Eucommia ulmoides Flavones as Potential Alternatives to Antibiotic Growth Promoters in a Low-Protein Diet Improve Growth Performance and Intestinal Health in Weaning Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Protocol and Dietary Treatment
2.2. Growth Performance and Diarrhea Index
2.3. Intestinal Morphology Evaluation
2.4. Serum Biochemical Parameters Determination
2.5. Microbiota Composition Analysis
2.6. Statistical Analysis
3. Results
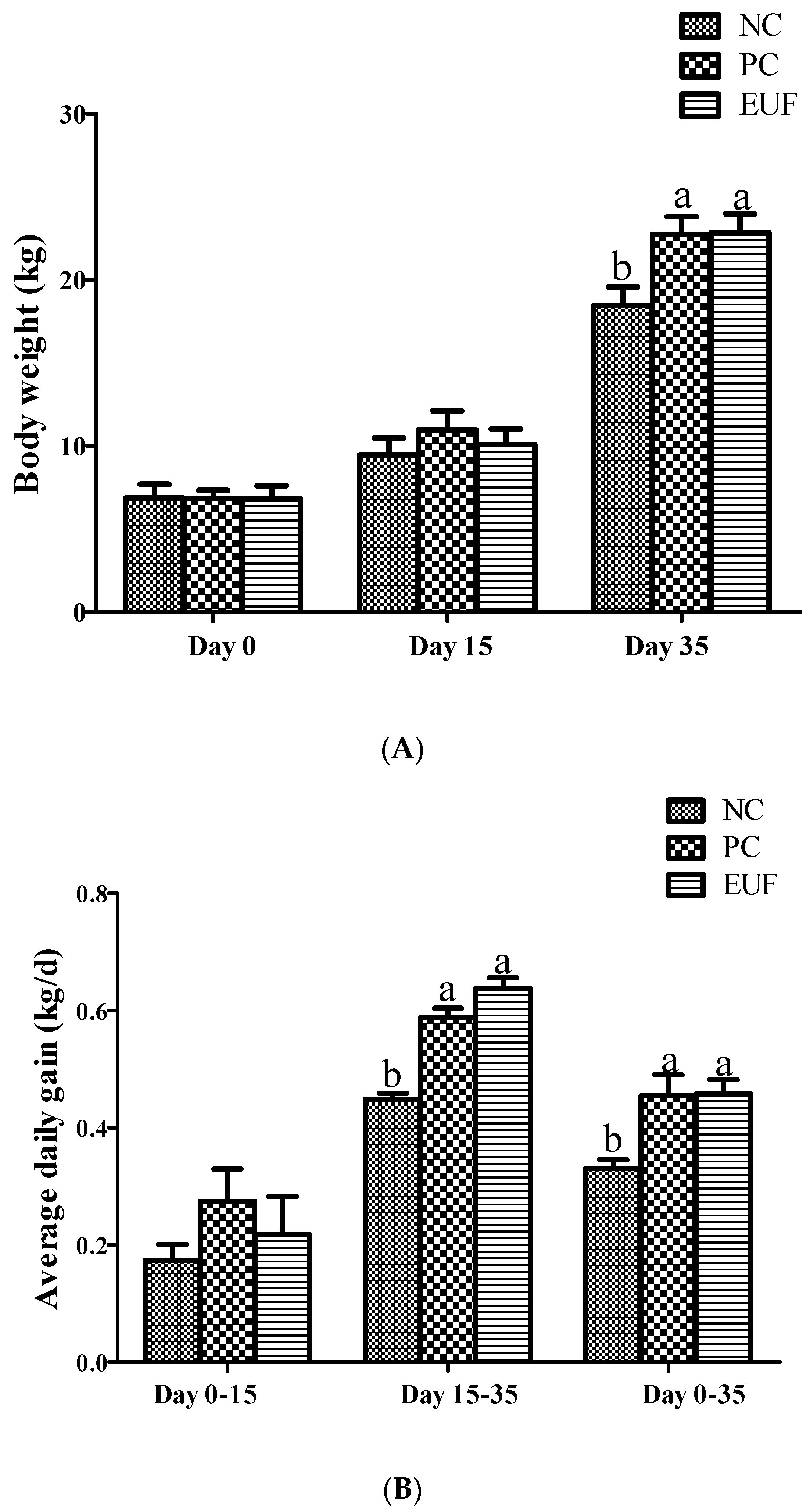
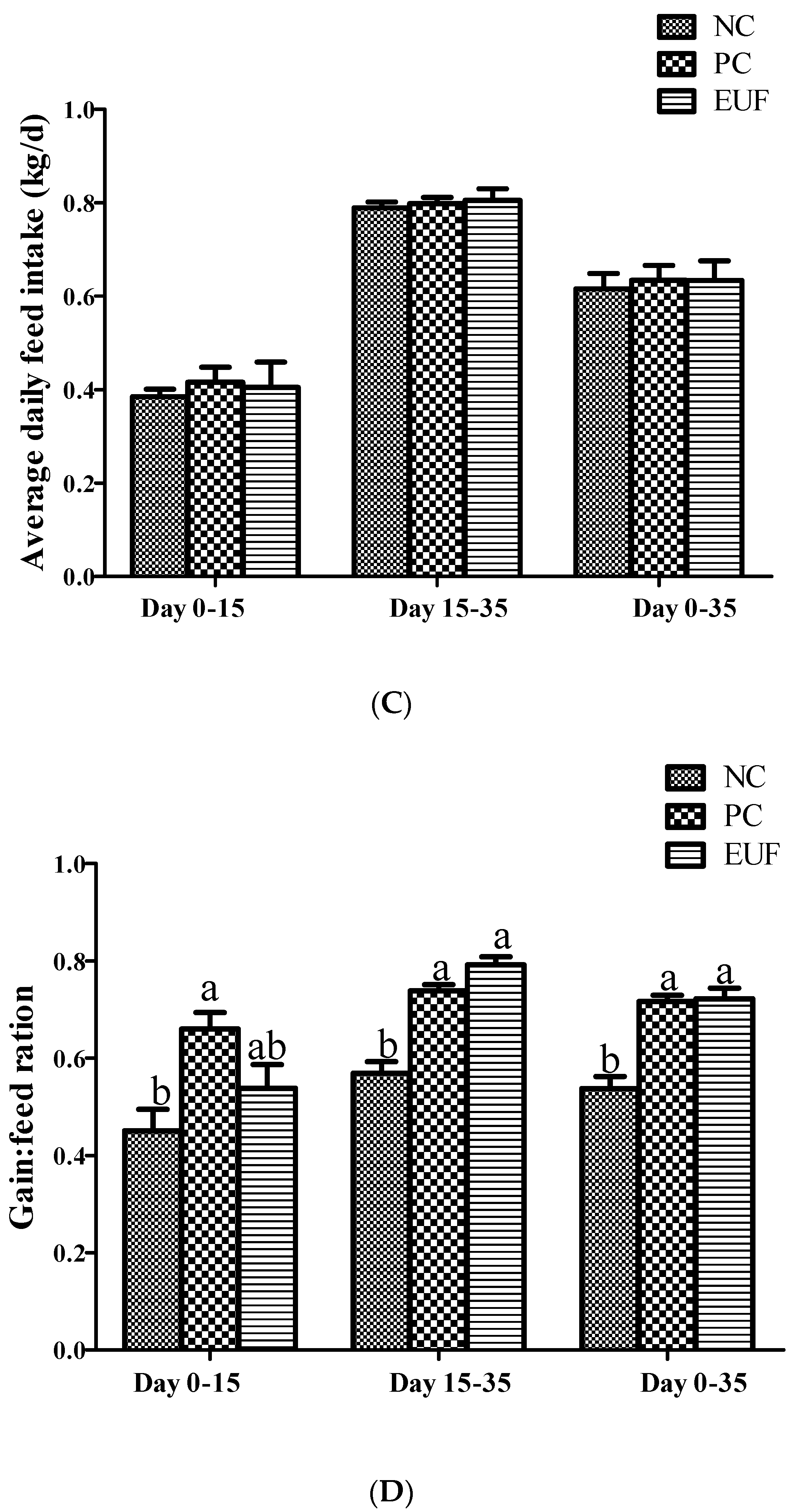
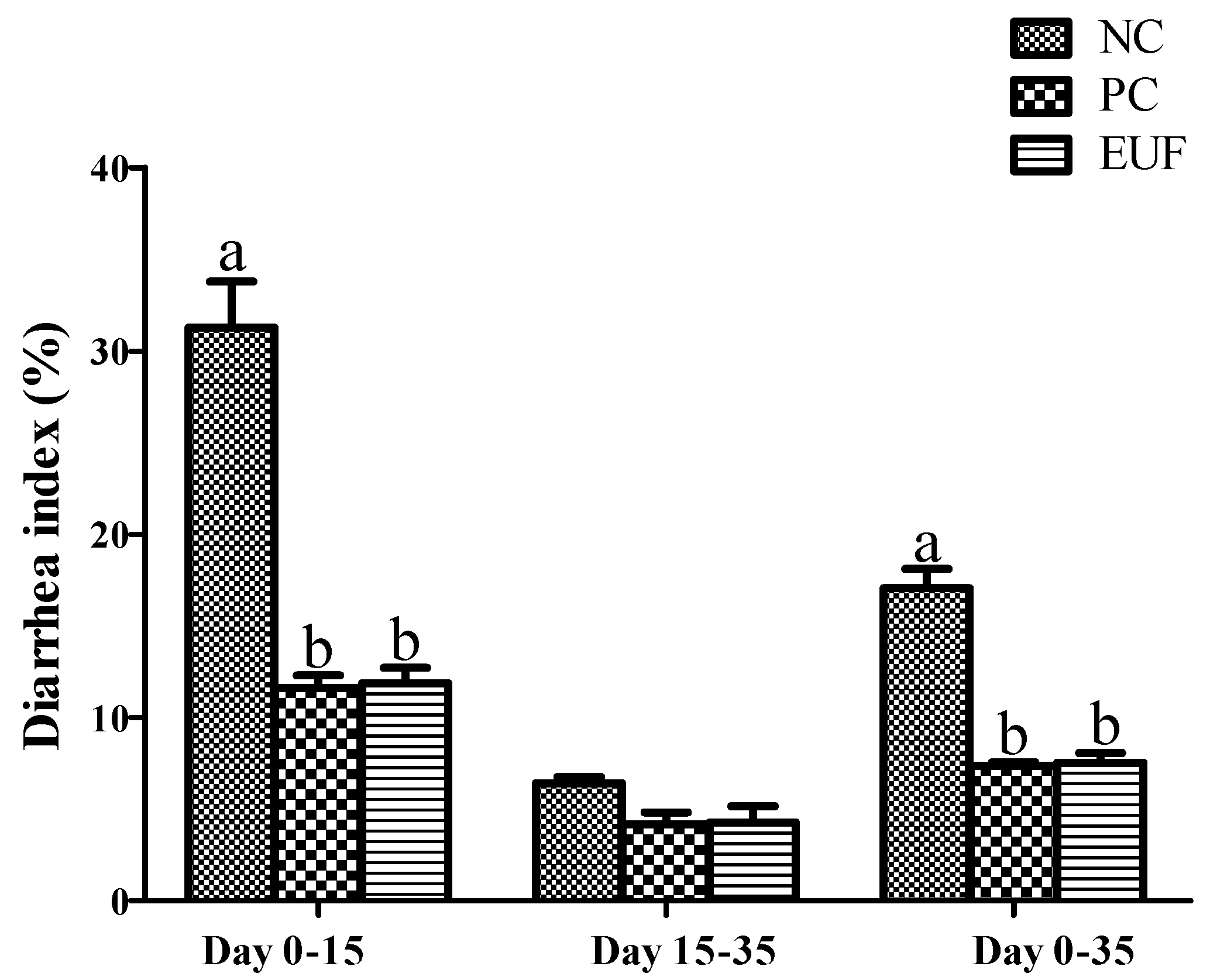
3.1. Growth Performance and Diarrhea Index
3.2. Serum Biochemical Parameters
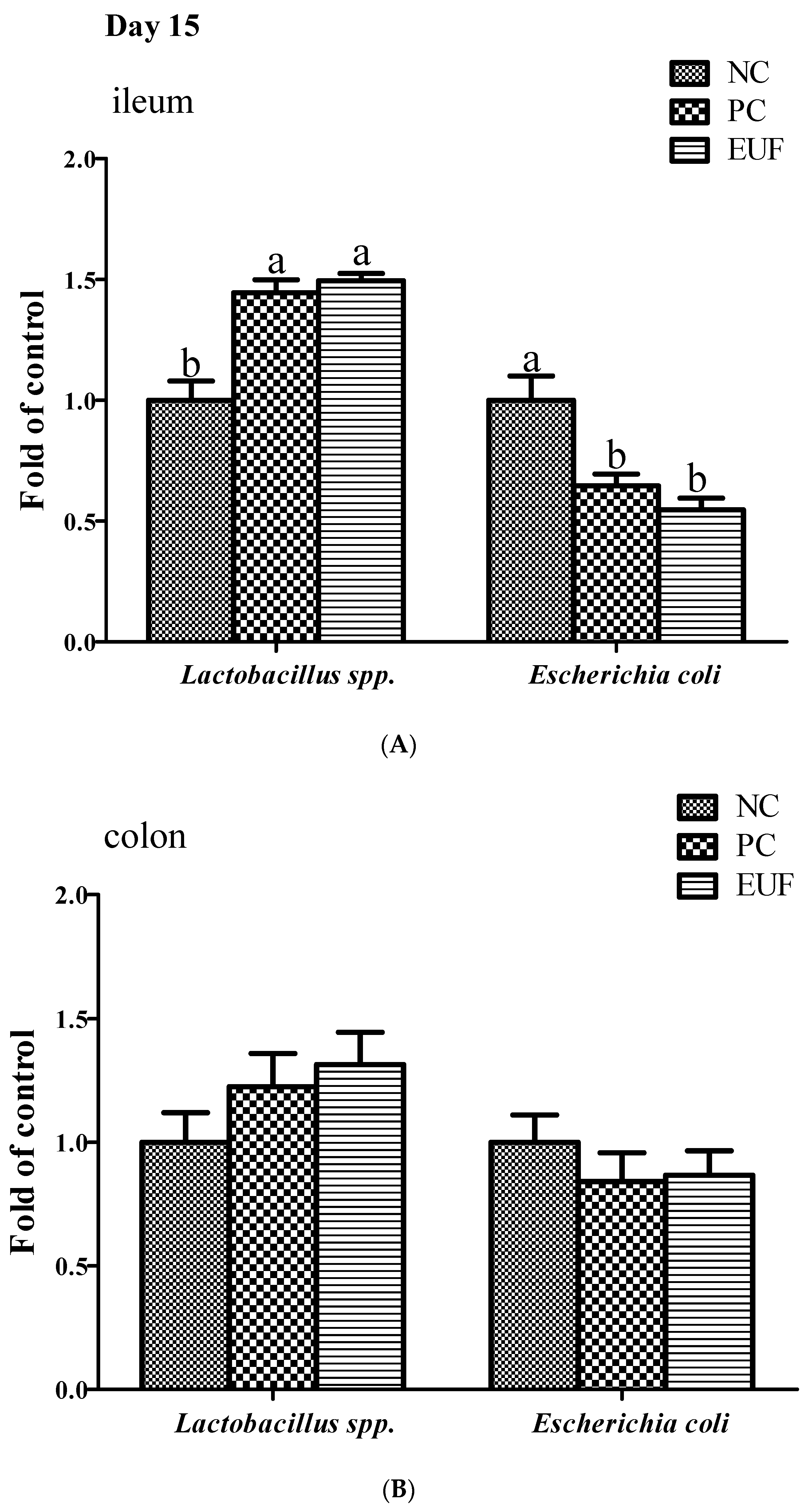
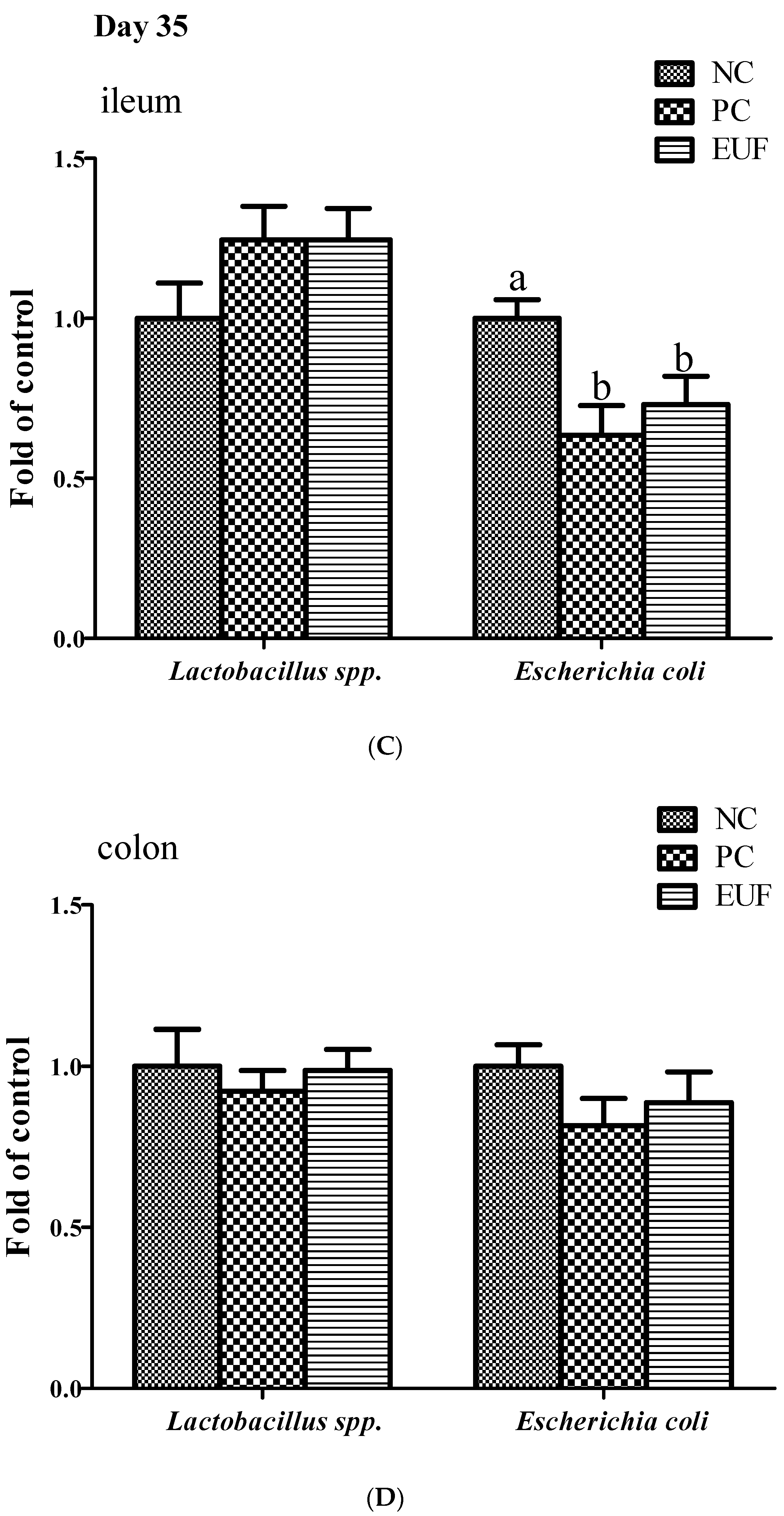
3.3. Microbiota Composition in Ileum and Colon
3.4. Intestinal Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aarestrup, F.M. Occurrence, selection and spread of resistance to antimicrobial agents used for growth promotion for food animals in Denmark. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2000, 108, 1–48. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Britti, M.S.; Bosi, P.; Oswald, I.; Mengheri, E. Alternatives to in-feed antibiotics in pigs: Evaluation of probiotics, zinc or organic acids as protective agents for the intestinal mucosa. A comparison of in vitro and in vivo results. Anim. Res. 2005, 54, 203–218. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Johnson, L.R. Expression of protooncogenes c-fos and c-myc in healing of gastric mucosal stress ulcers. Am. J. Physiol. Gastrointest. Liver Physiol. 1994, 266, G878–G886. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-H. Formulation and Evaluation of Multi-particulate Antibiotic Alternatives for Oral Delivery to Livestock Animals to Target Gut Pathogens. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2016. [Google Scholar]
- Li, S.; Zheng, J.; Deng, K.; Chen, L.; Zhao, X.L.; Jiang, X.; Fang, Z.; Che, L.; Xu, S.; Feng, B. Supplementation with organic acids showing different effects on growth performance, gut morphology, and microbiota of weaned pigs fed with highly or less digestible diets. J. Anim. Sci. 2018, 96, 3302–3318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, S.; Xu, Y.; Pan, L.; Wang, Q.; Wang, C.; Wu, J.; Wu, Y.; Han, Y.; Yun, C.; Piao, X. Mixed organic acids as antibiotic substitutes improve performance, serum immunity, intestinal morphology and microbiota for weaned piglets. Anim. Feed Sci. Technol. 2018, 235, 23–32. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.E.; Liu, G.; Oladele, O.A.; Rahu, N.; Tossou, M.; Yin, Y. Health-promoting properties of Eucommia ulmoides: A review. Evid. Based Complementary Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Su, Y.-Q.; Yang, F.-X.; Peng, J.-N.; Li, X.-H.; Sun, R.-C. Antioxidative activity of water extracts from leaf, male flower, raw cortex and fruit of Eucommia ulmoides Oliv. For. Prod. J. 2007, 57, 74–79. [Google Scholar]
- Park, S.A.; Choi, M.-S.; Jung, U.J.; Kim, M.-J.; Kim, D.J.; Park, H.-M.; Park, Y.B.; Lee, M.-K. Eucommia ulmoides Oliver leaf extract increases endogenous antioxidant activity in type 2 diabetic mice. J. Med. Food 2006, 9, 474–479. [Google Scholar] [CrossRef]
- Wlodarska, M.; Willing, B.; Keeney, K.; Menendez, A.; Bergstrom, K.; Gill, N.; Russell, S.; Vallance, B.; Finlay, B. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun. 2011, 79, 1536–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlett, J.; Shanmugam, K.; Münch, G.; Wu, M.J. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Hussain, T.; Tan, B.; Liu, Y.; Ji, P.; Yin, Y. The evaluation of antioxidant and anti-inflammatory effects of Eucommia ulmoides flavones using diquat-challenged piglet models. Oxidative Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Xiang, Q.; Wang, J.; Peng, J.; Wei, H. Oregano essential oil improves intestinal morphology and expression of tight junction proteins associated with modulation of selected intestinal bacteria and immune status in a pig model. BioMed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, D.; Yuan, D.; Tan, B.; Wang, J.; Liu, Y.; Tan, B. The role of Nrf2 signaling pathway in eucommia ulmoides flavones regulating oxidative stress in the intestine of piglets. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, Y.; Zeng, X.; Zhang, T.; Li, P.; Yao, B.; Wang, L.; Qiao, S.; Zeng, X. Effect of antibiotic-free, low-protein diets with specific amino acid compositions on growth and intestinal flora in weaned pigs. Food Funct. 2020, 11, 493–507. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Zhu, X.; Han, H.; Ren, W.; Chen, S.; Bin, P.; Liu, G.; Huang, X.; Fang, R. Effects of long-term protein restriction on meat quality, muscle amino acids, and amino acid transporters in pigs. J. Agric. Food Chem. 2017, 65, 9297–9304. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, L.; Tan, B.; Li, G.; Huang, B.; Xiong, X.; Li, F.; Kong, X.; Liu, G.; Yin, Y. Developmental changes in intercellular junctions and Kv channels in the intestine of piglets during the suckling and post-weaning periods. J. Anim. Sci. Biotechnol. 2016, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.; Liu, S.; Chen, S.; Zhang, F.; Li, N.; Yin, J.; Peng, Y.; Wu, L.; Liu, G.; Yin, Y. Dietary L-glutamine supplementation increases Pasteurella multocida burden and the expression of its major virulence factors in mice. Amino Acids 2013, 45, 947–955. [Google Scholar] [CrossRef]
- Castillo, M.; Martín-Orúe, S.M.; Manzanilla, E.G.; Badiola, I.; Martín, M.; Gasa, J. Quantification of total bacteria, enterobacteria and lactobacilli populations in pig digesta by real-time PCR. Vet. Microbiol. 2006, 114, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.-F.; Yin, F.-G.; Liu, H.-J.; Yin, Y.-L.; Xing, F.-F.; He, Q.-H.; Li, T.-J.; Huang, R.-L.; Zhang, P. Changes of physiological and biochemical parameters and viscera indexes in early-weaned piglets. Acta Lab. Anim. Sci. Sin. 2006, 4. [Google Scholar] [CrossRef]
- Ding, H.; Cao, A.; Li, H.; Zhao, Y.; Feng, J. Effects of Eucommia ulmoides leaf extracts on growth performance, antioxidant capacity and intestinal function in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2020. [Google Scholar] [CrossRef]
- Pieper, R.; Tudela, C.V.; Taciak, M.; Bindelle, J.; Pérez, J.F.; Zentek, J. Health relevance of intestinal protein fermentation in young pigs. Anim. Health Res. Rev. 2016, 17, 137–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wu, C.; Feng, J. Effect of dietary antibacterial peptide and zinc-methionine on performance and serum biochemical parameters in piglets. Czech J. Anim. Sci. 2011, 56, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Obaleye, J.; Akinremi, C.; Balogun, E.; Adebayo, J. Toxicological studies and antimicrobial properties of some Iron (III) complexes of Ciprofloxacin. Afr. J. Biotechnol. 2007, 6. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, T.; Stoll, M.; Blomberg, L.A.; Caperna, T. Regulation of fetuin A gene expression in the neonatal pig liver 1. Animal 2018, 12, 288–294. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Zhang, T.; Yang, Y.; Yang, F.; Gao, X. Effects of dietary copper on nutrient digestibility, tissular copper deposition and fur quality of growing-furring mink (Mustela vison). Biol. Trace Elem. Res. 2014, 158, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Amacher, D.E. A toxicologist’s guide to biomarkers of hepatic response. Hum. Exp. Toxicol. 2002, 21, 253–262. [Google Scholar] [CrossRef]
- Bussy, F.; Salmon, H.; Delaval, J.; Berri, M.; Pi, N.C. Immunomodulating effect of a seaweed extract from Ulva armoricana in pig: Specific IgG and total IgA in colostrum, milk, and blood. Vet. Anim. Sci. 2019, 7, 100051. [Google Scholar] [CrossRef]
- Ohsaki, A.; Venturelli, N.; Buccigrosso, T.M.; Osganian, S.K.; Lee, J.; Blumberg, R.S.; Oyoshi, M.K. Maternal IgG immune complexes induce food allergen–specific tolerance in offspring. J. Exp. Med. 2018, 215, 91–113. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Yuan, D.; Tan, B.; Murtaza, G.; Rahu, N.; Kalhoro, M.S.; Kalhoro, D.H.; Yin, Y. Eucommia ulmoides flavones (EUF) abrogated enterocyte damage induced by LPS involved in NF-κB signaling pathway. Toxicol. Vitr. 2020, 62, 104674. [Google Scholar] [CrossRef]
- Wang, S.; Blachier, F.; Zhao, F.; Yin, Y. Intestinal microbiota: Development, metabolism and functions. J. Food Agric. Environ. 2011, 9, 121–129. [Google Scholar]
- Walsh, M.; Sholly, D.; Hinson, R.; Saddoris, K.; Sutton, A.; Radcliffe, J.; Odgaard, R.; Murphy, J.; Richert, B. Effects of water and diet acidification with and without antibiotics on weanling pig growth and microbial shedding. J. Anim. Sci. 2007, 85, 1799–1808. [Google Scholar] [CrossRef]
- Yeo, S.; Lee, S.; Park, H.; Shin, H.; Holzapfel, W.; Huh, C.S. Development of putative probiotics as feed additives: Validation in a porcine-specific gastrointestinal tract model. Appl. Microbiol. Biotechnol. 2016, 100, 10043–10054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thu, T.; Loh, T.C.; Foo, H.; Yaakub, H.; Bejo, M. Effects of liquid metabolite combinations produced by Lactobacillus plantarum on growth performance, faeces characteristics, intestinal morphology and diarrhoea incidence in postweaning piglets. Trop. Anim. Health Prod. 2011, 43, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Han, M.; Zhang, G.; Qiao, S.; Li, D.; Ma, X. The signal pathway of antibiotic alternatives on intestinal microbiota and immune function. Curr. Protein Pept. Sci. 2016, 17, 785–796. [Google Scholar] [CrossRef]
- Lai, P.; Roy, J. Antimicrobial and chemopreventive properties of herbs and spices. Curr. Med. Chem. 2004, 11, 1451–1460. [Google Scholar] [CrossRef]
- Cho, I.; Yamanishi, S.; Cox, L.; Methé, B.A.; Zavadil, J.; Li, K.; Gao, Z.; Mahana, D.; Raju, K.; Teitler, I. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012, 488, 621–626. [Google Scholar] [CrossRef]
- Farhadi, A.; Banan, A.; Fields, J.; Keshavarzian, A. Intestinal barrier: An interface between health and disease. J. Gastroenterol. Hepatol. 2003, 18, 479–497. [Google Scholar] [CrossRef]
- Lillehoj, H.S.; Kim, D.K.; Bravo, D.M.; Lee, S.H. Effects of dietary plant-derived phytonutrients on the genome-wide profiles and coccidiosis resistance in the broiler chickens. In Proceedings of the International Symposium on Animal Genomics for Animal Health (AGAH 2010), Paris, France, 31 May–2 June 2010; p. S34. [Google Scholar]
- Montagne, L.; Boudry, G.; Favier, C.; Le Huërou-Luron, I.; Lalles, J.-P.; Seve, B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 2007, 97, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Opapeju, F.; Pluske, J.; Kim, J.; Hampson, D.; Nyachoti, C. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
| Ingredients, % | Phase 1 | Phase 2 |
|---|---|---|
| Corn | 57 | 60 |
| Expended maize | 5 | 5 |
| Soybean meal (43% CP) | 22 | 22 |
| Rice bran meal | 5 | 5 |
| Broken rice | 5 | 5 |
| Fish meal | 2 | / |
| Sucrose | 1 | / |
| Calcium lactate | 0.3 | 0.3 |
| Calcium hydrogen phosphate | 1 | 1 |
| Limestone powder | 0.1 | 0.1 |
| Premix 1 | 1 | 1 |
| 98% lysine | 0.4 | 0.4 |
| Threonine | 0.1 | 0.1 |
| Chemical composition 2 | ||
| Dry matter | 87.86 | 88.58 |
| Crude protein | 17.4 | 16.3 |
| Calculated DE, kcal/kg | 3466 | 3420 |
| Lysine | 0.79 | 0.71 |
| Calcium | 0.68 | 0.63 |
| Total phosphorus | 0.53 | 0.46 |
| Items | NC | PC | EUF | Pooled-SEM | p-Value |
|---|---|---|---|---|---|
| Day 15 | |||||
| Total protein, g/L | 48.29 b | 50.21 b | 59.82 a | 2.30 | 0.006 |
| Albumin, g/L | 32.15 | 35.94 | 37.45 | 3.04 | 0.552 |
| Blood urea nitrogen, mmol/L | 4.21 | 4.53 | 4.78 | 0.16 | 0.075 |
| Glucose, mmol/L | 6.78 | 6.49 | 6.53 | 0.20 | 0.629 |
| Alanine transaminase, U/L | 32.38 b | 38.59 ab | 47.37 a | 2.73 | 0.006 |
| Aspartate aminotransferase, U/L | 30.24 | 34.27 | 29.57 | 3.18 | 0.608 |
| Alkaline phosphatase, U/L | 214.24 ab | 199.27 b | 237.57 a | 9.20 | 0.029 |
| Immunoglobulin G, g/L | 1.36 b | 1.76 ab | 1.97 a | 0.15 | 0.036 |
| Immunoglobulin M, g/L | 0.36 | 0.45 | 0.48 | 0.04 | 0.121 |
| Day 35 | |||||
| Total protein, g/L | 45.26 b | 49.67 ab | 52.98 a | 1.95 | 0.046 |
| Albumin, g/L | 28.56 | 29.79 | 32.97 | 3.21 | 0.631 |
| Blood urea nitrogen, mmol/L | 4.39 | 4.06 | 3.78 | 0.18 | 0.083 |
| Glucose, mmol/L | 6.87 | 6.79 | 6.94 | 0.16 | 0.838 |
| Alanine transaminase, U/L | 35.19 | 39.46 | 41.38 | 2.81 | 0.306 |
| Aspartate aminotransferase, U/L | 28.49 | 31.05 | 29.87 | 1.84 | 0.642 |
| Alkaline phosphatase, U/L | 197.56 | 214.56 | 227.69 | 9.33 | 0.104 |
| Immunoglobulin G, g/L | 1.27 | 1.58 | 1.87 | 0.18 | 0.088 |
| Immunoglobulin M, g/L | 0.29 | 0.32 | 0.28 | 0.03 | 0.710 |
| Log10 cfu/g | NC | PC | EUF | Pooled-SEM | p-Value | |
|---|---|---|---|---|---|---|
| Day 15 | ||||||
| Lactic acid bacteria | Ileum | 6.59 b | 7.35 ab | 7.49 a | 0.13 | <0.001 |
| Colon | 7.28 | 7.18 | 7.29 | 0.21 | 0.933 | |
| Coliform bacteria | Ileum | 5.59 a | 4.58 b | 4.76 b | 0.19 | 0.003 |
| Colon | 4.54 | 4.28 | 4.32 | 0.22 | 0.687 | |
| Day 35 | ||||||
| Lactic acid bacteria | Ileum | 6.27 | 5.98 | 6.18 | 0.12 | 0.240 |
| Colon | 7.15 | 6.87 | 7.09 | 0.25 | 0.721 | |
| Coliform bacteria | Ileum | 4.79 a | 4.15 b | 4.06 b | 0.15 | 0.001 |
| Colon | 4.35 | 4.28 | 4.21 | 0.13 | 0.751 | |
| Items | NC | PC | EUF | Pooled-SEM | p-Value | |
|---|---|---|---|---|---|---|
| Day 15 | ||||||
| Villus height, μm | Jejunum | 246.47 b | 274.32 a | 276.35 a | 7.80 | 0.023 |
| Ileum | 197.89 b | 241.32 a | 242.86 a | 12.79 | 0.035 | |
| Crypt depth, μm | Jejunum | 105.62 | 89.57 | 102.45 | 7.85 | 0.348 |
| Ileum | 86.57 | 74.54 | 78.54 | 7.22 | 0.551 | |
| Villus height: Crypt depth | Jejunum | 2.33 b | 3.06 a | 2.70 ab | 0.16 | 0.020 |
| Ileum | 2.29 b | 3.24 a | 3.09 ab | 0.24 | 0.028 | |
| Day 35 | ||||||
| Villus height, μm | Jejunum | 197.23 | 226.89 | 234.35 | 12.37 | 0.151 |
| Ileum | 168.54 | 187.43 | 198.48 | 14.91 | 0.362 | |
| Crypt depth, μm | Jejunum | 112.54 | 105.84 | 104.58 | 7.94 | 0.777 |
| Ileum | 98.57 | 99.45 | 97.58 | 4.13 | 0.951 | |
| Villus height: Crypt depth | Jejunum | 1.75 b | 2.14 ab | 2.24 a | 0.11 | 0.017 |
| Ileum | 1.71 | 1.89 | 2.03 | 0.20 | 0.542 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, D.; Wang, J.; Xiao, D.; Li, J.; Liu, Y.; Tan, B.; Yin, Y. Eucommia ulmoides Flavones as Potential Alternatives to Antibiotic Growth Promoters in a Low-Protein Diet Improve Growth Performance and Intestinal Health in Weaning Piglets. Animals 2020, 10, 1998. https://doi.org/10.3390/ani10111998
Yuan D, Wang J, Xiao D, Li J, Liu Y, Tan B, Yin Y. Eucommia ulmoides Flavones as Potential Alternatives to Antibiotic Growth Promoters in a Low-Protein Diet Improve Growth Performance and Intestinal Health in Weaning Piglets. Animals. 2020; 10(11):1998. https://doi.org/10.3390/ani10111998
Chicago/Turabian StyleYuan, Daixiu, Jing Wang, Dingfu Xiao, Jiefeng Li, Yanhong Liu, Bie Tan, and Yulong Yin. 2020. "Eucommia ulmoides Flavones as Potential Alternatives to Antibiotic Growth Promoters in a Low-Protein Diet Improve Growth Performance and Intestinal Health in Weaning Piglets" Animals 10, no. 11: 1998. https://doi.org/10.3390/ani10111998
APA StyleYuan, D., Wang, J., Xiao, D., Li, J., Liu, Y., Tan, B., & Yin, Y. (2020). Eucommia ulmoides Flavones as Potential Alternatives to Antibiotic Growth Promoters in a Low-Protein Diet Improve Growth Performance and Intestinal Health in Weaning Piglets. Animals, 10(11), 1998. https://doi.org/10.3390/ani10111998






