell by semi-heterogeneous conditions. Sci. Asia 2003, 29, 115–120. [Google Scholar] [CrossRef]
- Alban, S.; Schauerte, A.; Franz, G. Anticoagulant sulfated polysaccharides: Part I. Synthesis and structure–activity relationships of new pullulan sulfates. Carbohydr. Polym. 2002, 47, 267–276. [Google Scholar] [CrossRef]
- Huang, R.; Du, Y.; Zheng, L.; Liu, H.; Fan, L. A new approach to chemically modified chitosan sulfates and study of their influences on the inhibition of Escherichia coli and Staphylococcus aureus growth. React. Funct. Polym. 2004, 59, 41–51. [Google Scholar] [CrossRef]
- Desai, U.R. New antithrombin-based anticoagulants. Med. Res. Rev. 2004, 24, 151–181. [Google Scholar] [CrossRef]
- Drozd, N.N.; Sher, A.I.; Makarov, V.A.; Vikhoreva, G.A.; Gorbachiova, I.N. Comparison of antitrombin activity of the polysulphate chitosan derivatives in vitro and in vivo system. Thromb. Res. 2001, 102, 445–455. [Google Scholar] [CrossRef]
- Horton, D.; Just, E.K. Preparation from Chitin of 2-Amino-2-deoxy-(1-64)-ß glucopyranuronan and its 2-Sulfoamino Analog Having Blood-anticoagulant Properties. Carbohydr. Res. 1973, 29, 173–180. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C. Chitosan Chemistry: Relevance to the Biomedical Sciences. Adv. Polym. Sci. 2005, 186, 151–209. [Google Scholar]
- Sashiwa, H.; Makimura, Y.; Roy, R.; Shigemasa, Y. Chemical modification of chitosan: Preparation of chitosan–sialic acid branched polysaccharide hybrids. Chem. Commun. 2000, 909–910. [Google Scholar] [CrossRef]
- Chokradjaroen, C.; Rujiravanit, R.; Theeramunkong, S.; Saito, N. Effect of electrical discharge plasma on cytotoxicity against cancer cells of N, O-carboxymethyl chitosan-stabilized gold nanoparticles. Carbohydr. Polym. 2020, 237, 116162. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Carboxymethylated Chitins and Chitosans. Carbohydr. Polym. 1988, 8, 1–21. [Google Scholar]
- Chen, S.C.; Wu, Y.C.; Mi, F.L.; Lin, Y.H.; Yu, L.C.; Sung, H.W. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef]
- Thanou, M.; Nihot, M.T.; Jansen, M.; Verhoef, J.C.; Junginger, H.E. Mono-N-Carboxymethyl Chitosan (MCC), a polyampholytic chitosan derivative, enhances the intestinal absorption of low molecular weight heparin across intestinal epithelia in vitro and in vivo. J. Pharm. Sci. 2001, 90, 38–46. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, H.M. Crosslinked carboxymethylchitosan-g-poly(acrylic acid) copolymer as a novel superabsorbent polymer. Carbohydr. Res. 2006, 341, 887–896. [Google Scholar] [CrossRef]
- Sui, W.; Wang, S.; Chen, G.; Xu, G. Surface and aggregate properties of an amphiphilic derivative of carboxymethylchitosan. Carbohydr. Res. 2004, 339, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Aiping, Z.; Jianhong, L.; Wenhui, Y. Effective loading and controlled release of camptothecin by O-carboxymethylchitosan aggregates. Carbohydr. Polym. 2006, 63, 89–96. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Tanfani, F.; Emanuelli, M.; Mariotti, S. N-(Carboxymethylidene) chitosans and N-(carboxymethyl)chitosans: Novel chelating polyampholytes obtained from chitosan glyoxylate. Carbohydr. Res. 1982, 107, 199–214. [Google Scholar] [CrossRef]
- Shigemasa, Y.; Ishida, A.; Sashiwa, H.; Saimoto, H.; Okamoto, Y.; Minami, S.; Matsuhashi, A. Synthesis of a new chitin derivative, (1-carboxyethyl)chitosan. Chem. Lett. 1995, 24, 623–624. [Google Scholar] [CrossRef]
- Rinaudo, R.; Desbrieres, J.; Le Dung, P.; Thuy Binh, P.; Dong, N.T. NMR investigation of chitosan derivatives formed by the reaction of chitosan with levulinic acid. Carbohydr. Polym. 2001, 46, 339–348. [Google Scholar] [CrossRef]
- Biagini, G.; Bertani, A.; Muzzarelli, R.A.A.; Damadei, A.; Di Benedetto, G.; Belligolli, A.; Riccoti, G.; Zucchini, C.; Rizzoli, C. Wound management with N-carboxybutyl chitosan. Biomaterials 1991, 12, 281–286. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C.; Tarsi, R.; Miliani, M.; Gabbanelli, F.; Cartolari, M. Fungistatic activity of modified chitosans against Saprolegnia parasitica. Biomacromolecules 2001, 2, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Kuo, T.-H. O-carboxymethyl chitosan/fucoidan nanoparticles increase cellular curcumin uptake. Food Hydrocoll. 2016, 53, 261–269. [Google Scholar] [CrossRef]
- Sashiwa, H.; Kawasaki, N.; Nakayama, A.; Muraki, E.; Yamamoto, N.; Aiba, S. Chemical modification of chitosan. 14:(1) Synthesis of water-soluble chitosan derivatives by simple acetylation. Biomacromolecules 2002, 3, 1126–1128. [Google Scholar] [CrossRef]
- Sashiwa, H.; Kawasaki, N.; Nakayama, A.; Muraki, E.; Yamamoto, N.; Zhu, H.; Nagano, H.; Omura, Y.; Saimoto, H.; Shigemasa, Y.; et al. Chemical modification of chitosan. 13.(1) Synthesis of organo-soluble, palladium adsorbable, and biodegradable chitosan derivatives toward the chemical plating on plastics. Biomacromolecules 2002, 3, 1120–1125. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; Rabea, E.I.; Rogge, T.M.; Stevens, C.V.; Smagghe, G.; Steurbaut, W.; Hofte, M. Synthesis and fungicidal activity of new N,O-Acyl chitosan derivatives. Biomacromolecules 2004, 5, 589–595. [Google Scholar] [CrossRef]
- Hirano, S.; Midorikawa, T. Novel method for the preparation of N-acylchitosan fiber and N-acylchitosan-cellulose fiber. Biomaterials 1998, 19, 293–297. [Google Scholar] [CrossRef]
- Rathke, T.D.; Hudson, S.M. Review of Chitin and Chitosan as Fiber and Film Formers. J. Macromol. Sci. Part C Polym. Rev. 1994, 34, 375–437. [Google Scholar] [CrossRef]
- Agboh, O.C.; Qin, Y. Chitin and Chitosan Fibers. Polym. Adv. Technol. 1997, 8, 355–365. [Google Scholar] [CrossRef]
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering. Bioprinting 2019, 17. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Chang, C.P.; Lin, S.M. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloids Surf. B Biointerfaces 2007, 57, 250–255. [Google Scholar] [CrossRef]
- Chow, K.S.; Khor, E. Novel Fabrication of Open-Pore Chitin Matrixes. Biomacromolecules 2000, 1, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sainitya, R.; Sriram, M.; Kalyanaraman, V.; Dhivya, S.; Saravanan, S.; Vairamani, M.; Sastry, T.P.; Selvamurugan, N. Scaffolds containing chitosan/carboxymethyl cellulose/mesoporous wollastonite for bone tissue engineering. Int. J. Biol. Macromol. 2015, 80, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Olad, A.; Azhar, F.F. The synergetic effect of bioactive ceramic and nanoclay on the properties of chitosan–gelatin/nanohydroxyapatite–montmorillonite scaffold for bone tissue engineering. Ceram. Int. 2014, 40, 10061–10072. [Google Scholar] [CrossRef]
- Kavya, K.C.; Jayakumar, R.; Nair, S.; Chennazhi, K.P. Fabrication and characterization of chitosan/gelatin/nSiO2 composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2013, 59, 255–263. [Google Scholar] [CrossRef]
- Serra, I.R.; Fradique, R.; Vallejo, M.C.; Correia, T.R.; Miguel, S.P.; Correia, I.J. Production and characterization of chitosan/gelatin/β-TCP scaffolds for improved bone tissue regeneration. Mater. Sci. Eng. C 2015, 55, 592–604. [Google Scholar] [CrossRef]
- Puvaneswary, S.; Talebian, S.; Raghavendran, H.B.; Murali, M.R.; Mehrali, M.; Afifi, A.M.; Kasim, N.H.B.A.; Kamarul, T. Fabrication and in vitro biological activity of βTCP-Chitosan-Fucoidan composite for bone tissue engineering. Carbohydr. Polym. 2015, 134, 799–807. [Google Scholar] [CrossRef]
- Sajesh, K.M.; Jayakumar, R.; Nair, S.V.; Chennazhi, K.P. Biocompatible conducting chitosan/polypyrrole–alginate composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2013, 62, 465–471. [Google Scholar] [CrossRef]
- De la Riva, B.; Nowak, C.; Sánchez, E.; Hernández, A.; Schulz-Siegmund, M.; Pec, M.K.; Delgado, A.; Évora, C. VEGF-controlled release within a bone defect from alginate/chitosan/PLA-H scaffolds. Eur. J. Pharm. Biopharm. 2009, 73, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Fan, Y.; Liu, X.; Li, X.; Li, P.; Wang, J.; Sha, Z.; Feng, Q. Repair of bone defect in femoral condyle using microencapsulated chitosan, nanohydroxyapatite/collagen and poly (L-lactide)-based microsphere-scaffold delivery system. Artif. Organs 2011, 35, E119–E128. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geol. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Suh, J.K.F.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar]
- Austin, K. Scaffold Design: Use of Chitosan in cartilage tissue engineering. Mmg 445 Basic Biotechnol. eJ. 2007, 3, 62–66. [Google Scholar]
- Cabuk, M.; Alan, Y.; Yavuz, M.; Unal, H.I. Synthesis, characterization and antimicrobial activity of biodegradable conducting polypyrrole-graft-chitosan copolymer. Appl. Surf. Sci. 2014, 318, 168–175. [Google Scholar] [CrossRef]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Jayakumar, R.; Chennazhi, K.P.; Srinivasan, S.; Nair, S.V.; Furuike, T.; Tamura, H. Chitin scaffolds in tissue engineering. Int. J. Mol. Sci. 2011, 12, 1876–1887. [Google Scholar] [CrossRef]
- Deepthi, S.; Venkatesan, J.; Kim, S.K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93 Pt B, 1338–1353. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Mahony, O.; Tsigkou, O.; Ionescu, C.; Minelli, C.; Ling, L.; Hanly, R.; Smith, M.E.; Stevens, M.M.; Jones, J.R. Silica-Gelatin Hybrids with Tailorable Degradation and Mechanical Properties for Tissue Regeneration. Adv. Funct. Mater. 2010, 20, 3835–3845. [Google Scholar] [CrossRef]
- Heinemann, S.; Heinemann, C.; Bernhardt, R.; Reinstorf, A.; Nies, B.; Meyer, M.; Worch, H.; Hanke, T. Bioactive silica-collagen composite xerogels modified by calcium phosphate phases with adjustable mechanical properties for bone replacement. Acta Biomater. 2009, 5, 1979–1990. [Google Scholar] [CrossRef] [PubMed]
- Poologasundarampillai, G.; Ionescu, C.; Tsigkou, O.; Murugesan, M.; Hill, R.G.; Stevens, M.M.; Hanna, J.V.; Smith, M.E.; Jones, J.R. Synthesis of bioactive class II poly (γ-glutamic acid)/silica hybrids for bone regeneration. J. Mater. Chem. 2010, 20, 8952–8961. [Google Scholar] [CrossRef]
- Toskas, G.; Cherif, C.; Hund, R.-D.; Laourine, E.; Mahltig, B.; Fahmi, A.; Heinemann, C.; Hanke, T. Chitosan(PEO)/silica hybrid nanofibers as a potential biomaterial for bone regeneration. Carbohydr. Polym. 2013, 94, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Deville, S.; Saiz, E.; Nalla, R.K.; Tomsia, A.P. Strong Biomimetic Hydroxyapatite Scaffolds. Adv. Sci. Technol. 2006, 49, 148–152. [Google Scholar] [CrossRef]
- Pighinelli, L.; Kucharska, M. Chitosan-hydroxyapatite composites. Carbohydr. Polym. 2013, 93, 256–262. [Google Scholar] [CrossRef]
- Chen, P.; Liu, L.; Pan, J.; Mei, J.; Li, C.; Zheng, Y. Biomimetic composite scaffold of hydroxyapatite/gelatin-chitosan core-shell nanofibers for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 97, 325–335. [Google Scholar] [CrossRef]
- Oliveira, S.S.L.; Oliveira, H.M.L.; Fook, M.V.L. Arcabouços obtidos por agregação de esferas de quitosana/hidroxiapatita. Rev. Eletrônica De Mater. E Process. 2015, 10, 128–136. [Google Scholar]
- Liu, X.; Yu, B.; Huang, Q.; Liu, R.; Feng, Q.; Cai, Q.; Mi, S. In vitro BMP-2 peptide release from thiolated chitosan based hydrogel. Int. J. Biol. Macromol. 2016, 93, 314–321. [Google Scholar] [CrossRef]
- Cui, Z.K.; Kim, S.; Baljon, J.J.; Wu, B.M.; Aghaloo, T.; Lee, M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019, 10, 3523. [Google Scholar] [CrossRef]
- Gritsch, L.; Maqbool, M.; Mourino, V.; Ciraldo, F.E.; Cresswell, M.; Jackson, P.R.; Lovell, C.; Boccaccini, A.R. Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: Copper and strontium. J. Mater. Chem. B 2019, 7, 6109–6124. [Google Scholar] [CrossRef]
- Jahan, K.; Mekhail, M.; Tabrizian, M. One-step fabrication of apatite-chitosan scaffold as a potential injectable construct for bone tissue engineering. Carbohydr. Polym. 2018, 203, 60–70. [Google Scholar] [CrossRef]
- Lu, H.T.; Lu, T.W.; Chen, C.H.; Mi, F.L. Development of genipin-crosslinked and fucoidan-adsorbed nano-hydroxyapatite/hydroxypropyl chitosan composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 128, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Matinfar, M.; Mesgar, A.S.; Mohammadi, Z. Evaluation of physicochemical, mechanical and biological properties of chitosan/carboxymethyl cellulose reinforced with multiphasic calcium phosphate whisker-like fibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Nezafati, N.; Faridi-Majidi, R.; Pazouki, M.; Hesaraki, S. Synthesis and characterization of a novel freeze-dried silanated chitosan bone tissue engineering scaffold reinforced with electrospun hydroxyapatite nanofiber. Polym. Int. 2019, 68, 1420–1429. [Google Scholar] [CrossRef]
- Baysal, K.; Aroguz, A.Z.; Adiguzel, Z.; Baysal, B.M. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int. J. Biol. Macromol. 2013, 59, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Simsek-Ege, F.A.; Bond, G.M.; Stringer, J. Polyelectrolye Complex Formation Between Alginate and Chitosan as a Function of pH. J. Appl. Polym. Sci. 2003, 88, 346–351. [Google Scholar] [CrossRef]
- Iwasaki, N.; Yamane, S.-T.; Majima, T.; Kasahara, Y.; Minami, A.; Harada, K.; Nishimura, S.-I. Feasibility of Polysaccharide Hybrid Materials for Scaffolds in Cartilage Tissue Engineering: Evaluation of Chondrocyte Adhesion to Polyion Complex Fibers Prepared from Alginate and Chitosan. Biomacromolecules 2004, 5, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.W.; Yu, H.N. Coelectrospinning of chitosan/alginate fibers by dual-jet system for modulating material surfaces. Carbohydr. Polym. 2013, 95, 716–727. [Google Scholar] [CrossRef]
- Hyland, L.L.; Taraban, M.B.; Hammouda, B.; Bruce Yu, Y. Mutually reinforced multicomponent polysaccharide networks. Biopolymers 2011, 95, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, T.I.; Montaser, A.S.; Li, S. Effect of cellulose nanocrystals on scaffolds comprising chitosan, alginate and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2018, 121, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Toloue, E.B.; Karbasi, S.; Salehi, H.; Rafienia, M. Potential of an electrospun composite scaffold of poly (3-hydroxybutyrate)-chitosan/alumina nanowires in bone tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1075–1091. [Google Scholar] [CrossRef] [PubMed]
- Saekhor, K.; Udomsinprasert, W.; Honsawek, S.; Tachaboonyakiat, W. Preparation of an injectable modified chitosan-based hydrogel approaching for bone tissue engineering. Int. J. Biol. Macromol. 2019, 123, 167–173. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, H.; Trinh, P.; Heilshorn, S.C.; Yang, F. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials 2017, 127, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Nesic, D.; Whiteside, R.; Brittberg, M.; Wendt, D.; Martin, I.; Mainil-Varlet, P. Cartilage tissue engineering for degenerative joint disease. Adv. Drug Deliv. Rev. 2006, 58, 300–322. [Google Scholar] [CrossRef]
- Liao, J.; Tian, T.; Shi, S.; Xie, X.; Ma, Q.; Li, G.; Lin, Y. The fabrication of biomimetic biphasic CAN-PAC hydrogel with a seamless interfacial layer applied in osteochondral defect repair. Bone Res. 2017, 5, 1–15. [Google Scholar] [CrossRef]
- McCullen, S.D.; Autefage, H.; Callanan, A.; Gentleman, E.; Stevens, M.M. Anisotropic fibrous scaffolds for articular cartilage regeneration. Tissue Eng. Part A 2012, 18, 2073–2083. [Google Scholar] [CrossRef]
- Steele, J.A.M.; McCullen, S.D.; Callanan, A.; Autefage, H.; Accardi, M.A.; Dini, D.; Stevens, M.M. Combinatorial scaffold morphologies for zonal articular cartilage engineering. Acta Biomater. 2014, 10, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Mellati, A.; Fan, C.M.; Tamayol, A.; Annabi, N.; Dai, S.; Bi, J.; Jin, B.; Xian, C.; Khademhosseini, A.; Zhang, H. Microengineered 3D cell-laden thermoresponsive hydrogels for mimicking cell morphology and orientation in cartilage tissue engineering. Biotechnol. Bioeng. 2017, 114, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Chuah, Y.J.; Peck, Y.; Lau, J.E.J.; Hee, H.T.; Wang, D.-A. Hydrogel based cartilaginous tissue regeneration: Recent insights and technologies. Biomater. Sci. 2017, 5, 613–631. [Google Scholar] [CrossRef]
- Qu, D.; Mosher, C.Z.; Boushell, M.K.; Lu, H.H. Engineering complex orthopaedic tissues via strategic biomimicry. Ann. Biomed. Eng. 2015, 43, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2017, 33, 59. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Lahiji, A.; Sohrabi, A.; Hungerford, D.S.; Frondoza, C.G. Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J. Biomed. Mater. Res. 2000, 51, 586–595. [Google Scholar] [CrossRef]
- Vårum, K.M.; Myhr, M.M.; Hjerde, R.J.N.; Smidsrød, O. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr. Res. 1997, 299, 99–101. [Google Scholar] [CrossRef]
- Pangburn, S.H.; Trescony, P.V.; Heller, J. Lysozyme degradation of partially deacetylated chitin, its films and hydrogels. Biomaterials 1982, 3, 105–108. [Google Scholar] [CrossRef]
- Moss, J.M.; Van Damme, M.-P.I.; Murphy, W.H.; Stanton, P.G.; Thomas, P.; Preston, B.N. Purification, characterization, and biosynthesis of bovine cartilage lysozyme isoforms. Arch. Biochem. Biophys. 1997, 339, 172–182. [Google Scholar] [CrossRef]
- Greenwald, R.A.; Josephson, A.S.; Diamond, H.S.; Tsang, A. Human cartilage lysozyme. J. Clin. Investig. 1972, 51, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Sechriest, V.F.; Miao, Y.J.; Niyibizi, C.; Westerhausen-Larson, A.; Matthew, H.W.; Evans, C.H.; Fu, F.H.; Suh, J.K. GAG-augmented polysaccharide hydrogel: A novel biocompatible and biodegradable material to support chondrogenesis. J. Biomed. Mater. Res. 2000, 49, 534–541. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.S.M.; Dijkstra, P.J.; Karperien, M.; van Blitterswijk, C.A.; Zhong, Z.Y.; Feijen, J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2544–2551. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugam, A.; Kumar, R.A.; Priya, M.V.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Tan, H.; Marra, K.G. Injectable, biodegradable hydrogels for tissue engineering applications. Materials 2010, 3, 1746–1767. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef]
- Grabovac, V.; Guggi, D.; Bernkop-Schnürch, A. Comparison of the mucoadhesive properties of various polymers. Adv. Drug Deliv. Rev. 2005, 57, 1713–1723. [Google Scholar] [CrossRef]
- Lee, Y.; Chung, H.J.; Yeo, S.; Ahn, C.-H.; Lee, H.; Messersmith, P.B.; Park, T.G. Thermo-sensitive, injectable, and tissue adhesive sol–gel transition hyaluronic acid/pluronic composite hydrogels prepared from bio-inspired catechol-thiol reaction. Soft Matter 2010, 6, 977–983. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, Y.; Kong, W.H.; Kim, T.G.; Park, T.G.; Lee, H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromolecules 2011, 12, 2653–2659. [Google Scholar] [CrossRef]
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef]
- Roughley, P.; Hoemann, C.; DesRosiers, E.; Mwale, F.; Antoniou, J.; Alini, M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials 2006, 27, 388–396. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Sun, J.; Legare, A.; McKee, M.D.; Buschmann, M.D. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthr. Cartil. 2005, 13, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, K.; Zhou, X.; Li, T.; Xu, Y.; Qiang, L.; Peng, M.; Xu, Y.; Xie, L.; He, C.; et al. Controllable fabrication of hydroxybutyl chitosan/oxidized chondroitin sulfate hydrogels by 3D bioprinting technique for cartilage tissue engineering. Biomed. Mater. 2018, 14, 025006. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, K.; Zhao, S.; Zhang, C.; Li, J.; Yang, H.; Liu, X.; Yin, X.; Chen, D.; Xu, W.; et al. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2018, 108, 383–390. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B.; Li, M.; Li, J.; Wan, Y. Enhanced dual network hydrogels consisting of thiolated chitosan and silk fibroin for cartilage tissue engineering. Carbohydr. Polym. 2020, 227, 115335. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, D.; Karbasi, S.; Razavi, S.; Mohammadi, S.; Shokrgozar, M.A.; Bonakdar, S. Electrospun poly(hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, F.; Wang, Q.; Wu, X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Shamekhi, M.A.; Mirzadeh, H.; Mahdavi, H.; Rabiee, A.; Mohebbi-Kalhori, D.; Baghaban Eslaminejad, M. Graphene oxide containing chitosan scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2019, 127, 396–405. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA approved guidance conduits and wraps for peripheral nerve injury: A review of materials and efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef]
- Ciardelli, G.; Chiono, V. Materials for peripheral nerve regeneration. Macromol. Biosci. 2006, 6, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, S.; Serra, S.C.; Ribeiro-Samy, S.; Sousa, N.; Heimann, C.; Barwig, C.; Grothe, C.; Salgado, A.J.; Haastert-Talini, K. In vitro evaluation of cell-seeded chitosan films for peripheral nerve tissue engineering. Tissue Eng. Part A 2014, 20, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Freier, T.; Montenegro, R.; Koh, H.S.; Shoichet, M.S. Chitin-based tubes for tissue engineering in the nervous system. Biomaterials 2005, 26, 4624–4632. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ushida, T.; Tateishi, T. Development of biodegradable porous scaffolds for tissue engineering. Mater. Sci. Eng. C 2001, 17, 63–69. [Google Scholar] [CrossRef]
- Chandy, T.; Sharma, C.P. Chitosan-as a Biomaterial. Biomater. Artif. Cells Artif. Organs 1990, 18, 1–24. [Google Scholar] [CrossRef]
- Yamaguchi, I.; Itoh, S.; Suzuki, M.; Osaka, A.; Tanaka, J. The chitosan prepared from crab tendons: II. The chitosan/apatite composites and their application to nerve regeneration. Biomaterials 2003, 24, 3285–3292. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, P.; Yang, Y.; Wang, X.; Gu, X. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials 2004, 25, 4273–4278. [Google Scholar] [CrossRef]
- Ishikawa, N.; Suzuki, Y.; Ohta, M.; Cho, H.; Suzuki, S.; Dezawa, M.; Ide, C. Peripheral nerve regeneration through the space formed by a chitosan gel sponge. J. Biomed. Mater. Res. Part A 2007, 83, 33–40. [Google Scholar] [CrossRef]
- Wang, W.; Itoh, S.; Matsuda, A.; Ichinose, S.; Shinomiya, K.; Hata, Y.; Tanaka, J. Influences of mechanical properties and permeability on chitosan nano/microfiber mesh tubes as a scaffold for nerve regeneration. J. Biomed. Mater. Res. Part A 2008, 84, 557–566. [Google Scholar]
- Lauto, A.; Foster, L.J.; Avolio, A.; Sampson, D.; Raston, C.; Sarris, M.; McKenzie, G.; Stoodley, M. Sutureless nerve repair with laser-activated chitosan adhesive: A pilot in vivo study. Photomed. Laser Surg. 2008, 26, 227–234. [Google Scholar] [CrossRef]
- Sun, L.P.; Wang, S.; Zhang, Z.W.; Wang, X.Y.; Zhang, Q.Q. Biological evaluation of collagen–chitosan scaffolds for dermis tissue engineering. Biomed. Mater. 2009, 4, 055008. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.C.C.C.; Silva, G.A.B.; Hell, R.C.R.; Martins, M.D.; Alves, J.B.; Goes, A.M. Three-dimensional culture of rat BMMSCs in a porous chitosan-gelatin scaffold: A promising association for bone tissue engineering in oral reconstruction. Arch. Oral Biol. 2011, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Thein-Han, W.W.; Saikhun, J.; Pholpramoo, C.; Misra, R.D.K.; Kitiyanant, Y. Chitosan–gelatin scaffolds for tissue engineering: Physico-chemical properties and biological response of buffalo embryonic stem cells and transfectant of GFP–buffalo embryonic stem cells. Acta Biomater. 2009, 5, 3453–3466. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, X.-L.; Lin, X.-M.; Liu, T.-Q.; Ma, X.-H.; Cui, Z.-F. Chitosan/gelatin porous scaffolds containing hyaluronic acid and heparan sulfate for neural tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 999–1014. [Google Scholar] [CrossRef]
- Hajiabbas, M.; Mashayekhan, S.; Nazaripouya, A.; Naji, M.; Hunkeler, D.; Rajabi Zeleti, S.; Sharifiaghdas, F. Chitosan-gelatin sheets as scaffolds for muscle tissue engineering. Artif. Cells Nanomed. Biotechnol. 2015, 43, 124–132. [Google Scholar] [CrossRef]
- Jridi, M.; Hajji, S.; Ayed, H.B.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin–chitosan composite edible films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef]
- Sarem, M.; Moztarzadeh, F.; Mozafari, M.; Shastri, V.P. Optimization strategies on the structural modeling of gelatin/chitosan scaffolds to mimic human meniscus tissue. Mater. Sci. Eng. C 2013, 33, 4777–4785. [Google Scholar] [CrossRef]
- Martín-López, E.; Alonso, F.R.; Nieto-Díaz, M.; Nieto-Sampedro, M. Chitosan, gelatin and poly (L-Lysine) polyelectrolyte-based scaffolds and films for neural tissue engineering. J. Biomater. Sci. Polym. Ed. 2012, 23, 207–232. [Google Scholar] [CrossRef]
- Kamalesh, S.; Tan, P.; Wang, J.; Lee, T.; Kang, E.T.; Wang, C.H. Biocompatibility of electroactive polymers in tissues. J. Biomed. Mater. Res. 2000, 52, 467–478. [Google Scholar] [CrossRef]
- Burrows, M.C.; Zamarion, V.M.; Filippin-Monteiro, F.B.; Schuck, D.C.; Toma, H.E.; Campa, A.; Garcia, C.R.S.; Catalani, L.H. Hybrid scaffolds built from PET and collagen as a model for vascular graft architecture. Macromol. Biosci. 2012, 12, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Liow, S.S.; Loh, X.J. Biodegradable polymers for electrospinning: Towards biomedical applications. Mater. Sci. Eng. C 2014, 45, 659–670. [Google Scholar] [CrossRef]
- Baniasadi, H.; Ramazani, S.A.A.; Mashayekhan, S. Fabrication and characterization of conductive chitosan/gelatin-based scaffolds for nerve tissue engineering. Int. J. Biol. Macromol. 2015, 74, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, B.; Zarrintaj, P.; Surwase, S.S.; Baheiraei, N.; Saeb, M.R.; Mozafari, M.; Kim, Y.C.; Park, O.O. Self-gelling electroactive hydrogels based on chitosan-aniline oligomers/agarose for neural tissue engineering with on-demand drug release. Colloids Surf. B Biointerfaces 2019, 184, 110549. [Google Scholar] [CrossRef] [PubMed]
- Bolaina-Lorenzo, E.; Martínez-Ramos, C.; Monleón-Pradas, M.; Herrera-Kao, W.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Electrospun polycaprolactone/chitosan scaffolds for nerve tissue engineering: Physicochemical characterization and Schwann cell biocompatibility. Biomed. Mater. 2016, 12, 015008. [Google Scholar] [CrossRef]
- Sadeghi, A.; Moztarzadeh, F.; Aghazadeh Mohandesi, J. Investigating the effect of chitosan on hydrophilicity and bioactivity of conductive electrospun composite scaffold for neural tissue engineering. Int. J. Biol. Macromol. 2019, 121, 625–632. [Google Scholar] [CrossRef]
- Salehi, M.; Bagher, Z.; Kamrava, S.K.; Ehterami, A.; Alizadeh, R.; Farhadi, M.; Falah, M.; Komeili, A. Alginate/chitosan hydrogel containing olfactory ectomesenchymal stem cells for sciatic nerve tissue engineering. J. Cell. Physiol. 2019, 234, 15357–15368. [Google Scholar] [CrossRef]
- Dvorak, H.F. Vascular permeability to plasma, plasma proteins, and cells: An update. Curr. Opin. Hematol. 2010, 17, 225–229. [Google Scholar] [CrossRef]
- Loke, W.K.; Lau, S.K.; Yong, L.L.; Khor, E.; Sum, C.K. Wound dressing with sustained anti-microbial capability. J. Biomed. Mater. Res. 2000, 53, 8–17. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Morganti, P.; Morganti, G.; Palombo, P.; Palombo, M.; Biagini, G.; Mattioli Belmonte, M.; Giantomassi, F.; Orlandi, F.; Muzzarelli, C. Chitin nanofibrils/chitosan glycolate composites as wound medicaments. Carbohydr. Polym. 2007, 70, 274–284. [Google Scholar] [CrossRef]
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound Healing: Biologic Features and Approaches to Maximize Healing Trajectories. Curr. Probl. Surg. 2001, 38, 71–140. [Google Scholar] [CrossRef] [PubMed]
- Grose, R.; Werner, S. Wound-healing studies in transgenic and knockout mice. Mol. Biotechnol. 2004, 28, 147. [Google Scholar] [CrossRef]
- Nedelec, B.; De Oliveira, A.; Saint-Cyr, M.; Garrel, D.R. Differential effect of burn injury on fibroblasts from wounds and normal skin. Plast. Reconstr. Surg. 2007, 119, 2101–2109. [Google Scholar] [CrossRef]
- Ud-Din, S.; Bayat, A. Non-invasive objective devices for monitoring the inflammatory, proliferative and remodelling phases of cutaneous wound healing and skin scarring. Exp. Dermatol. 2016, 25, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.T.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P.K. In vivo evaluation of chitosan–PVP–titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef]
- Martins, E.A.N.; Invernizzi, M.S.; Campos, M.G.N.; Teodoro, P.A.; Contieri, M.B.; da Silva, L.C.L.C. Emprego de membrana de quitosana em feridas cutâneas induzidas experimentalmente em equinos. Ciência Rural 2013, 43, 1824–1830. [Google Scholar] [CrossRef]
- Liu, R.; Xu, X.; Zhuang, X.; Cheng, B. Solution blowing of chitosan/PVA hydrogel nanofiber mats. Carbohydr. Polym. 2014, 101, 1116–1121. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti-Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Silva, H.S.R.C.; dos Santos, K.S.; Ferreira, E.I. Quitosana: Derivados hidrossolúveis, aplicações farmacêuticas e avanços. Quim. Nova 2006, 29, 776–785. [Google Scholar] [CrossRef]
- Allan, G.G.; Atman, L.C.; Bensinger, R.E.; Ghosh, D.K.; Hirabayashi, Y.; Neogi, A.N.; Neogi, S. Biomedical applications of chitin and chitosan. In Chitin, Chitosan, and Related Enzymes; Zikakis, J.P., Ed.; Academic Press: Orlando, FL, USA, 1984; pp. 119–133. [Google Scholar]
- Okamoto, Y.; Shibazaki, K.; Minami, S.; Matsuhashi, A.; Tanioka, S.; Shigemasa, Y. Evaluation of chitin and chitosan on open wound healing in dogs. J. Vet. Med. Sci. 1995, 57, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Shigemasa, Y.; Minami, S. Applications of Chitin and Chitosan for Biomaterials. Biotechnol. Genet. Eng. Rev. 1996, 13, 383–420. [Google Scholar] [CrossRef] [PubMed]
- Pavis, H.; Wilcock, A.; Edgecombe, J.; Carr, D.; Manderson, C.; Church, A.; Fisher, A. Pilot study of nasal morphine-Chitosan for the relief of breakthrough pain in patients with cancer. J. Pain Symptom Manag. 2002, 24, 598–602. [Google Scholar] [CrossRef]
- Illum, L.; Farraj, N.F.; Davis, S.S. Chitosan as a novel nasal delivery system for peptide drugs. Pharm. Res. 1994, 11, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Illum, L.; Jabbal-Gill, I.; Hinchcliffe, M.; Fisher, A.N.; Davis, S.S. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev. 2001, 51, 81–96. [Google Scholar] [CrossRef]
- Shiraishi, S.; Arahira, M.; Imai, T.; Otagiri, M. Enhancement of dissolution rates of several drugs by low-molecular chitosan and alginate. Chem. Pharm. Bull. 1990, 38, 185–187. [Google Scholar] [CrossRef][Green Version]
- Maestrelli, F.; Zerrouk, N.; Chemtob, C.; Mura, P. Influence of chitosan and its glutamate and hydrochloride salts on naproxen dissolution rate and permeation across Caco-2 cells. Int. J. Pharm. 2004, 271, 257–267. [Google Scholar] [CrossRef]
- Zerrouk, N.; Mennini, N.; Maestrelli, F.; Chemtob, C.; Mura, P. Comparison of the effect of chitosan and polyvinylpyrrolidone on dissolution properties and analgesic effect of naproxen. Eur. J. Pharm. Biopharm. 2004, 57, 93–99. [Google Scholar] [CrossRef]
- Fráguas, R.M.; Rocha, D.A.; Queiroz, E.D.R.; De Abreu, C.M.P.; de Sousa, R.V.; de Oliveira Júnior, E.N. Caracterização química e efeito cicatrizante de quitosana, com baixos valores de massa molar e grau de acetilação, em lesões cutâneas. Polímeros 2015, 25, 205–211. [Google Scholar] [CrossRef]
- De Souza Costa, E., Jr.; Mansur, H.S. Preparação e caracterização de blendas de quitosana/poli (álcool vinílico) reticuladas quimicamente com glutaraldeído para aplicação em engenharia de tecido. Química Nova 2008, 31, 1460–1466. [Google Scholar] [CrossRef]
- Dos Santos, B.; da Silva, R.; Farias, I. Morfologia e propriedades térmicas de blendas de poli (álcool vinílico)/quitosana. Rev. Iberoam. Polímeros 2016, 17, 139–144. [Google Scholar]
- Morgado, P.I.; Miguel, S.P.; Correia, I.J.; Aguiar-Ricardo, A. Ibuprofen loaded PVA/chitosan membranes: A highly efficient strategy towards an improved skin wound healing. Carbohydr. Polym. 2017, 159, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Maegawa, T.; Takahashi, T. Glass transition temperature of chitosan and miscibility of chitosan/poly (N-vinyl pyrrolidone) blends. Polymer 2000, 41, 7051–7056. [Google Scholar] [CrossRef]
- Li, J.; Zivanovic, S.; Davidson, P.A.; Kit, K. Characterization and comparison of chitosan/PVP and chitosan/PEO blend films. Carbohydr. Polym. 2010, 79, 786–791. [Google Scholar] [CrossRef]
- De Albuquerque Santos Guimarães, A. Hidrogéis à Base de Quitosana/Poli(Álcool Vinílico) Para Liberação de Fármaco Visando Uso Potencial Como Curativo; Universidade Federal da Paraíba: João Pessoa, Brazil, 2018. [Google Scholar]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Souza, R.F.B.; Souza, F.C.B.; Moraes, Â.M. Incorporação de eritromicina a membranas de quitosana complexada com alginato ou xantana para a aplicação no tratamento de lesões de pele. In Proceedings of the XX Congresso Brasileiro de Engenharia Química, Florianópolis, SC, USA, 22 October 2014. [Google Scholar]
- Liu, J.; Huang, Y.; Kumar, A.; Tan, A.; Jin, S.; Mozhi, A.; Liang, X.-J. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 2014, 32, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liao, X.; Zhang, J.; Yang, F.; Fan, Z. Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2018, 119, 402–412. [Google Scholar] [CrossRef]
- Asadpour, S.; Kargozar, S.; Moradi, L.; Ai, A.; Nosrati, H.; Ai, J. Natural biomacromolecule based composite scaffolds from silk fibroin, gelatin and chitosan toward tissue engineering applications. Int. J. Biol. Macromol. 2020, 154, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Sivashankari, P.R.; Prabaharan, M. Three-dimensional porous scaffolds based on agarose/chitosan/graphene oxide composite for tissue engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef]
- Lyra, M.A.M.; Soares-Sobrinho, J.L.; Brasileiro, M.T.; de La Roca, M.F.; de Sá Barraza, J.A.; Viana, O.; Rolim-Neto, P.J. Sistemas matriciais hidrofílicos e mucoadesivos para liberação controlada de fármacos. Lat. Am. J. Pharm. 2007, 26, 784–793. [Google Scholar]
- Figueiredo, E.C.; Dias, A.C.B.; Arruda, M.A.Z. Impressão molecular: Uma estratégia promissora na elaboração de matrizes para a liberação controlada de fármacos. Rev. Bras. De Ciências Farm. 2008, 44, 361–375. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Stulzer, H.K.; Lacerda, L.; Tagliari, M.P.; Silva, M.A.S.; Fávere, V.T.; Laranjeira, M.C.M. Synthesis and characterization of cross-linked malonylchitosan microspheres for controlled release of acyclovir. Carbohydr. Polym. 2008, 73, 490–497. [Google Scholar] [CrossRef]
- Naskar, S.; Koutsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2019, 27, 379–393. [Google Scholar] [CrossRef]
- Miyazaki, S.; Yamaguchi, H.; Yokouchi, C.; Takada, M.; Hou, W.M. Sustained-release and Intragastric-Floating granules of indomethacin using chitosan in rabbits. Chem. Pharm. Bull. 1988, 36, 4033–4038. [Google Scholar] [CrossRef] [PubMed]
- Hillyard, I.W.; Doczi, J.; Kierna, P.B. Antacid and antiulcer properties of the polysaccharide chitosan in the rat. Exp. Biol. Med. 1964, 115, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Fuoss, R.M.; Sadek, H. Mutual Interaction of Polyelectrolyte. Science 1949, 110, 552–554. [Google Scholar] [CrossRef]
- Tsuchida, E.; Osada, Y.; Abe, K. Formation of polyion complexes between polycarboxylic acids and polycations carrying charges in the chain backbone. Die Makromol. Chem. 1974, 175, 583–592. [Google Scholar] [CrossRef]
- Michaels, A.S. Polyelectrolyte Complexes. Ind. Eng. Chem. 1965, 57, 32–40. [Google Scholar] [CrossRef]
- Bekturov, E.A.; Bimendina, L.A. Interpolymer Complexes. Adv. Polym. Sci. 1981, 41, 99–147. [Google Scholar]
- Hamman, J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Takayama, K.; Machida, Y.; Nagai, T. Characteristics of polyion complexes of chitosan with sodium alginate and sodium polyacrylate. Int. J. Pharm. 1990, 61, 35–41. [Google Scholar] [CrossRef]
- Takeuchi, H.; Yamamoto, H.; Niwa, T.; Hino, T.; Kawashima, Y. Enteral absorption of insulin in rats from mucoadhesive chitosan-coated liposomes. Pharm. Res. 1996, 13, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.H.; Liu, T.Y.; Chen, S.Y.; Liu, D.M. Drug release behavior of chitosan-montmorillonite nanocomposite hydrogels following electrostimulation. Acta Biomater. 2008, 4, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, C.; Sandri, G.; Bonferoni, C.; Cerezo, P.; Rossi, S.; Ferrari, F.; Caramella, C.; Viseras, C. Solid state characterisation of silver sulfadiazine loaded on montmorillonite/chitosan nanocomposite for wound healing. Colloids Surf. B Biointerfaces 2014, 113, 152–157. [Google Scholar] [CrossRef]
- Wang, S.; Jing, Y. Effects of formation and penetration properties of biodegradable montmorillonite/chitosan nanocomposite film on the barrier of package paper. Appl. Clay Sci. 2017, 138, 74–80. [Google Scholar] [CrossRef]
- Zheng, J.P.; Luan, L.; Wang, H.Y.; Xi, L.F.; Yao, K.D. Study on ibuprofen/montmorillonite intercalation composites as drug release system. Appl. Clay Sci. 2007, 36, 297–301. [Google Scholar] [CrossRef]
- Tavares, A.A.; Lima, P.H.C.; Pereira, C.A.B.; Leal, R.G.; Braga, C.R.C.; Canedo, E.L.; Silva, S.M.L. Liberação controlada de ibuprofeno a partir de filmes de quitosana/montmorilonita. In Proceedings of the 14° Congresso da Sociedade Latino Americana de Biomateriais, Orgãos Artificiais e Engenharia de Tecidos-SLABO, Maresias, Brail, 20–24 August 2017. [Google Scholar]
- Perumal, D. Microencapsulation of ibuprofen and Eudragit® RS 100 by the emulsion solvent diffusion technique. Int. J. Pharm. 2001, 218, 1–11. [Google Scholar] [CrossRef]
- Illum, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Pinto, J.F.; Muller, R.H. Pellets as carriers of solid lipid nanoparticles (SLN) for oral administration of drugs. Pharmazie 1999, 54, 506–509. [Google Scholar]
- Borchard, G.; Lueben, H.L.; de Boer, A.G.; Verhoef, J.C.; Lehr, C.M.; Junginger, H.E. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: Effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J. Control. Release 1996, 39, 131–138. [Google Scholar]
- Soane, R.J.; Frier, M.; Perkins, A.C.; Jones, N.S.; Davis, S.S.; Illum, L. Evaluation of the clearance characteristics of bioadhesive systems in humans. Int. J. Pharm. 1999, 178, 55–65. [Google Scholar] [CrossRef]
- Lehr, C.M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm. 1992, 78, 43–48. [Google Scholar] [CrossRef]
- Peppas, N.A.; Buri, P.A. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release 1985, 2, 257–275. [Google Scholar] [CrossRef]
- Park, H.; Amiji, M.; Park, K. Mucoadhesive hydrogels effective at neutral pH. Proc. Int. Symp. Control. Rel. Bioact. Mater. 1989, 16, 217–218. [Google Scholar]
- Pontillo, A.R.N.; Detsi, A. Nanoparticles for ocular drug delivery: Modified and non-modified chitosan as a promising biocompatible carrier. Nanomedicine 2019, 14, 1889–1909. [Google Scholar] [CrossRef]
- Fabiano, A.; Beconcini, D.; Migone, C.; Piras, A.M.; Zambito, Y. Quaternary Ammonium Chitosans: The Importance of the Positive Fixed Charge of the Drug Delivery Systems. Int. J. Mol. Sci. 2020, 21, 6617. [Google Scholar] [CrossRef]
- Lueßen, H.L.; de Leeuw, B.J.; Langemeßer, M.W.E.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Mucoadhesive Polymers in Peroral Peptide Drug Delivery. VI. Carbomer and Chitosan Improve the Intestinal Absorption of the Peptide Drug Buserelin In Vivo. Pharm. Res. 1996, 13, 1668–1672. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Reis, R.L.; Mano, J.F. Graft copolymerized chitosan—Present status and applications. Carbohydr. Polym. 2005, 62, 142–158. [Google Scholar] [CrossRef]
- Berscht, P.C.; Nies, B.; Liebendorfer, A.; Kreuter, J. In vitro evaluation of biocompatibility of different wound dressing materials. J. Mater. Sci. Mater. Med. 1995, 6, 201–205. [Google Scholar] [CrossRef]
- Kotzé, A.F.; Luessen, H.L.; de Leeuw, B.J.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Comparison of the effect of different chitosan salts and N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). J. Control. Release 1998, 51, 35–46. [Google Scholar] [CrossRef]
- Zhao, K.; Li, S.; Li, W.; Yu, L.; Duan, X.; Han, J.; Wang, X.; Jin, Z. Quaternized chitosan nanoparticles loaded with the combined attenuated live vaccine against Newcastle disease and infectious bronchitis elicit immune response in chicken after intranasal administration. Drug Deliv. 2017, 24, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Shuai, X.; Unger, F.; Simon, M.; Bi, D.; Kissel, T. The depolymerization of chitosan: Effects on physicochemical and biological properties. Int. J. Pharm. 2004, 281, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; El-Helw, A.M.; Sobahi, T.R.; Abdelaal, M.Y. Chitosan based atorvastatin nanocrystals: Effect of cationic charge on particle size, formulation stability, and in-vivo efficacy. Int. J. Nanomed. 2015, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Shiraishi, S.; Saitô, H.; Otagiri, M. Interaction of indomethacin with low molecular weight chitosan,and improvements of some pharmaceutical properties of indomethacin by low molecular weight chitosans. Int. J. Pharm. 1991, 67, 11–20. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Soppimath, K.S.; Aminabhavi, T.M. Synthesis and Characterization of Polyacrylamide-Grafted Chitosan Hydrogel Microspheres for the Controlled Release of Indomethacin. J. Appl. Polym. Sci. 2003, 87, 1525–1536. [Google Scholar] [CrossRef]
- Aiedeh, K.; Taha, M.O. Synthesis of chitosan succinate and chitosan phthalate and their evaluation as suggested matrices in orally administered, colon-specific drug delivery systems. Arch. Pharm. Pharm. Med. Chem. 1999, 332, 103–107. [Google Scholar] [CrossRef]
- Bigucci, F.; Luppi, B.; Cerchiara, T.; Sorrenti, M.; Bettinetti, G.; Rodriguez, L.; Zecchi, V. Chitosan/pectin polyelectrolyte complexes: Selection of suitable preparative conditions for colon-specific delivery of vancomycin. Eur. J. Pharm. Sci. 2008, 35, 435–441. [Google Scholar] [CrossRef]
- Nunthanid, J.; Luangtana-anan, M.; Sriamornsak, P.; Limmatvapirat, S.; Huanbutta, K.; Puttipipatkhachorn, S. Use of spray-dried chitosan acetate and ethylcellulose as compression coats for colonic drug delivery: Effect of swelling on triggering in vitro drug release. Eur. J. Pharm. Biopharm. 2009, 71, 356–361. [Google Scholar] [CrossRef]
- Calvo, P.; Vila-Jato, J.L.; Alonso, M.J. Evaluation of cationic polymer-coated nanocapsules as ocular drug carriers. Int. J. Pharm. 1997, 153, 41–50. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.; Ramasamy, M.; Suresh, B. Chitosan nanoparticles as a new delivery system for the anti-Alzheimer drug tacrine. Nanomedicine 2010, 6, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; El-Nahhas, S.A.; Ghorab, M.M.; Salama, A.H. Chitosan/cyclodextrin nanoparticles as drug delivery system. J. Incl. Phenom. Macrocycl. Chem. 2011, 72, 127–136. [Google Scholar] [CrossRef]
- Arora, S.; Gupta, S.; Narang, R.K.; Budhiraja, R.D. Amoxicillin loaded chitosan-alginate polyelectrolyte complex nanoparticles as mucopenetrating delivery system for h. Pylori. Sci. Pharm. 2011, 79, 673–694. [Google Scholar] [CrossRef]
- Liu, L.; Tang, X.; Wang, Y.; Guo, S. Smart gelation of chitosan solution in the presence of NaHCO3 for injectable drug delivery system. Int. J. Pharm. 2011, 414, 6–15. [Google Scholar] [CrossRef]
- Luo, Q.; Zhao, J.; Zhang, X.; Pan, W. Nanostructured lipid carrier (NLC) coated with Chitosan Oligosaccharides and its potential use in ocular drug delivery system. Int. J. Pharm. 2011, 403, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Saboktakin, M.R.; Tabatabaie, R.M.; Maharramov, A.; Ramazanov, M.A. Synthesis and in vitro evaluation of carboxymethyl starch-chitosan nanoparticles as drug delivery system to the colon. Int. J. Biol. Macromol. 2011, 48, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhu, Y. Chitosan enclosed mesoporous silica nanoparticles as drug nano-carriers: Sensitive response to the narrow pH range. Microporous Mesoporous Mater. 2012, 150, 83–89. [Google Scholar] [CrossRef]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Erythrocytes load of low molecular weight chitosan nanoparticles as a potential vascular drug delivery system. Colloids Surf. B Biointerfaces 2012, 95, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Popat, A.; Liu, J.; Lu, G.Q.; Qiao, S.Z. A pH-responsive drug delivery system based on chitosan coated mesoporous silica nanoparticles. J. Mater. Chem. 2012, 22, 11173–11178. [Google Scholar] [CrossRef]
- Arulmozhi, V.; Pandian, K.; Mirunalini, S. Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surf. B Biointerfaces 2013, 110, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Jiang, T.; Zheng, X.; Zhang, J.; Sun, J.; Sun, C.; Wang, S. Novel chitosan-functionalized spherical nanosilica matrix as an oral sustained drug delivery system for poorly water-soluble drug carvedilol. ACS Appl. Mater. Interfaces 2013, 5, 103–113. [Google Scholar] [CrossRef]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Goncalves, L.M.D. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef]
- Huang, J.; Ren, J.; Chen, G.; Li, Z.; Liu, Y.; Wang, G.; Wu, X. Tunable sequential drug delivery system based on chitosan/hyaluronic acid hydrogels and PLGA microspheres for management of non-healing infected wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 89, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Sun, J.; Tan, H.; Yuan, G.; Li, J.; Jia, Y.; Xiong, D.; Chen, G.; Lai, J.; Ling, Z.; et al. Covalently polysaccharide-based alginate/chitosan hydrogel embedded alginate microspheres for BSA encapsulation and soft tissue engineering. Int. J. Biol. Macromol. 2019, 127, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Ong, T.P.; Moreno, F.S.; Ross, S.A. Targeting the epigenome with bioactive food components for cancer prevention. J. Nutr. Nutr. 2011, 4, 275–292. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Bao, B.; Ahmad, A.; Sarkar, F.H. Induction of cancer cell death by isoflavone: The role of multiple signaling pathways. Nutrients 2011, 3, 877–896. [Google Scholar] [CrossRef]
- Fu, S.; Xia, J.; Wu, J. Functional Chitosan Nanoparticles in Cancer Treatment. J. Biomed. Nanotechnol. 2016, 12, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Balla, V.K. A review on the use of magnetic fields and ultrasound for non-invasive cancer treatment. J. Adv. Res. 2018, 14, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Bowan, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Wang, H.; Han, J.; Zhao, S.; Dumbleton, J.; Agarwal, P.; Zhang, W.; Zhao, G.; Yu, J.; Zynger, D.L.; et al. Chitosan-Decorated Doxorubicin-Encapsulated Nanoparticle Targets and Eliminates Tumor Reinitiating Cancer Stem-like Cells. ACS Nano 2015, 9, 5725–5740. [Google Scholar] [CrossRef]
- Qin, C.; Zhou, B.; Zeng, L.; Zhang, Z.; Liu, Y.; Du, Y.; Xiao, L. The physicochemical properties and antitumor activity of cellulase-treated chitosan. Food Chem. 2004, 84, 107–115. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Ahmed, O.A.A.; Kurakula, M.; Caruso, G.; Caraci, F.; Asfour, H.Z.; Alfarsi, A.; Eid, B.G.; Mohamed, A.I.; Alruwaili, N.K. Chitosan-based microparticles enhance ellagic acid’s colon targeting and proapoptotic activity. Pharmaceutics 2020, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.B.F.; Melo, B.M.; Costa, H.S.; Costa Júnior, E.S. Liberação controlada de cisplatina utilizando microesferas de Quitosana/PVA como veículo. In Proceedings of the 13º Congresso da Sociedade Latino Americana de Biomateriais, Orgãos Artificiais e Engenharia de Tecidos-SLABO, Foz do Iguaçu, Brazil, 24–27 August 2016. [Google Scholar]
- Tahir, N.; Madni, A.; Balasubramanian, V.; Rehman, M.; Correia, A.; Kashif, P.M.; Makila, E.; Salonen, J.; Santos, H.A. Development and optimization of methotrexate-loaded lipid-polymer hybrid nanoparticles for controlled drug delivery applications. Int. J. Pharm. 2017, 533, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Madni, A.; Torchilin, V.; Filipczak, N.; Pan, J.; Tahir, N.; Shah, H. Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Deliv. 2019, 26, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.S.; Park, K.; Lee, S.; Nam, H.Y.; Min, K.H.; Jo, H.G.; Park, J.H.; Choi, K.; Jeong, S.Y.; et al. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J. Control. Release 2008, 127, 41–49. [Google Scholar] [CrossRef]
- Miwa, A.; Ishibe, A.; Nakano, M.; Yamashira, T.; Itai, S.; Jinno, S.; Kawahara, H. Development of novel chitosan derivatives as micellar carriers of taxol. Pharm. Res. 1998, 15, 1844–1850. [Google Scholar] [CrossRef]
- Kozlovskaya, V.; Liu, F.; Xue, B.; Ahmad, F.; Alford, A.; Saeed, M.; Kharlampieva, E. Polyphenolic polymersomes of temperature-sensitive poly (N-vinylcaprolactam)-block-poly (N-vinylpyrrolidone) for anticancer therapy. Biomacromolecules 2017, 18, 2552–2563. [Google Scholar] [CrossRef]
- Niu, S.; Bremner, D.H.; Wu, J.; Wu, J.; Wang, H.; Li, H.; Qian, Q.; Zheng, H.; Zhu, L. l-Peptide functionalized dual-responsive nanoparticles for controlled paclitaxel release and enhanced apoptosis in breast cancer cells. Drug Deliv. 2018, 25, 1275–1288. [Google Scholar] [CrossRef]
- Niu, S.; Williams, G.R.; Wu, J.; Wu, J.; Zhang, X.; Chen, X.; Li, S.; Jiao, J.; Zhu, L.-M. A chitosan-based cascade-responsive drug delivery system for triple-negative breast cancer therapy. J. Nanobiotechnol. 2019, 17, 1–18. [Google Scholar] [CrossRef]
- Agudelo, D.; Sanyakamdhorn, S.; Nafisi, S.; Tajmir-Riahi, H.A. Transporting antitumor drug tamoxifen and its metabolites, 4-hydroxytamoxifen and endoxifen by chitosan nanoparticles. PLoS ONE 2013, 8, e60250. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Hu, Y.L.; Qi, W.; Han, F.; Shao, J.Z.; Gao, J.Q. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int. J. Nanomed. 2011, 6, 3351–3359. [Google Scholar]
- Loh, J.W.; Yeoh, G.; Saunders, M.; Lim, L.Y. Uptake and cytotoxicity of chitosan nanoparticles in human liver cells. Toxicol. Appl. Pharm. 2010, 249, 148–157. [Google Scholar] [CrossRef]
- Huang, M.; Khor, E.; Lim, L.-Y. Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation. Pharm. Res. 2004, 21, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.T.S.; Laskmanan, V.-K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wingett, D.; Engelhard, M.H.; Feris, K.; Reddy, K.M.; Turner, P.; Layne, J.; Hanley, C.; Bell, J.; Tenne, D. Fluorescent dye encapsulated ZnO particles with cell-specific toxicity for potential use in biomedical applications. J. Mater. Sci. Mater. Med. 2009, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Pandey, A.C.; Verma, S.P.; Das, P.; Tewari, R.P. Efficient water soluble nanostructured ZnO grafted O-carboxymethyl chitosan/curcumin-nanocomposite for cancer therapy. Process. Biochem. 2015, 50, 678–688. [Google Scholar] [CrossRef]
- Anitha, J.; Selvakumar, R.; Murugan, K. Chitosan capped ZnO nanoparticles with cell specific apoptosis induction through P53 activation and G2/M arrest in breast cancer cells-In vitro approaches. Int. J. Biol. Macromol. 2019, 136, 686–696. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Omer, A.M.; Lu, W.; Zhang, S.; Jiang, X.; Wu, H.; Yu, D.; Ouyang, X.K. pH-sensitive ZnO/carboxymethyl cellulose/chitosan bio-nanocomposite beads for colon-specific release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 128, 468–479. [Google Scholar] [CrossRef]
- Hong, H.; Shi, J.; Yang, Y.; Zhang, Y.; Engle, J.W.; Nickles, R.J.; Wang, X.; Cai, W. Cancer-targeted optical imaging with fluorescent zinc oxide nanowires. Nano Lett. 2011, 11, 3744–3750. [Google Scholar] [CrossRef]
- Ostrovsky, S.; Kazimirsky, G.; Gedanken, A.; Brodie, C. Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res. 2009, 2, 882–890. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Tomar, P.; Sharma, V.; Dixit, V.K. Development and characterization of chitosan coated poly-(ɛ-caprolactone) nanoparticulate system for effective immunization against influenza. Vaccine 2011, 29, 9026–9037. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Park, D.; Lee, Y.C. Chitosan combined with ZnO, TiO(2) and Ag nanoparticles for antimicrobial wound healing applications: A mini review of the research trends. Polymers 2017, 9, 21. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Limbach, L.K.; Wick, P.; Manser, P.; Grass, R.N.; Bruinink, A.; Stark, W.J. Exposure of engineered nanoparticles to human lung epithelial cells: Influence of chemical composition and catalytic activity on oxidative stress. Environ. Sci. Technol. 2007, 41, 4158–4163. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Choi, J.Y.; Ramasamy, T.; Truong, D.H.; Nguyen, C.N.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Hyaluronic acid-coated solid lipid nanoparticles for targeted delivery of vorinostat to CD44 overexpressing cancer cells. Carbohydr. Polym. 2014, 114, 407–415. [Google Scholar] [CrossRef]
- Ramasamy, T.; Tran, T.H.; Cho, H.J.; Kim, J.H.; Kim, Y.I.; Jeon, J.Y.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Chitosan-based polyelectrolyte complexes as potential nanoparticulate carriers: Physicochemical and biological characterization. Pharm. Res. 2014, 31, 1302–1314. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J. Chitosan-based zinc oxide nanoparticle for enhanced anticancer effect in cervical cancer: A physicochemical and biological perspective. Saudi Pharm. J. 2018, 26, 205–210. [Google Scholar] [CrossRef]
- Chakra, C.H.S.; Rajendar, V.; Rao, K.V.; Kumar, M. Enhanced antimicrobial and anticancer properties of ZnO and TiO2 nanocomposites. 3 Biotech 2017, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Sun, W.; Qian, W.; Ye, Y.; Ma, X. The synthesis of chitosan-based silver nanoparticles and their antibacterial activity. Carbohydr. Res. 2009, 344, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F.; Al-Lohedan, H.A.; Al-Haque, H.N. Chitosan-mediated fabrication of metal nanocomposites for enhanced biomedical applicationsf. Adv. Mater. Lett. 2016, 8, 89–100. [Google Scholar] [CrossRef]
- Li, Q.L.; Huang, N.; Chen, J.; Wan, G.; Zhao, A.; Chen, J.; Wang, J.; Yang, P.; Leng, Y. Anticoagulant surface modification of titanium via layer-by-layer assembly of collagen and sulfated chitosan multilayers. J. Biomed. Mater. Res. Part A 2009, 89, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Yang, M.H.; Chiu, W.T.; Chiu, C.H.; Yang, C.S.; Chen, Y.W.; Chen, K.C.; Peng, R.Y. Composite nano-titanium oxide–chitosan artificial skin exhibits strong wound-healing effect—An approach with anti inflammatory and bactericidal kinetics. Macromol. Biosci. 2008, 8, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Esparza, L.M.; Ruvalcaba-Gomez, J.M.; Maytorena-Verdugo, C.I.; Gonzalez-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Perez-Larios, A.; Montalvo-Gonzalez, A.E. Chitosan-TiO2: A versatile hybrid composite. Materials 2020, 13, 811. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Li, J.; Wu, Y.Y.; Liu, Y.M. Amperometric immunobiosensor for alpha-fetoprotein using Au nanoparticles/chitosan/TiO(2)-graphene composite based platform. Bioelectrochemistry 2013, 90, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Zamora-Mora, V.; Sibaja-Ballestero, M.; Vega-Baudrit, J.; Lopez, D.; Mijangos, C. Influence of iron oxide nanoparticles on the rheological properties of hybrid chitosan ferrogels. J. Colloid Interface Sci. 2009, 339, 53–59. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; Martínez-Castañón, G.A.; Martínez-Martínez, R.E.; Loyola-Rodríguez, J.P.; Patiño-Marín, N.; Reyes-Macías, J.F.; Ruiz, F. Antibacterial effect of silver nanoparticles against Streptococcus mutans. Mater. Lett. 2009, 63, 2603–2606. [Google Scholar] [CrossRef]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, M.; Li, X.; Huang, Y.; Liang, W. A hybrid thermo-sensitive chitosan gel for sustained release of Meloxicam. J. Biomater. Sci. Polym. Ed. 2008, 19, 1239–1247. [Google Scholar] [CrossRef]
- Fontana, C.R.; dos Santos Júnior, D.S.; Bosco, J.M.; Spolidorio, D.M.; Chierici Marcantonio, R.A. Evaluation of chitosan gel as antibiotic and photosensitizer delivery. Drug Deliv 2008, 15, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Eby, D.M.; Schaeublin, N.M.; Farrington, K.E.; Hussain, S.M.; Johnson, G.R. Lysozyme catalyzes the formation of antimicrobial silver nanoparticles. ACS Nano 2009, 3, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.-M.; Zhang, Q.-B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl. Mater. Interfaces 2011, 3, 218–228. [Google Scholar] [CrossRef]
- Asharani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Aliev, G.; Daza, J.; Lipsitt, A.; Martínez-Agüero, M.; Palacios, H.H.; Fischbach, K.; Obrenovich, M.E.; LaManna, J.C.; Bragin, V.; Morales, L. Silver nanoparticles as alternate strategies for drug delivery to Alzheimer brain. Alzheimers Dement. J. Alzheimers Assoc. 2009, 5, P324. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Sathishkumar, G.; Sivanandhan, G.; MubarakAli, D.; Rajesh, M.; Arun, R.; Kapildev, G.; Manickavasagam, M.; Thajuddin, N.; Premkumar, K. Biogenic silver nanoparticles for cancer treatment: An experimental report. J. Colloids Surf. B Biointerfaces 2013, 106, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Ganesan, S. Preparation and characterization of gold nanoparticles with different capping agents. Int. J. Green Nanotechnol. 2011, 3, 47–55. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Nunes, D.P.; Troxler, R.F.; Offner, G.D. Suppression of MUC1 synthesis downregulates expression of the epidermal growth factor receptor. Cancer Biol. Ther. 2005, 4, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Rani, N. Effect of PVP, PVA and POLE surfactants on the size of iridium nanoparticles. Open J. Inorg. Chem. 2012, 2, 67. [Google Scholar] [CrossRef]
- Nivethaa, E.A.K.; Dhanavel, S.; Rebekah, A.; Narayanan, V.; Stephen, A. A comparative study of 5-Fluorouracil release from chitosan/silver and chitosan/silver/MWCNT nanocomposites and their cytotoxicity towards MCF-7. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 244–250. [Google Scholar]
- Fukushima, S.; Kasai, T.; Umeda, Y.; Ohnishi, M.; Sasaki, T.; Matsumoto, M. Carcinogenicity of multi-walled carbon nanotubes: Challenging issue on hazard assessment. J. Occup. Health 2018, 60, 10–30. [Google Scholar] [CrossRef]
- González-Lavado, E.; Valdivia, L.; García-Castaño, A.; González, F.; Pesquera, C.; Valiente, R.; Fanarraga, M.F. Multi-walled carbon nanotubes complement the anti-tumoral effect of 5-Fluorouracil. Oncotarget 2019, 10, 2022–2029. [Google Scholar] [CrossRef]
- Das, M.; Datir, S.R.; Singh, R.P.; Jain, S. Augmented anticancer activity of a targeted, intracellularly activatable, theranostic nanomedicine based on fluorescent and radiolabeled, methotrexate-folic acid-multiwalled carbon nanotube conjugate. Mol. Pharm. 2013, 10, 2543–2557. [Google Scholar] [CrossRef]
- Datir, S.R.; Das, M.; Singh, R.P.; Jain, S. Hyaluronate tethered,“smart” multiwalled carbon nanotubes for tumor-targeted delivery of doxorubicin. Bioconjugate Chem. 2012, 23, 2201–2213. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef]
- Elgrabli, D.; Floriani, M.; Abella-Gallart, S.; Meunier, L.; Gamez, C.; Delalain, P.; Rogerieux, F.; Boczkowski, J.; Lacroix, G. Biodistribution and clearance of instilled carbon nanotubes in rat lung. Part. Fibre Toxicol. 2008, 5, 20. [Google Scholar] [CrossRef]
- Arya, N.; Arora, A.; Vasu, K.S.; Sood, A.K.; Katti, D.S. Combination of single walled carbon nanotubes/graphene oxide with paclitaxel: A reactive oxygen species mediated synergism for treatment of lung cancer. Nanoscale 2013, 5, 2818–2829. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Wang, Y.; Chen, Y.; Jin, Q.; Ji, J. Doxorubicin conjugated phospholipid prodrugs as smart nanomedicine platforms for cancer therapy. J. Mater. Chem. B 2015, 3, 3297–3305. [Google Scholar] [CrossRef]
- Singh, R.P.; Sharma, G.; Sonali; Singh, S.; Bharti, S.; Pandey, B.L.; Koch, B.; Muthu, M.S. Chitosan-folate decorated carbon nanotubes for site specific lung cancer delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Karnati, K.R.; Wang, Y. Understanding the co-loading and releasing of doxorubicin and paclitaxel using chitosan functionalized single-walled carbon nanotubes by molecular dynamics simulations. Phys. Chem. Chem. Phys. 2018, 20, 9389–9400. [Google Scholar] [CrossRef]
- Cao, N.; Feng, S.-S. Doxorubicin conjugated to D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS): Conjugation chemistry, characterization, in vitro and in vivo evaluation. Biomaterials 2008, 29, 3856–3865. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Kohay, H.; Sarisozen, C.; Sawant, R.; Jhaveri, A.; Torchilin, V.P.; Mishael, Y.G. PEG-PE/clay composite carriers for doxorubicin: Effect of composite structure on release, cell interaction and cytotoxicity. J. Acta Biomater. 2017, 55, 443–454. [Google Scholar] [CrossRef]
- Liao, L.; Liu, J.; Dreaden, E.C.; Morton, S.W.; Shopsowitz, K.E.; Hammond, P.T.; Johnson, J.A. A convergent synthetic platform for single-nanoparticle combination cancer therapy: Ratiometric loading and controlled release of cisplatin, doxorubicin, and camptothecin. J. Am. Chem. Soc. 2014, 136, 5896–5899. [Google Scholar] [CrossRef]
- Loira-Pastoriza, C.; Todoroff, J.; Vanbever, R. Delivery strategies for sustained drug release in the lungs. J. Adv. Drug Deliv. Rev. 2014, 75, 81–91. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Shim, G.; Kim, M.-G.; Park, J.Y.; Oh, Y.-K. Graphene-based nanosheets for delivery of chemotherapeutics and biological drugs. Adv. Drug Deliv. Rev. 2016, 105, 205–227. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 2010, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Huh, M.S.; Lee, S.Y.; Min, S.; Lee, S.; Koo, H.; Chu, J.U.; Lee, K.E.; Jeon, H.; Choi, Y. Tumor-homing poly-siRNA/glycol chitosan self-cross-linked nanoparticles for systemic siRNA delivery in cancer treatment. Angew. Chem. Int. Ed. 2012, 51, 7203–7207. [Google Scholar] [CrossRef]
- Ardeshirzadeh, B.; Anaraki, N.A.; Irani, M.; Rad, L.R.; Shamshiri, S. Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 48, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.K.; Choi, M.-C.; Kong, J.-Y.; Kim, G.Y.; Kim, M.J.; Kim, S.-H.; Mishra, S.; Singh, R.P.; Ha, C.-S. Synthesis and Drug-Delivery Behavior of Chitosan-Functionalized Graphene Oxide Hybrid Nanosheets. Macromol. Mater. Eng. 2011, 296, 131–140. [Google Scholar] [CrossRef]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-functionalized graphene oxide as a nanocarrier for drug and gene delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. JNCI J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.B. Photodynamic therapy: The sensitization of cancer cells to light. J. Chem. Educ. 1999, 76, 592. [Google Scholar] [CrossRef]
- Babilas, P.; Karrer, S.; Sidoroff, A.; Landthaler, M.; Szeimies, R.M. Photodynamic therapy in dermatology–an update. Photodermatol. Photoimmunol. Photomed. 2005, 21, 142–149. [Google Scholar] [CrossRef]
- Szaciłowski, K.; Macyk, W.; Drzewiecka-Matuszek, A.; Brindell, M.; Stochel, G. Bioinorganic photochemistry: Frontiers and mechanisms. Chem. Rev. 2005, 105, 2647–2694. [Google Scholar] [CrossRef] [PubMed]
- Creixell, M.; Bohórquez, A.C.; Torres-Lugo, M.; Rinaldi, C. EGFR-Targeted Magnetic Nanoparticle Heaters Kill Cancer Cells without a Perceptible Temperature Rise. ACS Nano 2011, 5, 7124–7129. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, A.; Toliopoulos, I.; Giotis, C.; Metsios, A.; Verginadis, I.; Simos, Y.; Havelas, K.; Hadziaivazis, G.; Karkabounas, S. Functionality of natural killer cells from end-stage cancer patients exposed to coherent electromagnetic fields. Electromagn. Biol. Med. 2011, 30, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Van de Walle, C.G. Hydrogen as a cause of doping in zinc oxide. Phys. Rev. Lett. 2000, 85, 1012. [Google Scholar] [CrossRef]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef]
- Wang, H.; Xie, C.; Zhang, W.; Cai, S.; Yang, Z.; Gui, Y. Comparison of dye degradation efficiency using ZnO powders with various size scales. J. Hazard. Mater. 2007, 141, 645–652. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, P.; Navrotsky, A.; Yuan, Z.-Y.; Ren, T.-Z.; Halasa, M.; Su, B.-L. Hierarchically assembled porous ZnO nanoparticles: Synthesis, surface energy, and photocatalytic activity. Chem. Mater. 2007, 19, 5680–5686. [Google Scholar] [CrossRef]
- Guo, D.; Wu, C.; Jiang, H.; Li, Q.; Wang, X.; Chen, B. Synergistic cytotoxic effect of different sized ZnO nanoparticles and daunorubicin against leukemia cancer cells under UV irradiation. J. Photochem. Photobiol. B Biol. 2008, 93, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, W.; Wang, S.; Liu, Y.; Pope, C. Phototoxicity of zinc oxide nanoparticle conjugatesin human ovarian cancer NIH: OVCAR-3 cells. J. Biomed. Nanotechnol. 2008, 4, 432–438. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Bandara, S.; Carnegie, C.; Johnson, C.; Akindoju, F.; Williams, E.; Swaby, J.M.; Oki, A.; Carson, L.E. Synthesis and characterization of Zinc/Chitosan-Folic acid complex. Heliyon 2018, 4, e00737. [Google Scholar] [CrossRef]
- Mathew, M.E.; Mohan, J.C.; Manzoor, K.; Nair, S.V.; Tamura, H.; Jayakumar, R. Folate conjugated carboxymethyl chitosan–manganese doped zinc sulphide nanoparticles for targeted drug delivery and imaging of cancer cells. Carbohydr. Polym. 2010, 80, 442–448. [Google Scholar] [CrossRef]
- Yuan, Q.; Hein, S.; Misra, R.D.K. New generation of chitosan-encapsulated ZnO quantum dots loaded with drug: Synthesis, characterization and in vitro drug delivery response. Acta Biomater. 2010, 6, 2732–2739. [Google Scholar] [CrossRef]
- Arias, J.L.; Reddy, L.H.; Couvreur, P. Fe3O4/chitosan nanocomposite for magnetic drug targeting to cancer. J. Mater. Chem. 2012, 22, 7622–7632. [Google Scholar] [CrossRef]
- Ma, M.; Wu, Y.; Zhou, J.; Sun, Y.; Zhang, Y.; Gu, N. Size dependence of specific power absorption of Fe3O4 particles in AC magnetic field. J. Magn. Magn. Mater. 2004, 268, 33–39. [Google Scholar] [CrossRef]
- Babincova, M.; Sourivong, P.; Leszczynska, D.; Babinec, P. Blood-specific whole-body electromagnetic hyperthermia. Med. Hypotheses 2000, 55, 459–460. [Google Scholar] [CrossRef]
- Liu, Z.; Lammers, T.; Ehling, J.; Fokong, S.; Bornemann, J.; Kiessling, F.; Gätjens, J. Iron oxide nanoparticle-containing microbubble composites as contrast agents for MR and ultrasound dual-modality imaging. Biomaterials 2011, 32, 6155–6163. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Yang, X.; Yin, M.; Ren, J.; Qu, X. The use of multifunctional magnetic mesoporous core/shell heteronanostructures in a biomolecule separation system. Biomaterials 2011, 32, 4683–4690. [Google Scholar] [CrossRef]
- Satarkar, N.S.; Hilt, J.Z. Hydrogel nanocomposites as remote-controlled biomaterials. Acta Biomater. 2008, 4, 11–16. [Google Scholar] [CrossRef]
- Kawashita, M.; Kawamura, K.; Li, Z. PMMA-based bone cements containing magnetite particles for the hyperthermia of cancer. Acta Biomater. 2010, 6, 3187–3192. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, K.N.; Kim, K.M.; Lee, Y.K. Targeting to carcinoma cells with chitosan-and starch-coated magnetic nanoparticles for magnetic hyperthermia. J. Biomed. Mater. Res. Part A 2009, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Su, Z. Characterization and application of high magnetic property chitosan particles. J. Appl. Polym. Sci. 2001, 81, 1175–1181. [Google Scholar] [CrossRef]
- Denkbaş, E.B.; Kiliçay, E.; Birlikseven, C.; Öztürk, E. Magnetic chitosan microspheres: Preparation and characterization. React. Funct. Polym. 2002, 50, 225–232. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Q.; Fang, Z.; Zhang, X.; Zhang, B. Magnetic chitosan nanocomposites: A useful recyclable tool for heavy metal ion removal. Langmuir 2009, 25, 3–8. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Hu, S.-H.; Liu, T.-Y.; Liu, D.-M.; Chen, S.-Y. Magnetic-sensitive behavior of intelligent ferrogels for controlled release of drug. Langmuir 2006, 22, 5974–5978. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, B.; Zhou, Y.; Jia, D. Chitosan-induced synthesis of magnetite nanoparticles via iron ions assembly. J. Polym. Adv. Technol. 2008, 19, 1256–1261. [Google Scholar] [CrossRef]
- Zhao, D.-L.; Wang, X.-X.; Zeng, X.-W.; Xia, Q.-S.; Tang, J.-T. Preparation and inductive heating property of Fe3O4–chitosan composite nanoparticles in an AC magnetic field for localized hyperthermia. J. Alloys Compd. 2009, 477, 739–743. [Google Scholar] [CrossRef]
- Thu, H.P.; Huong, L.T.T.; Nhung, H.T.M.; Tham, N.T.; Tu, N.D.; Thi, H.T.M.; Hanh, P.T.B.; Nguyet, T.T.M.; Quy, N.T.; Nam, P.H.; et al. Fe3O4/o-Carboxymethyl Chitosan/Curcumin-based Nanodrug System for Chemotherapy and Fluorescence Imaging in HT29 Cancer Cell Line. Chem. Lett. 2011, 40, 1264–1266. [Google Scholar] [CrossRef]
- Lin, T.C.; Lin, F.H.; Lin, J.C. In vitro feasibility study of the use of a magnetic electrospun chitosan nanofiber composite for hyperthermia treatment of tumor cells. Acta Biomater. 2012, 8, 2704–2711. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, J.; Zhang, R.; Wang, J.; Su, T.; Tian, Z.; Han, D.; Zhao, C.; Fan, M.; Li, C.; et al. Quaternized Chitosan/Alginate-Fe3O4 Magnetic Nanoparticles Enhance the Chemosensitization of Multidrug-Resistant Gastric Carcinoma by Regulating Cell Autophagy Activity in Mice. J. Biomed. Nanotechnol. 2016, 12, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, A.G.; Narsireddy, A.; Rao, N.M.; Manorama, S.V. A simple approach to design chitosan functionalized Fe3O4 nanoparticles for pH responsive delivery of doxorubicin for cancer therapy. J. Magn. Magn. Mater. 2018, 448, 199–207. [Google Scholar]
- Liao, H.; Qi, R.; Shen, M.; Cao, X.; Guo, R.; Zhang, Y.; Shi, X. Improved cellular response on multiwalled carbon nanotube-incorporated electrospun polyvinyl alcohol/chitosan nanofibrous scaffolds. Colloids Surf. B Biointerfaces 2011, 84, 528–535. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Pang, D.W.-P.; Hwang, S.-M.; Tuan, H.-Y.; Hu, Y.-C. A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials 2012, 33, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Ju, E.; Ren, J.; Qu, X. Immunostimulatory oligonucleotides-loaded cationic graphene oxide with photothermally enhanced immunogenicity for photothermal/immune cancer therapy. Biomaterials 2014, 35, 9963–9971. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, Y.; Han, P.; Li, D.; Gui, X.; Zhang, Z.; Fu, K.; Chu, M. Graphitic carbon nanocages as new photothermal agent and drug Carrier for 980-nm-laser-driven cancer therapy. Carbon 2018, 136, 234–247. [Google Scholar] [CrossRef]
- Chen, G.Y.; Chen, C.L.; Tuan, H.-Y.; Yuan, P.X.; Li, K.C.; Yang, H.J.; Hu, Y.C. Graphene oxide triggers toll-like receptors/autophagy responses in vitro and inhibits tumor growth in vivo. Adv. Healthc. Mater. 2014, 3, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Cheon, Y.A.; Bae, J.H.; Chung, B.G. Reduced graphene oxide nanosheet for chemo-photothermal therapy. Langmuir 2016, 32, 2731–2736. [Google Scholar] [CrossRef]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Wu, F.; Yuan, P.; Chi, C.; Zhou, N. Magnetic and fluorescent carbon nanotubes for dual modal imaging and photothermal and chemo-therapy of cancer cells in living mice. Carbon 2017, 123, 70–83. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, S.; Jiang, Y.; Ma, H.; Shi, M.; Wang, Q.; Zhong, W.; Liao, W.; Xing, M.M.Q. Doxorubicin-loaded single wall nanotube thermo-sensitive hydrogel for gastric cancer chemo-photothermal therapy. Adv. Funct. Mater. 2015, 25, 4730–4739. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, F.; Zhao, C.; Lv, Y.; Ma, G.; Wei, W.; Tian, Z. Beyond a carrier: Graphene quantum dots as a probe for programmatically monitoring anti-cancer drug delivery, release, and response. ACS Appl. Mater. Interfaces 2017, 9, 27396–27401. [Google Scholar] [CrossRef]
- Geng, B.; Yang, D.; Pan, D.; Wang, L.; Zheng, F.; Shen, W.; Zhang, C.; Li, X. NIR-responsive carbon dots for efficient photothermal cancer therapy at low power densities. Carbon 2018, 134, 153–162. [Google Scholar] [CrossRef]
- Li, Z.; Jaroniec, M.; Papakonstantinou, P.; Tobin, J.M.; Vohrer, U.; Kumar, S.; Attard, G.; Holmes, J.D. Supercritical fluid growth of porous carbon nanocages. Chem. Mater. 2007, 19, 3349–3354. [Google Scholar] [CrossRef]
- Li, W.; Han, P.; Chen, Y.; Guo, Y.; Li, D.; Wu, Y.; Yue, Y.; Chu, M. Drug-Loaded Polymer-Coated Graphitic Carbon Nanocages for Highly Efficient in Vivo Near-Infrared Laser-Induced Synergistic Therapy through Enhancing Initial Temperature. ACS Appl. Mater. Interfaces 2018, 10, 31186–31197. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, Y.; Han, P.; Liu, Y.; Li, W.; Zhu, F.; Fu, K.; Chu, M. Biocompatible chitosan-carbon nanocage hybrids for sustained drug release and highly efficient laser and microwave co-irradiation induced cancer therapy. Acta Biomater. 2019, 103, 237–246. [Google Scholar] [CrossRef]
- Yin, Z.F.; Wu, L.; Yang, H.G.; Su, Y.H. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858. [Google Scholar] [CrossRef]
- Ren, W.; Zeng, L.; Shen, Z.; Xiang, L.; Gong, A.; Zhang, J.; Mao, C.; Li, A.; Paunesku, T.; Woloschak, G.E. Enhanced doxorubicin transport to multidrug resistant breast cancer cells via TiO2 nanocarriers. RSC Adv. 2013, 3, 20855–20861. [Google Scholar] [CrossRef]
- Du, Y.; Ren, W.; Li, Y.; Zhang, Q.; Zeng, L.; Chi, C.; Wu, A.; Tian, J. The enhanced chemotherapeutic effects of doxorubicin loaded PEG coated TiO 2 nanocarriers in an orthotopic breast tumor bearing mouse model. J. Mater. Chem. B 2015, 3, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ji, Y.; Chen, Q.; Zhu, X.; Zhang, X.; Tan, Z.; Tian, Q.; Yang, X.; Zhang, Z. In vitro and in vivo chemo-phototherapy of magnetic TiO2 drug delivery system formed by pH-sensitive coordination bond. J. Biomater. Appl. 2016, 31, 568–581. [Google Scholar] [CrossRef]
- Shen, S.; Wu, L.; Liu, J.; Xie, M.; Shen, H.; Qi, X.; Yan, Y.; Ge, Y.; Jin, Y. Core-shell structured Fe3O4@TiO2-doxorubicin nanoparticles for targeted chemo-sonodynamic therapy of cancer. Int. J. Pharm. 2015, 486, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Samadi, S.; Moradkhani, M.; Beheshti, H.; Irani, M.; Aliabadi, M. Fabrication of chitosan/poly(lactic acid)/graphene oxide/TiO2 composite nanofibrous scaffolds for sustained delivery of doxorubicin and treatment of lung cancer. Int. J. Biol. Macromol. 2018, 110, 416–424. [Google Scholar] [CrossRef]
- Kim, S.; Nishimoto, S.K.; Bumgardner, J.D.; Haggard, W.O.; Gaber, M.W.; Yang, Y. A chitosan/beta-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials 2010, 31, 4157–4166. [Google Scholar] [CrossRef] [PubMed]
- Malesu, V.K.; Sahoo, D.; Nayak, P.L. Chitosan–sodium alginate nanocomposites blended with cloisite 30b as a novel drug delivery system for anticancer drug curcumin. Int. J. Appl. Biol. Pharm. Technol. 2011, 2, 402–411. [Google Scholar]
- Xie, Y.T.; Du, Y.Z.; Yuan, H.; Hu, F.Q. Brain-targeting study of stearic acid-grafted chitosan micelle drug-delivery system. Int. J. Nanomed. 2012, 7, 3235–3244. [Google Scholar]
- Vivek, R.; Nipun Babu, V.; Thangam, R.; Subramanian, K.S.; Kannan, S. pH-responsive drug delivery of chitosan nanoparticles as Tamoxifen carriers for effective anti-tumor activity in breast cancer cells. Colloids Surf. B Biointerfaces 2013, 111, 117–123. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Kong, L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS Pharmscitech 2013, 14, 585–592. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Kong, M.; An, Y.; Liu, Y.; Li, J.J.; Zhou, X.; Feng, C.; Li, J.; Jiang, S.Y.; Cheng, X.J.; et al. Hydroxybutyl chitosan thermo-sensitive hydrogel: A potential drug delivery system. J. Mater. Sci. 2013, 48, 5614–5623. [Google Scholar] [CrossRef]
- Anitha, A.; Deepa, N.; Chennazhi, K.P.; Lakshmanan, V.K.; Jayakumar, R. Combinatorial anticancer effects of curcumin and 5-fluorouracil loaded thiolated chitosan nanoparticles towards colon cancer treatment. Biochim. Biophys. Acta 2014, 1840, 2730–2743. [Google Scholar] [CrossRef] [PubMed]
- Unsoy, G.; Khodadust, R.; Yalcin, S.; Mutlu, P.; Gunduz, U. Synthesis of Doxorubicin loaded magnetic chitosan nanoparticles for pH responsive targeted drug delivery. Eur. J. Pharm. Sci. 2014, 62, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Vivek, R.; Thangam, R.; Nipunbabu, V.; Ponraj, T.; Kannan, S. Oxaliplatin-chitosan nanoparticles induced intrinsic apoptotic signaling pathway: A “smart” drug delivery system to breast cancer cell therapy. Int. J. Biol. Macromol. 2014, 65, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, B.; Zou, M.; Cheng, G. Cyclic RGD-modified chitosan/graphene oxide polymers for drug delivery and cellular imaging. Colloids Surf. B Biointerfaces 2014, 122, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Lv, Y.; Williams, G.R.; Tao, L.; Yang, H.; Li, H.; Zhu, L. Lactobionic acid and carboxymethyl chitosan functionalized graphene oxide nanocomposites as targeted anticancer drug delivery systems. Carbohydr. Polym. 2016, 151, 812–820. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y.; Han, X. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2018, 536, 224–234. [Google Scholar] [CrossRef]
- Amini-Fazl, M.S.; Mohammadi, R.; Kheiri, K. 5-Fluorouracil loaded chitosan/polyacrylic acid/Fe3O4 magnetic nanocomposite hydrogel as a potential anticancer drug delivery system. Int. J. Biol. Macromol. 2019, 132, 506–513. [Google Scholar] [CrossRef]
- Chen, X.; Fan, M.; Tan, H.; Ren, B.; Yuan, G.; Jia, Y.; Li, J.; Xiong, D.; Xing, X.; Niu, X.; et al. Magnetic and self-healing chitosan-alginate hydrogel encapsulated gelatin microspheres via covalent cross-linking for drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zeng, X.; Zheng, Y.; Wang, X.; Tang, R. Surface-fluorinated and pH-sensitive carboxymethyl chitosan nanoparticles to overcome biological barriers for improved drug delivery in vivo. Carbohydr. Polym. 2019, 208, 59–69. [Google Scholar] [CrossRef]
- Chi, J.; Jiang, Z.; Qiao, J.; Peng, Y.; Liu, W.; Han, B. Synthesis and anti-metastasis activities of norcantharidin-conjugated carboxymethyl chitosan as a novel drug delivery system. Carbohydr. Polym. 2019, 214, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Park, M.H.; Jo, G.; Kim, S.Y.; Chun, H.J.; Yang, D.H. Photo-Cured Glycol Chitosan Hydrogel for Ovarian Cancer Drug Delivery. Mar. Drugs 2019, 17, 41. [Google Scholar] [CrossRef]
- Jaiswal, S.; Dutta, P.K.; Kumar, S.; Koh, J.; Pandey, S. Methyl methacrylate modified chitosan: Synthesis, characterization and application in drug and gene delivery. Carbohydr. Polym. 2019, 211, 109–117. [Google Scholar] [CrossRef]
- Habibi Jouybari, M.; Hosseini, S.; Mahboobnia, K.; Boloursaz, L.A.; Moradi, M.; Irani, M. Simultaneous controlled release of 5-FU, DOX and PTX from chitosan/PLA/5-FU/g-C3N4-DOX/g-C3N4-PTX triaxial nanofibers for breast cancer treatment in vitro. Colloids Surf. B Biointerfaces 2019, 179, 495–504. [Google Scholar] [CrossRef]
- Perez-Alvarez, L.; Ruiz-Rubio, L.; Artetxe, B.; Vivanco, M.D.; Gutierrez-Zorrilla, J.M.; Vilas-Vilela, J.L. Chitosan nanogels as nanocarriers of polyoxometalates for breast cancer therapies. Carbohydr. Polym. 2019, 213, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Rengifo, A.F.C.; Stefanes, N.M.; Toigo, J.; Mendes, C.; Argenta, D.F.; Dotto, M.E.R.; da Silva, M.C.S.; Nunes, R.J.; Caon, T.; Parize, A.L.; et al. PEO-chitosan nanofibers containing carboxymethyl-hexanoyl chitosan/dodecyl sulfate nanoparticles loaded with pyrazoline for skin cancer treatment. Eur. Polym. J. 2019, 119, 335–343. [Google Scholar] [CrossRef]
- Shafiee, S.; Ahangar, H.A.; Saffar, A. Taguchi method optimization for synthesis of Fe3O4 @chitosan/Tragacanth Gum nanocomposite as a drug delivery system. Carbohydr. Polym. 2019, 222, 114982. [Google Scholar] [CrossRef] [PubMed]
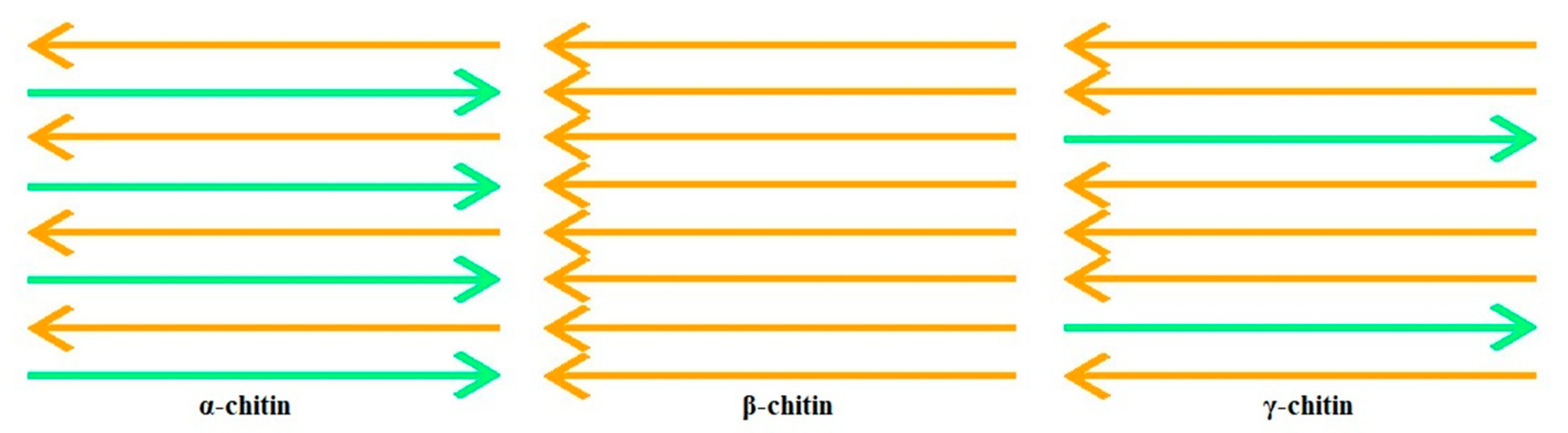
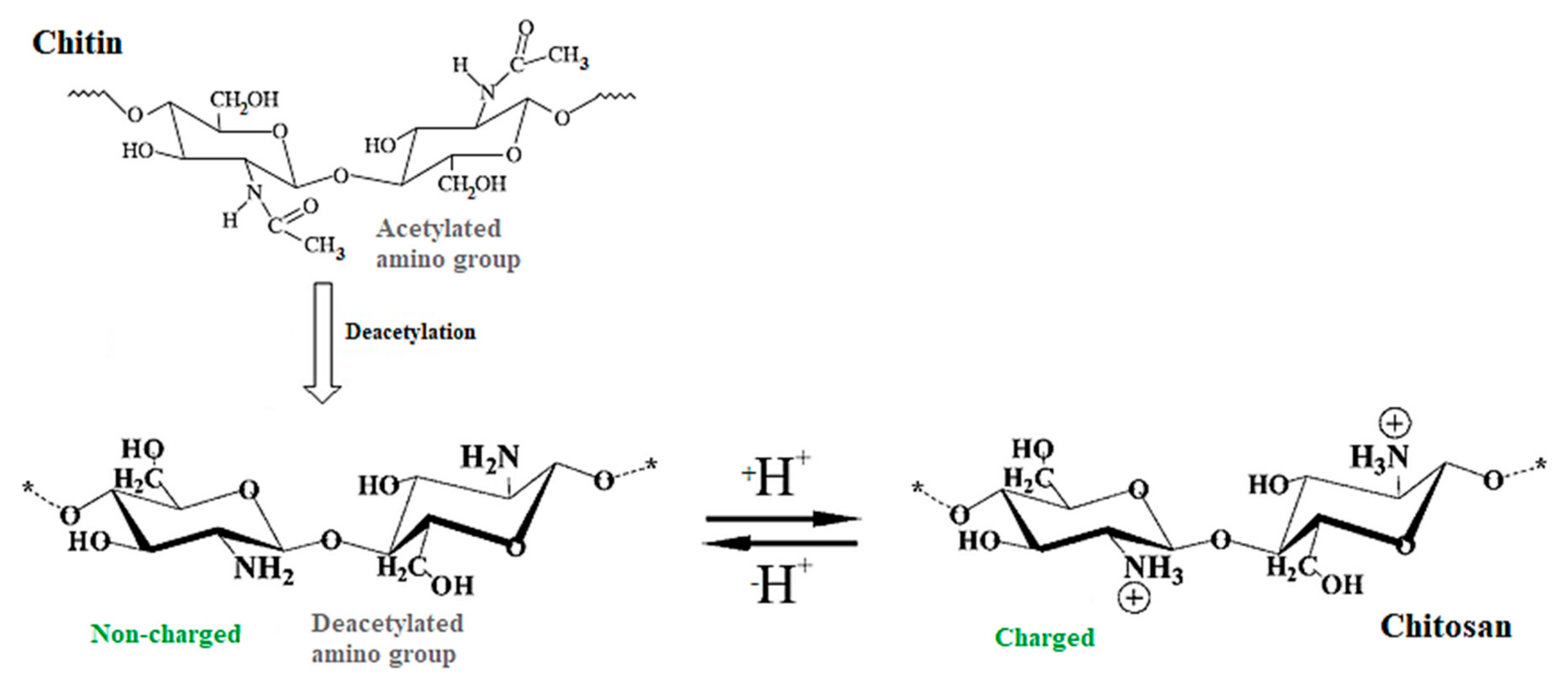
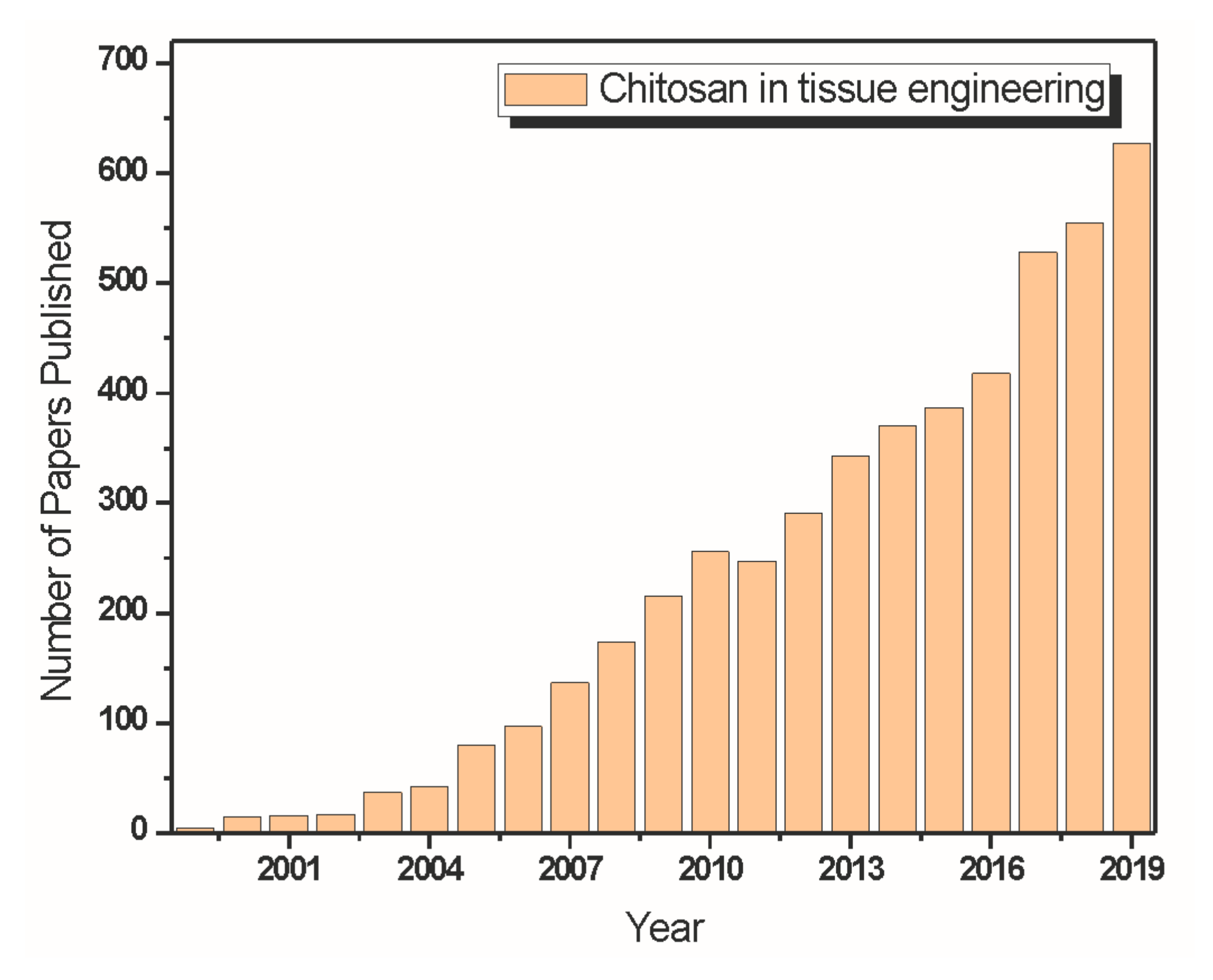
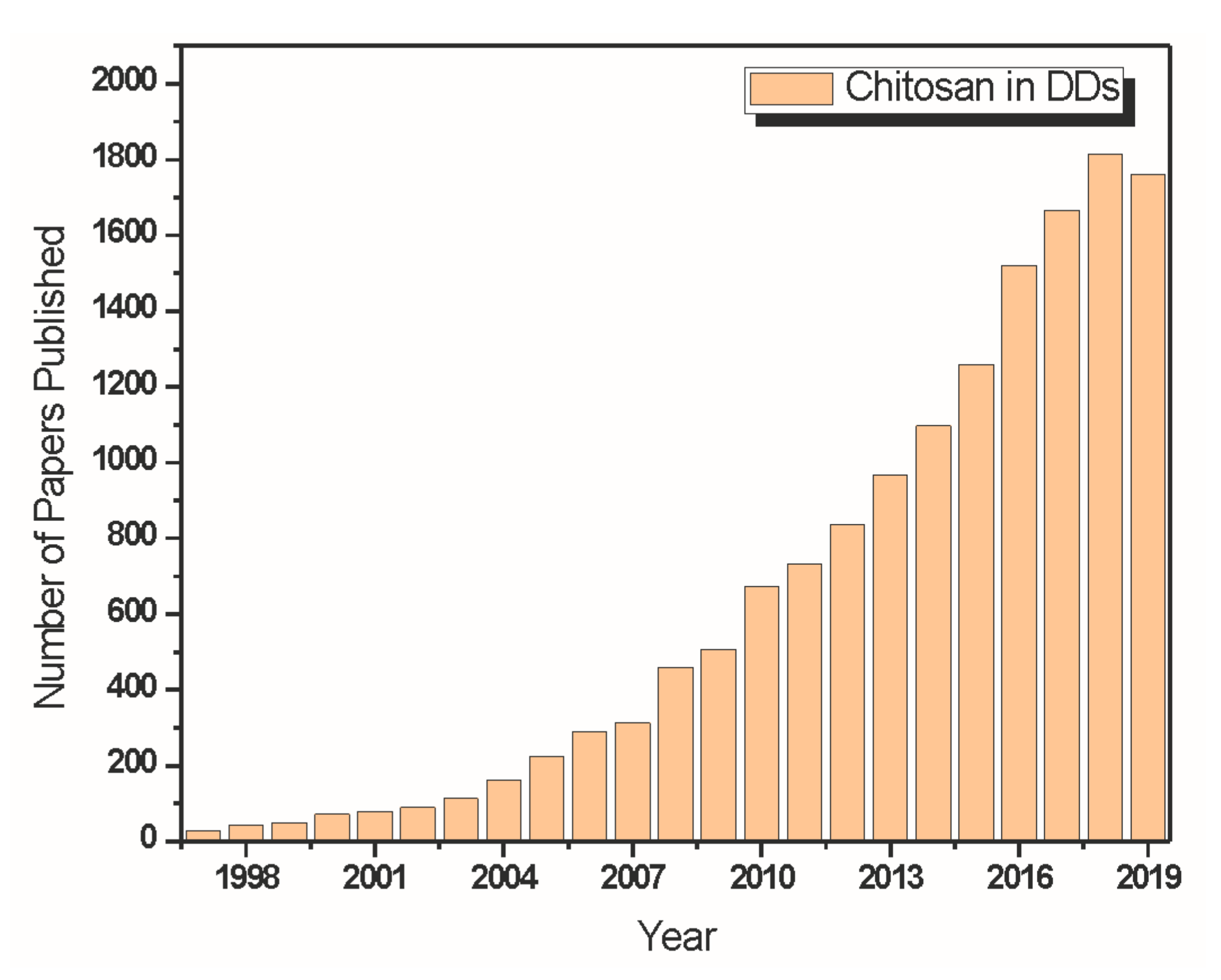
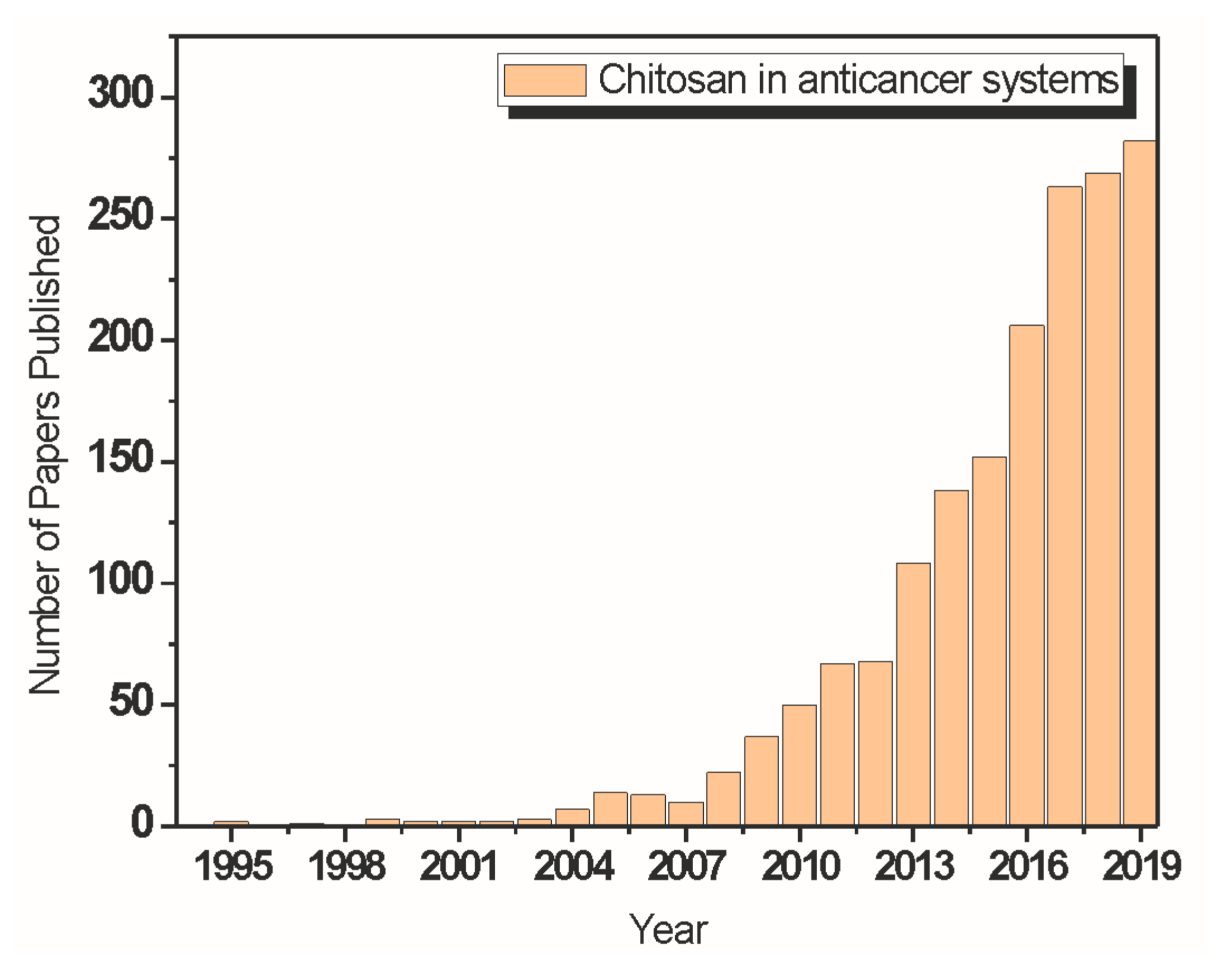
| System | Field of Study | Application | Relevant Results | Reference |
|---|---|---|---|---|
| CS film | Wound healing | CS film on wound repair in distal horse limb | CS membrane accentuated the formation of granulation tissue, emphasizing a potential repairing effect | Martins et al., 2013 [191] |
| CS/PVA hydrogel nanofiber mats | Wound healing | Nanofiber mats and hydrogel application in wound healing | The nanofiber mats showed good bactericidal activity against Escherichia coli, combining the advantages of nanofibrous mats and hydrogel dressing | Liu et al., 2014 [213] |
| CS–Alg and CS-xanthan polyelectrolyte complexes incorporated with erythromycin | Wound healing | CS membranes complexed with alginate or xanthan for the treatment of skin lesions | The release of the erythromycin molecule from the matrices was slow, requiring less frequent changes and resulting in less traumatic and more comfortable treatment for the patient | Souza et al., 2014 [212] |
| CS | Wound healing | Healing effect of low molar mass and degree of acetylation chitosan on skin lesions of rats | CS plays an important role in the recovery of acute skin lesions in rats, accelerating the healing process and providing a reduction in the width of the lesion | Fráguas et al., 2015 [204] |
| CS/gelatin /PVA-based hydrogels | Wound healing | Hydrogels for wound dressing applications | Hydrogels analyzed rapidly absorbed blood from the damaged tissue and adhered to the dermal surface, blocking broken blood vessels, and stimulating platelet release | Fan et al., 2016 [211] |
| CS-Ag hydrogel | Wound healing | Novel chitosan hydrogels reinforced with silver NPs for accelerating wound healing | The hydrogel exhibited a porous 3D network and ultrahigh mechanical properties due to the intermolecular and intramolecular interactions. Ag NPs integrated into CS hydrogel enhanced the antibacterial properties and also reinforced its mechanical properties | Xie et al., 2018 [214] |
| CS/PEO/silica hybrid nanofibers | Bone tissue engineering | Hybrid nanofibers as a potential biomaterial for bone repair and regeneration | Proved to be cytocompatible, promoting the attachment and proliferation of murine osteoblast-like 7F2-cells, and also enhance a fast apatite formation by incorporation of Ca2+ ions and subsequent immersion in modified simulated body fluid (m-SBF) | Toskas et al., 2013 [100] |
| CS–Alg and CS–Alg–fucoidan scaffolds | Bone tissue engineering | Biocomposite containing fucoidan for bone tissue engineering | In vitro studies using the MG-63 cell line revealed a good cytocompatibility, increased alkaline phosphatase secretion, and enhanced cell proliferation in the CS–Alg–fucoidan scaffold, and two times higher protein adsorption and mineralization in comparison with CS–Alg scaffold | Venkatesan et al., 2014 [117] |
| CS/ Hydroxyapatite(HA)/gelatin scaffolds | Bone tissue engineering | Scaffolds obtained by aggregation of CS/ HA spheres for bone tissue regeneration | A 3D structure was formed, and the HA acts as reinforcement until the concentration of 3% | Oliveira et al., 2015 [104] |
| HA/gelatin–CS core-shell nanofibers | Bone tissue engineering | Biomimetic composite scaffolds for bone tissue engineering | Gelatin-CS core-shell structured nanofibers improved HA’s mineralization efficiency and formed a homogeneous HAP deposit compared with CS and CS-gelatin nanofibers. When Human osteoblast-like cell line (MG-63) were cultured on the materials, the results showed that HA deposited on the Gelatin-CS core-shell nanofibers could further enhance osteoblast cell proliferation | Chen et al., 2018 [103] |
| Apatite-CS scaffold | Bone tissue engineering | A composite scaffold as a potential injectable construct for bone tissue engineering | The scaffold presented an osteogenic-like microenvironment adequate for biomineralization. MC3T3 preosteoblast cells differentiation and subsequent biomineralization, revealed by ALP enzymatic activities over seven days of culture and in vitro studies, showed good morphology and cell adhesion | Jahan et al., 2018 [108] |
| Cellulose nanocrystals (CNC) on CS–Alg-HA scaffolds | Bone tissue engineering | Composite scaffold for bone tissue engineering | Scaffolds containing CNC exhibited an enhancement in swelling, porosity, and compression strength. The results also evoked that the scaffold comprising CNC has a promising cell growth and cell adherence | Shaheen et al., 2018 [118] |
| Microporous methacrylated glycol CS montmorillonite nanocomposite | Bone tissue engineering | Nanocomposite hydrogel for bone tissue engineering | The hydrogels formed an interconnected microporous network and promoted the proliferation and the attachment and induced the differentiation of encapsulated mesenchymal stem cells in vitro | Cui et al., 2019 [106] |
| Composite scaffolds of Cu–CS derivative and a Sr-substituted HA | Bone tissue engineering | Material platforms containing therapeutic ions for bone tissue engineering | While Sr is uniformly released to assist the regeneration process for an extensive time, Cu has an explosive release from CS, enable possible bacterial colonization to be quickly stopped | Gritsch et al., 2019 [107] |
| Genipin-crosslinked and fucoidan (FD)-adsorbed nano-hydroxyapatite/hydroxypropyl chitosan | Bone tissue engineering | Composite scaffolds for bone tissue engineering | The scaffolds showed an open structure with interconnected pores and a rough morphology. The incorporation of n-HA and adsorption of FD increased ALP activity in 7F2 osteoblast cells and promoted their mineralization | Lu et al., 2019 [109] |
| CS/carboxymethyl cellulose reinforced with multiphasic calcium phosphate | Bone tissue engineering | Whisker-like fibers for bone tissue engineering | The composite scaffolds exhibited desirable microstructures with high porosity and interconnected pores. Scaffolds presented an improved in vitro proliferation, attachment, and mineralization of MG63 cells, and triphasic fibers were more effective in reinforcing the scaffolds and resulted in higher cell viability | Matinfar et al., 2019 [110] |
| Novel freeze-dried silanated CS scaffold reinforced with HA nanofiber | Bone tissue engineering | Novel freeze-dried scaffold for bone tissue engineering | CGH25 scaffold (15% of GPTMS and 25% of n-Has) presented better mechanical properties and exhibited a uniform distribution of n-HAs with an appropriate porous microstructure, porosity percentage, and water uptake. It was also observed a proper biomineralization behavior after 14 days of soaking in SBF solution and the viability of MG-63 osteoblastic cells improved concerning chitosan scaffold for up to 72 h of cell culture | Nezafati et al., 2019 [111] |
| Poly (3-hydroxybutyrate)- CS/alumina nanowires | Bone tissue engineering | Composite scaffold for bone tissue engineering applications | A formation of calcium phosphate sediments on the surface of alumina containing scaffolds after 7 and 28 days of immersion in SBF was observed. Proliferation and viability of MG-63 cells and alkaline phosphatase secretion are significantly higher on scaffolds containing alumina than that of the PHB or PHB-CS | Toloue et al., 2019 [119] |
| CS-based hydrogel | Bone tissue engineering | Injectable hydrogel for bone tissue engineering | It was observed on the surface and in the hydrogel’s cross-section, an interconnected porous structure suitable for transporting extracellular fluid, nutrients, and hormones to cells and waste removal. Moreover, the SaOS-2 cells adhered and proliferated well on the hydrogel | Saekhor et al., 2019 [120] |
| Electrospun poly(hydroxybutyrate)/chitosan fibrous scaffolds | Cartilage tissue engineering | Composite scaffold for cartilage tissue engineering | The results evidenced an increase in the hydrophilicity and the mass loss percentage, and the scaffolds’ rate with CS. They maintained the mechanical properties in an appropriate range for the cartilage tissue engineering application. Additionally, in PHB/CS scaffolds, the chondrocytes attached well, spread, and slightly penetrated the polymer matrix of the fibers | Sadeghi et al., 2016 [152] |
| Chitosan/Poly(vinyl alcohol) (PVA)/graphene oxide (GO) composite nanofibers | Cartilage tissue engineering | Composite nanofibers for cartilage tissue engineering | The addition of GO increased the mechanical properties of the nanofibers. A cell proliferation assay was performed after day 14, and the results showed that the chitosan/PVA/GO (6% by weight) composition provides the most suitable environment for the growth of ATDC5 cells | Cao et al., 2017 [153] |
| A thermoresponsive chitosan-g-poly(N-isopropylacrylamide) (CS-g-PNIPAAm) copolymer | Cartilage tissue engineering | Thermoresponsive hydrogels for cartilage tissue engineering applications | The microengineering constructions mimicked the shape and organization of the superficial zone of cartilage. After 28 days of incubation in a chondrogenic medium, the mesenchymal stem cells (MSCs) encapsulated in the synthesized hydrogel showed a 6 and 7 fold increase in the secretion of glycosaminoglycans (GAGs) and total collagen. | Mellati et al., 2017 [126] |
| Hydroxybutyl CS (HBC)/oxidized chondroitin sulfate (OCS) hydrogels | Cartilage tissue engineering | Hydrogels fabricated by 3D bioprinting technique for cartilage tissue engineering | Human adipose-derived mesenchymal stem cells could be 3D cultured in HBC/OCS hydrogel, maintaining good viability. Moreover, the hydrogels were found to trigger the least amount of pro-inflammatory gene expression of macrophage and to inhibit acute immune responses in 7 days | Li et al., 2018 [149] |
| Photocrosslinkable maleilated chitosan (MCS)/ methacrylated silk fibroin (MSF) micro/nanocomposite hydrogels | Cartilage tissue engineering | Micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering | The hydrogel exhibited the compressive modulus of 0.32 ± 0.07 MPa when the MSF content was 0.1%, a value in the articular cartilage compressive modulus range. The results of in vitro cytotoxic evaluation and cell culture evidenced the biocompatibility of the hydrogels with TGF-β1 to mouse articular chondrocytes and the capacity to support cells attachment well | Zhou et al., 2018 [150] |
| Graphene oxide (GO)-CS scaffolds | Cartilage tissue engineering | Composite scaffolds for cartilage tissue engineering | As GO concentration increases, it was observed an improvement in the physical and mechanical properties and an augmentation in the proliferation of human articular chondrocytes in the nanocomposite scaffold, especially in extended cultivation periods (14 days). Additionally, it was observed a more spherical morphology of the human articular chondrocyte on the cross-linked scaffolds for 21 days of culture in vitro | Shamekhi et al., 2019 [154] |
| Thiolated chitosan (CS-NAC) and silk fibroin (SF) hydrogels | Cartilage tissue engineering | Dual network hydrogels for cartilage tissue engineering | Examinations of dry CS-NAC/SF gels revealed a highly porous structure with well-interconnected pores. Cell culture demonstrated that CS-NAC/SF gels supported the growth of chondrocytes while effectively maintaining their phenotype | Liu et al., 2020 [151] |
| Silk Fibroin-Gelatin-CS composite scaffolds | Tissue engineering applications | Composite scaffold for tissue engineering applications | The addition of silk fibroin to the composites increased the mechanical strength and degradation rate. The composite scaffolds improved endothelial cell attachment and growth compare to silk fibroin alone | Asadpour et al., 2019 [215] |
| Agarose/CS/GO (ACGO) composite | Tissue engineering applications | Composite scaffold for tissue engineering applications | The ACGO composite scaffolds exhibited the well -defined interconnected pores with rough surface morphology and showed adequate hemocompatibility and Vero cell proliferation ability. Additionally, the GO present in the composite scaffolds provided a favorable environment for cell attachment and proliferation | Sivashankari and Prabaharan 2019 [216] |
| Cs/gelatin/hyaluronic acid (HA)/heparan sulfate (HS) composite scaffolds | Engineering of neural tissue | Composite scaffold for neural tissue engineering | The presence of HA and HS in the scaffolds promoted a relevant initial NS/PCs adhesion and supported the long-term growth in a 3D environment. Additionally, NS/PCs in the Cs/Gel/HA/HS scaffold maintained multilinear differentiation potentials with increased neuronal differentiation | Guan et al., 2013 [169] |
| Cell-Seeded Chitosan Films | Engineering of neural tissue | Films for peripheral nerve tissue engineering | Results demonstrate an excellent cytocompatibility of the chitosan substrate, which allowed neurites to grow from dissociated sensory neurons, what is additionally supported in films preseeded with rat Schwann cells (SCs)- rat bone-marrow-derived mesenchymal stromal cells (BMSC) co-cultures | Wrobel et al., 2014 [157] |
| Chitosan/gelatin-based scaffolds | Engineering of neural tissue | Conductive scaffold for nerve tissue engineering | An increase in the scaffolds’ electrical and mechanical properties was observed with the incorporation of polyaniline/ graphene (PAG) NPs, and a decrease in the porosity and the water retention capacity. Additionally, conductive scaffolds exhibited a relatively slower degradation, and among the PAG- incorporated scaffolds, the largest number of attached Schwann cells was observed in the scaffolds with 2.5 wt.% PAG (C2G6-PAG2.5). | Baniasadi et al., 2015 [177] |
| Polycaprolactone (PCL)/chitosan (CS) scaffold | Engineering of neural tissue | Composite scaffolds for nerve tissue engineering | The adherence of Schwann cells (SC) to the scaffolds was proven, and the scaffold containing 5 wt.% of CS exhibited a more significant proliferation on days 1 and 3. Additionally, the scaffolds proved to be non-cytotoxic, and when compared to pure PCL or CS scaffolds, the CS/PCL scaffolds showed better cell distribution, and the cells exhibited an SC phenotype | Bolaina-Lorenzo et al., 2016 [179] |
| CS–aniline oligomer and agarose hydrogel | Engineering of neural tissue | Self-gelling electroactive hydrogel for neural tissue engineering with on-demand drug release | It was observed that the hydrogels’ conductivity was −10−4 S/cm, which can mimic the conductivity of the tissue. The aniline oligomer prolonged the release rate of the drug and rendered electroactivity to the hydrogel, increasing cellular activities, such as proliferation. Additionally, the hydrophobic nature of the aniline oligomer repels water and biological fluids, thus decreasing the swelling rate and the degradation rate. | Bagheri et al., 2019 [178] |
| Poly (ε-caprolactone) (PCL)/CS/PolyPyrrole (PPy) nanofibrous scaffold | Engineering of neural tissue | Composite scaffold for neural tissue engineering | Chitosan promoted a considerable increase in the hydrophilicity of the scaffold and a decrease in the fiber diameter. The combination of the nanofibrous structure with a greater hydrophilicity favors neural growth, cell attachment, and proliferation. In-vitro studies using PC12 cells revealed that the PCL/CS/ PPy scaffold supports cell attachment, spreading and revealed a significant increase in proliferation up to 356% in comparison to Pure PCL and neurite extension of PC12 | Sadeghi et al., 2019 [180] |
| Alg/CS hydrogel containing olfactory ectomesenchymal stem cells (OE-MSCs) | Engineering of neural tissue | Composite hydrogel for sciatic nerve tissue engineering | Results confirmed that the hydrogel could provide a suitable substrate for cell survival. Additionally, Alg/CS hydrogel with OE-MSCs applied to the sciatic nerve defect enhances regeneration compared to the control group and hydrogel without cells. | Salehi et al., 2019 [181] |
| System | Drug Loaded | Relevant Results | Reference |
|---|---|---|---|
| CS NPs | Tacrine | The particles showed adequate drug-loading capacity and in vitro release studies showed an initial burst release, followed by a continuous and slow release of the drug. Additionally, polysorbate 80 coating of NPs slightly reduced the drug diffusion-controlled release, and followed the Fickian mechanism | Wilson et al., 2010 [259] |
| CS-cyclodextrin, carboxymethyl-b-cyclodextrin (CM-b-CD) hybrid nanocarrier | Sulindac | The formation of NPs and encapsulation of sulindac was confirmed through DSC and FTIR analysis. Release profiles indicate a continuous release of the drug throughout 24 h. However, the rate of release was faster during the first hours, being about 55–90% of the drug released after 3 h | Ammar et al., 2011 [260] |
| CS–Alg Polyelectrolyte Complex NPs | Amoxicillin | The release mechanism of Amoxicillin from the CS-ALG PEC NPS was a combination of both diffusion and erosion. The in vitro drug release studies for 6 hrs revealed the PEC system’s gastroprotective nature and diffusion through the swollen polymeric complex as the main drug release mechanism (Higuchi model), which may significantly reduce the chances of incomplete eradication and systemic side effects. | Arora et al., 2011 [261] |
| CS/NaHCO3 system | Dipyridamole (DP) | The release profiles exhibited a fast release rate on the first day, then a gently stable release in 16 days, followed by an accelerating linear release over 30 days. For the gelling systems of three formulations in this study, the chitosan hydrogel from one with moderate NaHCO3 concentration at 0.10 mol/L had the lowest DP release rate, which was mainly attributed to its stronger gel structure | Liu et al., 2011 [262] |
| CS Oligosaccharides (COS)-coated NLC (nanostructured lipid carrier) | Flurbiprofen | The particle was coated with the COS polymer surrounding the surface uniformly, which positively charged NLC dispersions, providing a longer retention time by interacting with the negative mucous. It is also noticed the superior mucoadhesive properties of COS-coated NLCs, beneficial to the ocular drug delivery system. | Luo et al., 2011 [263] |
| CS–carboxymethyl starch (CMS) NPs | 5-aminosalicylic acid (5-ASA) | An initial burst release was observed, which can be attributed to the desorption of loosely attached 5-ASA from the matrix polymers’ surface. The greatest release for 5-ASA-loaded CS–CMS NPs occurred in the release media of high ionic strength, and a significantly small portion of 5-ASA was released in water. Additionally, the amount of drug released from NPs decreased on increasing cross-linking time | Saboktakin et al., 2011 [264] |
| CS enclosed mesoporous silica NPs (MSN) | Ibuprofen (IBU) | In vitro release properties of samples in a pH range from pH 7.4 to 6.8 was observed, and the release of IBU showed sensitivity to the release medium pH value. The great amount of IBU released was achieved in pH 6.8, pH value around some tumor cells and inflammatory tissues | Chen et al., 2012 [265] |
| Low molecular weight (LMW) CS NPs | Erythrocytes | LMW CS NPs showed good compatibility with erythrocytes and can be easily attached to the erythrocyte membrane’s surface, suggesting a potential vascular drug delivery system. The composite drug delivery system contains no harmful components and is entirely biodegradable. | Fan et al., 2012 [266] |
| CS-coated mesoporous silica nanospheres | Ibuprofen (IBU) | A pH-responsive release of IBU has been achieved by varying the shell structure of positively charged CS in the designed pH 4.0–7.4 solution. Under basic conditions, CS forms a gel-like structure that is insoluble and prevents IBU release at pH 7.4. When the pH is below its isoelectric point (6.3), the drug has been released due to protonation of the amino group on CS | Popat et al., 2012 [267] |
| Ellagic acid encapsulated CS NPs (EA@CS-NP) | Ellagic acid (EA) | The drug-encapsulation and loading-efficiency of the NPs were 94 ± 1.03% and 33 ± 2.15%, respectively. The drug is entirely released in a phosphate buffer medium by 48 h at room temperature, indicating a sustained drug release pattern of EA from EA@CS-NP. | Arulmozhi et al., 2013 [268] |
| Novel CS-Functionalized Spherical Nanosilica Matrix (CTS-SNM) | Carvedilol (CAR) | The release manner showed a pH dependency, and after the burst release of drug adsorbed on the CTS-SNM surface or mixed with the CS shell, the release pattern of CAR from the CAR-CTS-SNM pores took place in pulses with a rise in pH all along the gastrointestinal tract, regulated by the swelling effect of CS under acidic environment and the shrinking effect in a relatively alkaline environment | Sun et al., 2013 [269] |
| CS/sodium tripolyphosphate (TPP)-hyaluronic acid-based NPs | Ceftazidime (CFT) | In vitro release and permeation studies showed a prolonged drug release profile from the CS/TPP-hyaluronic acid NPs gel formulation. Furthermore, the gel formulations were not cytotoxic on ARPE-19 and HEK293T cell lines. The formulation was stable, and the drug activity, as shown by microbiological studies | Silva et al., 2017 [270] |
| CS/montmorillonite films | Ibuprofen (IBU) | Homogeneous systems with good drug dispersion were obtained, and a slower and more controlled release rate of the drug was observed, indicating that the development of this bio-nanocomposite is promising for DDS | Tavares et al., 2017 [235] |
| CS/hyaluronic acid hydrogel | Vancomycin | Vancomycin-loaded hydrogels were more reliable and elastic, and the gelation time was shortened. Additionally, the delivery of vancomycin was sustainable for 7 d, reducing the abuse of antibiotics, and the releasing rate was dependent on pH levels | Huang et al., 2018 [271] |
| Alg/CS hydrogel embedded alginate microspheres | Bull Serum Albumin (BSA) | The controlled release of BSA encapsulated within composite hydrogels showed a significantly lower rate when compared with control hydrogel or microspheres alone, what indicates that this hydrogel/microsphere composite system shows excellent potential for a variety of controlled drug delivery applications, especially in protein delivery | Xing et al., 2019 [272] |
| Material | Application | Relevant Results | Reference |
|---|---|---|---|
| CS/β-glycerophosphate (β-GP) thermo-sensitive gel | Delivery of ellagic acid (EA) for brain cancer treatment | In the anti-tumor activity study, the results showed that the CS/β-GP gel loaded with EA could inhibit cancer cells’ growth in an EA concentration-dependent manner. Additionally, the CS gels containing 1% (w/v) of EA significantly reduced viability of U87 7 human glioblastoma cells and C6 rat glioma cell line compared with the CS gels without the presence of EA at 3 days incubation | Kim et al., 2010 [414] |
| CS/GO composite loaded with anticancer drug camptothecin (CPT) | Treatment of HepG2 and HeLa cancer cells | The results demonstrated a higher load capacity of GO-CS for CPT and a considerably elevated cytotoxicity in the HepG2 and HeLa cell lines exhibited by the GO-CS-CPT complexes when correlated to the pure drug. GO-CS is additionally capable of condensing plasmid DNA into stable nanosized complexes, and the resultant GO-CS/pDNA NPs demonstrate moderate transfection efficiency in HeLa cells in specific proportions of nitrogen/phosphate | Bao et al., 2011 [358] |
| CS–Alg/Cloisite 30B nanocomposites | Delivery of Curcumin for cancer treatment | The drug was released in a controlled manner, with a much more pronounced release in the basic medium than the acidic medium, and this controlled delivery device presents the advantage of using biodegradable polymers, what implies in no need for surgical removal | Malesu et al., 2011 [415] |
| CS-functionalized graphene oxides (FGOCs) | Investigate the release behavior of 5-fluorouracil and ibuprofen | IBU and 5-FU were loaded successfully on FGOCs sheets, despite lower numbers of π electrons, and it was observed a controlled release behavior and long- term biocompatibility of FGOCs graphene sheets. Additionally, 5-FU loaded graphene sheets (FGOCs/5-FU) induced significant MCF-7 cancer cell death and presented reduced toxicity to non-targeted cells. | Rana et al., 2011 [357] |
| CS nanocarrier (NC)-based delivery of Ag NPs | Cytotoxic efficiency of the Ag-CS NC system in human colon cancer cells (HT 29) to cancer therapy | The 50% decrease in HT 29 cells’ viability is achieved for an Ag NPs concentration of 0.33 μg mL−1. Ag-CS NCs treatment increased the production of intracellular ROS, indicating that the increase in apoptosis induction in HT 29 cells can be obtained by oxidative stress, moreover to the classic caspase signaling pathway | Sanpui et al., 2011 [326] |
| CS nanofibers including Fe3O4 and Fe2+ magnetic NPs | Hyperthermia treatment of tumor cells | This material proved to be non-cytotoxic according to in vitro cell incubation experiments, and by applying a magnetic field, they would be able to enhance the temperature of the solution to 45 °C and effectively reduce the rate of proliferation/growth of tumor cells | Lin et al., 2012 [391] |
| Stearic acid–grafted chitosan (CS-SA) | Carrier for a brain-targeting drug delivery system. | DOX was entrapped into CS-SA micelles with a high drug-loading capacity and exhibited slow drug-release behavior, with a cumulative release up to 72% within 48 h in vitro. CS-SA/DOX micelles showed a therapeutic effect on C6 in vitro, and a relatively high amount of DOX was found in the brain, while a lower amount of drug was accumulated in the heart | Xie et al., 2012 [416] |
| Ellagic acid encapsulated CS NPs (EA@CS-NP) | DDS in a human oral cancer cell line (KB) | EA@CS-NP exhibit significant cytotoxicity in KB cells in a dose-dependent manner with a very low IC50 value compared to the free EA. In vitro anticancer studies showed a significant antiproliferative effect on the human oral cancer KB cell line, suggesting that CS acted as a promising cancer drug carrier that enhanced the anticancer property of the polyphenolic compound EA. | Arulmozhi et al., 2013 [268] |
| Au/CS/TiO2-Gr composite | An amperometric immunobiosensor for the detection of α-fetoprotein | This immunobiosensor was considered a new tactic for AFP detecting with great sensitivity, bioactivity, and selectivity with suitable storage stability | Huang et al., 2013 [316] |
| CS NPs | Tamoxifen (Tam) carriers for effective anti-tumor activity in breast cancer cells | Tam-loaded CS NPs induced remarkable improvement in anticancer activity, as demonstrated by MTT-assay, AO/EtBr, and Hoechst nuclear staining. In human breast cancer MCF-7 cells, it was demonstrated that Tam-loaded CS NPs increases the intracellular concentration of Tam and enhance its anticancer efficiency by inducing apoptosis in a caspase-dependent manner | Vivek et al., 2013 [417] |
| CS-modified PLGA NPs | Surface for Improved Drug Delivery of 5-fluorouracil (5-FU) | After introducing chitosan, the zeta potential of the PLGA nanoparticle surface changed from negative charge to positive one, making the drug carriers more affinity to cancer cells. The positive charge and hydrophilic property induced a moderate and prolonged drug release and a high cumulative drug release. CS-modified PLGA NPs showed the versatility of surface and a possible improvement in the efficacy of current PLGA-based DDS | Wang et al., 2013 [418] |
| Hydroxybutyl chitosan (HBC) | Thermo-sensitive hydrogel to deliver Doxorubicine hydrochloride (DOX-HCl) for cancer treatment | The in vitro study showed that the HBC solution had low cytotoxicity. The DOX-HCl (1 mg/mL) loaded HBC gels displayed slow release rates independent of the HBC concentration and significantly reduced viability of 4T-1 cells compared with the HBC gels after 1-day incubation. The DOX_HCl loaded HBC gel inhibited rat breast cancer cell growth in a DOX_HCl concentration-dependent manner. | Wang et al., 2013 [419] |
| Thiolated CS (TCS) NPs loaded with curcumin/5-FU | Colon cancer treatment | 5-FU-TCS-NPs and CRC-TCS-NPs systems showed an initial burst release at pH 4.5 and 7.4, followed by a sustained pattern. The in vitro studies proved the enhanced anticancer effects (2.5 to 3 fold) of combined treatment compared to the individual and control bare drugs. The in vivo studies proved the improved plasma half-life of nanoentrapped 5-FU and the CRC up to 72 h | Anitha et al., 2014 [420] |
| CS coated magnetic nanoparticles (CS MNPs) | Target of DOX to the tumor site under a magnetic field | Fluorescence microscopy images have revealed that DOX loaded CS MNPs were taken up by the cells and accumulated around the nucleus so that DOX is successfully delivered near to the nucleus, where the drug shows its anti-cancer activity. Additionally, DOX loaded CS MNPs were more toxic on Doxorubicin resistant MCF-7 cells than the free drug. | Unsoy et al., 2014 [421] |
| CS NPs | Oxaliplatin drug delivery system to breast cancer cell therapy | A faster release from the DDS was observed at pH 4.5 than at pH 7.4, a desirable characteristic for tumor-targeted drug delivery. It was found that expression of Bax, Bik, cytochrome C, caspase-9, and -3 was significantly up-regulated while the Bcl-2 and Survivin were inhibited in breast cancer MCF-7 cells. These cells cultured with NPs for 48 h became apoptotic in a caspase-dependent manner indicate that CS NPs could act as an efficient DDS importing Oxalipaltin to target cancer cells | Vivek et al., 2014 [422] |
| Cyclic RGD-modified CS/graphene oxide (GO) | Drug delivery system of Doxorubicin (DOX) for hepatocellular carcinoma-targeted therapy and imaging | Cellular uptake and proliferation studies using hepatoma cells (Bel-7402, SMMC-7721, HepG2) indicated that the cRGD-modified CS/GO could recognize hepatoma cells and promote drug uptake by the cells. The system exhibits a pH-responsive behavior due to the hydrogen bonding interaction between GO and RC, with a slow-release at a lower pH (tumor environment). A higher pH leads to a weaker hydrophobic interaction and hydrogen bonding, resulting in a faster release rate. | Wang et al., 2014 [423] |
| Nanofibrous scaffolds of PEO/CS/GO | Controlled release of DOX for lung cancer treatment | The π-π stacking interaction among DOX and GO with thin pores of nanofiber scaffolds demonstrated a more significant drug load (98%) and controlled release of DOX. Instability can be observed under acidic conditions of the hydrogen bonding interaction between GO and DOX, resulting in an accelerated drug release at pH 5.3 | Ardeshirzadeh et al., 2015 [356] |
| CS-decorated DOX-encapsulated NP | Target and elimination of tumor reinitiating cancer stem-like cells | CS can eliminate tumor-initiating cancer stem cells by a DOX delivery to the tumor microenvironment. It was observed a 6 times increase in the cytotoxicity of the DOX when compared to the use of free DOX for eliminating CD44+ cancer stem-like cells residing in 3D mammary tumor spheroids and a reduction in the size of the tumor in an orthotopic xenograft tumor model with no evident systemic toxicity | Rao et al., 2015 [278] |
| Nanocomposites of CS/Ag and CS/Ag/MWCNT encapsulated with 5-FU | Study the release profile and cytotoxicity of this system towards an MCF-7 cell line | It was observed the encapsulation of 5-FU and sustained and prolonged release of this drug in the case of the CS/Ag/MWCNT nanocomposite, and more significant cytotoxicity with an IC50 of 50 μg/mL when related to the CS/Ag system | Nivethaa et al., 2016 [333] |
| Graphene oxide (GO)-carboxymethyl chitosan (CMC)-fluorescein isothiocyanate- lactobionic acid (LA) | Targeted anticancer drug delivery systems with DOX | It was observed a high drug loading content and efficiency (>96%) and pH-sensitive release, being faster at the mildly acidic pH typical of the tumor microenvironment and slow and sustained manner at the normal physiological pH. In vitro tests on a liver cancer cell line demonstrated that DOX-loaded systems effectively induce cell death, being almost as potent as the free drug. Further, the DOX-loaded LA conjugated GO system was able to selectively induce the death of cancerous cells and also was non-toxic to a non-cancerous cell line | Pan et al., 2016 [424] |
| CS/PVA | Potential use in the controlled release of cisplatin | The amount of Cisplatin incorporated in the chitosan/PVA beads was 5mg per mg of beads. Authors concluded that the chitosan/PVA system presented a vehicle behavior for drug release of cisplatin | Souza et al., 2016 [281] |
| CS-folate (FA) conjugated MWCNT | Efficient and safe platforms in the delivery of docetaxel (DTX) for lung cancer therapy | This material showed an up to 79% efficiency of encapsulation of the drug, and a sustained release of drugs was revealed. Compared to that of DocelTM, after 24 h of incubation with A549 cells, DTX-CS-FA-CNAC achieved a reduction of up to 89 times in the IC50 value. Additionally, DTXCHI-FA-CNAC evidenced increased cytotoxicity and low toxicity when correlated to DTX-CNAC, DTX-CS-CNAC, and DocelTM | Singh et al., 2017 [342] |
| CS-coated GCNCs | Photothermal cancer therapy (PTT) | The phototoxicity of GCNCs/CS further damages the tumors after loading with 5-FU. Tumors are no longer detected after 6 days of 5FU-GCNCs/CS treatment under irradiation, promoting efficient death of nasopharyngeal carcinoma cells and inhibiting tumor growth | Li et al., 2018 [406] |
| CS | Enhancement of the anti-tumor activity of natural killer (NK) cells by activating dendritic cells (DC) | They found that DCs are directly activated by CS, what improve the effector functions of human NK cells, promoted the survival of these cells, and enhanced their cytotoxicity averse to leukemia cells, implying in a better antitumor activity in vivo | Li et al., 2018 [11] |
| CS-grafted-dihydrocaffeic acid and oxidized pullulan hydrogels | pH-responsive injectable hydrogels for localized delivery of Doxorubicin (DOX) | The hydrogels showed good DOX release, effectively killing colon tumor cells (HCT116 cells) and good antibacterial properties against E. coli and S. aureus in vitro when the antibacterial model drug amoxicillin was encapsulated in the hydrogels, suggesting that this material is a candidate for the development of colon cancer drug delivery carriers | Liang et al., 2018 [425] |
| PLA/CS/TiO2/DOX/GO | Controlled release of DOX for lung cancer treatment | The results indicated a lower DOX release rate due to the improvement in PLA/CS content, TiO2, and GO/TiO2/DOX ratio. With the increment of the DOX ratio, there was an increase in the multiplication curbing effect on cancer cell targeting. The use of this nanofiber and an external magnetic field implied in increased killing of A549 cancer cell | Samadi et al., 2018 [413] |
| CS/polyacrylic acid/Fe3O4 magnetic nanocomposite hydrogel | Potential anticancer drug delivery system of 5-fluorouracil (5-FU) | The drug release tests showed that the CS/PAA/Fe3O4 enhanced the stability of drug dosing for a long time with controlled releases in the colon and rectal conditions, indicating a potential use of this material in DDS as a colon and rectal administration of 5-FU | Amini-Fazl et al., 2019 [426] |
| CS–Alg hydrogel encapsulated with magnetic gelatin microspheres (MGM) | Injectable hydrogel scaffolds for anti-cancer drug delivery of 5-fluorouracil (5-FU) | With a 30 mg/mL concentration of MGMs, the composite hydrogel provided a suitable performance and showed excellent self-healing ability under physiological conditions. Moreover, this composite hydrogel showed the sustained in vitro 5-FU release compared with control MGMs and carboxyethyl chitosan (CEC)-oxidized alginate (OAlg) hydrogel. So, this magnetic gel scaffold can render the formulation of a therapeutically effective platform for cancer treatment | Chen et al., 2019 [427] |
| CMCS-based nanoparticles (AM NPs) with N-(3-Aminopropyl)-imidazole (AM NPs) and perfluorobutyric anhydride (fluorinated nanoparticles -FM NPs) | pH-sensitive NPs for the delivery of Doxorubicin (DOX) | The concentration of DOX in blood for AM or FM NPs formulations was higher than that of free DOX, and it is also observed a decline of drug level in plasma with a prolonged time. In vivo DOX distribution and antitumor experiment further verified that surface-fluorinated NPs could deliver more DOX into tumor area than non-modified NPs, leading to the best tumor suppression. CMCS-based nano-carriers could prolong the blood circulation time of DOX and improve the bio-availability of the drug in vivo | Cheng et al., 2019 [428] |
| Norcantharidin-conjugated carboxymethyl chitosan (CMCS-NCTD) | Biopolymer carrier for a useful anti-tumor compound with severe nephrotoxicity | CMCS-NCTD could significantly inhibit tumor cells’ migration and displayed excellent anti-metastasis effects both in vitro and in vivo in a dose-dependent manner (P < 0.05). The enhanced antitumor effects of CMCS-NCTD were confirmed by inhibiting the growth of solid tumors and extending the survival time of tumor-bearing mice. CMCS-NCTD could inhibit tumor angiogenesis and reduce degradation of the extracellular matrix by regulating the expressions of VEGF, MMP-9, and TIMP-1 | Chi et al., 2019 [429] |
| CS-GCNCs loaded with 5-FU | Photothermal cancer therapy (PTT) | After coating with CS, there was a significant reduction in the cytotoxicity of GCNCs, cell cycle defects, and in the 5-FU release rate of CS-GCNCs, implying in a sustained release, which could be up by 808 nm laser and microwave co-irradiation. Additionally, it was observed an enhance in the level of caspase-3 expression due to the high retention in cancer cell killing bioactivity by the 5-FU in CS-GCNCs, which implies in a higher apoptosis-related cysteine peptidase | Guo et al., 2019 [407] |
| Light-cured glycol chitosan (GC) hydrogel containing paclitaxel (PTX)-complexed beta-cyclodextrin (β-CD) (GC/CD/PTX) | Ovarian cancer (OC) therapy | The tumor volumes in the control and free PTX-treated samples increased gradually, whereas GC/PTX- and GC/CD/PTX-treated samples had a gradual decrease for 7 days, resulting in overall necrosis tumor tissue. A single local administration of GC/PTX showed a superior antitumor effect in the OC mouse model than that of systemic administration of free PTX solution via intravenous injection | Hyun et al., 2019 [430] |
| Methyl methacrylate (MMA) modified chitosan (CS) conjugate (CSMMA) | Potential gene and drug delivery agent of Curcumin | In vitro drug release study of curcumin loaded CSMMA nanoparticles demonstrated an initial burst release due to the drug entrapped near the surface of NPs, followed by a controlled release of curcumin. Additionally, pH 5.0 was more favorable than the physiological pH for the drug release. CSMMA proved to be a reasonably good gene delivery agent based on transfection efficiency studies in mammalian cancer cell lines (A549, HeLa, and HepG2) | Jaiswal et al., 2019 [431] |
| CS/PLA/5-FU/g-C3N4-DOX/g-C3N4-PTX triaxial nanofibers | Simultaneous controlled release of 5- fluorouracil (5-FU), Doxorubicin (DOX), and Paclitaxel (PTX) for breast cancer treatment | The maximum MCF-7 breast cancer cells killing was found to be 94.2% using tri-layer nanofibers containing intermediate and shell flow rates of 1 mL/h and 0.5 mL/h, respectively after 14 days incubation time | Jouybari et al., 2019 [432] |
| Hybrid lipid-CS NPs loaded with cisplatin | A platform for the controlled release of cisplatin in cancer therapy | It was confirmed the cytotoxic effect on the A2780 ovarian cancer cell line over 48 h and an increase in cellular uptake of LPHNPs. Controlled release behavior of cisplatin with a longer average residence time and half-life was evidenced, and toxicity studies in rats supplied a safety profile of LPHNPs | Khan et al., 2019 [283] |
| CS nanogels | Nanocarriers of polyoxometalates (POM) for breast cancer therapies | The nanocomposites have demonstrated a pH-responsive release capability of POMs, which lets the delivery of polyanions at acidic pH. So, CS nanocarriers would present significant potential for POM delivery into tumoral cells due to their pH-triggered deliverability that inhibits cytotoxic drug release at physiological pH | Pérez-Álvarez et al., 2019 [433] |
| PEO-CS nanofibers containing carboxymethyl-hexanoyl CS/ dodecyl sulfate NPs loaded with pyrazoline H3TM04 | Skin cancer treatment | When utilizing PEO-CS nanofibers as carriers, it was observed an increased transport rate of H3TM04 through the epidermis. In in vitro cytotoxicity assays, the incorporation of H3TM04 into the nanocarriers increased its cytotoxic effect toward B16F10 melanoma cells | Rengifo et al., 2019 [434] |
| Fe3O4 @chitosan/Tragacanth Gum nanocomposite | Nanocomposite for Curcumin delivery | The release behavior of curcumin was studied in two different pH 7.4 and 3.4 and 37 °C and 40 °C. The nanocomposite showed a higher swelling ratio at pH 3.4 and temperature 40 °C with suitable pH and thermosensitive in vitro drug release profiles, indicating a good potential for delivery of chemotherapeutic drugs | Shafiee et al., 2019 [435] |
| Bio-nanocomposite beads of ZnO/CMC/CS | Environmentally friendly intelligent vehicles for colon-specific drug delivery | This nanocomposite was effectively loaded with 5-FU and showed pH-sensitivity, biodegradation capacity, and self-sustaining release behavior, relying on the content of CMC, CS, and ZnO nanoparticles | Sun et al., 2019 [299] |
| Ellagic acid (EA) encapsulated in CS and coated by eudragit S100 (ES100) microparticles | Improve the target and cytotoxic effect of EA on colon cancer cells | Results indicated that the generated material exhibits improved colon targeting and enhanced cytotoxic and proapoptotic activity against HCT 116 colon cancer when compared with the administration of raw EA | Alhakamy et al., 2020 [280] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa Victor, R.; Marcelo da Cunha Santos, A.; Viana de Sousa, B.; de Araújo Neves, G.; Navarro de Lima Santana, L.; Rodrigues Menezes, R. A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials 2020, 13, 4995. https://doi.org/10.3390/ma13214995
de Sousa Victor R, Marcelo da Cunha Santos A, Viana de Sousa B, de Araújo Neves G, Navarro de Lima Santana L, Rodrigues Menezes R. A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials. 2020; 13(21):4995. https://doi.org/10.3390/ma13214995
Chicago/Turabian Stylede Sousa Victor, Rayssa, Adillys Marcelo da Cunha Santos, Bianca Viana de Sousa, Gelmires de Araújo Neves, Lisiane Navarro de Lima Santana, and Romualdo Rodrigues Menezes. 2020. "A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment" Materials 13, no. 21: 4995. https://doi.org/10.3390/ma13214995
APA Stylede Sousa Victor, R., Marcelo da Cunha Santos, A., Viana de Sousa, B., de Araújo Neves, G., Navarro de Lima Santana, L., & Rodrigues Menezes, R. (2020). A Review on Chitosan’s Uses as Biomaterial: Tissue Engineering, Drug Delivery Systems and Cancer Treatment. Materials, 13(21), 4995. https://doi.org/10.3390/ma13214995