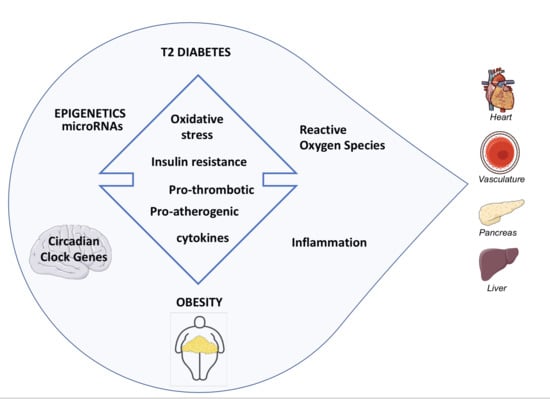
Prevention of Diabetes and Cardiovascular Disease in Obesity
Abstract
1. Introduction
2. Pathogenesis of Diabetes in Obesity
2.1. Non-Esterified Fatty Acids (NEFAs) and Nitric Oxide (NO)
2.2. Clock Genes
2.3. Insulin Resistance (IR) and Reactive Oxygen Species (ROS)
2.4. Insulin Resistance (IR) and Inflammation
- the activation of transcription factor NF-κB, which is involved in insulin-sensitivity, and Inhibitor of Nuclear Factor Kappa B Kinase Subunit β (IKKβ), and
- by directly inhibiting IRS-1 with phosphorylation on its serine residues [41].
3. Progression of IGT to T2D in Obesity: From Epigenetics to the Role of miRNAs
3.1. Novel miRNA-Based Approaches for the Prevention of Diabetes Using Bariatric Metabolic Surgery (BMS)
3.2. Epigenetics as a Driving Force Controlling Obesity-Related Genes Toward Diabetes
4. Obesity as a Major Risk Factor for Cardiovascular Disease
5. Available Means to Prevent the Progression of Prediabetes to Diabetes: Lifestyle, Pharmaceutical, and Other Approaches
6. Long-Term Prevention of Diabetes and Cardiovascular Disease in Obesity
7. Long-Term Prevention of Mortality in Obesity
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ARIC | Atherosclerosis Risk in Communities |
| ARNTL | Aryl Hydrocarbon Receptor Nuclear Translocator Like |
| BMI | Body Mass Index |
| BMS | Bariatric Metabolic Surgery |
| CLOCK | Circadian Locomotor Output Cycles Kaput |
| CpG | Cytosine Guanine island |
| CVD | Cardiovascular Disease |
| DPP | Diabetes Prevention Program |
| FTO | Fat Mass and Obesity-Associated Protein |
| GWA | Genome Wide Association |
| HF | Heart Failure |
| IGT | Impaired Glucose Tolerance |
| IKKβ | Inhibitor of Nuclear Factor Kappa B Kinase Subunit β |
| IL-6 | Interleukin 6 |
| IR | Insulin Resistance |
| IRS-1 | Insulin Receptor Substrate 1 |
| IRS-2 | Insulin Receptor Substrate 2 |
| KCNJ11 | KATP Channel |
| KLF14 | Krupper-Like Factor 14 |
| MC4R | Melanocortin 4 Receptor |
| MetS | Metabolic Syndrome |
| miR | MicroRNA |
| NF-κB | Nuclear Factor kappa B |
| NLRP3 | Nod-Like Receptor Family Pyrin Domain Containing 3 |
| NOD | Non-Obese Diabetic |
| T2D | Type 2 Diabetes |
| TNF-α | Tumor Necrosis Factor |
| VAT | Visceral Adipose Tissue |
| WHO | World Health Organization |
Appendix A
References
- Abarca-Gómez, L.; Abdeen, Z.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Schnurr, T.M.; Jakupović, H.; Carrasquilla, G.D.; Ängquist, L.; Grarup, N.; Sørensen, T.I.A.; Tjønneland, A.; Overvad, K.; Pedersen, O.; Hansen, T.; et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetology 2020, 63, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Pizzocri, P.; Librenti, M.C.; Vedani, P.; Marchi, M.; Cucchi, E.; Orena, C.; Paganelli, M.; Giacomelli, M.; Ferla, G.; et al. Laparoscopic adjustable gastric banding for the treatment of morbid (grade 3) obesity and its metabolic complications: A three-year study. J. Clin. Endocrinol. Metab. 2002, 87, 3555–3561. [Google Scholar] [CrossRef]
- Fuller, J.H.; Shipley, M.J.; Rose, G.; Jarrett, R.J.; Keen, H. Mortality from coronary heart disease and stroke in relation to degree of glycaemia: The Whitehall study. BMJ 1983, 287, 867–870. [Google Scholar] [CrossRef]
- Twig, G.; Afek, A.; Derazne, E.; Tzur, D.; Cukierman-Yaffe, T.; Gerstein, H.C.; Tirosh, A. Diabetes risk among overweight and obese metabolically healthy young adults. Diabetes Care 2014, 37, 2989–2995. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, L.G.; Jensen, B.W.; Ängquist, L.; Osler, M.; Sørensen, T.I.A.; Baker, J.L. Change in overweight from childhood to early adulthood and risk of Type 2 diabetes. N. Engl. J. Med. 2018, 378, 1302–1312. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef]
- Lindström, J.; Ilanne-Parikka, P.; Peltonen, M.; Aunola, S.; Eriksson, J.G.; Hemiö, K.; Hämäläinen, H.; Härkönen, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368, 1673–1679. [Google Scholar] [CrossRef]
- Hermanides, J.; Cohn, D.M.; Devries, J.H.; Kamphuisen, P.W.; Huijgen, R.; Meijers, J.C.M.; Hoekstra, J.B.L.; Buller, H.R. Venous thrombosis is associated with hyperglycemia at diagnosis: A case-control study. J. Thromb. Haemost. 2009, 7, 945–949. [Google Scholar] [CrossRef][Green Version]
- La Sala, L.; Prattichizzo, F.; Ceriello, A. The link between diabetes and atherosclerosis. Eur. J. Prev. Cardiol. 2019, 26, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Jonk, A.M.; Houben, A.J.H.M.; De Jongh, R.T.; Serné, E.H.; Schaper, N.C.; Stehouwer, C.D.A. Microvascular Dysfunction in Obesity: A potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology 2007, 22, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Avogaro, A.; De Kreutzenberg, S.V. Mechanisms of endothelial dysfunction in obesity. Clin. Chim. Acta 2005, 360, 9–26. [Google Scholar] [CrossRef]
- Stapleton, P.A.; James, M.E.; Goodwill, A.G.; Frisbee, J.C. Obesity and vascular dysfunction. Pathophysiology 2008, 15, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Price, T.B.; Perseghin, G.; Petersen, K.F.; Rothman, D.L.; Cline, G.W.; Shulman, G.I. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Investig. 1996, 97, 2859–2865. [Google Scholar] [CrossRef]
- De Nigris, V.; Pujadas, G.; La Sala, L.; Testa, R.; Genovese, S.; Ceriello, A. Short-term high glucose exposure impairs insulin signaling in endothelial cells. Cardiovasc. Diabetol. 2015, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nat. Cell Biol. 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Lamia, K.A.; Storch, K.-F.; Weitz, C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 2008, 105, 15172–15177. [Google Scholar] [CrossRef]
- McHill, A.W.; Melanson, E.L.; Higgins, J.; Connick, E.; Moehlman, T.M.; Stothard, E.R.; Wright, K.P. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc. Natl. Acad. Sci. USA 2014, 111, 17302–17307. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Yi, C.-X.; La Fleur, S.E.; Fliers, E. The hypothalamic clock and its control of glucose homeostasis. Trends Endocrinol. Metab. 2010, 21, 402–410. [Google Scholar] [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Shimba, S.; Ishii, N.; Ohta, Y.; Ohno, T.; Watabe, Y.; Hayashi, M.; Wada, T.; Aoyagi, T.; Tezuka, M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 12071–12076. [Google Scholar] [CrossRef] [PubMed]
- Rudic, R.D.; McNamara, P.; Curtis, A.-M.; Boston, R.C.; Panda, S.; HogenEsch, J.B.; Fitzgerald, G.A. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004, 2, e377. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Yamada, T.; Tsukita, S.; Takahashi, K.; Ishigaki, Y.; Oka, Y.; Katagiri, H. Obesity alters circadian expressions of molecular clock genes in the brainstem. Brain Res. 2009, 1263, 58–68. [Google Scholar] [CrossRef]
- Yang, Q.; Graham, T.E.; Mody, N.; Preitner, F.; Peroni, O.D.; Zabolotny, J.M.; Kotani, K.; Quadro, L.; Kahn, B.B. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nat. Cell Biol. 2005, 436, 356–362. [Google Scholar] [CrossRef]
- Vieira, E.; Ruano, E.G.; Figueroa, A.L.C.; Aranda, G.; Momblan, D.; Carmona, F.; Gomis, R.; Vidal, J.; Hanzu, F.A. Altered clock gene expression in obese visceral adipose tissue is associated with metabolic syndrome. PLoS ONE 2014, 9, e111678. [Google Scholar] [CrossRef]
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Kojetin, D.J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Noel, R.; et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nat. Cell Biol. 2012, 485, 62–68. [Google Scholar] [CrossRef]
- Sitaula, S.; Billon, C.; Kamenecka, T.M.; Solt, L.A.; Burris, T.P. Suppression of atherosclerosis by synthetic REV-ERB agonist. Biochem. Biophys. Res. Commun. 2015, 460, 566–571. [Google Scholar] [CrossRef]
- La Sala, L.; Cattaneo, M.; De Nigris, V.; Pujadas, G.; Testa, R.; Bonfigli, A.R.; Genovese, S.; Ceriello, A. Oscillating glucose induces microRNA-185 and impairs an efficient antioxidant response in human endothelial cells. Cardiovasc. Diabetol. 2016, 15, 71. [Google Scholar] [CrossRef]
- La Sala, L.; Mrakic-Sposta, S.; Micheloni, S.; Prattichizzo, F.; Ceriello, A. Glucose-sensing microRNA-21 disrupts ROS homeostasis and impairs antioxidant responses in cellular glucose variability. Cardiovasc. Diabetol. 2018, 17, 1–14. [Google Scholar] [CrossRef]
- La Sala, L.; Mrakic-Sposta, S.; Tagliabue, E.; Prattichizzo, F.; Micheloni, S.; Sangalli, E.; Specchia, C.; Uccellatore, A.C.; Lupini, S.; Spinetti, G.; et al. Circulating microRNA-21 is an early predictor of ROS-mediated damage in subjects with high risk of developing diabetes and in drug-naïve T2D. Cardiovasc. Diabetol. 2019, 18, 1–12. [Google Scholar] [CrossRef]
- La Sala, L.; Pujadas, G.; De Nigris, V.; Canivell, S.; Novials, A.; Genovese, S.; Ceriello, A. Oscillating glucose and constant high glucose induce endoglin expression in endothelial cells: The role of oxidative stress. Acta Diabetol. 2014, 52, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rizky, L.; Stefanovic, N.; Tate, M.; Ritchie, R.H.; Ward, K.W.; De Haan, J.B. The nuclear factor (erythroid-derived 2)-like 2 (Nrf2) activator dh404 protects against diabetes-induced endothelial dysfunction. Cardiovasc. Diabetol. 2017, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Siow, R.; Sugden, D.; Gao, L.; Cheng, X.; Mann, G. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: A role for Nrf2 in vascular protection in diabetes. Nutr. Metab. Cardiovasc. Dis. 2010, 21, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Leiter, L.A.; Ceriello, A.; Davidson, J.A.; Hanefeld, M.; Monnier, L.; Owens, D.; Tajima, N.; Tuomilehto, J. Postprandial glucose regulation: New data and new implications. Clin. Ther. 2005, 27, S42–S56. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’Ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 4, 851–863. [Google Scholar] [CrossRef]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rotellar, F.; Silva, C.; Rodríguez, A.; Salvador, J.; Gil, M.; Cienfuegos, J.A.; Frühbeck, G. Validation of endogenous control genes in human adipose tissue: Relevance to obesity and obesity-associated Type 2 diabetes mellitus. Horm. Metab. Res. 2007, 39, 495–500. [Google Scholar] [CrossRef]
- Luft, V.C.; Schmidt, M.I.; Pankow, J.S.; Couper, D.; Ballantyne, C.; Young, J.H.; Duncan, B.B. Chronic inflammation role in the obesity-diabetes association: A case-cohort study. Diabetol. Metab. Syndr. 2013, 5, 31. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Gao, Z.; Hwang, D.; Bataille, F.; Lefevre, M.; York, D.; Quon, M.J.; Ye, J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J. Biol. Chem. 2002, 277, 48115–48121. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Yuan, M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity-and diet-induced insulin resistance. Int. J. Obes. Relat. Metab. Disord. 2003, 27, S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Green, E.A.; Eynon, E.E.; Flavell, R.A. Local expression of TNFalpha in neonatal NOD mice promotes diabetes by enhancing presentation of islet antigens. Immunity 1998, 9, 733–743. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nat. Cell Biol. 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- McGeary, S.E.; Lin, K.S.; Shi, C.Y.; Pham, T.M.; Bisaria, N.; Kelley, G.M.; Bartel, D.P. The biochemical basis of microRNA targeting efficacy. Science 2019, 366, eaav1741. [Google Scholar] [CrossRef]
- La Sala, L.; Tagliabue, E.; De Candia, P.; Prattichizzo, F.; Ceriello, A. One-hour plasma glucose combined with skin autofluorescence identifies subjects with pre-diabetes: The DIAPASON study. BMJ Open Diabetes Res. Care 2020, 8, e001331. [Google Scholar] [CrossRef]
- De Candia, P.; Spinetti, G.; Specchia, C.; Sangalli, E.; La Sala, L.; Uccellatore, A.; Lupini, S.; Genovese, S.; Matarese, G.; Ceriello, A. A unique plasma microRNA profile defines type 2 diabetes progression. PLoS ONE 2017, 12, e0188980. [Google Scholar] [CrossRef]
- Mysore, R.; Zhou, Y.; Sädevirta, S.; Savolainen-Peltonen, H.; Haridas, P.N.; Soronen, J.; Leivonen, M.; Sarin, A.-P.; Fischer-Posovszky, P.; Wabitsch, M.; et al. MicroRNA-192* impairs adipocyte triglyceride storage. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2016, 1861, 342–351. [Google Scholar] [CrossRef]
- Belarbi, Y.; Mejhert, N.; Lorente-Cebrián, S.; Dahlman, I.; Arner, P.; Rydén, M.; Kulyté, A. MicroRNA-193b controls adiponectin production in human white adipose tissue. J. Clin. Endocrinol. Metab. 2015, 100, E1084–E1088. [Google Scholar] [CrossRef]
- Párrizas, M.; Brugnara, L.; Esteban, Y.; González-Franquesa, A.; Canivell, S.; Murillo, S.; Gordillo-Bastidas, E.; Cussó, R.; Cadefau, J.A.; García-Roves, P.M.; et al. Circulating miR-192 and miR-193b are markers of prediabetes and are modulated by an exercise intervention. J. Clin. Endocrinol. Metab. 2015, 100, E407–E415. [Google Scholar] [CrossRef]
- Jimenez-Lucena, R.; Alcala-Diaz, J.F.; Roncero-Ramos, I.; Lopez-Moreno, J.; Camargo, A.; Gomez-Delgado, F.; Quintana-Navarro, G.M.; Vals-Delgado, C.; Rodriguez-Cantalejo, F.; Luque, A.R.M.; et al. MiRNAs profile as biomarkers of nutritional therapy for the prevention of Type 2 diabetes mellitus: From the CORDIOPREV study. Clin. Nutr. 2020. [Google Scholar] [CrossRef]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Leccesi, L.; Nanni, G.; Pomp, A.; Castagneto, M.; Ghirlanda, G.; et al. Bariatric surgery versus conventional medical therapy for Type 2 diabetes. N. Engl. J. Med. 2012, 366, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, H.; Suzuki, H.I. Systems and synthetic microRNA biology: From biogenesis to disease pathogenesis. Int. J. Mol. Sci. 2019, 21, 132. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A.; Wicik, Z.; Owczarz, M.; Jonas, M.I.; Kotlarek, M.; Świerniak, M.; Lisik, W.; Jonas, M.; Noszczyk, B.; Puzianowska-Kuźnicka, M. NGS reveals molecular pathways affected by obesity and weight loss-related changes in miRNA levels in adipose tissue. Int. J. Mol. Sci. 2017, 19, 66. [Google Scholar] [CrossRef]
- Wu, Q.; Li, J.V.; Seyfried, F.; Le Roux, C.W.; Ashrafian, H.; Athanasiou, T.; Fenske, W.; Darzi, A.; Nicholson, J.K.; Holmes, E.; et al. Metabolic phenotype-microRNA data fusion analysis of the systemic consequences of Roux-en-Y gastric bypass surgery. Int. J. Obes. 2015, 39, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Hubal, M.J.; Nadler, E.P.; Ferrante, S.C.; Barberio, M.D.; Suh, J.-H.; Wang, J.; Dohm, G.L.; Pories, W.J.; Mietus-Snyder, M.; Freishtat, R.J. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity 2017, 25, 102–110. [Google Scholar] [CrossRef]
- Bae, Y.; Kim, Y.; Lee, H.; Kim, H.; Jeon, J.S.; Noh, H.; Han, D.C.; Ryu, S.; Kwon, S.H. Bariatric surgery alters microRNA content of circulating exosomes in patients with obesity. Obesity 2019, 27, 264–271. [Google Scholar] [CrossRef]
- Alkandari, A.; Ashrafian, H.; Sathyapalan, T.; Darzi, A.; Holmes, E.; Athanasiou, T.; Atkin, S.L.; Gooderham, N.J. Bariatric surgery modulates urinary levels of microRNAs involved in the regulation of renal function. Front. Endocrinol. 2019, 10, 319. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.-S.; Cheng, Y.-S.; Jia, B.-L.; Yu, G.; Yin, X.-Q.; Wang, Y. Expression of microRNA-448 and SIRT1 and prognosis of obese Type 2 diabetic mellitus patients after laparoscopic bariatric surgery. Cell. Physiol. Biochem. 2018, 45, 935–950. [Google Scholar] [CrossRef]
- Said, M.A.; Verweij, N.; Van Der Harst, P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK biobank study. JAMA Cardiol. 2018, 3, 693–702. [Google Scholar] [CrossRef]
- Oluwagbemigun, K.; Buyken, A.E.; Alexy, U.; Schmid, M.; Herder, C.; Nöthlings, U. Developmental trajectories of body mass index from childhood into late adolescence and subsequent late adolescence–young adulthood cardiometabolic risk markers. Cardiovasc. Diabetol. 2019, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.B.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef]
- Zeggini, E.; Weedon, M.N.; Lindgren, C.M.; Frayling, T.M.; Elliott, K.S.; Lango, H.; Timpson, N.J.; Perry, J.R.B.; Rayner, N.W.; Freathy, R.M.; et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007, 316, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M. Genome–wide association studies provide new insights into type 2 diabetes aetiology. Nat. Rev. Genet. 2007, 8, 657–662. [Google Scholar] [CrossRef]
- Kim, A.Y.; Park, Y.J.; Pan, X.; Shin, K.C.; Kwak, S.-H.; Bassas, A.F.; Sallam, R.M.; Park, K.S.; Alfadda, A.A.; Xu, A.; et al. Obesity-induced DNA hypermethylation of the adiponectin gene mediates insulin resistance. Nat. Commun. 2015, 6, 7585. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Castillo, D.; Queipo-Ortuño, M.I.; Sanchez-Alcoholado, L.; Ramos-Molina, B.; Alcaide-Torres, J.; Morcillo, S.; Ocaña-Wilhelmi, L.; Tinahones, F.; Queipo-Ortuño, M.I.; Cardona, F. Altered adipose tissue DNA methylation status in metabolic syndrome: Relationships between global DNA methylation and specific methylation at adipogenic, lipid metabolism and inflammatory candidate genes and metabolic variables. J. Clin. Med. 2019, 8, 87. [Google Scholar] [CrossRef]
- Dick, K.J.; Nelson, C.P.; Tsaprouni, L.; Sandling, J.K.; Aïssi, D.; Wahl, S.; Meduri, E.; Morange, P.-E.; Gagnon, F.; Grallert, H.; et al. DNA methylation and body-mass index: A genome-wide analysis. Lancet 2014, 383, 1990–1998. [Google Scholar] [CrossRef]
- Hidalgo, B.; Irvin, M.R.; Sha, J.; Zhi, D.; Aslibekyan, S.; Absher, D.; Tiwari, H.K.; Kabagambe, E.K.; Ordovas, J.M.; Arnett, D.K. Epigenome-wide association study of fasting measures of glucose, insulin, and HOMA-IR in the genetics of lipid lowering drugs and diet network study. Diabetes 2013, 63, 801–807. [Google Scholar] [CrossRef]
- Larsen, L.H.; Echwald, S.M.; Sørensen, T.I.A.; Andersen, T.; Wulff, B.S.; Pedersen, O. Prevalence of mutations and functional analyses of melanocortin 4 receptor variants identified among 750 men with Juvenile-onset obesity. J. Clin. Endocrinol. Metab. 2005, 90, 219–224. [Google Scholar] [CrossRef]
- Altshuler, D.; Hirschhorn, J.N.; Klannemark, M.; Lindgren, C.M.; Vohl, M.-C.; Nemesh, J.; Lane, C.R.; Schaffner, S.F.; Bolk, S.; Brewer, C.; et al. The common PPARgamma Pro12Ala polymorphism is associated with decreased risk of type 2 diabetes. Nat. Genet. 2000, 26, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Gloyn, A.L.; Weedon, M.N.; Owen, K.R.; Turner, M.J.; Knight, B.A.; Hitman, G.; Walker, M.; Levy, J.C.; Sampson, M.; Halford, S.; et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 2003, 52, 568–572. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.I. Genomics, type 2 diabetes, and obesity. N. Engl. J. Med. 2010, 363, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Voight, B.F.; Scott, L.J.; Steinthorsdottir, V.; Morris, A.P.; Dina, C.; Welch, R.P.; Zeggini, E.; Huth, C.; Aulchenko, Y.S.; The MAGIC Investigators; et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 2010, 42, 579–589. [Google Scholar] [CrossRef]
- Small, K.S.; Hedman, Å.K.; Grundberg, E.; Nica, A.C.; Thorleifsson, G.; Kong, A.; Thorsteindottir, U.; Shin, S.-Y.; Richards, H.B.; Soranzo, N.; et al. Identification of an imprinted master trans regulator at the KLF14 locus related to multiple metabolic phenotypes. Nat. Genet. 2011, 43, 561–564. [Google Scholar] [CrossRef]
- Nicoletti, C.F.; Cortes-Oliveira, C.; Noronha, N.Y.; Pinhel, M.A.S.; Dantas, W.S.; Jácome, A.; Marchini, J.S.; Gualano, B.; Crujeiras, A.B.; Nonino, C.B. DNA methylation pattern changes following a short-term hypocaloric diet in women with obesity. Eur. J. Clin. Nutr. 2020, 74, 1345–1353. [Google Scholar] [CrossRef]
- Sziráki, A.; Tyshkovskiy, A.; Gladyshev, V.N. Global remodeling of the mouse DNA methylome during aging and in response to calorie restriction. Aging Cell 2018, 17, e12738. [Google Scholar] [CrossRef]
- Wiebe, N.; Stenvinkel, P.; Tonelli, M. Associations of chronic inflammation, insulin resistance, and severe obesity with mortality, myocardial infarction, cancer, and chronic pulmonary disease. JAMA Netw. Open 2019, 2, e1910456. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the risk of heart failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Manson, J.E.; Colditz, G.A.; Stampfer, M.J.; Willett, W.C.; Rosner, B.; Monson, R.R.; Speizer, F.E.; Hennekens, C.H. A prospective study of obesity and risk of coronary heart disease in women. N. Engl. J. Med. 1990, 322, 882–889. [Google Scholar] [CrossRef]
- Calle, E.E.; Thun, M.J.; Petrelli, J.M.; Rodriguez, C.; Heath, C.W. Body-mass index and mortality in a prospective cohort of U.S. adults. N. Engl. J. Med. 1999, 341, 1097–1105. [Google Scholar] [CrossRef]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C.; Diabetes Control and Complications Trial Research Group. The Effect of Intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Bendor, C.D.; Bardugo, A.; Pinhas-Hamiel, O.; Afek, A.; Twig, G. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc. Diabetol. 2020, 19, 1–14. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, B.; Snetselaar, L.G.; Wallace, R.B.; Caan, B.J.; Rohan, T.E.; Neuhouser, M.L.; Shadyab, A.H.; Chlebowski, R.T.; Manson, J.E.; et al. Association of normal-weight central obesity with all-cause and cause-specific mortality among postmenopausal women. JAMA Netw. Open 2019, 2, e197337. [Google Scholar] [CrossRef] [PubMed]
- Eckel, N.; Meidtner, K.; Kalle-Uhlmann, T.; Stefan, N.; Schulze, M.B. Metabolically healthy obesity and cardiovascular events: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2015, 23, 956–966. [Google Scholar] [CrossRef]
- Lee, Y.-B.; Kim, D.H.; Kim, S.M.; Kim, N.H.; Choi, K.M.; Baik, S.H.; Park, Y.G.; Han, K.; Yoo, H.J. Hospitalization for heart failure incidence according to the transition in metabolic health and obesity status: A nationwide population-based study. Cardiovasc. Diabetol. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Jiang, L.; Shi, K.; Guo, Y.-K.; Ren, Y.; Li, Z.-L.; Xia, C.-C.; Li, L.; Liu, X.; Xie, L.-J.; Gao, Y.; et al. The additive effects of obesity on myocardial microcirculation in diabetic individuals: A cardiac magnetic resonance first-pass perfusion study. Cardiovasc. Diabetol. 2020, 19, 1–13. [Google Scholar] [CrossRef]
- Fumagalli, C.; Maurizi, N.; Day, S.M.; Ashley, E.A.; Michels, M.; Colan, S.D.; Jacoby, D.; Marchionni, N.; Vincent-Tompkins, J.; Ho, C.Y.; et al. Association of Obesity with Adverse Long-term Outcomes in Hypertrophic Cardiomyopathy. JAMA Cardiol. 2019, 5, 1–8. [Google Scholar] [CrossRef]
- Linssen, P.B.C.; Veugen, M.G.J.; Henry, R.M.A.; Van Der Kallen, C.J.H.; Kroon, A.A.; Schram, M.T.; Rocca, H.-P.B.-L.; Stehouwer, C.D.A. Associations of (pre)diabetes with right ventricular and atrial structure and function: The Maastricht Study. Cardiovasc. Diabetol. 2020, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Han, K.; Koh, E.S.; Kim, E.S.; Lee, M.-K.; Nam, G.E.; Kwon, H.-S. Weight change and mortality and cardiovascular outcomes in patients with new-onset diabetes mellitus: A nationwide cohort study. Cardiovasc. Diabetol. 2019, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pagidipati, N.J.; Zheng, Y.; Green, J.B.; McGuire, D.K.; Mentz, R.J.; Shah, S.; Aschner, P.; Delibasi, T.; Rodbard, H.W.; Westerhout, C.M.; et al. Association of obesity with cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease: Insights from TECOS. Am. Hear J. 2020, 219, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Stokes, M.; Elliott, A.; Munawar, D.A.; Khokhar, K.B.; Thiyagarajah, A.; Hendriks, J.; Linz, D.; Gallagher, C.; Kaye, D.; et al. Complex interaction of obesity, intentional weight loss and heart failure: A systematic review and meta-analysis. Heart 2019, 106, 58–68. [Google Scholar] [CrossRef]
- Tsatsoulis, A.; Paschou, S.A. Metabolically Healthy Obesity: Criteria, Epidemiology, Controversies, and Consequences. Curr. Obes. Rep. 2020, 9, 109–120. [Google Scholar] [CrossRef]
- Silveira, E.A.; Kliemann, N.; Noll, M.; Sarrafzadegan, N.; De Oliveira, C. Visceral obesity and incident cancer and cardiovascular disease: An integrative review of the epidemiological evidence. Obes. Rev. 2020. [Google Scholar] [CrossRef]
- Henriksson, H.; Henriksson, P.; Tynelius, P.; Ekstedt, M.; Berglind, D.; Labayen, I.; Ruiz, J.R.; Lavie, C.J.; Ortega, F.B. Cardiorespiratory fitness, muscular strength, and obesity in adolescence and later chronic disability due to cardiovascular disease: A cohort study of 1 million men. Eur. Heart J. 2020, 41, 1503–1510. [Google Scholar] [CrossRef]
- Merlotti, C.; Morabito, A.; Ceriani, V.; Pontiroli, A.E. Prevention of type 2 diabetes in obese at-risk subjects: A systematic review and meta-analysis. Acta Diabetol. 2014, 51, 853–863. [Google Scholar] [CrossRef]
- Merlotti, C.; Morabito, A.; Pontiroli, A.E. Prevention of type 2 diabetes; a systematic review and meta-analysis of different intervention strategies. Diabetes Obes. Metab. 2014, 16, 719–727. [Google Scholar] [CrossRef]
- Prattichizzo, F.; La Sala, L.; Rydén, L.; Marx, N.; Ferrini, M.; Valensi, P.; Ceriello, A. Glucose-lowering therapies in patients with type 2 diabetes and cardiovascular diseases. Eur. J. Prev. Cardiol. 2019, 26, 73–80. [Google Scholar] [CrossRef]
- Bays, H.E.; Weinstein, R.; Law, G.; Canovatchel, W. Canagliflozin: Effects in overweight and obese subjects without diabetes mellitus. Obesity 2013, 22, 1042–1049. [Google Scholar] [CrossRef]
- Lundkvist, P.; Pereira, M.J.; Katsogiannos, P.; Sjöström, C.D.; Johnsson, E.; Eriksson, J.W. Dapagliflozin once daily plus exenatide once weekly in obese adults without diabetes: S ustained reductions in body weight, glycaemia and blood pressure over 1 year. Diabetes Obes. Metab. 2017, 19, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Lundkvist, P.; Sjöström, C.D.; Amini, S.; Pereira, M.J.; Johnsson, E.; Eriksson, J.W. Dapagliflozin once-daily and exenatide once-weekly dual therapy: A 24-week randomized, placebo-controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes. Metab. 2016, 19, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, C.W.; Astrup, A.; Fujioka, K.; Greenway, F.L.; Lau, D.C.W.; Van Gaal, L.; Ortiz, R.M.V.; Wilding, J.; Skjøth, T.V.; Manning, L.S.; et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: A randomised, double-blind trial. Lancet 2017, 389, 1399–1409. [Google Scholar] [CrossRef]
- Guardado-Mendoza, R.; Salazar-López, S.S.; Álvarez-Canales, M.F.D.L.L.; Vázquez, D.F.; Martínez-López, Y.E.; Jiménez-Ceja, L.M.; Suarez, E.; Angulo-Romero, F.; Evia-Viscarra, M.L.; De Oca-Loyola, M.L.M.; et al. The combination of linagliptin, metformin and lifestyle modification to prevent type 2 diabetes (PRELLIM). A randomized clinical trial. Metabolism 2020, 104, 154054. [Google Scholar] [CrossRef]
- Pollack, R.M.; Donath, M.Y.; Leroith, D.; Leibowitz, G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care 2016, 39, S244–S252. [Google Scholar] [CrossRef]
- Everett, B.M.; Donath, M.Y.; Pradhan, A.D.; Thuren, T.; Pais, P.; Nicolau, J.C.; Glynn, R.J.; Libby, P.; Ridker, P.M. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J. Am. Coll. Cardiol. 2018, 71, 2392–2401. [Google Scholar] [CrossRef]
- Cameron, A.R.; Morrison, V.L.; Levin, D.; Mohan, M.; Forteath, C.; Beall, C.; McNeilly, A.D.; Balfour, D.J.K.; Savinko, T.; Wong, A.K.F.; et al. Anti-inflammatory effects of metformin irrespective of diabetes status. Circ. Res. 2016, 119, 652–665. [Google Scholar] [CrossRef]
- Borzouei, S.; Sheikh, V.; Ghasemi, M.; Zamani, A.; Telikani, Z.; Zareighane, Z.; Salehi, I.; Mozayanimonfared, A.; Amirzargar, M.A.; Alahgholi-Hajibehzad, M. Anti-inflammatory effect of combined sitagliptin and vitamin d3 on cytokines profile in patients with type 2 diabetes mellitus. J. Interf. Cytokine Res. 2019, 39, 293–301. [Google Scholar] [CrossRef]
- Foschi, D.; Sorrentino, L.; Tubazio, I.; Vecchio, C.; Vago, T.; Bevilacqua, M.; Rizzi, A.; Corsi, F. Ileal interposition coupled with duodenal diverted sleeve gastrectomy versus standard medical treatment in type 2 diabetes mellitus obese patients: Long-term results of a case–control study. Surg. Endosc. 2018, 33, 1553–1563. [Google Scholar] [CrossRef]
- Gloy, V.L.; Briel, M.; Bhatt, D.L.; Kashyap, S.R.; Schauer, P.R.; Mingrone, G.; Bucher, H.C.; Nordmann, A.J. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ 2013, 347, f5934. [Google Scholar] [CrossRef]
- Boido, A.; Ceriani, V.; Cetta, F.; Lombardi, F.; Pontiroli, A.E. Bariatric surgery and prevention of cardiovascular events and mortality in morbid obesity: Mechanisms of action and choice of surgery. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 437–443. [Google Scholar] [CrossRef]
- Merlotti, C.; Ceriani, V.; Morabito, A.; Pontiroli, A.E. Subcutaneous fat loss is greater than visceral fat loss with diet and exercise, weight-loss promoting drugs and bariatric surgery: A critical review and meta-analysis. Int. J. Obes. 2017, 41, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Camastra, S.; Muscelli, E.; Gastaldelli, A.; Holst, J.J.; Astiarraga, B.; Baldi, S.; Nannipieri, M.; Ciociaro, D.; Anselmino, M.; Mari, A.; et al. Long-term effects of bariatric surgery on meal disposal and beta-cell function in diabetic and nondiabetic patients. Diabetes 2013, 62, 3709–3717. [Google Scholar] [CrossRef][Green Version]
- Astiarraga, B.; Gastaldelli, A.; Muscelli, E.; Baldi, S.; Camastra, S.; Mari, A.; Papadia, F.; Camerini, G.; Adami, G.; Scopinaro, N.; et al. Biliopancreatic diversion in nonobese patients with type 2 diabetes: Impact and mechanisms. J. Clin. Endocrinol. Metab. 2013, 98, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Look Ahead Research Group. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016, 4, 913–921. [Google Scholar] [CrossRef]
- Nathan, D.M.; Barrett-Connor, E.; Crandall, J.; Edelstein, S.L.; Goldberg, R.; Horton, E.S.; Knowler, W.; Mather, K.J.; Orchard, T.J.; Pi-Sunyer, X.; et al. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar] [CrossRef]
- Gong, Q.; Zhang, P.; Wang, J.; Ma, J.; An, Y.; Chen, Y.; Zhang, B.; Feng, X.; Li, H.; Chen, X.; et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019, 7, 452–461. [Google Scholar] [CrossRef]
- Lindström, J.; Peltonen, M.; Eriksson, J.G.; Ilanne-Parikka, P.; Aunola, S.; Keinänen-Kiukaanniemi, S.; Uusitupa, M.; Tuomilehto, J.; Finnish Diabetes Prevention Study (DPS). Improved lifestyle and decreased diabetes risk over 13 years: Long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetology 2012, 56, 284–293. [Google Scholar] [CrossRef]
- Peradze, N.; Farr, O.M.; Perakakis, N.; Lázaro, I.; Sala-Vila, A.; Mantzoros, C.S. Short-term treatment with high dose liraglutide improves lipid and lipoprotein profile and changes hormonal mediators of lipid metabolism in obese patients with no overt type 2 diabetes mellitus: A randomized, placebo-controlled, cross-over, double-blind clinical trial. Cardiovasc. Diabetol. 2019, 18, 141. [Google Scholar] [CrossRef]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Sjöström, C.D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, Å.; Bengtsson, C.; Bergmark, G.; et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012, 307, 56–65. [Google Scholar] [CrossRef]
- Carlsson, L.M.S.; Sjöholm, K.; Karlsson, C.; Jacobson, P.; Andersson-Assarsson, J.C.; Svensson, P.-A.; Larsson, I.; Hjorth, S.; Neovius, M.; Taube, M.; et al. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: A post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2017, 5, 271–279. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Zakaria, A.S.; Mantegazza, E.; Morabito, A.; Saibene, A.; Mozzi, E.; Micheletto, G. Long-term mortality and incidence of cardiovascular diseases and type 2 diabetes in diabetic and nondiabetic obese patients undergoing gastric banding: A controlled study. Cardiovasc. Diabetol. 2016, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pontiroli, A.E.; Ceriani, V.; Sarro, G.; Micheletto, G.; Giovanelli, A.; Zakaria, A.S.; Fanchini, M.; Osio, C.; Nosari, I.; Veronelli, V.A.; et al. Incidence of diabetes mellitus, cardiovascular diseases, and cancer in patients undergoing malabsorptive surgery (biliopancreatic diversion and biliointestinal bypass) vs medical treatment. Obes. Surg. 2018, 29, 935–942. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Ceriani, V.; Tagliabue, E.; Zakaria, A.S.; Veronelli, A.; Folli, F.; Zanoni, I. Bariatric surgery, compared to medical treatment, reduces morbidity at all ages but does not reduce mortality in patients aged <43 years, especially if diabetes mellitus is present: A post hoc analysis of two retrospective cohort studies. Acta Diabetol. 2019, 57, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.F.; Thompson, T.J.; Thun, M.; Flanders, D.; Pamuk, E.; Byers, T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000, 23, 1499–1504. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Morabito, A. Long-term prevention of mortality in morbid obesity through bariatric surgery. A systematic review and meta-analysis of trials performed with gastric banding and gastric bypass. Ann. Surg. 2011, 253, 484–487. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Zakaria, A.S.; Fanchini, M.; Osio, C.; Tagliabue, E.; Micheletto, G.; Saibene, A.; Folli, F. A 23-year study of mortality and development of co-morbidities in patients with obesity undergoing bariatric surgery (laparoscopic gastric banding) in comparison with medical treatment of obesity. Cardiovasc. Diabetol. 2018, 17, 161. [Google Scholar] [CrossRef]
- Ceriani, V.; Sarro, G.; Micheletto, G.; Giovanelli, A.; Zakaria, A.S.; Fanchini, M.; Osio, C.; Nosari, I.; Morabito, A.; Pontiroli, A.E.; et al. Long-term mortality in obese subjects undergoing malabsorptive surgery (biliopancreatic diversion and biliointestinal bypass) versus medical treatment. Int. J. Obes. 2018, 43, 1147–1153. [Google Scholar] [CrossRef]
- Davidson, L.E.; Adams, T.D.; Kim, J.; Jones, J.L.; Hashibe, M.; Taylor, D.; Mehta, T.; McKinlay, R.; Simper, S.C.; Smith, S.C.; et al. Association of patient age at gastric bypass surgery with long-term all-cause and cause-specific mortality. JAMA Surg. 2016, 151, 631–637. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Ceriani, V.; Tagliabue, E. Compared with Controls, bariatric surgery prevents long-term mortality in Persons with obesity only above median age of cohorts: A systematic review and meta-analysis. Obes. Surg. 2020, 30, 2487–2496. [Google Scholar] [CrossRef]
- Blundell, J.E.; Dulloo, A.G.; Salvador, J.; Frühbeck, G. Beyond BMI—Phenotyping the obesities. Obes. Facts 2014, 7, 322–328. [Google Scholar] [CrossRef]
- Toplak, H.; Woodward, E.; Yumuk, V.; Oppert, J.-M.; Halford, J.C.; Frühbeck, G. 2014 EASO position statement on the use of anti-obesity drugs. Obes. Facts 2015, 8, 166–174. [Google Scholar] [CrossRef]
| Prevention of T2D | Prevention of CVD | Prevention of Mortality | Prevention of Complications of Diabetes | Other Preventions (Cancer, Kidney Disease) | |
|---|---|---|---|---|---|
| Diet | + | not shown | not yet shown | not yet shown | not yet shown |
| Drugs | ++ | liraglutide | not yet shown | not yet shown | not yet shown |
| Surgery | +++ | ++ | ++ | ++ | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Sala, L.; Pontiroli, A.E. Prevention of Diabetes and Cardiovascular Disease in Obesity. Int. J. Mol. Sci. 2020, 21, 8178. https://doi.org/10.3390/ijms21218178
La Sala L, Pontiroli AE. Prevention of Diabetes and Cardiovascular Disease in Obesity. International Journal of Molecular Sciences. 2020; 21(21):8178. https://doi.org/10.3390/ijms21218178
Chicago/Turabian StyleLa Sala, Lucia, and Antonio E. Pontiroli. 2020. "Prevention of Diabetes and Cardiovascular Disease in Obesity" International Journal of Molecular Sciences 21, no. 21: 8178. https://doi.org/10.3390/ijms21218178
APA StyleLa Sala, L., & Pontiroli, A. E. (2020). Prevention of Diabetes and Cardiovascular Disease in Obesity. International Journal of Molecular Sciences, 21(21), 8178. https://doi.org/10.3390/ijms21218178